Significance Statement
Studies of the adverse renal consequences of AKI have almost exclusively focused on eGFR changes, whereas few studies have examined AKI’s effects on proteinuria. The authors analyzed data from two prospective cohort studies that assessed urine protein-to-creatinine ratio, BP, eGFR, medication use and other important covariates annually per research protocol and tracked interim episodes of hospitalization for AKI. They found that an episode of hospitalized AKI was independently and significantly associated with increased proteinuria. Further research is needed to examine worsening proteinuria as a potential mechanism by which AKI leads to accelerated loss of renal function. The authors’ findings also suggest that routine monitoring of proteinuria after AKI may be warranted, and highlight the need for research to determine how to best manage proteinuria post-AKI.
Keywords: acute renal failure, proteinuria, renal injury
Visual Abstract
Abstract
Background
Prior studies of adverse renal consequences of AKI have almost exclusively focused on eGFR changes. Less is known about potential effects of AKI on proteinuria, although proteinuria is perhaps the strongest risk factor for future loss of renal function.
Methods
We studied enrollees from the Assessment, Serial Evaluation, and Subsequent Sequelae of AKI (ASSESS-AKI) study and the subset of the Chronic Renal Insufficiency Cohort (CRIC) study enrollees recruited from Kaiser Permanente Northern California. Both prospective cohort studies included annual ascertainment of urine protein-to-creatinine ratio, eGFR, BP, and medication use. For hospitalized participants, we used inpatient serum creatinine measurements obtained as part of clinical care to define an episode of AKI (i.e., peak/nadir inpatient serum creatinine ≥1.5). We performed mixed effects regression to examine change in log-transformed urine protein-to-creatinine ratio after AKI, controlling for time-updated covariates.
Results
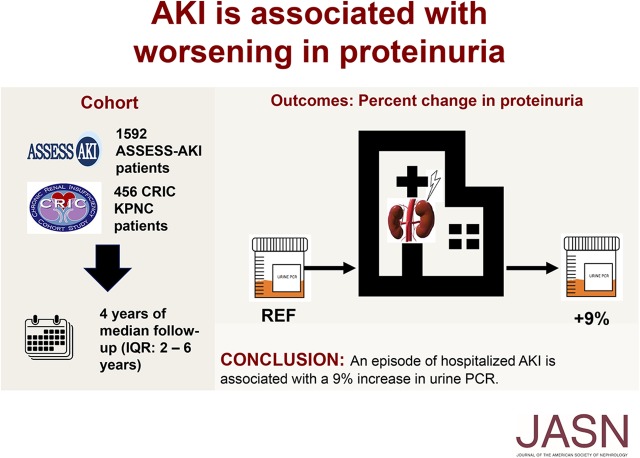
At cohort entry, median eGFR was 62.9 ml/min per 1.73 m2 (interquartile range [IQR], 46.9–84.6) among 2048 eligible participants, and median urine protein-to-creatinine ratio was 0.12 g/g (IQR, 0.07–0.25). After enrollment, 324 participants experienced at least one episode of hospitalized AKI during 9271 person-years of follow-up; 50.3% of first AKI episodes were Kidney Disease Improving Global Outcomes stage 1 in severity, 23.8% were stage 2, and 25.9% were stage 3. In multivariable analysis, an episode of hospitalized AKI was independently associated with a 9% increase in the urine protein-to-creatinine ratio.
Conclusions
Our analysis of data from two prospective cohort studies found that hospitalization for an AKI episode was independently associated with subsequent worsening of proteinuria.
There is substantial recent interest in defining the extent to which episodes of acute kidney injury (AKI) may independently contribute to the development or acceleration of chronic kidney disease (CKD).1,2 Prior studies of the consequences of AKI on subsequent kidney function have almost exclusively focused on changes in estimated glomerular filtration rate (eGFR). Few studies have examined the effect of AKI on subsequent change in the level of proteinuria,2,3 although proteinuria is an important measure of kidney damage, a key parameter for diagnosis and classification of CKD severity,4 and perhaps, the strongest risk factor for future loss of renal function.5
Animal models of renal ischemia-reperfusion injury demonstrate a higher risk of proteinuria after AKI.6 Increased levels of proteinuria after AKI in humans could reflect residual renal tubular damage, including impaired proximal tubular uptake of filtered albumin, because albumin and other low molecular weight proteins that reach the tubular lumen are normally reabsorbed by tubular cells.7,8 In addition, lack of full recovery from tubular injury may lead to higher levels of brush border components of cellular enzymes in the urine.9 Finally, physicians may avoid use of medications, such as angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs), after AKI out of concerns that ACE-I/ARB will predispose to recurrent AKI; this together with less strict blood pressure (BP) control may also lead to higher levels of proteinuria after AKI.
A recent study suggested that AKI is a risk factor for incident or worsening proteinuria among US veterans.10 However, those results were only on the basis of semiquantitative dipstick proteinuria measurements obtained as part of routine clinical care, and therefore, there was likely ascertainment bias in addition to greater measurement error. Use or nonuse of ACE-I/ARB after AKI was also not systematically assessed.10 In contrast, a prospective study of 131 pediatric patients undergoing cardiac surgery found that those who experienced mild to moderate AKI did not subsequently have greater albuminuria compared with those who did not experience AKI.11
The goal of this study was to quantify rigorously the association between an episode of hospitalized AKI and changes in the level of proteinuria in adults. To achieve this, we analyzed data from two contemporary prospective cohort studies.
Methods
To increase sample size and generalizability, including encompassing a wider range of eGFR, we a priori planned to combine data from participants of two prospective National Institutes of Health–sponsored cohort studies: all enrollees from the Assessment, Serial Evaluation, and Subsequent Sequelae of AKI (ASSESS-AKI) study and the subset of the Chronic Renal Insufficiency Cohort (CRIC) study enrollees recruited from Kaiser Permanente Northern California, a large integrated health care delivery system in which episodes of AKI are comprehensively captured through their electronic health record system. To study if AKI is associated with greater urinary protein excretion, we conducted a longitudinal analysis of within-person change in urine protein-to-creatinine ratio before and after an episode of hospitalized AKI among these ASSESS-AKI study and CRIC study participants.
Study Sample
The ASSESS-AKI study is a prospective, parallel-matched cohort study of patients discharged from the hospital.12 Briefly, a total of 1603 adults ages 18–89 years old were enrolled from 2009 to 2015 at four Clinical Research Centers. At entry, participants were required to have an eGFR≥15 ml/min per 1.73 m2 (no upper limit to eGFR) and no history of maintenance hemodialysis or peritoneal dialysis. All ASSESS-AKI study participants were enrolled for long-term follow-up starting 3 months after initial hospitalization. In addition to annual in-person study visits during which serum creatinine (SCr) and urine protein-to-creatinine ratio were quantified, BP was measured using a standardized protocol, and self-reported medication use was recorded; there were interim 6-month telephone contacts. At each contact, the occurrence of any hospitalizations was ascertained by self-report and/or surveillance of electronic medical record systems, with validation of clinical outcomes through physician adjudication of medical records using standardized criteria.12 Approximately one half of the ASSESS-AKI study participants experienced hospitalized AKI before the start of long-term follow-up, but we did not include that AKI episode in our analysis due to lack of quantification of proteinuria before study enrollment.
We also studied the subset of 456 CRIC study participants enrolled from Kaiser Permanente Northern California. The CRIC study design and enrollee characteristics have been previously published.13−15 Briefly, adult patients with eGFR 20–70 ml/min per 1.73 m2 were initially enrolled from seven clinical centers (13 recruitment sites) throughout the United States, with one of the recruitment sites being Kaiser Permanente Northern California. Important exclusion criteria included polycystic kidney disease, multiple myeloma, or glomerulonephritis on active immunosuppression. All CRIC study participants are scheduled for annual in-person study visits and contacted every 6 months by phone. At the yearly study visit, SCr and urine protein-to-creatinine ratio were quantified; BP was measured using a standardized protocol, and self-reported medication use was recorded. Occurrence of medical events of interest was determined by participant self-report and adjudicated by study physicians after manual review of relevant medical records. The subset of CRIC study participants enrolled from Kaiser Permanente Northern California received care in a large integrated health care delivery system, where essentially all hospitalizations (approximately 95%) occur at Kaiser Permanente–owned hospitals. There were four Kaiser Permanente members who were enrolled in both the ASSESS-AKI study and the CRIC study, and for this analysis, they were counted as CRIC study participants.
Ascertainment of Hospitalized AKI Episodes during Follow-Up
The hospitalized AKI episodes included in this analysis occurred after enrollment in each study on the basis of identified hospitalizations that included measurements of inpatient SCr. Thus, we did not include the original AKI episode present in approximately one half of the ASSESS-AKI study participants at study entry, which was integral to the sampling approach in that study. We used only inpatient SCr measurements to define AKI in our analysis, because we wanted to reduce the chances of mistaking rapid progression of CKD for AKI. For ASSESS-AKI study enrollees, obtaining inpatient medical records, including inpatient SCr values, was specified in the study protocol. For the Kaiser Permanente Northern California enrollees who were a part of the CRIC study, we were able to extract all inpatient SCr values from the Kaiser Permanente electronic medical record (an activity outside of the parent CRIC study core protocol).16,17
We adapted consensus definitions18 and defined AKI as a ≥50% relative difference between the peak and nadir inpatient SCr concentrations during a single hospitalization. We did not use an absolute change of ≥0.3 mg/dl only to identify patients with AKI due to concerns about possible misclassification.19 We further defined the severity of the AKI episode as follows comparing the ratio of peak SCr with the nadir SCr: stage 1 (relative difference of 50%–199%), stage 2 (relative difference between 200% and 299%), and stage 3 (relative difference ≥300%, a peak SCr ≥4.0, and/or having received acute renal replacement therapy [RRT]).
Follow-Up and Outcome
Participants were followed from enrollment into the ASSESS-AKI study or the CRIC study until the last research study visit date with a research protocol–obtained urine protein-to-creatinine ratio through September 11, 2017. Follow-up also stopped at the onset of ESRD (defined as the date of initiation of maintenance dialysis or receipt of kidney transplant) or death from any cause.
Proteinuria was quantified as urine protein-to-creatinine ratio (grams per gram) measured at the University of Minnesota (the ASSESS-AKI study central laboratory) or the University of Pennsylvania William Pepper Laboratory (the CRIC study central laboratory) using urine samples collected at the annual in-person study visits. Random spot urine samples were used in the ASSESS-AKI study; either 24-hour urine collections or random spot urine samples were used in the CRIC study. No clinically obtained proteinuria measurements were included for analysis.
In the ASSESS-AKI study, urine total protein was measured using a turbidimetric method, and urine creatinine was measured using the Roche enzymatic method on a Roche Modular P analyzer from the beginning of the study through December 2013 and a Roche Cobas 6000 analyzer after January 2014. In the CRIC study, total protein in urine was measured using a turbidimetric method on a Roche Modular P from the beginning of the study through October 2008. Subsequently, it was measured using quantitation of colorimetric reaction on a Beckman Coulter UniCel DxC-800. Urine creatinine in the CRIC study was measured using a Jaffe reaction on a BioTek microplate reader through October 2008 and using a Jaffe rate reaction on a Beckman Coulter UniCel DxC-800 after that date.
Covariates
Data on patient characteristics and relevant comorbid conditions were ascertained from the ASSESS-AKI study and the CRIC study research databases. These included demographics (age, sex, and race/ethnicity) and diabetes mellitus status at each study visit defined according to the ASSESS-AKI study protocols and the CRIC study protocols.13−15 Repeated annual study visit eGFR level was assessed using the SCr-based Chronic Kidney Disease Epidemiology Collaboration equation20 for the ASSESS-AKI study participants and the SCr- and cystatin C–based CRIC GFR estimating equation for the CRIC study participants.21 No clinically obtained outpatient SCr measurements were included for analysis. Measured systolic BP (SBP), number of BP medications, and use of ACE-I or ARB were updated at the annual study visit.22,23 For missing values in time-updated covariates in models, we used a last value carry-forward approach (e.g., we use the level of SBP from the most recent prior study visit to carry forward to the current visit if SBP is missing at the current visit). Seven ASSESS-AKI study participants with completely missing SBP readings were excluded from analysis.
Statistical Approach
Analyses were performed using SAS software, version 9.3 (Cary, NC). We compared differences in characteristics at cohort entry between those who did and those who did not experience an episode of AKI during follow-up. For continuous variables, we used either the t test or the Wilcoxon rank sum test, and for discrete variables, we used the chi-squared test.
To evaluate whether an episode of hospitalized AKI was independently associated with a change in the level of proteinuria, we performed mixed effects regression to examine the change in natural logarithm–transformed urine protein-to-creatinine ratio (log urine protein-to-creatinine ratio):`
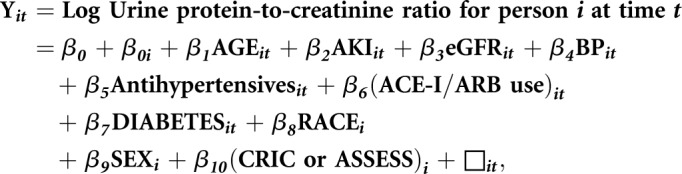 |
where β0 is the mean log urine protein-to-creatinine ratio for the entire cohort, β0i is a random effect for the difference in mean log urine protein-to-creatinine ratio for person i from the cohort mean urine protein-to-creatinine ratio, AGEit is the age of person i at time t, and AKIit is a binary indicator of whether person i experienced AKI between time t − 1 and t. eGFRit, BPit, Antihypertensivesit, (ACE-I/ARB use)it, and DIABETESit are the measured eGFR, SBP, use of antihypertensive medications, use of ACE-I/ARB, and presence of diabetes mellitus for person i at time t, respectively. RACEi, SEXi, and (CRIC or ASSESS)i indicate the race, sex, and study enrollment of person i, respectively. εit is a random error term for person i at time t.
We chose to analyze the urine protein-to-creatinine ratio on the log scale, because the distribution for raw urine protein-to-creatinine ratio values was highly skewed, and residuals from the log urine protein-to-creatinine ratio model were more normally distributed. Furthermore, much of the published literature shows that the association between proteinuria level and future adverse outcomes is linear on the log scale.24,25
This mixed effects modeling approach easily accommodates repeated episodes of hospitalized AKI that occurred between different annual study visits for a given study participant. For example, if a participant had an AKI episode between year 1 study visit and year 2 study visit and another episode between year 3 study visit and year 4 study visit, both episodes were included. Because urine protein-to-creatinine ratio was obtained yearly in the ASSESS-AKI study and the CRIC study, for the purposes of our analysis, if more than one episode of hospitalized AKI occurred in between consecutive annual study visits, it was considered only a single episode (this scenario only affected 42 participants).
To assess the robustness of our findings, we conducted a secondary analysis, which required fewer modeling assumptions but discarded selected available information. For participants who experienced AKI, we examined log urine protein-to-creatinine ratio measured at the closest annual study visit before the first episode of AKI and log urine protein-to-creatinine ratio measured at the closest annual study visit after the first episode of AKI and calculated the change. We then compared this with change in log urine protein-to-creatinine ratio between two consecutive annual study visits for randomly selected participants who did not experience AKI matched for calendar year. Using mixed effects regression, we analyzed the difference in the absolute change in log urine protein-to-creatinine ratio for those who did versus did not experience hospitalized AKI, with adjustment for the CRIC study or the ASSESS study enrollment, age, sex, race, eGFR, SBP, use of any antihypertensive medications, use of ACE-I/ARBs, and presence of diabetes mellitus with time updating of covariates as appropriate. We accounted for within-person variation by specifying a random intercept on the individual, and we accounted for within-matched pairs correlation by specifying a random residual on the matched pair.
We in addition examined whether more severe AKI was associated with larger changes in urine protein-to-creatinine ratio. Finally, we conducted exploratory analyses into whether baseline proteinuria was an effect modifier of any association between AKI and changes in urine protein-to-creatinine ratio.
Results
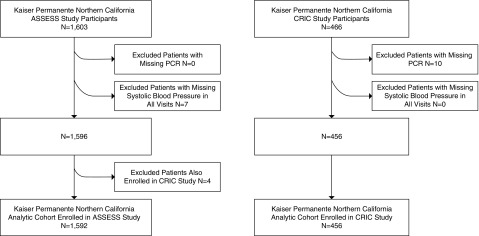
Our final cohort included 2048 study participants with available follow-up data on the occurrence of AKI episodes and longitudinal research measurements of urine protein-to-creatinine ratio (1592 from the ASSESS-AKI study and 456 from the CRIC study who were members of Kaiser Permanente Northern California) (Figure 1). The characteristics of our study population overall and by study are shown in Table 1. Among the 1592 ASSESS-AKI study participants, 266 were observed to have experienced at least one episode of hospitalized AKI after enrollment into the ASSESS-AKI study during a total follow-up of 5368 person-years. Among the 456 Kaiser Permanente Northern California CRIC study participants, 58 were observed to have experienced at least one episode of hospitalized AKI after enrollment during a total follow-up of 3903 person-years. Among first episodes of hospitalized AKI, 50.3% were Kidney Disease Improving Global Outcomes (KDIGO) stage 1 in severity, 23.8% were KDIGO stage 2, and 25.9% were KDIGO stage 3.
Figure 1.
The final study population was assembled from ASSESS-AKI and CRIC Kaiser Permanente Northern California study enrollees with few exclusions. ASSESS, Assessment, Serial Evaluation, and Subsequent Sequelae; CRIC, Chronic Renal Insufficiency Cohort, PCR, protein-to-creatinine ratio.
Table 1.
Characteristics of participants at cohort entry overall and stratified by the presence or absence of an episode of AKI during follow-up
| Characteristics of participants | Total, n=2048 | AKI during Follow-Up, n=324 | Non-AKI during Follow-Up, n=1724 | P Value |
|---|---|---|---|---|
| Characteristics at cohort entry | ||||
| Age at cohort entry, mean (±SD), yr | 63.3 (±12.4) | 63.9 (±11.6) | 63.2 (±12.5) | 0.35 |
| Women, N (%) | 900 (43.9%) | 145 (44.8%) | 755 (43.8%) | 0.75 |
| Black race, N (%) | 361 (17.6%) | 59 (18.2%) | 302 (17.5%) | 0.76 |
| eGFR at cohort entry, ml/min per 1.73 m2 | ||||
| Median [interquartile range] | 62.9 [46.9–84.6] | 57.1 [43.8–81.0] | 63.9 [48.3–85.1] | <0.01 |
| Urine protein-to-creatinine ratio at cohort entry (g/g) | ||||
| Median [interquartile range] | 0.12 [0.07–0.25] | 0.17 [0.09–0.42] | 0.11 [0.07–0.23] | <0.001 |
| Diabetes mellitus, N (%) | 805 (39.3) | 186 (57.4) | 619 (35.9) | <0.001 |
| Use of ACE-I or ARB at cohort entry, N (%) | 989 (48.3) | 175 (54.0) | 814 (47.2) | <0.05 |
| No. of BP medications at cohort entry | ||||
| Median [interquartile range] | 2 [1–2] | 2 [1–3] | 2 [1–2] | <0.001 |
| Systolic BP at cohort entry, mm Hg | ||||
| Mean (±SD) | 126 (±21) | 129 (±23) | 126 (±21) | <0.05 |
| Diastolic BP at cohort entry, mm Hg | ||||
| Mean (±SD) | 71 (±14) | 71 (±14) | 71 (±13) | 0.87 |
| Follow-up characteristics | ||||
| Follow-up time (year) | ||||
| Median [interquartile range] | 3.9 [2.0–5.8] | 4.0 [2.8–5.7] | 3.9 [1.9–5.8] | 0.12 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
One quarter of participants who experienced hospitalized AKI (81 of 324) had more than one episode of AKI during the study observation. Of these 81 individuals, the median number of additional AKI episodes was 1 (interquartile range [IQR], 1–2).
The median number of urine protein-to-creatinine ratio measurements among the 1592 ASSESS-AKI study participants was five (IQR, three to six). The median number of urine protein-to-creatinine ratio measurements among the 456 CRIC study participants was 11 (IQR, 6–13). The median number of urine protein-to-creatinine ratio measurements among the persons who experienced an episode of AKI was two (the ASSESS-AKI study) or four (the CRIC study) before the first AKI episode and two (the ASSESS-AKI study) or five (the CRIC study) after the first AKI episode. Table 2 shows the average time gaps between episodes of hospitalized AKI and pre- and post-proteinuria quantification associated with the first episode of AKI.
Table 2.
Distribution of urine protein-to-creatinine ratio measurements overall and relative to AKI episodes
| Total, n=2048 | ||
|---|---|---|
| AKI during Follow-Up, n=324 | Non-AKI during Follow-Up, n=1724 | |
| No. of urine protein-to-creatinine ratio measurements overall | ||
| Mean (±SD) no. of urine protein-to-creatinine ratio measurements | 6 (±3) | 5 (±3) |
| Median [interquartile range] number of urine protein-to-creatinine ratio measurements | 5 [4–7] | 5 [3–7] |
| Urine protein-to-creatinine ratio measurements relative to AKI episode | ||
| Number of urine protein-to-creatinine ratio measurements pre-AKI | ||
| Mean (±SD) | 3 (±2) | |
| Median [interquartile range] | 2 [1–3] | |
| Time between last pre-AKI urine protein-to-creatinine ratio and AKI episode | ||
| Mean (±SD) days | 199 (±131) | |
| Median [interquartile range] days | 190 [100–283] | |
| No. of urine protein-to-creatinine ratio measurements post-AKI | ||
| Mean (±SD) | 3 (±2) | |
| Median [interquartile range] | 2 [1–3] | |
| Time between AKI episode and first post-AKI urine protein-to-creatinine ratio | ||
| Mean (±SD) days | 194 (±138) | |
| Median [interquartile range] days | 170 [96–265] | |
In fully adjusted models, we observed that, after an episode of hospitalized AKI, there was a 9% increase in urine protein-to-creatinine ratio (1.09-fold increase; 95% confidence interval [95% CI], 1.02 to 1.16; P=0.01) in our primary analysis (Table 3). In our secondary analysis using the matched participants, similar results were seen, with an 11% increase in urine protein-to-creatinine ratio after an episode of hospitalized AKI (1.11-fold increase; 95% CI, 1.02 to 1.21; P=0.02) (Table 4). (Raw urine protein-to-creatinine ratio values are shown in Supplemental Table 3.)
Table 3.
Multivariable association of an episode of AKI and change (fold increase) in level of urine protein-to-creatinine ratio overall and analyses that exclude receipt of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or systolic BP level
| Comparisons | Full Model (Shown in Supplemental Table 1) | Model Excluding Receipt of ACE-I/ARB | Model Excluding Systolic BP Level | |||
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | |
| AKI between study visits (versus no AKI) | 1.09 (1.02 to 1.16) | 0.01 | 1.09 (1.02 to 1.17) | 0.01 | 1.07 (1.00 to 1.14) | 0.04 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval.
Table 4.
Multivariable association of an episode of AKI and change (fold increase) in level of urine protein-to-creatinine ratio using an AKI versus matched non-AKI episode modeling approach (i.e., comparing only a single pair of pre- and poststudy visits urine protein-to-creatinine ratios) overall and analyses that exclude receipt of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or systolic BP level
| Comparisons | Full Model (Shown in Supplemental Table 1) | Model Excluding Receipt of ACE-I/ARB | Model Excluding Systolic BP Level | |||
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | |
| AKI between study visits (versus no AKI) | 1.11 (1.02 to 1.21) | 0.02 | 1.11 (1.02 to 1.21) | 0.02 | 1.08 (0.99 to 1.18) | 0.09 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval.
To explore whether less use of ACE-I/ARB or higher SBP after AKI may explain some of the higher urine protein-to-creatinine ratio levels after AKI, we excluded these terms from the models. In both instances, the association was not strengthened (Tables 3 and 4).
We also noted that more severe AKI was associated with greater changes in urine protein-to-creatinine ratio (Tables 5 and 6). For example, in our primary analysis, stage 3 AKI was associated with a 24% increase in urine protein-to-creatinine ratio (1.24-fold increase; 95% CI, 1.09 to 1.40; P<0.001).
Table 5.
Multivariable association of severity of AKI and change (fold increase) in level of urine protein-to-creatinine ratio overall and analyses that exclude receipt of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or systolic BP level
| Comparisons | Full Model | Model Excluding Receipt of ACE-I/ARB | Model Excluding Systolic BP Level | |||
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | |
| AKI stage 1 between study visits (versus no AKI) | 1.06 (0.97 to 1.16) | 0.22 | 1.06 (0.97 to 1.16) | 0.21 | 1.04 (0.95 to 1.14) | 0.35 |
| AKI stage 2 between study visits (versus no AKI) | 1.01 (0.89 to 1.15) | 0.88 | 1.01 (0.88 to 1.15) | 0.92 | 0.99 (0.87 to 1.13) | 0.89 |
| AKI stage 3 between study visits (versus no AKI) | 1.24 (1.09 to 1.40) | <0.001 | 1.25 (1.10 to 1.42) | <0.001 | 1.21 (1.06 to 1.37) | <0.01 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval.
Table 6.
Multivariable association of severity of AKI and change (fold increase) in level of urine protein-to-creatinine ratio using an AKI versus matched non-AKI episode modeling approach (i.e., comparing only a single pair of pre- and post-study visits urine protein-to-creatinine ratios) overall and analyses that exclude receipt of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or systolic BP level
| Comparisons | Full Model | Model Excluding Receipt of ACE-I/ARB | Model Excluding Systolic BP Level | |||
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | |
| AKI stage 1 between study visits (versus no AKI) | 1.03 (0.92 to 1.16) | 0.59 | 1.03 (0.92 to 1.16) | 0.57 | 1.00 (0.88 to 1.12) | 0.95 |
| AKI stage 2 between study visits (versus no AKI) | 1.07 (0.91 to 1.26) | 0.43 | 1.07 (0.90 to 1.26) | 0.44 | 1.06 (0.90 to 1.26) | 0.48 |
| AKI stage 3 between study visits (versus no AKI) | 1.32 (1.12 to 1.55) | <0.001 | 1.32 (1.13 to 1.56) | <0.001 | 1.29 (1.09 to 1.53) | <0.01 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval.
In exploratory analyses, we evaluated for a potential interaction between baseline proteinuria level (>0.5 or <0.5 g/g) and AKI and found a statistically significant interaction in our primary analysis (P<0.01). In stratified models, the association of AKI with change in urine protein-to-creatinine ratio was more pronounced among those with baseline urine protein-to-creatinine ratio <0.5 g/g (adjusted relative risk, 1.14; 95% CI, 1.06 to 1.22) than those with baseline urine protein-to-creatinine ratio ≥0.5 g/g (adjusted relative risk, 0.93; 95% CI, 0.78 to 1.10). Similar results were noted in our secondary analysis (results are not shown).
Results of the full multivariable mixed effects models are shown in Supplemental Table 1. As expected, time-updated diabetes mellitus, higher SBP, and lower eGFR were all risk factors for more proteinuria, but the parent study was not associated with the outcome in the primary analysis. Supplemental Table 2 shows the results of the full multivariable mixed effects models for our sensitivity analysis.
Among patients with hospitalized AKI, mean SBP measured at the closest annual study visit before the AKI episode was 127±22 mm Hg; mean SBP measured at the closest annual study visit after the AKI episode was 130±23 mm Hg (P=0.10). After an episode of AKI, the mean number of antihypertensive medications decreased (from 1.6±1.4 to 1.1±1.4; P<0.001). Prevalence of ACE-I/ARB use was 41.4% pre-AKI and 39.2% post-AKI (P=0.58).
Discussion
In this unique prospective analysis of two distinct patient populations—one consisting of patients discharged from the hospital (approximately one half of whom had an episode of hospitalized AKI shortly before study entry) and another consisting of community-dwelling persons with CKD—we found that hospitalized AKI was independently associated with a subsequent increase in proteinuria.
Examining proteinuria as a sequela of AKI is a novel dimension to understanding whether and how AKI may accelerate the development or progression of CKD. Prior studies of the adverse renal consequences of AKI have almost exclusively focused on changes in SCr-based eGFR.2,3 Such a focus on SCr may underestimate the potential renal sequelae of AKI, because even if an episode of AKI does lead to nephron loss, total GFR may be sustained due to compensatory increase in single-nephron GFR.26,27 Patient series have reported relatively high incidence of proteinuria among adult survivors of severe AKI (e.g., patients requiring acute RRT).28,29 However, these studies did not include non-AKI comparator groups, and there was no information on whether proteinuria was present before the AKI episode. In a recent parallel cohort study, patients who had experienced AKI had more proteinuria than matched controls who did not.30 However, there was no assessment of proteinuria before the AKI episode, and therefore, it was not clear if the patients with AKI merely had higher levels of proteinuria before the AKI episode. This is an important consideration, because proteinuria is itself a risk factor for AKI31,32; therefore, any differences in levels of proteinuria observed post-AKI could have been present pre-AKI.
Our study findings are concordant with and extend those of Parr et al.,10 who recently reported that AKI is a risk factor for worsening of proteinuria in a retrospective cohort study of 90,614 US veterans who were hospitalized between 2004 and 2012. A major advantage of our study compared with that of Parr et al.10 includes our use of research-grade quantification of urine protein-to-creatinine ratio (rather than semiquantitative readings from dipstick urinalysis obtained as part of routine clinical care). In addition, there was research protocol–driven rigorous and systematic prospective ascertainment of important covariates, such as BP and receipt of ACE-I/ARB concurrent in time with proteinuria assessment. Our study design focusing on within-person changes in urine protein-to-creatinine ratio level also reduces the potential effect of confounding and ascertainment bias. We defined AKI on the basis of actual observed acute changes in SCr, which are much more accurate than reliance on administrative diagnostic codes.33−37 The inclusion of enrollees from two cohorts increases the external validity of our study.
We note that, when SBP was excluded from the mixed effects model, AKI was persistently associated with worsening proteinuria, and the magnitude of effect was not greater (specifically, 1.07- versus 1.09-fold increase in urine protein-to-creatinine ratio) (Table 3). Thus, this did not support the hypothesis that increased proteinuria after AKI reflected higher BP after AKI. The lack of change in the urine protein-to-creatinine ratio parameter estimate with exclusion of the term for receipt of ACE-I/ARB from the mixed effects model (1.09 versus 1.09 in Table 3) suggests that change in renin-angiotensin system blockade is also not an explanation.
Our study extends the growing literature concerning the bidirectional association between AKI and proteinuria. Research in the past decade has clearly established that proteinuria (similar to reduced eGFR) is a strong risk factor for AKI.25,31,32 A recent study reported that proteinuria (similar to reduced eGFR) is an independent risk factor for nonrecovery from severe dialysis-requiring AKI.38 Demonstrating that proteinuria worsens after AKI and rigorously quantifying the magnitude of this in patients with mostly mild to moderate AKI add importantly to complete the understanding of how AKI and CKD, two of the major clinical problems in nephrology, are interconnected.39,40 Our results and those of Parr et al.10 make contributions in at least several areas. First, they give pathophysiologic insights (e.g., worsening proteinuria as a potential mechanism by which AKI leads to accelerated loss of renal function). Second, these data have clinical practice implications (e.g., physicians should not only determine eGFR but also, monitor proteinuria among patients who experienced AKI). Third, they highlight important related research questions (e.g., how should proteinuria, BP medications, and target BP level be managed after AKI?). Fourth, results of our exploratory analysis could generate mechanistic hypotheses useful for additional research.
Limitations of our study include the relatively small number of patients with dialysis-requiring AKI, which precluded our ability to explore the effect of AKI of the greatest severity on subsequent proteinuria levels. Because of changes in the study protocol over time and enrollee willingness to collect timed urine specimens repeatedly, both 24-hour urine collections and random urine samples were used to determine urine protein-to-creatinine ratio in the CRIC study, which may contribute to measurement variation. The central laboratory methodology for quantification of urine protein and urine creatinine changed over time for both the ASSESS-AKI study and the CRIC study, but these changes should affect both study participants who did and study participants who did not experience AKI, and it should not contribute additional bias. Proteinuria was only assessed once yearly, and there are known substantial day-to-day fluctuations in this parameter.41 We could not analyze longitudinal changes in urine albumin-to-creatinine ratio, because proteinuria in the CRIC study participants during follow-up was quantified only as total proteinuria, consistent with prior studies that have included patients with CKD without diabetes.42 In general, urine protein-to-creatinine ratio and albumin-to-creatinine ratio are strongly correlated, and study conclusions are very similar when either was analyzed.43−46 There are theoretical advantages to assessing total (i.e., albumin and nonalbumin) protein excretion in the urine47,48 when assessing renal tubular damage. However, because we did not have the ratio of urine albumin to urine total protein, we are unable to shed more light on the nature of proteinuria. Combining two different study cohorts augmented study power and external validity but also, introduced more heterogeneity; however, the ASSESS-AKI study and the CRIC study were designed to have similar follow-up visit schedules and protocol elements. We chose to focus only on hospitalized AKI episodes, because given the currently available research and nonresearch data sources, it is difficult methodologically to define with confidence outpatient AKI episodes unrelated to hospitalizations. It can be particularly challenging to distinguish potential outpatient AKI episodes from nonlinear eGFR trajectories in CKD or rapid progression of CKD.49
In conclusion, on the basis of our analyses of at-risk adults with pre- and post-AKI urine protein-to-creatinine ratio measurements in two prospective research cohorts, AKI is an independent risk factor for worsening proteinuria. Future studies are needed to better define how different treatment strategies affecting parameters, such as post-AKI proteinuria, may affect prognosis among patients who experienced AKI to inform best practices.
Disclosures
Dr. Liu reports grants from the National Institutes of Health (NIH), National Heart, Lung and Blood Institute; grants from the NIH, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK); personal fees from Durect; personal fees from Z S Pharma; personal fees from Theravance; personal fees from Quark; personal fees from Potrero Med; other from Amgen; personal fees from American Society of Nephrology; personal fees from National Kidney Foundation; personal fees from National Policy Forum on Critical Care and ARF; personal fees from Baxter; and personal fees from Astra Zeneca outside the submitted work. Dr. Anderson reports grants from the NIH during the conduct of the study. Dr. Chinchilli reports grants from the NIH, NIDDK during the conduct of the study. Dr. Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research. Dr. Kaufman reports personal fees from the NIDDK during the conduct of the study. Dr. Parikh reports personal fees from AbbVie Pharmaceutical Research and Development; personal fees from Genfit Biopharmaceutical Company; other from Renalytix AI; grants from the NIDDK; and grants from the National Heart, Lung and Blood Institute outside the submitted work. Dr. Saab reports grants from the NIDDK during the conduct of the study and serves as a medical director for Fresenius Medical Care outside the submitted work. Dr. Go reports grants from the NIDDK during the conduct of the study. Dr. Feldman reports personal fees from Kyowa Hakko Kirin Co., Ltd. and grants from NIH. Dr. Siew received honorarium for an educational talk provided at DaVita. All other remaining authors have nothing to disclose.
Funding
Dr. Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research. We acknowledge funding support from NIDDK grants R01DK098233, R01DK101507, R01DK114014, K23DK100468, and R03DK111881. In addition, this work was supported in part by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland General Clinical Research grant M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago CTSA grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco Clinical and Translational Science Institute grant UL1 RR-024131. The Assessment, Serial Evaluation, and Subsequent Sequelae of AKI (ASSESS-AKI) study was supported by cooperative agreements from National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK082223, U01DK082185, U01DK082192, and U01DK082183. The Chronic Renal Insufficiency Cohort (CRIC) study was supported by cooperative agreements from National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902.
Supplementary Material
Acknowledgments
The Clinical and Translational Science Awards study investigators are Lawrence J. Appel (Johns Hopkins University), Dr. Feldman, Dr. Go, Jiang He (Tulane University), James P. Lash (University of Illinois), Panduranga S. Rao (University of Michigan), Mahboob Rahman (Case Western Reserve University), and Raymond R. Townsend (University of Pennsylvania). The ASSESS study investigators are Study Chair: Dr. Kaufman; Penn State (Data Coordinating Center): Dr. Chinchilli, Nasrollah Ghahramani, W. Brian Reeves, Lan Kong, Ming Wang, and Elana Farace; Kaiser Permanente Northern California: Dr. Go, Dr. C.-y. Hsu, Dr. R. K. Hsu, Thida Tan, Juan D. Ordonez, and Sijie Zheng; Vanderbilt: T. Alp Ikizler, Dr. Siew, Julia B. Lewis, and Lorraine Ware, Yale: Dr. Parikh, Steven Coca, and Dennis G. Moledina; London, Canada: Dr. Garg; Cincinnati: Prasad Devarajan; Montreal, Canada: Michael Zappitelli; University of Washington: Dr. Himmelfarb and Mark Wurfel; and National Institute of Diabetes and Digestive and Kidney Diseases: Paul L. Kimmel and Marva Moxey-Mims.
The opinions expressed in this article are the authors’ own and do not reflect the view of the NIH, the Department of Health and Human Services, or the U.S. Government.
Dr. C. Hsu, Dr. R. Hsu, Dr. Liu, Dr. Anderson, Dr. Chen, Dr. Chinchilli, Dr. Feldman, Dr. Garg, Dr. Hamm, Dr. Himmelfarb, Dr. Kaufman, Dr. Kusek, Dr. Parikh, Dr. Siew, Dr. W. Yang, and Dr. Go designed the study. Dr. C. Hsu, Dr. Anderson, Dr. J. Yang, Dr. Chen, Dr. Chinchilli, Dr. Feldman, Dr. Garg, Dr. Hamm, Dr. Himmelfarb, Dr. Kaufman, Dr. Kusek, Dr. Parikh, Dr. Ricardo, Dr. Rosas, Dr. Saab, Dr. Siew, Dr. Sondheimer, Dr. Taliercio, and Dr. Go acquired the data. All authors interpreted the data. Dr. C. Hsu drafted the paper, and all authors revised it critically for important intellectual content. All authors gave final approval of the version to be published.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
on behalf of the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators and the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Study:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, James P. Lash, Panduranga S. Rao, Mahboob Rahman, Raymond R. Townsend, James Kaufman, Vernon M. Chinchilli, Nasrollah Ghahramani, W. Brian Reeves, Lan Kong, Ming Wang, Elana Farace, Alan Go, Chi-yuan Hsu, Raymond Hsu, Thida Tan, Juan D. Ordonez, Sijie Zheng, T. Alp Ikizler, Edward D. Siew, Julia B. Lewis, Lorraine Ware, Chirag Parikh, Steven Coca, Dennis G. Moledina, Amit Garg, Prasad Devarajan, Michael Zappitelli, Jonathan Himmelfarb, Mark Wurfel, Paul L Kimmel, and Marva Moxey Mims
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101036/-/DCSupplemental.
Supplemental Table 1. Multivariable association between AKI and change in level of urine protein-to-creatinine ratio: full model and analyses that exclude receipt of ACE-I/ARB or systolic BP level.
Supplemental Table 2. Multivariable association between AKI and change in level of urine protein-to-creatinine ratio using an AKI versus matched non-AKI episode modeling approach (i.e., comparing only a single pair of pre- and poststudy visits urine protein-to-creatinine ratio): full model and analyses that exclude receipt of ACE-I/ARB or systolic BP level.
Supplemental Table 3. Distributions of absolute levels of urine protein-to-creatinine ratio (in units of grams per gram) at two consecutive study visits with and without intervening AKI.
References
- 1.Hsu CY: Yes, AKI truly leads to CKD. J Am Soc Nephrol 23: 967–969, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Hsu RK, Hsu CY: The role of acute kidney injury in chronic kidney disease. Semin Nephrol 36: 283–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 5.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL: Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, et al.: Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, et al.: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Raab WP: Diagnostic value of urinary enzyme determinations. Clin Chem 18: 5–25, 1972 [PubMed] [Google Scholar]
- 10.Parr SK, Matheny ME, Abdel-Kader K, Greevy RA Jr., Bian A, Fly J, et al.: Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 93: 460–469, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, et al.: TRIBE-AKI Consortium : Kidney outcomes 5 years after pediatric cardiac surgery: The TRIBE-AKI study. JAMA Pediatr 170: 1071–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Parikh CR, Ikizler TA, Coca S, Siew ED, Chinchilli VM, et al.: Assessment Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury Study Investigators : The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: Design and methods. BMC Nephrol 11: 22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, et al.: CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Hsu C-Y, Yang J, Tan TC, Zheng S, Ordonez JD, et al.: Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 13: 833–841, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL: Kidney disease: Improving Global Outcomes (KDIGO) AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 19.Lin J, Fernandez H, Shashaty MGS, Negoianu D, Testani JM, Berns JS, et al.: False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol 10: 1723–1731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.: CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al.: CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal N, McCulloch CE, Rahman M, Kusek JW, Anderson AH, Xie D, et al.: CRIC Study Investigators : Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: The chronic renal insufficiency cohort study. Hypertension 65: 93–100, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal N, McCulloch CE, Lin F, Robinson-Cohen C, Rahman M, Kusek JW, et al.: CRIC Study Investigators : Different components of blood pressure are associated with increased risk of atherosclerotic cardiovascular disease versus heart failure in advanced chronic kidney disease. Kidney Int 90: 1348–1356, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al.: Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al.: Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn WF: Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ Res 46: 440–448, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Husain-Syed F, Ferrari F, Sharma A, Hinna Danesi T, Bezerra P, Lopez-Giacoman S, et al.: Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant 34: 308–317, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, et al.: POST-RENAL Study Investigators and the ANZICS Clinical Trials Group : Long-term survival and dialysis dependency following acute kidney injury in intensive care: Extended follow-up of a randomized controlled trial. PLoS Med 11: e1001601, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolanos JA, Yuan CM, Little DJ, Oliver DK, Howard SR, Abbott KC, et al.: Outcomes after post-traumatic AKI requiring RRT in United States military service members. Clin J Am Soc Nephrol 10: 1732–1739, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horne KL, Shardlow A, Taal MW, Selby NM: Long term outcomes after acute kidney injury: Lessons from the ARID study. Nephron 131: 102–106, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu RK, Hsu CY: Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr Opin Nephrol Hypertens 20: 211–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, et al.: Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, et al.: CDC CKD Surveillance Team : Validation of CKD and related conditions in existing data sets: A systematic review. Am J Kidney Dis 57: 44–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, et al.: Validity of administrative database coding for kidney disease: A systematic review. Am J Kidney Dis 57: 29–43, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Hwang YJ, Shariff SZ, Gandhi S, Wald R, Clark E, Fleet JL, et al.: Validity of the international classification of diseases, tenth revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open 2: pii:e001821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J: Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9: 682–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BJ, Go AS, Parikh R, Leong TK, Tan TC, Walia S, et al.: Pre-admission proteinuria impacts risk of non-recovery after dialysis-requiring acute kidney injury. Kidney Int 93: 968–976, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb EJ, MacKenzie F, Stevens PE: How should proteinuria be detected and measured? Ann Clin Biochem 46: 205–217, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, et al.: AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease : Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Methven S, MacGregor MS, Traynor JP, Hair M, O’Reilly DS, Deighan CJ: Comparison of urinary albumin and urinary total protein as predictors of patient outcomes in CKD. Am J Kidney Dis 57: 21–28, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Fisher H, Hsu CY, Vittinghoff E, Lin F, Bansal N: Comparison of associations of urine protein-creatinine ratio versus albumin-creatinine ratio with complications of CKD: A cross-sectional analysis. Am J Kidney Dis 62: 1102–1108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al.: Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuhrman DY, Schneider MF, Dell KM, Blydt-Hansen TD, Mak R, Saland JM, et al.: Albuminuria, proteinuria, and renal disease progression in children with CKD. Clin J Am Soc Nephrol 12: 912–920, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrader J, Lüders S, Kulschewski A, Hammersen F, Züchner C, Venneklaas U, et al.: MARPLE Study Group : Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: Final results of a prospective long-term study (MARPLE study). J Hypertens 24: 541–548, 2006 [DOI] [PubMed] [Google Scholar]
- 48.McTaggart MP, Stevens PE, Price CP, Newall RG, Pinnock RG, Lamb EJ: Investigation of apparent non-albuminuric proteinuria in a primary care population. Clin Chem Lab Med 51: 1961–1969, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, et al.: Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59: 504–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.