Significance Statement
Although mucosal-associated invariant T (MAIT) cells are emerging as a key player in chronic inflammatory diseases, their role in CKD’s hallmark fibrosis is unclear. Using multicolor flow cytometry and immunofluorescence, the authors identified tissue-resident MAIT cells in healthy kidneys and demonstrated that absolute numbers of activated tissue-resident MAIT cells within the tubulointerstitial compartment of fibrotic human kidneys correlate with histologic severity of CKD (levels of interstitial fibrosis). In addition, using an in vitro mechanistic model of human renal fibrosis, they found that hypoxia-damaged proximal tubular epithelial cells are potent drivers of MAIT cell activation and cytotoxicity within the inflammatory and fibrotic microenvironment. These findings suggest that kidney MAIT cells are a potential therapeutic target for the treatment of CKD.
Keywords: mucosal-associated invariant T cells, chronic kidney disease, fibrosis, lymphocytes, Chronic inflammation
Visual Abstract
Abstract
Background
Mucosal-associated invariant T (MAIT) cells represent a specialized lymphocyte population associated with chronic inflammatory disorders. Little is known, however, about MAIT cells in diseases of the kidney, including CKD.
Methods
To evaluate MAIT cells in human native kidneys with tubulointerstitial fibrosis, the hallmark of CKD, we used multicolor flow cytometry to identify, enumerate, and phenotype such cells from human kidney tissue biopsy samples, and immunofluorescence microscopy to localize these cells. We cocultured MAIT cells and human primary proximal tubular epithelial cells (PTECs) under hypoxic (1% oxygen) conditions to enable examination of mechanistic tubulointerstitial interactions.
Results
We identified MAIT cells (CD3+ TCR Vα7.2+ CD161hi) in healthy and diseased kidney tissues, detecting expression of tissue-resident markers (CD103/CD69) on MAIT cells in both states. Tissue samples from kidneys with tubulointerstitial fibrosis had significantly elevated numbers of MAIT cells compared with either nonfibrotic samples from diseased kidneys or tissue samples from healthy kidneys. Furthermore, CD69 expression levels, also an established marker of lymphocyte activation, were significantly increased on MAIT cells from fibrotic tissue samples. Immunofluorescent analyses of fibrotic kidney tissue identified MAIT cells accumulating adjacent to PTECs. Notably, MAIT cells activated in the presence of human PTECs under hypoxic conditions (modeling the fibrotic microenvironment) displayed significantly upregulated expression of CD69 and cytotoxic molecules perforin and granzyme B; we also observed a corresponding significant increase in PTEC necrosis in these cocultures.
Conclusions
Our findings indicate that human tissue-resident MAIT cells in the kidney may contribute to the fibrotic process of CKD via complex interactions with PTECs.
The global burden of CKD has risen dramatically in recent years, largely driven by demographic expansion (population growth and ageing) and the increased prevalence of diabetes and hypertension worldwide.1 Regardless of its origins, CKD is characterized pathobiologically by fibrosis within the tubulointerstitial compartment, the interstitial tissue adjoining the renal tubules. The pathology of tubulointerstitial fibrosis is underpinned by the sustained presence of inflammatory immune cells within the local microenvironment.2 Most studies of CKD immunobiology have focused on conventional T lymphocytes reactive to classic MHC-peptide antigen complexes. However, less is known about the contribution and function of unconventional T cell subsets in driving this pathogenic fibrotic process in CKD.
Mucosal-associated invariant T (MAIT) cells are a specialized subset of unconventional (non–MHC-restricted) T cells that have emerged as key players in immunity and pathologic inflammation. MAIT cells are characterized by a highly restricted αβ T cell receptor (TCR) that recognizes small molecule antigens in the context of the nonclassic MHC-related molecule 1 (MR1).3–5 Human MAIT cells express a semi-invariant TCRα chain (Vα7.2 coupled with restricted Jα segments) associated with a limited repertoire of TCRβ chains (including Vβ13 and Vβ2).6 They are classically defined in humans by their coexpression of TCR Vα7.2 and high levels of the C-type lectin receptor CD161.7 Human MAIT cells also express several cytokine receptors under steady-state conditions, including IL-7Rα, IL-12R, and IL-18Rα, allowing them to respond to innate IL in a TCR-independent manner.8,9 Once activated in a TCR-dependent and/or -independent manner, MAIT cells display immediate effector function through the production of cytotoxic effector molecules (perforin and granzyme B). The majority of MAIT cells in humans also express CD8,10 a signature consistent with their potent cytotoxic activity against target cells.11
MAIT cells are abundant within human peripheral tissues, particularly in the liver and mucosal tissues, such as lung and gut.12 Indeed, MAIT cells have a distinct chemokine receptor profile, including CCR5 and CXCR3 expression, consistent with their capacity to home to peripheral tissues.13 Moreover, MAIT cells expressing markers compatible with tissue-resident lymphocytes (CD103 and CD69) have been identified in human peripheral tissue,14 highlighting their immunologic importance in the local microenvironment under healthy and diseased conditions. In healthy individuals, MAIT cells contribute to local protective immunity by maintaining epithelial and mucosal layer integrity and eliciting antimicrobial responses.8,15,16 However, recent studies have suggested a pathogenic function for these cells in chronic inflammatory diseases, with increased numbers of MAIT cells identified in tissue lesions of patients with inflammatory bowel disease,17 obesity,18 psoriasis,19 multiple sclerosis,20 and rheumatoid arthritis.21 In contrast, their functional contributions to the pathogenesis of the tubulointerstitial fibrosis of human CKD have not been studied.
The functions of MAIT cells in established mouse models of kidney disease are unknown because of their low prevalence in common laboratory mouse strains,6 underlining the importance of investigating these unconventional T cells in clinical (human) biospecimens. However, human studies have also been hampered by limited access to kidney tissue samples. To date, MAIT cells have only been detected in human kidney tissue by the expression of MAIT cell–specific TCR transcripts (Vα7.2-Jα33 and Vα7.2-Jα12).22,23
In this study, we show that MAIT cells are present in healthy human kidney tissue and display a tissue-resident phenotype. We demonstrate significantly increased numbers of MAIT cells in diseased native biopsy samples with tubulointerstitial fibrosis compared with nonfibrotic biopsy samples and healthy kidney tissue. Moreover, the accumulation of MAIT cells in fibrotic kidneys is restricted to the tubulointerstitial compartment, often in direct contact with proximal tubular epithelial cells (PTECs). Critically, our data point to damaged PTECs as critical drivers of MAIT cell activation and cytotoxicity within the inflammatory/fibrotic microenvironment and, thus, progression to CKD.
Methods
Kidney Tissue Specimens
Kidney cortical tissue was obtained with informed patient consent from the macroscopically/microscopically healthy portion of tumor nephrectomies or native diseased biopsy samples, after approval by the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (2002/011 and 2006/072). Healthy cortical tissue was obtained from 11 donors (three females/eight males) of mean age 59±9, whereas diseased clinical biopsy samples were obtained from 47 donors (18 females/29 males) of mean age 50±17. A range of primary diagnoses were sampled, including 18 glomerular immune-mediated (lupus nephritis, crescentic GN, membranoproliferative GN, pauci-immune GN, IgA nephropathy, membranous nephropathy, and minimal change disease), 20 glomerular non–immune-mediated (fibrillary GN, FSGS, renal amyloidosis, and diabetic nephropathy), and nine nonglomerular (interstitial nephritis, arterionephrosclerosis, and hypertensive nephropathy) causes (Supplemental Table 1).
Fresh biopsy samples were taken with a 16-gauge biopsy needle (Biopsybell, Mirandola, Italy) and immediately divided for: (1) tissue dissociation (1–5 mm of a core biopsy sample); (2) freezing in Tissue-Tek OCT compound (Sakura, Torrance, CA) for immunofluorescence (IF) analysis; and (3) fixation in formalin for assessing levels of interstitial fibrosis/tubular atrophy by renal histopathologists blinded to experimental results. For assessment of renal interstitial fibrosis, formalin-fixed 4-μm sections were stained with Masson's trichrome, and the proportion of fibrotic area in the cortex was quantified over 20 high-power fields. Biopsy samples displaying ≥5% interstitial fibrosis were deemed fibrotic, on the basis of the Banff 97 working classification of renal pathology.24 According to this criterion, diseased specimens were then grouped into biopsy samples without (n=17; five females/12 males; mean age 44±17; mean eGFR 69±27 ml/min per 1.73 m2) or with interstitial fibrosis (n=30; 13 females/17 males; mean age 53±16; mean eGFR 38±23 ml/min per 1.73 m2). Kidney function (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration method by AUSLAB (Queensland Health, Brisbane, Australia).
Tissue Dissociation for Flow Cytometric Analysis
Healthy kidney tissue and diseased biopsy samples were dissociated and processed for flow cytometric analysis according to our published methodology.25 Briefly, kidney tissue was digested with 1 mg/ml collagenase P (Roche, Mannheim, Germany) in the presence of 20 μg/ml DNase I (Roche) (250-µl volume) for 15 minutes. Supernatant from this initial dissociation step was collected for cytokine analysis. Dissociated tissue was then further digested with 10 μg/ml trypsin/4 μg/ml EDTA (Invitrogen, Grand Island, NY) (500-µl volume) for 10 minutes.
Flow Cytometry
Single cell suspensions were initially stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Life Technologies) to exclude nonviable cells. Cells were then incubated with Human TruStain FcX Blocking Solution (BioLegend, San Diego, CA) at room temperature for 5–10 minutes and then stained on ice for 30 minutes with combinations of test (Supplemental Table 2) or isotype-matched control antibodies in cold FACS buffer (0.5% BSA [Sigma, St Louis, MO] and 0.02% sodium azide [Sigma] in PBS). Flow-Count Fluorospheres (Beckman Coulter, Brea, CA) were used for direct determination of absolute counts as outlined in our published methodology.25 Cell acquisition was performed on an LSR Fortessa (BD Biosciences, San Jose, CA) and data analyzed with FlowJo software (TreeStar, Ashland, OR).
Cytokine Detection
Dissociation supernatants were harvested and levels of soluble proteins were determined using the LEGENDplex Human Inflammation Panel multiplex bead-based assay (BioLegend) according to the manufacturer’s instructions. Cytokine values were normalized to picograms per centimeter cubed of tissue.
IF Staining with Tyramide Signal Amplification
Frozen 7-μm tissue sections were fixed with 25% ethanol/75% acetone at room temperature for 5 minutes. Endogenous peroxidase activity was quenched with BLOXALL Endogenous Peroxidase and Alkaline Phosphatase Blocking Solution (Vector Laboratories, Burlingame, CA) at room temperature for 15 minutes, followed by a protein block with 10% Donkey Serum (Merck-Millipore, Burlington, MA) at room temperature for 20 minutes. Sections were sequentially probed with anti-TCR Vα7.2 (monoclonal mouse IgG1; Clone 3C10; BioLegend) and anti–Aquaporin-1 (rabbit polyclonal IgG; Santa Cruz, Dallas, TX) or isotype-matched control antibodies at room temperature for 30 minutes. Fluorescent detection was obtained by incubation with horseradish peroxidase–conjugated F(ab’)2 fragment donkey anti-mouse IgG and donkey anti-rabbit IgG secondary antibodies (both from Jackson ImmunoResearch, West Grove, PA) at room temperature for 20 minutes, followed by addition of Tyramide Signal Amplification (TSA) Plus Cyanine 3 (Cy3) and TSA Plus Cyanine 5 (Cy5) (both from Perkin Elmer, Waltham, MA) at room temperature for 10 minutes. Nuclei were stained with DAPI (Sigma). Slides were coverslipped in fluorescence mounting medium (Agilent Technologies, Santa Clara, CA). A Zeiss 780 NLO confocal microscope (Carl Zeiss, Hamburg, Germany) was used for fluorescence microscopy. Image acquisition and analysis were performed using ZEN software (Carl Zeiss). Quantitative analysis of MAIT cells was undertaken from healthy kidney tissue (n=4), nonfibrotic kidney biopsy samples (n=6), and fibrotic kidney biopsy samples (n=5), counting TCR Vα7.2+ cells in a randomly selected 1-mm2 area from four separate slides for each donor. The final count presented for each donor is the mean value (mean cells/mm2) from the four separate slides.
Isolation and Culture of Human Primary PTECs
PTECs were purified from the macroscopically/microscopically healthy portion of tumor nephrectomies following the method of Glynne and Evans26 and cultured in defined medium (DM) as previously described.27 All PTECs underwent no more than three passages before use in this study.
Hypoxic Treatment of Human Primary PTECs to Mimic the Fibrotic Microenvironment
PTECs were cultured in DM in 96-well flat-bottom plates to 70%–80% confluence. To prevent further proliferation, PTECs were irradiated with 3000 cGy and then further cultured for 72 hours in 200 µl fresh DM for normoxic PTECs (21% O2) or, for hypoxic PTECs (1% O2), in 200 µl fresh DM for 72 hours in an Invivo2 1000 Hypoxia Workstation (Ruskinn Laftec, Bayswater North, Victoria, Australia). For hypoxic conditions, DM was pretreated at 1% O2 for 24 hours before use. Expression of hypoxia inducible factor 1α (HIF-1α) by hypoxic PTECs alone was confirmed by western blotting as previously described.28
Human MAIT Cell Isolation
Leukocyte-rich buffy coats were obtained from healthy blood donors (Australian Red Cross Blood Service). Mononuclear cells were isolated from buffy coats using SepMate isolation tubes (Stemcell Technologies, Vancouver, Canada) and Ficoll Paque Plus density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). MAIT cells were enriched by positive immuno-magnetic selection using the EasySep Human CD8 Positive Selection Kit II (Stemcell Technologies) and were further purified by staining and flow cytometry sorting of live CD3-FITC+ (BD Biosciences), CD8-PerCP-Cy5.5+, TCR Vα7.2-Brilliant Violet 605+, and CD161-Brilliant Violet 785+ (all from BioLegend) events. These procedures routinely yielded MAIT cell preparations of >99% purity.
PTEC-MAIT Cell Cocultures
Human MAIT cells were resuspended at 1×106 cells/ml in complete medium consisting of RPMI 1640 supplemented with 30% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10 mM HEPES buffer solution (all from Invitrogen), and 50 µM 2-mercaptoethanol (Sigma). MAIT cells (100,000 cells in complete medium, 100-µl volume) were added to the preconditioned PTECs (without removal of PTEC culture medium) and cocultured for 24 hours under normoxic or hypoxic conditions (10% FBS final concentration; 300-µl final volume). Where indicated, recombinant human IL-12p70 (10 ng/ml; BioLegend), IL-15 (100 ng/ml; BioLegend), and IL-18 (50 ng/ml; R&D Systems, Minneapolis, MN) were added to cultures.
Culture supernatants were harvested and levels of soluble proteins (perforin and granzyme B) were determined using the LEGENDplex Human CD8/NK Panel multiplex bead-based assay. Cells were harvested and stained with LIVE/DEAD Fixable Near-IR Dead Cell reagent (to exclude dead cells), CD45-Brilliant Violet 510 (to exclude PTECs), and CD69-PE antibodies (BD Biosciences) or appropriate isotype controls for flow cytometric assessment of surface antigen expression on live MAIT cells. A fold change in protein expression under normoxic or hypoxic conditions was calculated as the value in the presence of PTECs/the value in the absence of PTECs.
PTECs from in vitro experiments were harvested by trypsin treatment and stained for Annexin-V and propidium iodide (PI) using the Annexin-V Apoptosis Detection kit I (BD Biosciences) according to the manufacturer’s instructions. The percentage of Annexin-V+ PI+ necrotic cells was determined by flow cytometry. A fold change in PTEC death under normoxic or hypoxic conditions was calculated as the value in the presence of MAIT cells/the value in the absence of MAIT cells.
Statistical Analyses
All statistical tests were performed using Prism 7.0 analysis software (GraphPad Software, La Jolla, CA). Comparisons between paired groups were performed using a Wilcoxon matched-pairs signed rank test and multiple comparisons were performed using a Kruskal–Wallis test with Dunn’s post-test. Absolute cell numbers were correlated with patient eGFR and levels of interstitial fibrosis in diseased biopsy samples by Spearman correlation analysis. P values ≤0.05 were considered statistically significant.
Results
Identification of Tissue-Resident MAIT Cells in Healthy Human Kidney Tissue
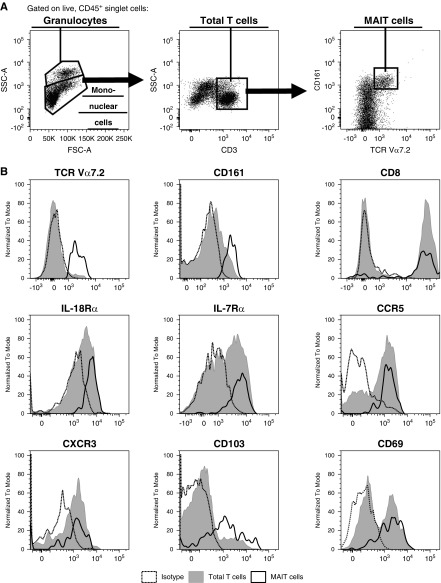
Healthy kidney tissue was enzymatically digested to obtain single cells for flow cytometric analysis. Using the gating strategy outlined in Figure 1A, we were able to separate CD45+ leukocytes into granulocytes with higher side scatter (SSC) and mononuclear cells. Total T cells were then defined as CD3+ mononuclear cells. Within this T cell compartment, MAIT cells were identified as a discrete TCR Vα7.2+ CD161hi population.
Figure 1.
Identification of tissue-resident MAIT cells in healthy human kidney tissue. (A) Gating strategy used to identify MAIT cells (CD3+ TCR Vα7.2+ CD161hi mononuclear cells) in healthy human kidney tissue. Representative flow cytometric data from one of 11 individual donors are shown. (B) Relative expression of TCR Vα7.2, CD161, CD8, IL-18Rα, IL-7Rα, CCR5, CXCR3, CD103, and CD69 by MAIT cells (black unfilled) and total T cells (gray filled) compared with isotype (dashed). Representative data from four to 11 individual donors are shown. FSC-A, forward scatter-area; SSC-A, side scatter-area.
Phenotypic analysis showed MAIT cells in healthy human kidney tissue to be predominantly CD8+ and express signature cytokine receptors IL-18Rα (CD218a) and IL-7Rα (CD127) and chemokine receptors CCR5 (CD195) and CXCR3 (CD183) (Figure 1B). In humans, the expression of CD103 and CD69 has been used to discriminate tissue-resident from circulating lymphocytes.29,30 Notably, expression of both markers was identified on kidney MAIT cells (Figure 1B). These results demonstrate, for the first time, that human MAIT cells are present in healthy kidney tissue and display a tissue-resident phenotype.
Absolute Numbers of Human MAIT Cells Correlate Significantly with Loss of Kidney Function
An identical gating strategy to that used for healthy kidney tissue was applied to identify MAIT cells in diseased biopsy samples. Notably, we observed equivalent flow cytometric profiles in diseased biopsy samples to those presented in Figure 1A for healthy kidney tissue (data not shown).
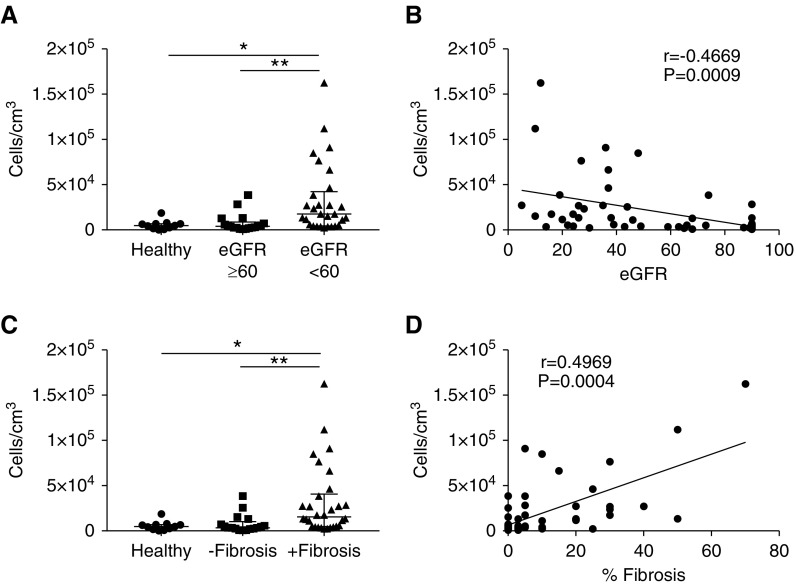
We enumerated MAIT cells in healthy and diseased kidney tissue, with diseased biopsy samples stratified on the basis of patient kidney function (eGFR). Results revealed a significant increase in MAIT cell numbers in diseased biopsy samples from patients with reduced kidney function (eGFR<60 ml/min per 1.73 m2) compared with diseased biopsy samples from patients with normal kidney function (eGFR≥60 ml/min per 1.73 m2) and healthy kidney tissue (Figure 2A). Further analysis showed significant negative correlations between patient eGFR and MAIT cell numbers (r=−0.47, P<0.001) (Figure 2B).
Figure 2.
MAIT cell numbers correlate significantly with loss of kidney function and degree of interstitial fibrosis. (A) Absolute numbers of MAIT cells in healthy kidney tissue and diseased biopsy samples with eGFR≥60 and eGFR<60. Values for individual donors are presented; horizontal bars represent medians, with interquartile range also presented. *P<0.05, **P<0.01, Kruskal–Wallis test with Dunn’s post-test. (B) Spearman correlation analysis of absolute numbers of MAIT cells versus patient eGFR. (C) Absolute numbers of MAIT cells in healthy kidney tissue and diseased biopsy samples without and with fibrosis. Values for individual donors are presented; horizontal bars represent medians, with interquartile range also presented. *P<0.05, **P<0.01, Kruskal–Wallis test with Dunn’s post-test. (D) Spearman correlation analysis of absolute numbers of MAIT cells versus percentages of interstitial fibrosis in diseased biopsy samples.
Significantly Elevated Numbers of Human MAIT Cells in Diseased Biopsy Samples with Interstitial Fibrosis
In addition to kidney function, diseased biopsy samples were grouped on the basis of the histologic absence or presence of interstitial fibrosis, the characteristic feature of all patterns of CKD. Significantly elevated numbers of MAIT cells were identified in diseased biopsy samples with interstitial fibrosis compared with diseased biopsy samples without fibrosis and healthy kidney tissue (Figure 2C). Notably, a significant correlation between MAIT cell numbers and the degree of interstitial fibrosis was also observed (Figure 2D). Collectively, these results associate MAIT cells with CKD pathogenesis and loss of kidney function.
We also correlated MAIT cell numbers to primary diagnoses of patients, with diseased biopsy samples stratified into glomerular immune-mediated, glomerular non–immune-mediated, and nonglomerular diseases. However, no significant differences in MAIT cell numbers between disease groupings were observed (Supplemental Figure 1A).
Significantly Elevated CD69 Expression on MAIT Cells in Diseased Biopsy Samples with Interstitial Fibrosis
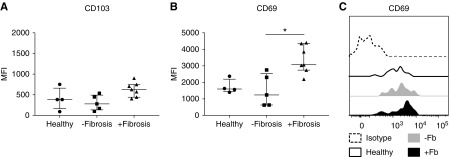
We next examined the phenotypes of kidney MAIT cells in healthy and diseased kidney tissue. Expression levels of surface markers TCR Vα7.2, CD161, CD8, IL-18Rα, IL-7Rα, CCR5, and CXCR3 on MAIT cells were comparable between healthy and diseased kidney tissue (data not shown). As for healthy kidneys, MAIT cells in diseased kidney biopsy samples displayed a tissue-resident phenotype, with expression of both CD103 and CD69 (Figure 3). Although CD103 expression levels were similar between healthy and diseased kidney tissue (Figure 3A), MAIT cell expression of CD69, a marker of tissue-residence, but also lymphocyte activation, was significantly elevated in diseased biopsy samples with interstitial fibrosis compared with nonfibrotic biopsy samples (Figure 3, B and C). No significant differences in CD103 or CD69 expression levels were observed when diseased biopsy samples were stratified on the basis of primary diagnoses of patients (Supplemental Figure 1, B and C). These results indicate that the local environment within fibrotic kidneys directs MAIT cells toward a more activated state.
Figure 3.
MAIT cells in diseased biopsy samples with interstitial fibrosis display a more activated phenotype. (A and B) Surface expression of (A) CD103 and (B) CD69 on MAIT cells in healthy kidney tissue and diseased biopsy samples without and with fibrosis. Values of median fluorescence intensity (MFI) for individual donors are shown; horizontal bars represent medians, with interquartile range also presented. *P<0.05, Kruskal–Wallis test with Dunn’s post-test. (C) Representative histogram of CD69 expression on MAIT cells from healthy kidney tissue (black unfilled) and diseased biopsy samples without (gray filled) and with (black filled) interstitial fibrosis compared with isotype (dashed).
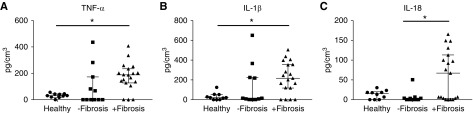
Significantly Elevated IL-18 in Diseased Biopsy Samples with Interstitial Fibrosis
The supernatant from dissociated tissue samples was analyzed for cytokine levels. Proinflammatory cytokines TNF-α (Figure 4A) and IL-1β (Figure 4B) were significantly elevated in the supernatant of dissociated fibrotic biopsy samples compared with healthy tissue. The critical role of innate cytokines, including IL-18, is established in MAIT cell activation.31 Notably, IL-18 levels were significantly increased in diseased biopsy samples with interstitial fibrosis compared with nonfibrotic biopsy samples (Figure 4C). Our data suggest that the inflammatory microenvironment within fibrotic kidneys contributes to MAIT cell activation.
Figure 4.
The proinflammatory microenvironment in diseased biopsy samples with interstitial fibrosis may direct MAIT cells toward a more activated state. (A–C) Levels of (A) TNF-α, (B) IL-1β, and (C) IL-18 in the supernatant of dissociated healthy kidney tissue (n=10) and diseased biopsy samples stratified on the basis of the absence (n=11) or presence of interstitial fibrosis (n=19). Values for individual donors are presented; horizontal bars represent medians, with interquartile range also presented. *P<0.05, Kruskal–Wallis test with Dunn’s post-test.
Colocalization of MAIT Cells with PTECs in Fibrotic Kidney Tissue
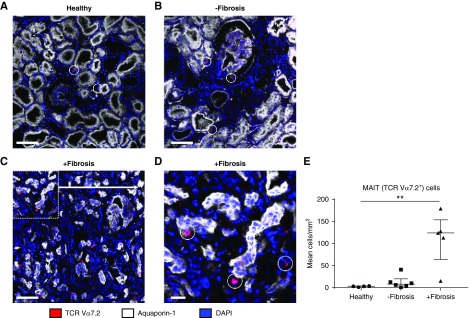
We next examined the localization of MAIT cells in human kidney tissue using TCR Vα7.2 IF staining. TCR Vα7.2+ cells were identified in healthy kidney tissue (Figure 5A), supporting the concept of tissue-resident MAIT cells. Consistent with our flow cytometric quantitative data, an increased presence of MAIT cells was detected in diseased biopsy samples with interstitial fibrosis (Figure 5, C and D) compared with nonfibrotic diseased biopsy samples (Figure 5B) and healthy kidney tissue (Figure 5A). Quantitative analysis was performed, confirming a significantly increased accumulation of MAIT cells in fibrotic biopsy samples (Figure 5E). Notably, MAIT cells were identified within the tubulointerstitial compartment, adjacent to PTECs, defined as tubular cells expressing aquaporin-1.32 Moreover, in fibrotic kidney tissue, MAIT cells localized to sites of tubulointerstitial injury (inflammation, tubular atrophy), suggesting that these cells are positioned to play a role during fibrotic disease progression.
Figure 5.
Colocalization of human MAIT cells with PTECs in fibrotic kidney tissue suggests complex pathogenic interactions between the cell populations. (A–D) IF staining of frozen sections from (A) healthy kidney tissue, (B) nonfibrotic diseased kidney tissue, and (C and D) fibrotic diseased kidney tissue stained for TCR Vα7.2 (red), Aquaporin-1 (white), and DAPI (blue). MAIT cells are circled. Scale bars represent (A–C) 100 μm and (D) 20 μm. (E) Quantification of MAIT cells from healthy kidney tissue (n=4), nonfibrotic kidney biopsy samples (n=6), and fibrotic kidney biopsy samples (n=5). Values for individual donors are presented; horizontal bars represent medians, with interquartile range also presented. **P<0.01, Kruskal–Wallis test with Dunn’s post-test.
MAIT Cells Activated in the Presence of Hypoxic PTECs Produce Significantly Increased Levels of Perforin and Granzyme B
Renal hypoxia is an established driver of tubulointerstitial inflammation/fibrosis and, thus, progression to CKD.33 We postulated that the activated phenotype of MAIT cells in fibrotic kidney tissue may be driven by adjacent PTECs in the hypoxic microenvironment. Therefore, we established an in vitro coculture system to model this human CKD setting and, thus, investigate the functional capacity of hypoxic PTECs to activate MAIT cells.
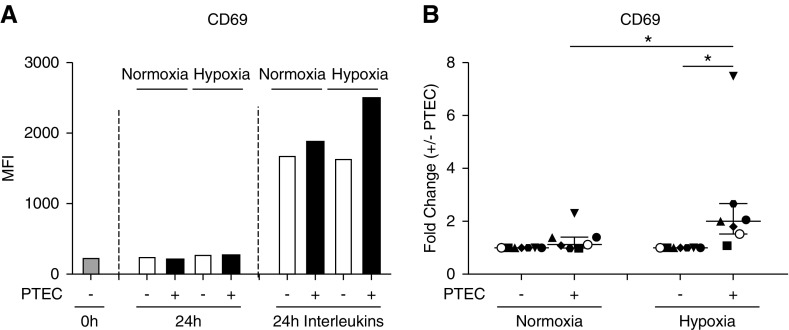
We isolated human MAIT cells by immuno-magnetic selection and flow cytometry sorting and examined CD69 expression levels on MAIT cells cocultured with preconditioned normoxic (21% O2) or hypoxic (1% O2) PTECs in the absence or presence of IL (IL-12p70, IL-15, IL-18) stimulation. There was minimal expression of CD69 on freshly isolated MAIT cells and MAIT cells cultured in the absence of IL stimulation (Figure 6A). MAIT cell expression of CD69 was upregulated in response to IL stimulation, with highest levels detected in hypoxic PTEC cocultures (Figure 6A). Over seven individual PTEC-MAIT cell coculture experiments, the fold change in MAIT cell CD69 expression (MFI in the presence of PTECs/MFI in the absence of PTECs) was significantly increased under hypoxic conditions, but not normoxic conditions (Figure 6B). Moreover, the fold change in MAIT cell CD69 expression under hypoxic conditions was significantly elevated compared with normoxic conditions (Figure 6B).
Figure 6.
MAIT cells activated in the presence of hypoxic PTECs display significantly upregulated expression of CD69. (A) CD69 expression by MAIT cells freshly isolated (0 hours) or after 24-hour culture without (−) or with (+) preconditioned PTECs under normoxic or hypoxic conditions in the absence (24-hour) or presence (24-hour IL) of IL-12p70, IL-15, and IL-18. Surface expression was measured by flow cytometry (gated on live, single, CD45+ cells) and expressed as the median fluorescence intensity (MFI). One representative donor experiment is shown. (B) Fold changes (MFI in the presence of PTECs/MFI in the absence of PTECs; +/− PTECs) in CD69 levels on IL-stimulated MAIT cells under normoxic and hypoxic conditions for seven individual donor PTEC experiments. Symbols represent individual donor PTEC experiments; the representative donor experiment from (A) is identified using open circles. Horizontal bars represent medians, with interquartile range also presented. *P<0.05, Wilcoxon matched-pairs signed-rank test.
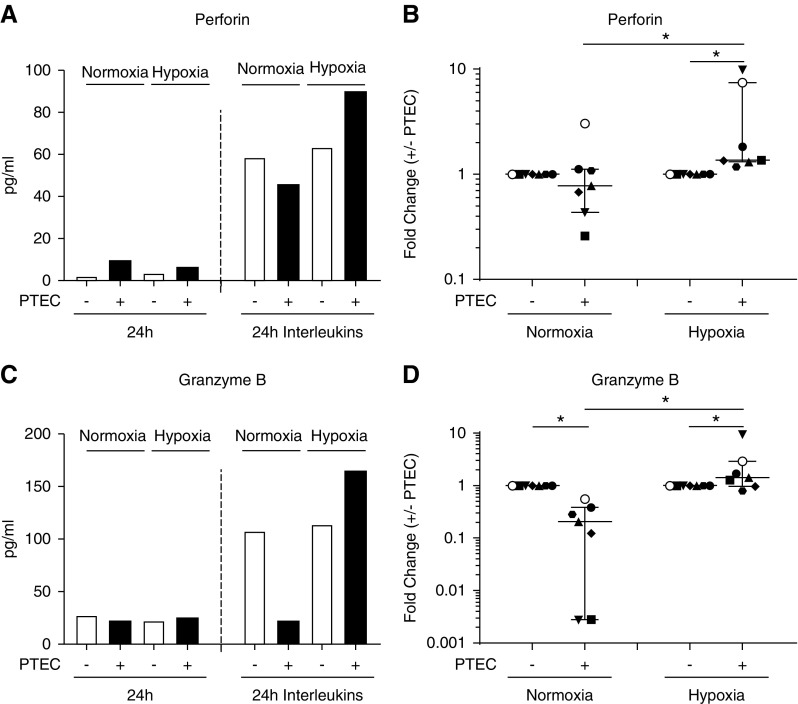
The production of cytotoxic effector molecules (perforin and granzyme B) by MAIT cells was also examined. MAIT cells activated in the presence of hypoxic PTECs secreted the greatest levels of both perforin and granzyme B (Figure 7, A and C). Notably, the fold change in MAIT cell production of perforin and granzyme B (concentration in the presence of PTECs/concentration in the absence of PTECs) was significantly elevated under hypoxic conditions (Figure 7, B and D). For both cytotoxic molecules, the fold change under hypoxic conditions was also significantly increased compared with the fold change under normoxic conditions (Figure 7, B and D). No significant differences between hypoxic and normoxic conditions were observed for other detectable analytes (granulysin, granzyme A, IFN-γ, IL-17A) (data not shown).
Figure 7.
MAIT cells activated in the presence of hypoxic PTECs produce significantly increased levels of cytotoxic molecules. (A and C) (A) Perforin and (C) granzyme B production by MAIT cells after 24-hour culture without (−) or with (+) preconditioned PTECs under normoxic or hypoxic conditions in the absence (24-hour) or presence (24-hour IL) of IL-12p70, IL-15, and IL-18. One representative donor experiment is shown. (B and D) Fold changes (concentration in the presence of PTECs/concentration in the absence of PTECs; +/− PTECs) in cytotoxic molecule production by IL-stimulated MAIT cells under normoxic and hypoxic conditions for seven individual donor PTEC experiments. Symbols represent individual donor PTEC experiments; the representative donor experiment from (A and C) is identified using filled triangles. Horizontal bars represent medians, with interquartile range also presented. *P<0.05, Wilcoxon matched-pairs signed-rank test.
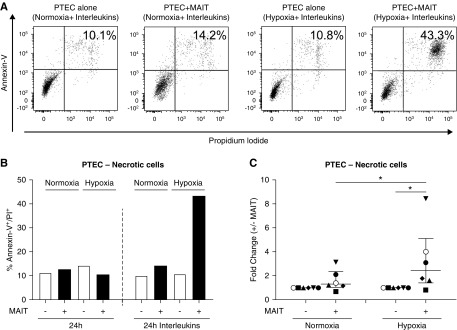
Significantly Increased PTEC Necrosis in Inflammatory/Hypoxic Cocultures
We next examined the possible functional role of these cytotoxic molecules in driving PTEC damage. PTEC injury was assessed in this in vitro coculture model by Annexin-V/PI staining, with highest levels of PTEC necrosis (percentage of Annexin-V+ PI+ cells) detected in hypoxic cocultures in the presence of IL stimulation (Figure 8, A and B). The fold change in PTEC necrosis (percentage of Annexin-V+ PI+ cells in the presence of MAIT cells/percentage of Annexin-V+ PI+ cells in the absence of MAIT cells) was significantly elevated under hypoxic conditions, but not normoxic conditions (Figure 8C). Furthermore, the fold change in PTEC necrosis under hypoxic conditions was significantly elevated compared with normoxic conditions (Figure 8C). Taken together, these data suggest that hypoxic PTECs activate cytotoxic MAIT cells within the inflammatory/fibrotic tubulointerstitium, which, in turn, leads to PTEC damage/necrosis.
Figure 8.
Significantly increased PTEC killing by activated cytotoxic MAIT cells in inflammatory/hypoxic cocultures. (A and B) PTEC viability after 24-hour culture without (−) or with (+) MAIT cells under normoxic or hypoxic conditions in the absence (24-hour) or presence (24-hour IL) of IL-12p70, IL-15, and IL-18. The percentage of Annexin-V+ PI+ necrotic cells was determined by flow cytometry. Results from one representative donor experiment are presented in the (A) dot plots and (B) by bar graph. (C) Fold changes (percentage of Annexin-V+ PI+ cells in the presence of MAIT cells/percentage of Annexin-V+ PI+ cells in the absence of MAIT cells) in PTEC necrosis (in the presence of IL) under normoxic and hypoxic conditions for six individual donor PTEC experiments. Symbols represent individual donor PTEC experiments; the representative donor experiment from (A and B) is identified using open circles. Horizontal bars represent medians, with interquartile range also presented. *P<0.05, Wilcoxon matched-pairs signed-rank test.
Discussion
MAIT cells have been previously identified in various human peripheral organs, with preferential enrichment within the liver and at mucosal barriers, including the gastrointestinal tract.8,10 Initial reports of MAIT cells in human kidney tissue have been made, although only by detection of MAIT cell–specific TCR transcripts (Vα7.2-Jα33 and Vα7.2-Jα12).22 Our study is the first to unequivocally identify MAIT cells, on the basis of cell surface expression of both TCR Vα7.2 and high levels of CD161, in healthy and diseased human kidney tissue.
Our phenotypic data report that human kidney MAIT cells are a population of tissue-resident lymphocytes. They coexpress tissue-residency markers CD103 (the α chain of the αEβ7 integrin) and CD69 (a type II C-lectin receptor), both molecules directly involved in tissue accumulation and retention.34,35 Tissue-resident cells represent a distinct population of lymphocytes that are noncirculating and establish residency in peripheral tissues under steady-state conditions. These tissue-resident lymphocytes self-renew independently of circulating precursors and serve as local sentinels in response to pathogens and stress ligands.36 Indeed, tissue-resident lymphocytes have been described in almost every peripheral organ, with evidence that residents greatly outnumber recirculating cells within mouse nonlymphoid tissues, including the kidney.37
Kidney-residing lymphocytes have been examined in experimental murine studies,38–43 yet translation of this work to human clinical samples remains limited. A longitudinal examination of repeat kidney biopsy samples from patients with lupus nephritis reported the expansion and persistence of individual T cell clones for up to 6 years, providing evidence of tissue-resident lymphocytes in lupus nephritis pathogenesis.44 We previously reported the accumulation of IFN-γ-producing CD69+ natural killer cells in kidney biopsy samples with interstitial fibrosis, proposing a functional role for these putative tissue-resident lymphocytes in human CKD.45 In this study, we provide the first evidence of tissue-resident MAIT cells in human kidney tissue, in line with published reports of tissue-resident MAIT cells in the human liver,46 gastric mucosa,14 and female genital mucosa.47
The formation of tissue-resident lymphocytes is largely dependent on local environmental cues, including cytokines IL-15 and TGF-β.48,49 In particular, a recent investigation documented a pivotal role for TGF-β in driving the development of kidney-resident lymphocytes in mice.40 In this study, Ma et al.40 showed that TGF-β promotes transendothelial migration of effector T cells and, thereby, residency in the kidney, by upregulating chemokine receptor CXCR3 on these lymphocytes. In our study, we have similarly identified strong expression of CXCR3 on tissue-resident MAIT cells in human kidney tissue. Given the central role of TGF-β in human kidney homeostasis and disease,50,51 we speculate that an analogous TGF-β–mediated pathway may promote the extravasation and subsequent formation of human kidney-resident MAIT cells.
Human MAIT cells have been linked to the immunopathogenesis of nonrenal chronic inflammatory diseases across multiple peripheral organs (gut, skin, brain).12,17,19,20,52 In particular, Hegde et al.53 recently highlighted a profibrogenic role for human MAIT cells in hepatic fibrosis, the final common pathway for chronic liver injury. Here, for the first time in a renal model, we demonstrate significant correlations between absolute MAIT cell numbers and both loss of kidney function (lower eGFR) and histologic severity of CKD (levels of interstitial fibrosis) in humans.
Although CD69 is constitutively expressed on resident lymphocytes to limit egress from peripheral tissues, the molecule is also established as a marker of activation on effector cells.54 Notably, we detected significantly elevated levels of CD69 on MAIT cells in diseased biopsy samples with interstitial fibrosis compared with nonfibrotic biopsy samples, with a concomitant increase in proinflammatory cytokines TNF-α, IL-1β, and IL-18 in fibrotic biopsy samples. These data are consistent with the concept that the inflammatory milieu within fibrotic kidneys skews MAIT cells toward an activated phenotype. A similar concept has been previously reported in patients with SLE, with the activation status of MAIT cells, on the basis of CD69 expression levels, shown to correlate with disease activity as well as plasma concentrations of inflammatory cytokines, including IL-18.55
The contribution of cytokine-mediated inflammation in the pathogenesis of CKD is well established. In particular, the literature ascribes functional roles to innate cytokines IL-12p70, IL-15, and IL-18 in renal pathobiology.56,57 These molecules are all potent proinflammatory cytokines secreted primarily by monocytes/macrophages and dendritic cells.58 Because of their constitutive expression of IL receptors, MAIT cells can be activated by combinations of these innate cytokines in the absence of TCR ligation.55,59 In turn, this leads to cytotoxic licensing of MAIT cells.60,61 However, until now, the role of MAIT cells in sensing these proinflammatory signals in CKD has not been evaluated.
In our fibrotic biopsy samples, MAIT cells were detected in areas of tubulointerstitial injury (inflammation/fibrosis), often in direct contact with PTECs. Renal hypoxia is a key pathobiologic driver of this tubulointerstitial injury in CKD, driven by a loss of peritubular capillaries, reduced efficiency of oxygen diffusion, and increased oxygen consumption in the diseased kidney.2,33 PTECs are particularly sensitive to hypoxia due to their dependence on aerobic oxidative metabolism,62 in turn leading to cellular stress and initiation of proinflammatory responses in the tubulointerstitial compartment.2,28 Thus, in order to define the functional interactions between colocalized PTECs and MAIT cells in human CKD, we established an in vitro coculture model under hypoxic conditions. Importantly, we showed that in association with proinflammatory IL, hypoxic PTECs dramatically enhance MAIT cell activation and cytotoxic licensing (perforin and granzyme B production). Furthermore, we demonstrated significantly increased PTEC necrosis in this inflammatory/hypoxic coculture. This supports the concept that tubulointerstitial MAIT cells are poised to sense PTEC-derived danger signals in the CKD microenvironment which, in turn, leads to their activation and cytotoxic activity (PTEC killing).
The perforin–granzyme B cytotoxic pathway has been implicated in the pathophysiology of chronic inflammatory diseases,63,64 and animal models of kidney disease have highlighted a role for the perforin–granzyme B pathway in promoting chronic tissue injury.65 Our data provide the first human evidence suggesting that MAIT cells respond to activatory signals from PTECs within the inflammatory/fibrotic kidney microenvironment by skewing to a cytotoxic (perforin- and granzyme B–producing) phenotype and function. Future studies of PTEC-MAIT cell interactions will be required to elucidate the relative contribution of TCR-dependent (triggered by MR1-antigen complexes) versus TCR-independent (cytokine-induced) signaling pathways in this activation process.
In contrast to these inflammatory/hypoxic conditions, we also showed that MAIT cells activated in the presence of normoxic (healthy) PTECs produce significantly reduced levels of granzyme B (Figure 7D). These findings are suggestive of an immuno-inhibitory role for healthy PTECs under homeostatic conditions and are reminiscent of previous studies from our group demonstrating PTEC-mediated immuno-regulation of T cell, B cell, and dendritic cell function.27,66–68
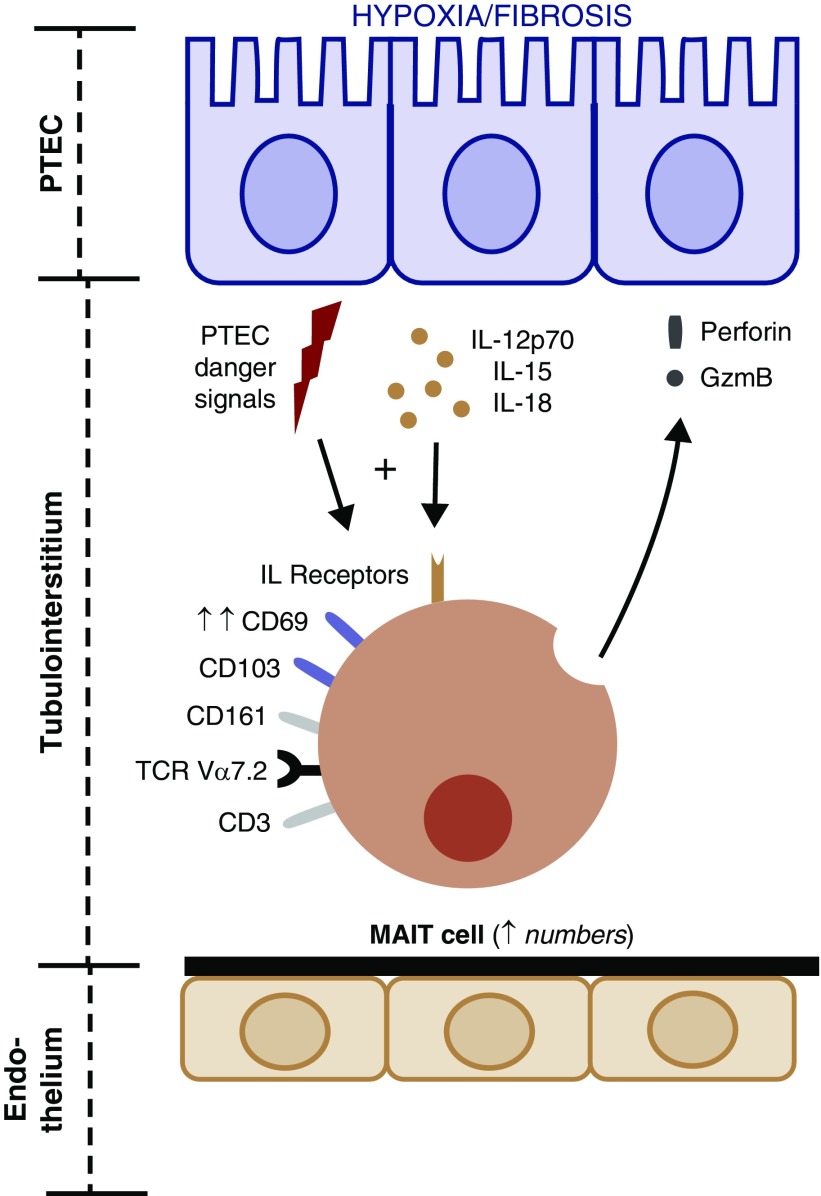
Collectively, these results provide the first comprehensive characterization of MAIT cells in healthy and diseased human kidneys. Our identification of tissue-resident MAIT cells in healthy kidneys suggests that they play an important role in homeostatic maintenance and protection against microbial infections (e.g., uropathogenic bacteria). In contrast, under the inflammatory/fibrotic conditions of CKD, we propose that kidney MAIT cells are activated by immuno-stimulatory danger signals that skew them toward a more pathogenic functionality (Figure 9). A deeper understanding of the mechanisms regulating kidney MAIT cell immunity in health and disease is now essential to stimulate the development of novel therapeutic strategies for the treatment of CKD. In particular, the crosstalk between these MAIT cells and other discrete immune cell populations that we have previously identified in inflammatory/fibrotic CKD (e.g., TGF-β–producing CD1c+ DCs,69 IFN-γ–secreting CD56bright natural killer cells,45 and IL-17A–expressing γδ T cells70) will be a significant area for future clinical investigation. Indeed, it will be critical to extend on the limitations of our studies and establish how these multiple immune cell populations collectively drive inflammatory/fibrotic CKD. Further functional examination of these distinct human immune cell populations (including MAIT cells) in renal fibrosis will be necessary to unequivocally define the unique, nonredundant role of MAIT cells in CKD pathogenesis.
Figure 9.
Human tissue-resident MAIT cells within the renal tubulointerstitium are poised to sense PTEC-derived danger signals in the inflammatory/fibrotic CKD micro-environment that, in turn, drives their activation (up-regulated expression of CD69) and cytotoxic activity (perforin/granzyme B production).
Disclosures
A/Prof. Healy reports grants from the National Health and Medical Research Council (GNT1099222), grants from Royal Brisbane & Women’s Hospital Foundation (project grant), grants and other from Royal Brisbane & Women’s Hospital (institutional grant), and other from Chemical Pathology, Pathology Queensland, during the conduct of the study.
Funding
The work was funded by Pathology Queensland, a Royal Brisbane and Women’s Hospital Research Grant, the Kidney Research Foundation, and National Health and Medical Research Council Project Grants (GNT1099222 and GNT1161319). Dr. Law was supported by a Pathology Queensland Study, Education, and Research Committee Scholarship. Mr. Giuliani was supported by an Australian Government Research Training Program Scholarship.
Supplementary Material
Acknowledgments
The authors would like to thank the tissue donors and clinicians, particularly renal histopathologist Dr. Leo Francis (Queensland Health), for assessment of interstitial fibrosis levels in kidney biopsy specimens.
Each author has participated sufficiently in the work to take public responsibility for the content. Dr. Law, A/Prof. Wilkinson, Prof. Beagley, A/Prof. Ungerer, A/Prof Healy, and Dr. Kassianos conceived and designed the study. Dr. Law, Ms. Wang, Dr. Kildey, Mr. Giuliani, and Dr. Kassianos carried out experiments and analyzed the data. Dr. Law, A/Prof. Wilkinson, A/Prof. Healy, and Dr. Kassianos drafted the paper. All authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “MAIT Cells as Drivers of Renal Fibrosis and CKD,” on pages 1145–1146.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/10.1681/ASN.2018101064/-/DCSupplemental.
Supplemental Table 1. Clinical and histologic features of patients at the time of kidney biopsy.
Supplemental Table 2. Antibodies used for flow cytometric staining.
Supplemental Figure 1. MAIT cell numbers and phenotype do not significantly correlate with primary diagnoses of patients. Absolute numbers of MAIT cells (A), and MAIT cell expression of CD103 (B) and CD69 (C) in healthy kidney tissue and diseased biopsy samples with glomerular immune-mediated (GI), glomerular non–immune-mediated (GNI) and nonglomerular (NG) primary diagnoses. Values for individual donors are presented; horizontal bars represent medians, with interquartile range also presented.
References
- 1.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al.: Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94: 567–581, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Kawakami T, Mimura I, Shoji K, Tanaka T, Nangaku M: Hypoxia and fibrosis in chronic kidney disease: Crossing at pericytes. Kidney Int Suppl (2011) 4: 107–112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L, et al.: Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem 280: 21183–21193, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Miley MJ, Truscott SM, Yu YY, Gilfillan S, Fremont DH, Hansen TH, et al.: Biochemical features of the MHC-related protein 1 consistent with an immunological function. J Immunol 170: 6090–6098, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al.: Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422: 164–169, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al.: An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 189: 1907–1921, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al.: Stepwise development of MAIT cells in mouse and human. PLoS Biol 7: e54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al.: Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117: 1250–1259, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Turtle CJ, Delrow J, Joslyn RC, Swanson HM, Basom R, Tabellini L, et al.: Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161(hi) CD8α+ semi-invariant T cells. Blood 118: 2752–2762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al.: Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 210: 2305–2320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, et al.: MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog 9: e1003681, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, Ahmad A: Role of MAIT cells in the immunopathogenesis of inflammatory diseases: New players in old game. Int Rev Immunol 37: 90–110, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Dias J, Leeansyah E, Sandberg JK: Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci U S A 114: E5434–E5443, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth JS, Salerno-Goncalves R, Blanchard TG, Patil SA, Kader HA, Safta AM, et al.: Mucosal-associated invariant T cells in the human gastric mucosa and blood: Role in Helicobacter pylori infection. Front Immunol 6: 466, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurioka A, Walker LJ, Klenerman P, Willberg CB: MAIT cells: New guardians of the liver. Clin Transl Immunology 5: e98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al.: Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11: 701–708, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, et al.: Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 176: 266–274, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, et al.: Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 125: 1752–1762, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teunissen MBM, Yeremenko NG, Baeten DLP, Chielie S, Spuls PI, de Rie MA, et al.: The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Invest Dermatol 134: 2898–2907, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, et al.: CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol 44: 3119–3128, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, et al.: Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol 193: 3891–3901, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al.: Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun 5: 3866, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, et al.: Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol 20: 1517–1525, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al.: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kildey K, Law B, Muczynski K, Wilkinson R, Healy H, Kassianos A: Identification and quantitation of leukocyte populations in human kidney tissue by multi-parameter flow cytometry. Bio Protoc 8: e2980, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glynne PA, Evans TJ: Inflammatory cytokines induce apoptotic and necrotic cell shedding from human proximal tubular epithelial cell monolayers. Kidney Int 55: 2573–2597, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kassianos AJ, Sampangi S, Wang X, Roper KE, Beagley K, Healy H, et al.: Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol Dial Transplant 28: 303–312, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Wilkinson R, Kildey K, Potriquet J, Mulvenna J, Lobb RJ, et al.: Unique molecular profile of exosomes derived from primary human proximal tubular epithelial cells under diseased conditions. J Extracell Vesicles 6: 1314073, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al.: Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al.: Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao X, Cai J: Mucosal-associated invariant T cells: New insights into antigen recognition and activation. Front Immunol 8: 1540, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedford JJ, Leader JP, Walker RJ: Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol 14: 2581–2587, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Mimura I, Nangaku M: The suffocating kidney: Tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol 6: 667–678, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al.: Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194: 2059–2063, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S: The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. J Immunol 159: 3748–3756, 1997 [PubMed] [Google Scholar]
- 36.Fan X, Rudensky AY: Hallmarks of tissue-resident lymphocytes. Cell 164: 1198–1211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, et al.: Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161: 737–749, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost EL, Kersh AE, Evavold BD, Lukacher AE: Cutting edge: Resident memory CD8 T cells express high-affinity TCRs. J Immunol 195: 3520–3524, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al.: Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma C, Mishra S, Demel EL, Liu Y, Zhang N: TGF-β controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J Immunol 198: 749–756, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, et al.: Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-asialo-GM1 antibody. J Immunol 195: 4973–4985, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC: Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al.: Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, et al.: Immunologic characteristics of intrarenal T cells: Trafficking of expanded CD8+ T cell β-chain clonotypes in progressive lupus nephritis. Arthritis Rheum 64: 1589–1600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law BMP, Wilkinson R, Wang X, Kildey K, Lindner M, Rist MJ, et al.: Interferon-γ production by tubulointerstitial human CD56bright natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int 92: 79–88, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al.: IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 190: 3142–3152, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, et al.: MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol 10: 35–45, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al.: T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43: 1101–1111, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al.: The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Sureshbabu A, Muhsin SA, Choi ME: TGF-β signaling in the kidney: Profibrotic and protective effects. Am J Physiol Renal Physiol 310: F596–F606, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kassianos AJ, Wang X, Sampangi S, Muczynski K, Healy H, Wilkinson R: Increased tubulointerstitial recruitment of human CD141(hi) CLEC9A(+) and CD1c(+) myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am J Physiol Renal Physiol 305: F1391–F1401, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Haga K, Chiba A, Shibuya T, Osada T, Ishikawa D, Kodani T, et al.: MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol 31: 965–972, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, et al.: Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun 9: 2146, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simms PE, Ellis TM: Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin Diagn Lab Immunol 3: 301–304, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiba A, Tamura N, Yoshikiyo K, Murayama G, Kitagaichi M, Yamaji K, et al.: Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res Ther 19: 58, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, et al.: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Azzi S, Gallerne C, Romei C, Le Coz V, Gangemi R, Khawam K, et al.: Human renal normal, tumoral, and cancer stem cells express membrane-bound interleukin-15 isoforms displaying different functions. Neoplasia 17: 509–517, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carroll HP, Paunovic V, Gadina M: Signalling, inflammation and arthritis: Crossed signals: The role of interleukin-15 and -18 in autoimmunity. Rheumatology (Oxford) 47: 1269–1277, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al.: CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 44: 195–203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sattler A, Dang-Heine C, Reinke P, Babel N: IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol 45: 2286–2298, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, et al.: MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol 8: 429–440, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein FH: Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Hiebert PR, Granville DJ: Granzyme B in injury, inflammation, and repair. Trends Mol Med 18: 732–741, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Boivin WA, Cooper DM, Hiebert PR, Granville DJ: Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab Invest 89: 1195–1220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujinaka H, Yamamoto T, Feng L, Nameta M, Garcia G, Chen S, et al.: Anti-perforin antibody treatment ameliorates experimental crescentic glomerulonephritis in WKY rats. Kidney Int 72: 823–830, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Wilkinson R, Wang X, Roper KE, Healy H: Activated human renal tubular cells inhibit autologous immune responses. Nephrol Dial Transplant 26: 1483–1492, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Sampangi S, Kassianos AJ, Wang X, Beagley KW, Klein T, Afrin S, et al.: The mechanisms of human renal epithelial cell modulation of autologous dendritic cell phenotype and function. PLoS One 10: e0134688, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sampangi S, Wang X, Beagley KW, Klein T, Afrin S, Healy H, et al.: Human proximal tubule epithelial cells modulate autologous B-cell function. Nephrol Dial Transplant 30: 1674–1683, 2015 [DOI] [PubMed] [Google Scholar]
- 69.Kassianos AJ, Wang X, Sampangi S, Afrin S, Wilkinson R, Healy H: Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney Int 87: 1153–1163, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Law BM, Wilkinson R, Wang X, Kildey K, Lindner M, Beagley K, et al.: Effector γδ T cells in human renal fibrosis and chronic kidney disease. Nephrol Dial Transplant 34: 40–48, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.