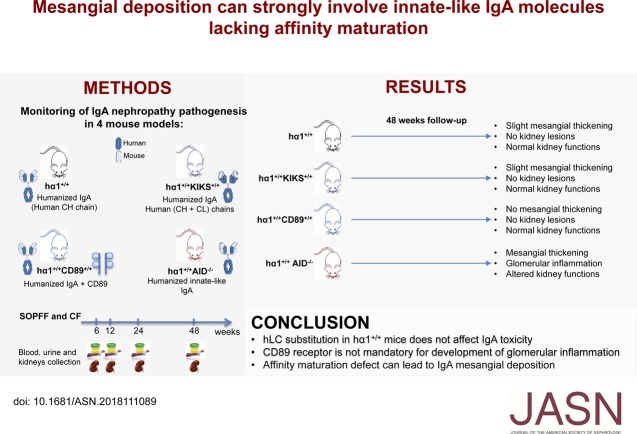
Significance Statement
IgA nephropathy (IgAN) is characterized by IgA glomerular mesangial deposition, but its pathogenesis remains unclear. Using humanized transgenic mouse models, the authors explored whether a hypogalactosylated hinge region (found in most mesangial IgA1 in human IgAN) is required for IgA deposition, demonstrating that hinge hypoglycosylation was not mandatory for deposition. To investigate whether low-affinity IgA produced by innate-like B cells might also yield mesangial deposits, they compared mice able to produce high-affinity mature IgA antibodies with mice lacking affinity maturation. They found that the low-affinity IgA can deposit in the mesangium and activate complement, that it is especially prone to induce glomerular cell thickening, and that it can initiate nephrotoxicity. These findings offer a new perspective regarding glomerular IgA deposits involving innate-like antibody responses.
Keywords: IgA nephropathy, IgA, Affinity, AID, Mouse model
Visual Abstract
Abstract
Background
IgA nephropathy (IgAN) often follows infections and features IgA mesangial deposition. Polymeric IgA deposits in the mesangium seem to have varied pathogenic potential, but understanding their pathogenicity remains a challenge. Most mesangial IgA1 in human IgAN has a hypogalactosylated hinge region, but it is unclear whether this is required for IgA deposition. Another important question is the role of adaptive IgA responses and high-affinity mature IgA antibodies and whether low-affinity IgA produced by innate-like B cells might also yield mesangial deposits.
Methods
To explore the effects of specific qualitative variations in IgA and whether altered affinity maturation can influence IgA mesangial deposition and activate complement, we used several transgenic human IgA1-producing models with IgA deposition, including one lacking the DNA-editing enzyme activation-induced cytidine deaminase (AID), which is required in affinity maturation. Also, to explore the potential role of the IgA receptor CD89 in glomerular inflammation, we used a model that expresses CD89 in a pattern observed in humans.
Results
We found that human IgA induced glomerular damage independent of CD89. When comparing mice able to produce high-affinity IgA antibodies with mice lacking AID-enabled Ig affinity maturation, we found that IgA deposition and complement activation significantly increased and led to IgAN pathogenesis, although without significant proteinuria or hematuria. We also observed that hinge hypoglycosylation was not mandatory for IgA deposition.
Conclusions
In a mouse model of IgAN, compared with high-affinity IgA, low-affinity innate-like IgA, formed in the absence of normal antigen-driven maturation, was more readily involved in IgA glomerular deposition with pathogenic effects.
IgA nephropathy (IgAN) is characterized by IgA mesangial deposition most often associated with complement deposition and extracellular matrix production. It is often accompanied by mesangial proliferative GN and stands as the most common pattern of primary GN in all countries where renal biopsy is widely practiced.1 It is implicit that not all IgA deposits possess equivalent pathogenic potential, because the glomerular landscape observed in different patients can vary along a broad spectrum from minimal or no lesions to severe sclerosis.2,3 The evolution of IgAN is highly variable; 20% of patients can develop kidney failure within 20 years, but the prognosis can also be excellent in cases showing discrete microscopic hematuria.4 Clinically silent IgA mesangial deposits, eventually associated with complement, can even be an incidental finding in 16% of the general population.3 IgAN may thus represent the emergent part of the IgA deposition iceberg, with a heterogeneity underscoring the multifactorial pathogenesis of the disease.
Accordingly, various inconstant abnormalities of deposited IgA have been reported in IgAN: antigen (Ag) or endogenous protein coprecipitation, negative electrostatic charges, and altered patterns of IgA glycosylation with hinge region hypogalactosylation and increased exposure of N-acetylglycosamine resulting in production of antihypogalactosylated hinge autoantibodies.5 Many experimental models have been developed, but none of them perfectly reproduces the disease.6 One study suggests that IgA deposits may be related to abnormal regulation of IgA synthesis and consequently, their affinity maturation.7 IgA affinity maturation is a process involving modification of the Ig V-region gene via somatic hypermutation (SHM), which takes place in the germinal center.8 We previously studied both polyclonal and monoclonal IgA in knock-in human constant α1 mice (hα1+/+ mice) lacking mIgM expression but producing human IgA1.9 We found that, in this model, deposition may involve both IgA primary structure features and a “variable domain disease.” Consequently, various changes during immune responses might modulate IgA deposition.10 We thus explored whether qualitative IgA variations related to IgA receptor expression and altered affinity maturation can influence IgA mesangial deposition and activate complement. To address these questions, we use different human IgA1-producing mouse models: hα1+/+, hα1+/+KIKS+/+, hα1+/+CD89+/+, and hα1+/+AID−/− mice (Table 1). We found that V regions with quasi-germline sequences and thus, lacking affinity maturation were more specifically involved in IgA deposition and glomerular disease.
Table 1.
Characteristics of used animal models
| Model | Genetic Feature | Key Features of the Model |
|---|---|---|
| hα1+/+ | Knock-in of the human Cα1 constant region coding gene9 | Production of human IgA1 heavy chain associated with mouse light chains |
| hα1+/+ KS+/+ | Knock-in of the human Cα1 constant region coding gene9 and knock-in of a functional human light chain coding gene11 | Production of IgA1 with fully human constant domains (heavy and light chains) |
| hα1+/+ CD89+/+ | Knock-in of the human Cα1 constant region coding gene9 and knock-in of the human IgA receptor (CD89 or FcαRI) coding gene12 | Production of human IgA1 and its receptor CD89 (FcαRI) |
| hα1+/+ AID−/− | Knock-in of the human Cα1 constant region coding gene9 and knock out of the AID coding gene13 | Production of low-affinity human IgA1 |
KS+/+, knock-in κ-switch; AID−/−, activation-induced cytidine deaminase.
Methods
Mouse Models
Animal experiments followed European regulations applied in France by decree no. 2013–118, February 1, 2013. All mouse models were housed in the conventional animal facility (CF) or specific and opportunistic pathogen-free facility (SOPFF) of Limoges University (agreement no. B87–085–05). The hα1+/+ mice9 were bred with the knock-in κ-switch (KS+/+) mice,11 the CD89+/+ mice (a gift from M.V.E.),12 and the AID−/− mice (a gift from T. Honjo).13 The obtained double mutants (Table 1) were compared with sex- and age-matched wild-type animals from a similar genetic background.
Kidney Function Parameters
Protein, albumin, and creatinine levels were measured in urine samples collected from mice using a Cobas 8000–0-1 analyzer. Plasma creatinine levels were measured by enzyme creatinine assay on a Konelab analyzer (Thermofisher). For hematuria, red cells were counted under light microscopy (Zeiss) in fresh urine mounted on Glasstic slides (KOVA).
In Vivo Experiments
To evaluate affinity of plasma hIgA1, 8-week-old hα1+/+ and hα1+/+AID−/− mice were immunized intraperitoneally with 50 μg ovalbumin (Sigma-Aldrich) and Addavax adjuvant (Invivogen) at days 0 and 14. Total and ovalbumin-specific hIgA were quantified in serum by ELISA.
Kidney Processing
Mice were euthanized by CO2, and kidneys were collected immediately. One kidney was snap frozen in isopentane using SNAPFROST (Excilone) and stored at −80°C. The other kidney was fixed in 4% paraformaldehyde for 24 hours before being stained for hematoxylin and eosin and Masson trichrome by the Anatomy-Histopathology Department, Hôpital Dupuytren, CHU Limoges. Blind histopathologic analyses were performed to identify kidney lesions. Glomerular cellularity was quantified by counting nuclei in each glomerulus.
Immunofluorescence on Kidney Sections
Acetone-fixed 8-μm kidney sections were incubated for 1 hour at room temperature with goat F(ab)′2 anti-human IgA (2052–01; Southern Biotech) or anti-murine C3 (ab11862; Abcam) and rabbit anti-podocin (P0372; Sigma-Aldrich) coupled to Alexa Fluor 568 (Invitrogen). hIgA was revealed using anti-goat Alexa Fluor 488 secondary antibody (A21467; Invitrogen), and mC3 was revealed using goat anti-rat Alexa Fluor 647 secondary antibody (A21247; Molecular Probes). Nuclei were stained with DAPI before mounting with Mowiol 4–88 solution (Sigma-Aldrich). Kidney sections were observed under an epifluorescence microscope (Nikon), and signals were quantified using Volocity software (Perkin Elmer). hIgA deposits represent the mean fluorescence intensity of hIgA in glomeruli × hIgA area normalized to the podocin area.
Plasma Igs
Plasma levels of different Ig isotypes and hIgA-CD89 complexes were quantified by ELISA. Briefly, 96-well plates (Nunc) were coated overnight with rabbit anti-human IgA (A0092; Dako), anti-human CD89 (555686; BD Biosciences), anti-mouse IgM, anti-mouse IgG, or anti-mouse IgA (1020–01, 1030–01, or 1040–01, respectively; Southern Biotech). Diluted plasma samples were incubated for 2 hours at 37°C. After washing, wells were incubated with alkaline phosphatase–conjugated goat anti-human IgA, anti-mouse IgM, anti-mouse IgG, or anti-mouse IgA (2050–04, 1021–04, 1030–04, or 1040–04, respectively). p-Nitrophenyl phosphate (1 mg/ml) was then added (Sigma-Aldrich), and alkaline phosphatase activity was blocked with 3 N NaOH. OD was measured at 405 nm using a Multiskan FC photometer (Thermo Scientific).
Lectin Binding Assays
Plates were coated with anti-human IgA. Mouse sera containing 100 ng hIgA diluted in 3% BSA-PBS were added for 2 hours at 37°C. After washing, wells were incubated with biotinylated lectins: Helix aspersa binding N-acetylglycosamine (EY Laboratories) and Maackia amurensis binding N-acetylneuraminic acid (Vector Laboratories). ExtrAvidin coupled to alkaline phosphatase (Sigma-Aldrich) was added for 1 hour at room temperature. Alkaline phosphatase activity was revealed as above. Lectin OD was normalized to the corresponding IgA OD, and results were expressed as [OD (Helix aspersa binding N-acetylglycosamine)/OD (IgA)] or [OD (Maackia amurensis binding N-acetylneuraminic acid)/OD (IgA)].
Western Blots
For detection of monomeric and polymeric IgA1, 30 ng plasma IgA1 was subjected to electrophoresis on a 12% polyacrylamide gel (Biorad) under nonreducing conditions. Proteins were then transferred to PVDF membranes (GE Healthcare). The human α-chain was detected using an HRP-linked goat anti-human IgA antibody (732605; Southern Biotech). Membranes were developed by enhanced chemiluminescence detection system according to the manufacturer’s instructions (Biorad).
Isolation of Kidney Glomeruli
Mice were anesthetized intraperitoneally (ketamine and 2% xylazine) and perfused with Dynabeads (Invitrogen). Briefly, 40 ml Dynabeads at a concentration of 3×107 beads per 1 ml HBSS (Gibco) were injected per mouse at a constant flow rate. After perfusion, kidneys were removed, minced into small pieces, and digested with Dnase I and collagenase V (1 mg/ml; Sigma-Aldrich) for 40 minutes at 37°C. Digested tissue was filtered through a 100-μm cell strainer (Fisherbrand). Extracted glomeruli containing Dynabeads were isolated by a magnetic particle concentrator (Invitrogen), washed, and then counted by light microscopy. One aliquot of extracted glomeruli was kept in QIAzol Lysis Reagent (QIAGEN) at −80°C until use. The other part was used for glomerular hIgA elution. Pooled glomeruli were lysed on ice using radioimmunoprecipitation assay buffer (Santa Cruz) containing a protein inhibitor cocktail (Sigma-Aldrich), and then, supernatants were collected after 15 minutes of centrifugation at 14,000×g at 4°C.
Autoimmunity, Autoreactivity, and Polyspecificity Tests
To test the presence of anti-dsDNA hIgA, hIgA polyspecificity, and autoreactivity, 0.5 μg/ml calf thymic DNA (Sigma-Aldrich), 10 μg/ml rabbit Ig fraction (X0903; Dako), or 10 µg/ml wild-type mouse kidney lysate, respectively, was coated on 96-well plates overnight at 4°C. Diluted serum samples and glomeruli lysates were added to plates and incubated at 37°C for 2 hours. Plates were washed, further incubated with AP-conjugated goat anti-mouse κ-light chain (1050–04; Southern Biotech) for 1 hour at room temperature, and revealed with p-nitrophenyl phosphate.
RNA Sequencing
Total RNA was extracted from purified kidney glomeruli using the miRNeasy mini kit (QIAGEN) according to the manufacturer’s protocol. Samples were sequenced on an Illumina NextSeq500 at the Nice-Sophia-Antipolis Functional Genomics Platform in France. Sequence reads were aligned with STAR on the mm10 genome. Biologic comparison was done using IPA (QIAGEN).
Statistics
Statistical analysis was performed using GraphPad Prism software. Data were shown as means ± SEM. Nonparametric Mann–Whitney test was performed, and P value <0.05 was considered significant. For RNA seq results, normalization and differential analysis were obtained using the DESeq2 package in R (Supplemental Files 1 and 2).
Results
Human Light Chain Cκ Domain Substitution in hα1+/+ Mice Does Not Affect IgA Toxicity
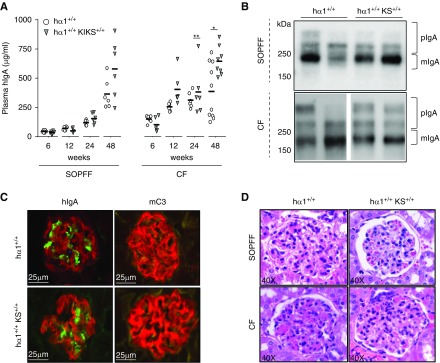
hα1+/+ mice produce the human IgA1 heavy chain (α1hHC) associated with mouse light chains. We asked if mispairing of the human constant Cα CH1 and the mouse Cκ domains might influence the tendency of chimeric IgA to generate deposits. To obtain an IgA closer to the human IgA structure, we bred hα1+/+ mice solely mutated at the IgH locus with KS+/+ mice that express a functional human light chain Cκ domain (hLC).11 Double-mutant hα1+/+KS+/+ mice producing IgA with fully human constant domains (Table 1) were bred in both a CF—in contact with endemic mouse pathogens and conventional immune stimulation—and an SOPFF. hα1+/+KS+/+ mice essentially produce IgA with human heavy chains and light chains together with minimal amounts of class-switched murine Igs but not mIgM (Supplemental Figure 1A). hIgA plasma levels increased with time, with higher quantities in CF mice than in SOPFF mice in both models. hIgA levels were significantly higher in hα1+/+KS+/+ than in hα1+/+ mice in CF (Figure 1A). In addition, polymeric IgA levels increased in an age-dependent manner in both mouse groups but to a higher extent in CF- than in SOPFF-housed animals (Supplemental Figure 1B); however, no difference in the monomeric-to-polymeric ratio was observed between the two models (Figure 1B). Glycosylation profiles of hIgA barely varied between mice at 24 weeks, except for a slight hypersialylation in hα1+/+KS+/+ compared with hα1+/+ mice (Supplemental Figure 1C). Kidney sections showed similar levels of IgA deposition in the mesangium in both models at 48 weeks (Figure 1C, Supplemental Figure 2). At the same age, small quantities of complement C3 deposits were detected in the mesangium of hα1+/+ and hα1+/+KS+/+ mice housed in CF (Figure 1C). Histologic analyses showed normal kidneys in SOPFF animals but slight mesangial expansion without cellular proliferation in kidneys from mice housed in CF (Figure 1D). Plasma creatinine and proteinuria levels remained normal in both models regardless of housing conditions (Supplemental Figure 3), and no hematuria was observed after 48 weeks follow-up.
Figure 1.
Human light chain Cκ domain (hLC) substitution does not affect IgA deposition in hα1+/+ knock-in κ-switch (KS+/+) mice. (A) Plasma hIgA levels in hα1+/+ and hα1+/+KS+/+ mice at 6, 12, 24, and 48 weeks in both a specific and opportunistic pathogen-free facility (SOPFF) and a conventional facility (CF). Results are the means ± SEM of n=6–10 mice per group. *P<0.05; **P<0.01. (B) Analysis of monomeric IgA (mIgA) and polymeric IgA (pIgA) by western blot at 48 weeks; molecular mass scale is expressed in kilodaltons. Blots are representative of n=6 mice per group, and two mice from each group are presented. (C) hIgA and mC3 deposits in mice kidneys at 48 weeks in CF (podocin in red, hIgA in green, and mC3 in yellow). (D) Hematoxylin and eosin staining of kidney sections from mice at 48 weeks. All pictures are representative for glomeruli found in kidney sections of n=6–12 mice per group. hIgA, human IgA.
Consequently, these data eliminate the hypothesis of mispaired heavy chains-light chains being responsible for chimeric IgA deposits in hα1+/+ mice.
CD89 Is Not Mandatory for Development of Glomerular Inflammation
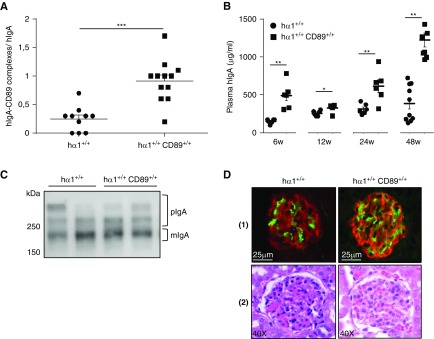
It was previously shown that α1KI-CD89 transgenic mice that express human CD89 (Table 2) on macrophages and monocytes developed pathogenic mesangial deposits. Soluble CD89 found in fractions containing high-molecular mass complexes was required for complement deposition, leukocyte infiltration, proteinuria, and hematuria.14 However, the role of CD89 was recently questioned in another study.15 To further explore this molecular aspect, we bred CD89+/+ transgenic mice in which CD89 expression on neutrophils as well as regulation and function of the receptor mimics the physiologic situation in humans12 with hα1+/+ mice (Table 1). We followed hα1+/+ and hα1+/+CD89+/+ mice in the CF for 48 weeks. Flow cytometry showed CD89 surface staining on murine blood neutrophils from hα1+/+CD89+/+ but not hα1+/+ mice (Supplemental Figure 4A). In addition, ELISA quantified the production of hIgA (Supplemental Figure 4B) and the presence of CD89-IgA complexes in hα1+/+CD89+/+ plasma (Figure 2A). hIgA plasma level was significantly higher in hα1+/+CD89+/+ than in hα1+/+ mice (Figure 2B). hIgA polymerization level increased with age in both groups, and there was globally no difference in the monomeric-to-polymeric ratio between the two groups (Figure 2C). hIgA galactosylation and sialylation profiles were similar (Supplemental Figure 4C). Only at 6 weeks of age was hIgA glomerular deposition significantly higher in hα1+/+CD89+/+ compared with hα1+/+ mice (Supplemental Figure 5). Afterward, this difference disappeared, and hIgA deposits were similar in both groups, with very slight mC3 deposits found at 48 weeks (Figure 2D, [1], Supplemental Figure 5). No mesangial cell proliferation was seen at 48 weeks in hα1+/+CD89+/+ mice (Figure 2D, [2]). Plasma creatinine and proteinuria levels did not significantly differ between both models at 48 weeks and remained in the normal range (Supplemental Figure 6); hematuria was not detected all along the study.
Table 2.
Key immunologic definitions
| Term | Definition |
|---|---|
| Affinity maturation | Process by which activated B cells can produce Igs with increased affinity; it is a direct result of somatic hypermutation |
| Somatic hypermutation | Cellular mechanism involving a programmed process of mutation affecting the variable regions of Ig genes |
| Activation-induced cytidine deaminase | Enzyme that creates DNA mutations during somatic hypermutation and affinity maturation |
| CD89 | Also called FcαRI; is the human receptor binding the IgA heavy chain constant region |
| Chimeric IgA | IgA composed of domains from distinct species (e.g., mouse and human) |
| Germline sequence | Inherited sequence before undergoing somatic mutation |
Figure 2.
Coexpression of human CD89 and IgA does not affect IgA toxicity in mice. (A) Plasma hIgA-CD89 levels in hα1+/+ and hα1+/+CD89+/+ mice at 12 weeks. OD of (hIgA-CD89 complexes) was normalized to OD of hIgA in each model (n=10–12 mice). (B) Plasma hIgA levels in mice at 6, 12, 24, and 48 weeks. (C) Analysis of hIgA circulating forms by western blot at 48 weeks. Blots are representative for n=6 mice per condition; two mice from each group are presented. (D, 1) hIgA deposits are in green. (D, 2) Hematoxylin and eosin staining of kidney sections from mice at 24 weeks. Pictures are representative for all glomeruli found in kidney sections of n=6–12 mice per condition. For numeric data, results are means ± SEM of n=6–10 mice per group. hIgA, human IgA. *P<0.05; **P<0.01; ***P<0.001.
We, therefore, conclude that, in such a model in our hands, IgA-CD89 complex formation does not increase the severity of IgA deposits or glomerular inflammation or increase the development of severe kidney lesions.
hα1+/+ AID−/− Mice Develop Mesangial Thickening and Complement Deposition
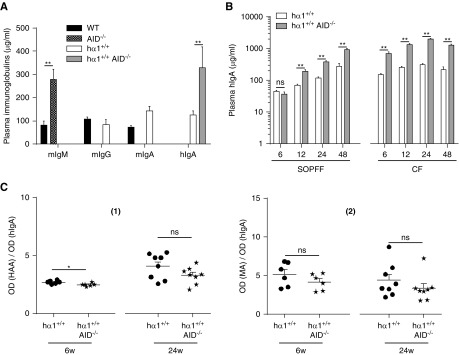
In a previous study with monoclonal IgA, we observed that IgA deposition clearly implicates not only the Cα constant region but also, the Ig variable domain.10 The sequence of this domain is edited during immune responses due to Ig affinity maturation and SHM (Table 2) of expressed Ig V(D)J genes. This process requires the DNA editing enzyme: AID. AID deficiency causes a complete defect in class switch recombination (CSR) and SHM.12,15 We thus bred the hα1+/+ model with the AID−/− mice;13 double-mutant hα1+/+AID−/− mice (Table 1) were housed in both SOPFF and CF. Results showed that hα1+/+AID−/− Ig production was restricted to hIgA, which is consistent with the AID deficiency and CSR defect (Figure 3A). B lymphocyte proportions in hα1+/+ and hα1+/+AID−/− mice were similar in both bone marrow and spleen. Interestingly, we observed a large increase in B1 lymphocyte percentage in the spleen of hα1+/+AID−/− mice (Supplemental Table 1). In addition, hIgA levels were significantly increased in hα1+/+AID−/− compared with hα1+/+ mice in an age-dependent manner regardless of housing conditions (Figure 3B). IgA galactosylation and sialylation profiles did not differ between the two groups (Figure 3C, [1] and [2]). To test hIgA affinity in these mice lacking AID expression, we injected intraperitoneally two sex- and age-matched hα1+/+ and hα1+/+AID−/− groups with 50 μg ovalbumin at day 0 followed by a boost at day 14. Despite the fact that total hIgA production was significantly higher in the hα1+/+AID−/− group, ovalbumin-specific hIgA production was absent (days 7 and 14) or significantly decreased (days 21 and 28) compared with the hα1+/+ group (Figure 4A, [1] and [2]). This confirmed the production of low-affinity hIgA in these mice as confirmed by repertoire analysis (Supplemental Figure 7). Different assays were then performed to test these low-affinity hIgAs for autoimmune properties. We failed to detect any anti-dsDNA (Supplemental Figure 8A) autoreactive or polyspecific hIgA in hα1+/+AID−/− sera (Supplemental Figure 8, B and C).
Figure 3.
Invalidation of activation-induced cytidine deaminase (AID) in hα1+/+ mice did not affect hIgA glycosylation profile. (A) Different isotype levels in wild-type (WT), AID−/−, hα1+/+, and hα1+/+AID−/− mice at 6 weeks. (B) Plasma hIgA levels in hα1+/+ and hα1+/+AID−/− mice at 6, 12, 24, and 48 weeks in both a specific and opportunistic pathogen-free facility (SOPFF) and a conventional facility (CF). (C) hIgA glycosylation levels at 6 and 24 weeks quantified by lectin-binding assay using (1) Helix aspersa binding N-acetylglycosamine (HAA) and (2) Maackia amurensis binding N-acetylneuraminic acid (MA) lectins; OD lectin per OD hIgA is presented. All data are expressed as means ± SEM of n=6–10 mice per group. hIgA, human IgA. *P<0.05; **P<0.01.
Figure 4.
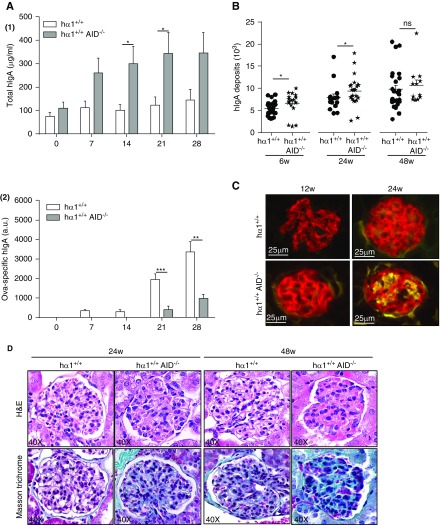
Low affinity-IgA deposits induce kidney lesions. (A, 1) Total hIgA and (A, 2) ovalbumin (OVA)-specific hIgA detected by ELISA in total serum after intraperitoneal OVA immunization of mice. (B) hIgA deposits quantified by image analysis using Volocity in conventional facility (CF) at 6, 24, and 48 weeks. Results are means ± SEM of n=150 glomeruli per condition. (C) mC3 deposits in kidney sections from mice in CF at 12 and 24 weeks. (D) Hematoxylin and eosin (H&E) and Masson trichrome staining of kidney sections from mice housed in CF. All pictures are representative for glomeruli found in kidney sections of n=6–12 mice per condition. Data are means ± SEM of n=6–10 mice per condition. hIgA, human IgA; AID, activation-induced cytidine deaminase. *P<0.05; **P<0.01; ***P<0.001.
Staining of kidney sections showed that IgA deposits were significantly heavier at 6 and 24 weeks of age in hα1+/+AID−/− compared with hα1+/+ mice raised in CF. This difference was not observed at 48 weeks of age or in animals housed in SOPFF (Figure 4B, Supplemental Figure 9, A and B). Importantly, in CF, slight mC3 deposition was detected after the age of 12 weeks in hα1+/+AID−/− mice and was more pronounced at 24 weeks. In hα1+/+ mice, mC3 deposits appeared later in CF and only faintly after 24 weeks (Figure 4C). Finally, in SOPFF, only hα1+/+AID−/− developed a discrete C3 deposit after 48 weeks (Supplemental Figure 9C). Moreover, histologic analyses early showed increased glomerular cell proliferation in 6-week-old hα1+/+AID−/− mice (50±8 versus 34±6 cells per glomerulus in hα1+/+ mice). Later, no difference in cell proliferation remained measurable, but hα1+/+AID−/− mice had developed considerable thickening of the mesangium at 24 and 48 weeks compared with hα1+/+ mice (Figure 4D). Plasma creatinine levels were significantly increased in hα1+/+AID−/− mice at 24 weeks, whereas proteinuria levels increased without reaching a significant difference (Supplemental Figure 10), and hematuria always remained undetectable.
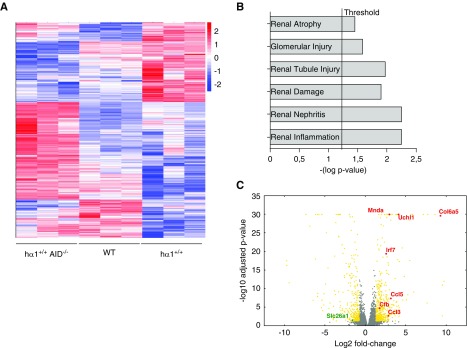
To characterize the molecular aspects of the more severe lesions seen in hα1+/+AID−/− mice, we conducted an exploratory transcriptome analysis by RNA sequencing comparing glomeruli isolated from wild-type, hα1+/+, and hα1+/+AID−/− mice at 24 weeks. Glomerular RNA profiling showed that, for 14,762 analyzed genes, the expression of 418 was significantly decreased (P<0.001), whereas 625 others were upregulated (P<0.001). In hα1+/+AID−/− mice, differentially expressed genes were clearly identified (Figure 5A, Supplemental Files 1 and 2). The top six nephrotoxicity markers upregulated in hα1+/+AID−/− mice are shown in Figure 5B. Notably, we observed significant upregulation of several genes related to the alternative and lectin pathways of complement (C3ar1, C3b, C4b, and Cfb). This was associated with significant upregulation of several pathways implicated in inflammatory responses and immune cell trafficking (Irf3 and Irf7); extracellular matrix organization (Col6a5); cell growth, proliferation, and cell cycle (Mnda and Uchl1); and nephrotoxicity parameters (Ccl5, Ccl3, and downregulated Slc26a1) in hα1+/+AID−/− versus hα1+/+ and wild-type mice (Figure 5C). Altogether, these data show that the low-affinity hIgAs, which are prominent in hα1+/+AID−/− mice, are able to deposit in glomerular mesangium and that they are especially prone to induce glomerular cell proliferation and thickening; also, they can initiate nephrotoxicity in mice.
Figure 5.
hα1+/+ and hα1+/+AID−/− mice present differential glomerular gene expression. (A) Heatmap of differentially expressed genes in wild-type (WT), hα1+/+, and hα1+/+AID−/− mice (n=3 mice per condition) on the basis of Z score. Red indicates upregulated genes; blue indicates downregulated genes. (B) Top six nephrotoxicity parameters upregulated in hα1+/+AID−/− compared with hα1+/+ mice. (C) Volcano plot for transcriptome profiling data presenting mRNA log2 fold changes in hα1+/+AID−/− versus hα1+/+ mice. Some significant upregulated and downregulated genes are depicted.
Discussion
IgA deposits in humans appear as a continuum ranging from isolated presence without clinical or biologic translation to the development of rapidly progressive proliferative glomerulopathy. The presence of clinical signs is probably only the tip of the iceberg. It is, therefore, important to understand how preclinical lesions can differ in terms of abundance, kinetics, and proinflammatory properties, notably depending on variations in the quality of circulating IgA during immune stimulations and ability of deposited IgA to recruit additional nephrotoxic activities. Among several existing IgAN experimental models, none have really succeeded in fully reproducing the disease.6 We show in this study that, unexpectedly, the potential of IgA to yield glomerular deposits and glomerular inflammation is highest when they carry unmutated V domains. This suggests that, in the burst of IgA secreted after immune stimulation, nephrotoxicity might strongly involve those IgA antibodies produced by bystander B cells or innate-like B cells rather than the high-affinity Ag-specific IgA antibodies selected by adaptive responses.
A major molecular aspect of IgA deposition concerns the potential role of IgA hinge region hypogalactosylation, which is commonly considered as promoting IgA deposits. Several findings supported a key role for galactose-deficient (Gd) IgA in renal aggression, because kidney biopsy eluates from patients with IgAN mainly contain negatively charged and polymeric IgA1.16,17 Free O-glycoside sites in the IgA1 hinge region are recognized as antigenic neoepitopes and targeted by autoantibodies. This results in the formation of bulky nephritogenic immune complexes.5 Several reports, however, suggested that the glycosylation deficiency of IgA often observed in IgAN is not the sole cause and that it is not a prerequisite for IgA deposition.10,18 In this study exploring different models, hα1+/+, hα1+/+KIKS+/+, hα1+/+CD89+/+, and hα1+/+AID−/−, we also observed that hinge hypoglycosylation was not mandatory for IgA deposition. Gd-IgA antibodies are often detected in healthy individuals after viral infection,19 and it has been demonstrated that these Gd-IgAs can play a protective role preventing IgA glomerular deposition.20 Furthermore, separate linkage studies in familial cases of IgAN and genome-wide association studies of both sporadic and familial IgAN cases suggest that there is a genetic component to IgAN.21 Several susceptibility loci have been identified, although interestingly, none of them implicated O-glycosylation genes.22 Finally, no difference in O-galactosylation activity was found in purified B cells isolated from blood and bone marrow of patients with IgAN.23
This study characterizes a model in which polyclonal chimeric IgA1 with a human α1 constant region is secreted and results in kidney deposition.9,10 Because a potential drawback of the hα1+/+ model might be instability of the Ig molecules assembled from both human and mouse constituents, we first confirmed that IgA deposition was unchanged in hα1+/+KIKS+/+ mice also expressing α-chains with a human constant domain.11 This validated that the ability to deposit such models is due to human Cα1 heavy chains independent of any misassociation with light chains.
We also explored the potential role of CD89 in our model by using a transgenic mouse model that precisely mimics the CD89 expression pattern observed in humans (because CD89 is normally absent in rodents). Forced expression of CD89 by monocytes/macrophages using the mouse CD11b gene promoter was shown to promote mouse IgA deposition and glomerular infiltration.23 Moreover, hα1+/+ mice bred with these mice synergistically developed earlier kidney lesions than single transgenic animals, consistent with the notion that human CD89 has increased affinity for human rather than mouse IgA and might accelerate hIgA deposition.14 Recently, the role of CD89 has been questioned. Using a mouse CD14 promoter, Xu et al.15 did not observe any IgA deposition or glomerular infiltration. In our study, we used a model mimicking CD89 expression seen in humans by using the CD89 gene promoter and regulatory elements and then restricting CD89 expression to the myeloid lineage. We did not observe any exacerbation of glomerular lesions in hα1+/+CD89+/+ double-mutant mice despite CD89 surface staining on blood neutrophils and the presence of circulating IgA-CD89 immune complexes. These discrepancies could be explained by a difference in the two model constructions. In one case, the CD89 expression was restricted to monocytes,23 and in the second case, it was mainly expressed by neutrophils, which is closer to human physiology.12,24 We thus cannot rule out differences in CD89 expression depending on the promoter used or the possibility that the shedding of soluble CD89 is lower from neutrophils than from monocytes.
Other than the contribution of IgA glycosylation or the presence of IgA-CD89 complexes to the process of deposition, the role of V regions can also be inferred from the observations made with monoclonal IgA.10 However, the contribution of V domains to the nephrotoxicity of polyclonal IgA is hard to appreciate in the context of their huge diversity. In the presence of Ags, mature B cells diversify their antibody repertoire through SHM and class switching processes. These processes are conditioned by environmental cues and strongly controlled by follicular dendritic cells and different subsets of CD4+ T cells, such as T follicular helper, T follicular regulatory, and T helper 17 cells.25 They also require the DNA editing enzyme AID, and its deficiency abrogates both CSR and SHM.13,26 A modification in this control system can curtail high affinity B cell selection. Interestingly, follicular dendritic cell secreted protein is a suppressive factor regulating IgA B cell responses, and follicular dendritic cell secreted protein deficient mice have deregulated IgA production in PP associated with IgA mesangial deposits.7 Another condition where IgA production is globally increased and might then become less dependent on Ag-specific stimulation and affinity for Ag is that of BAFF-overexpressing mice, which also present IgA deposits.27 Interestingly, in patients with IgAN, impaired affinity maturation of IgA was reported after vaccination with recall Ags.28 Our hα1+/+AID−/− model highlights two elements worthy of interest. The first is the production of low-affinity innate-like IgA leading to mesangial matrix expansion and fibrosis, and the second is that these IgA are capable of activating complement.
In the absence of AID, only unmutated Igs resembling natural antibodies are produced, which are notably characterized by their ability to bind self-Ags with low affinity and show polyreactivity.29 The process of affinity maturation due to AID-driven SHM of Ig V regions should thus be considered under two complementary aspects. First, it produces high levels of highly specific antibodies against immunizing Ags, and second, it terminates the production of Ig molecules with low affinity for Ag, self-reactivity, and polyreactivity.30 In hα1+/+AID−/− mice, natural polyreactive IgAs dominate the immune humoral response. Deposited IgAs in these mice can be considered as natural antibodies only encoded by the assembly of germline V(D)J segments. This also raises the question of the role of B1 lymphocytes in IgAN. B1 cell developmental origin, phenotype, and function are distinct from those of conventional B cells. They constitute a population of spontaneous natural Ig secreting cells.31 Although B1 cells are less clearly described in humans than in mice, recent studies suggest that human fetal-derived B1 cells exist32 and that a B1 cell equivalent is present in humans, notably in young individuals, and also exerts innate functions.33–35 Furthermore, it is noticeable that a subpopulation of B cells from mucosa-associated lymphoid tissues is related to a B1-like compartment, reacting to T-independent stimuli, but is not involved in the production of highly specific IgA against local bacteria.36,37
Activation of complement plays a key role in the pathogenesis and clinical expression of IgAN.38 Complement C3 is usually present in lesions with the same distribution as IgA. IgA has been shown to activate the alternative complement pathway in vitro independent from the hinge region, with higher activation by polymeric IgA.39 Polymeric IgAs also have the ability to activate the lectin pathway, because they can bind Mannose-Binding Lectin in vitro through the N-linked glycans.40 Interestingly, in our hα1+/+AID−/− model, C3 was markedly present in kidney lesions. Surprisingly, this complement activation was not associated with a higher proportion of polymeric IgA, which suggests that natural and very immature monomeric IgAs can also activate complement.
This study with different mouse models of IgAN, as for all other studies using mouse IgAN models, has several limitations. First, the hα1+/+ mice only mimic the initial stage of the human disease. In particular, we did not observe proteinuria or hematuria, including when complement was present in the glomerular mesangium. Transient hematuria is difficult to detect, and different mouse strains might have different susceptibilities to GN explaining differences in IgAN manifestations between models. Second, our study explores the mechanisms of IgA binding to the glomerular mesangium. A previous study suggested that the human IgA1 primary structure might, by itself, yield deposition-prone molecules in association with particular variable domains.10 Although this aspect cannot be easily measured, we might also speculate that natural IgA antibodies might differentially bind IgA receptors, such as CD71 or β1,4-glycosyltransferase on mesangial cells or mesangial matrix proteins.41 The accumulation of IgA might partly reflect saturation of the clearance function of these receptors. Mechanisms of this initial binding and its secondary consequences need additional investigation.
In conclusion, we demonstrated that IgA deposits and IgAN might notably implicate natural antibodies of the IgA class totally lacking affinity maturation. The conditions in which such unmutated IgAs may be produced in increased amounts in humans after poorly specific stimulations of B cells remain to be determined. A possible role for a B cell subpopulation from mucosa-associated lymphoid tissues related to a B1-like compartment and reacting to T-independent stimuli should be examined. On the basis of these data, the hypothesis of an imbalance in IgA production by the B1 versus B2 compartment of B cells will also deserve to be explored in human patients with IgAN.
Disclosures
None.
Funding
This study was supported by grants from Région Nouvelle-Aquitaine (appel d’offre 2017); Chaire d’Immuno-pathologie des Maladies Rénales, Limoges University; and Association Limousine pour l’Utilisation du Rein Artificiel à Domicile. Dr. Wehbi was supported by a doctoral fellowship from Lebanese University and Lebanese National Council for Scientific Research.
Supplementary Material
Acknowledgments
We thank Sylvie Desforges for excellent technical assistance, Cendrine LeCardeur for kidney section preparation, Claire Carrion for expert help with microscopy and image analysis, Dr. Hélène Chable from the Biochemistry Department in Centre Hospitalier Universitaire Dupuytren for urinalysis, Alain Chaunavel from the anatomy-pathology department for histology staining assistance, and Laetitia Magnol from Faculty of Sciences, Limoges University for creatinine analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Fatal Attraction: Immunoglobulin A and the Glomerular Mesangium,” on pages 1139–1141.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111089/-/DCSupplemental.
Supplemental Figure 1. hIgA production in hα1+/+KS+/+ mice.
Supplemental Figure 2. Mesangial deposits in hα1+/+KS+/+ mice.
Supplemental Figure 3. Kidney function parameters in hα1+/+KS+/+ mice.
Supplemental Figure 4. Human IgA and CD89 coexpression in mice.
Supplemental Figure 5. Mesangial deposits in hα1+/+CD89+/+ mice.
Supplemental Figure 6. Kidney function parameters in hα1+/+CD89+/+ mice.
Supplemental Figure 7. Absence of somatic hypermutation in hα1+/+AID−/− mice.
Supplemental Figure 8. Absence of autoimmunity in hα1+/+AID−/− mice.
Supplemental Figure 9. hIgA mesangial deposits in hα1+/+AID−/− mice.
Supplemental Figure 10. Kidney function parameters in hα1+/+AID−/− mice.
Supplemental File 1. Downregulated genes_HAIDS-HAM.
Supplemental File 2. Upregulated genes_HAIDS-HAM.
Supplemental Material. Methods.
Supplemental Table 1. Percentage of the various lymphoid populations in bone marrow and spleen of WT, hα1+/+, and hα1+/+AID−/− mice.
References
- 1.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Varis J, Rantala I, Pasternack A, Oksa H, Jäntti M, Paunu ES, et al.: Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol 46: 607–610, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, et al.: Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 23: 1753–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J: Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floege J, Moura IC, Daha MR: New insights into the pathogenesis of IgA nephropathy. Semin Immunopathol 36: 431–442, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Hou S, Landego I, Jayachandran N, Miller A, Gibson IW, Ambrose C, et al.: Follicular dendritic cell secreted protein FDC-SP controls IgA production. Mucosal Immunol 7: 948–957, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Bemark M, Hazanov H, Strömberg A, Komban R, Holmqvist J, Köster S, et al.: Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat Commun 7: 12698, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchez S, Amin R, Cogné N, Delpy L, Sirac C, Pascal V, et al.: Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci U S A 107: 3064–3069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oruc Z, Oblet C, Boumediene A, Druilhe A, Pascal V, Le Rumeur E, et al.: IgA structure variations associate with immune stimulations and IgA mesangial deposition. J Am Soc Nephrol 27: 2748–2761, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaud A, Lechouane F, Le Noir S, Monestier O, Cogné M, Sirac C: Efficient AID targeting of switch regions is not sufficient for optimal class switch recombination. Nat Commun 6: 7613, 2015 [DOI] [PubMed] [Google Scholar]
- 12.van Egmond M, van Vuuren AJ, Morton HC, van Spriel AB, Shen L, Hofhuis FM, et al.: Human immunoglobulin A receptor (FcalphaRI, CD89) function in transgenic mice requires both FcR gamma chain and CR3 (CD11b/CD18). Blood 93: 4387–4394, 1999 [PubMed] [Google Scholar]
- 13.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T: Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102: 553–563, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, et al.: Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209: 793–806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Li B, Huang M, Xie K, Li D, Li Y, et al. : Critical role of kupffer cell CD89 expression in experimental IgA nephropathy. PLoS One 11: e0159426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, et al.: The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7: 117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteiro RC, Halbwachs-Mecarelli L, Roque-Barreira MC, Noel LH, Berger J, Lesavre P: Charge and size of mesangial IgA in IgA nephropathy. Kidney Int 28: 666–671, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Boumediene A, Oblet C, Oruc Z, Duchez S, Morelle W, Huynh A, et al. : Gammopathy with IgA mesangial deposition provides a monoclonal model of IgA nephritogenicity and offers new insights into its molecular mechanisms. Nephrol Dial Transplant 26: 3930–3937, 2011 [DOI] [PubMed] [Google Scholar]
- 19.D’Arrigo I, Cló E, Bergström T, Olofsson S, Blixt O: Diverse IgG serum response to novel glycopeptide epitopes detected within immunodominant stretches of Epstein-Barr virus glycoprotein 350/220: Diagnostic potential of O-glycopeptide microarrays. Glycoconj J 30: 633–640, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Hiki Y, Takahashi K, Shimozato S, Odani H, Yamamoto K, Tomita M, et al.: Protective role of anti-synthetic hinge peptide antibody for glomerular deposition of hypoglycosylated IgA1. Clin Exp Nephrol 12: 20–27, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Buck KS, Smith AC, Molyneux K, El-Barbary H, Feehally J, Barratt J: B-cell O-galactosyltransferase activity, and expression of O-glycosylation genes in bone marrow in IgA nephropathy. Kidney Int 73: 1128–1136, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J: An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 81: 833–843, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, et al.: Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191: 1999–2009, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Egmond M, Hanneke van Vuuren AJ, van de Winkel JG: The human Fc receptor for IgA (Fc α RI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol Lett 68: 83–87, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mesin L, Ersching J, Victora GD: Germinal center B cell dynamics. Immunity 45: 471–482, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T: Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298: 1424–1427, 2002 [DOI] [PubMed] [Google Scholar]
- 27.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, et al.: Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layward L, Allen AC, Hattersley JM, Harper SJ, Feehally J: Low antibody affinity restricted to the IgA isotype in IgA nephropathy. Clin Exp Immunol 95: 35–41, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantaert T, Schickel J-N, Bannock JM, Ng YS, Massad C, Oe T, et al.: Activation-induced cytidine deaminase expression in human B cell precursors is essential for central B cell tolerance. Immunity 43: 884–895, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, et al.: Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360: 223–226, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgarth N: B-1 cell heterogeneity and the regulation of natural and antigen-induced IgM production. Front Immunol 7: 324, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA: Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335: 1195–1200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, et al.: A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum 60: 3734–3743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchanek O, Sadler R, Bateman EA, Patel SY, Ferry BL: Immunophenotyping of putative human B1 B cells in healthy controls and common variable immunodeficiency (CVID) patients. Clin Exp Immunol 170: 333–341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin DO, Holodick NE, Rothstein TL: Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70-. J Exp Med 208: 67–80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurnheer MC, Zuercher AW, Cebra JJ, Bos NA: B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J Immunol 170: 4564–4571, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Bahlburg A: B-1 cells as a source of IgA. Ann N Y Acad Sci 1362: 122–131, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, et al.: Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 26: 1503–1512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiemstra PS, Gorter A, Stuurman ME, Van Es LA, Daha MR: Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol 17: 321–326, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR: Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167: 2861–2868, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Molyneux K, Wimbury D, Pawluczyk I, Muto M, Bhachu J, Mertens PR, et al.: β1,4-galactosyltransferase 1 is a novel receptor for IgA in human mesangial cells. Kidney Int 92: 1458–1468, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.