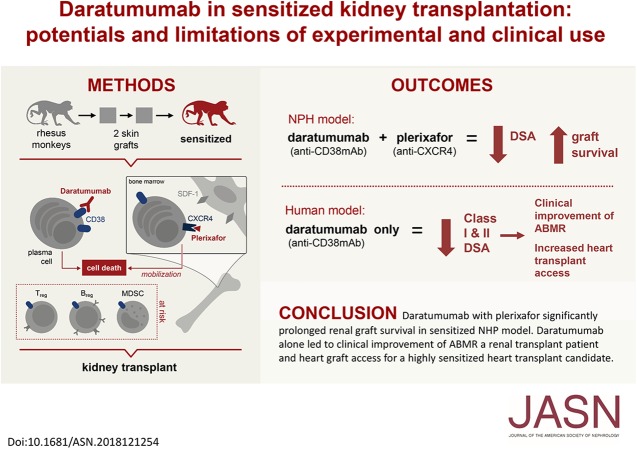
Significance Statement
Targeting plasma cells to reduce the risk of antibody-mediated rejection and decreased allograft survival due to anti-HLA donor-specific antibodies has not been explored in transplantation. After sensitizing eight rhesus macaques with two sequential mismatched skin allografts, the authors desensitized four with daratumumab (anti-CD38 mAb) and plerixafor (anti-CXCR4) before transplant. Compared with controls, the daratumumab-treated animals had significantly reduced donor-specific antibody levels and prolonged renal graft survival; however, this reduction was not maintained. Two patients treated with daratumumab—one with therapy-resistant acute kidney antibody-mediated rejection and a highly sensitized heart transplant candidate—exhibited a significant decrease in class 1 and 2 donor-specific antibodies that led to clinical improvement of antibody-mediated rejection and heart graft access. These findings suggest daratumumab merits investigation as a potential therapeutic strategy, although further research is needed.
Keywords: desensitization, daratumumab, plasma cell, antibody-mediated rejection, nonhuman primate
Visual Abstract
Abstract
Background
Donor-specific antibodies are associated with increased risk of antibody-mediated rejection and decreased allograft survival. Therefore, reducing the risk of these antibodies remains a clinical need in transplantation. Plasma cells are a logical target of therapy given their critical role in antibody production.
Methods
To target plasma cells, we treated sensitized rhesus macaques with daratumumab (anti-CD38 mAb). Before transplant, we sensitized eight macaques with two sequential skin grafts from MHC-mismatched donors; four of them were also desensitized with daratumumab and plerixafor (anti-CXCR4). We also treated two patients with daratumumab in the context of transplant.
Results
The animals treated with daratumumab had significantly reduced donor-specific antibody levels compared with untreated controls (57.9% versus 13% reduction; P<0.05) and prolonged renal graft survival (28.0 days versus 5.2 days; P<0.01). However, the reduction in donor-specific antibodies was not maintained because all recipients demonstrated rapid rebound of antibodies, with profound T cell–mediated rejection. In the two clinical patients, a combined heart and kidney transplant recipient with refractory antibody-mediated rejection and a highly sensitized heart transplant candidate, we also observed a significant decrease in class 1 and 2 donor-specific antibodies that led to clinical improvement of antibody-mediated rejection and to heart graft access.
Conclusions
Targeting CD38 with daratumumab significantly reduced anti-HLA antibodies and anti-HLA donor-specific antibodies in a nonhuman primate model and in two transplant clinical cases before and after transplant. This supports investigation of daratumumab as a potential therapeutic strategy; however, further research is needed regarding its use for both antibody-mediated rejection and desensitization.
Kidney transplantation is the preferred treatment for patients with ESRD because of improved patient survival, quality of life, and reduced cost compared with dialysis.1–3 However, donated organs are scarce, wait times are increasing, and many patients die awaiting transplantation. Highly sensitized patients have very low rates of eventual transplantation, and if they have donor-specific anti-HLA antibodies (DSAs), such patients have higher rates of antibody-mediated rejection (AMR) and early graft loss.4–7 Patients with prior organ transplantation, pregnancies, or blood transfusions have an increased likelihood of becoming sensitized by developing preformed anti-HLA antibodies. Thus, since the late 1990s, therapeutic strategies to desensitize and safely transplant this challenging patient population have been pursued.
Currently, variations of two protocols, high-dose intravenous Ig (IVIG) and/or plasmapheresis, are predominantly used in an attempt to desensitize potential recipients and allow successful transplantation across this barrier.8 Although desensitization decreases wait times and increases the rate of transplantation, these approaches are resource intense, AMR rates are high,9–13 and graft and patient survival are well below national averages for compatible live-donor transplantation.14 These early data underscore the urgent need for continued research into novel desensitization therapies. These observations are most likely due to a B cell–mediated response in the post-transplant period, leading to the production of de novo DSAs (dnDSAs). dnDSAs are the leading cause of late transplant failure after kidney transplantation and up to 30% of kidney allograft recipients develop dnDSAs. Despite numerous treatment strategies to boost conventional immunosuppression or to suppress B cell activity by targeting plasma cells (PCs), antibodies, and/or complement, no therapy reliably or satisfactorily reverses the effects of DSAs once established in the graft. Taken together, these detrimental effects of the humoral immune response strongly support the need for improved immune therapy that reduces pretransplant sensitization and better controls DSA-mediated AMR.
Daratumumab is an IgG1κ human mAb that binds to CD38 and inhibits the development of CD38-expressing cells via multiple mechanisms, including complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and apoptosis.15 CD38 is a transmembrane glycoprotein expressed on the surface of many immune cells—including PCs, plasmablasts, transitional cells—and is involved in functions such as receptor-mediated adhesion and signaling.16 It has been conjectured that PCs, not directly targeted by current desensitization methods, contribute to rebound humoral responses.17,18 Rituximab therapy in desensitization protocols aims to deplete B cells, thereby reducing antibody production.19 However, B cells lose expression of CD20 upon terminal differentiation to PCs, and rituximab consequently conveys limited efficacy with respect to PC depletion. In this context, two teams have analyzed the potential benefit of daratumumab to control PC production of anti-HLA antibodies in an experimental nonhuman primate (NHP) model and in two separate human clinical conditions. Using a rigorous sensitized NHP model, we hypothesized that CD38 targeting with daratumumab would be an effective means of PC depletion that would facilitate desensitization. In combination with daratumumab, we used plerixafor, a CXCR4 chemokine inhibitor, to increase mobilization of PCs from the marrow compartment. In this model, we demonstrate that this novel dual immunotherapy reduces levels of PCs and DSA, and significantly prolongs kidney allograft survival compared with nondesensitized controls. Concomitantly, daratumumab was used to clinically treat refractory life-threatening heart and kidney AMR and desensitize a candidate for heart transplant. In both cases, we observed a significant reduction of DSAs and panel-reactive antibodies (PRAs), improved allograft function, and finally patient and graft survival.
Methods
Experimental Model
Animals
We used eight male rhesus macaques (Macaca mulatta) from Alpha Genesis Inc. (Yemassee, SC). We paired donors and recipients for maximal mismatching of MHC class 1 and 2 loci. The Genetics Services Unit (Wisconsin National Primate Research Center; Madison, WI) performed the deep sequencing assays MHC genotyping. The Duke University Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines for the care and use of primates approved all medications, surgical procedures, and postoperative care of animals.
Sensitization, Desensitization, and Kidney Transplantation
For allosensitization, recipient animals received two serial skin grafts as previously reported.20,21 Donor cell flow crossmatch confirmed allosensitization. Briefly, we exchanged full-thickness skin grafts from the dorsum between donor-recipient pairs without immunosuppression. We performed the second skin graft 6 weeks after the first skin graft. Four animals received 16 mg/kg daratumumab (DARZALEX; Janssen Pharmaceutica NV, Beerse, Belgium) and 0.24 mg/kg plerixafor (Mozobil; Sanofi, Gentilly, France) approximately 8–12 weeks after the second skin transplantation once a week for 1 month while four animals (controls) received no desensitization. Lymph node and posterior-iliac-crest bone marrow biopsies were performed before (on the day of first infusion) and after desensitization therapy (a week after the final infusion). For induction, recipient animals received 50 mg/kg rhesus anti-CD4 mAb (CD4R1) intravenously a week before (day −7) kidney transplantation and 25 mg/kg rhesus anti-CD8 mAb (MT807R1) on the day of kidney transplantation (postoperative day 0) (both mAbs from National Institutes of Health [NIH] Nonhuman Primate Reagent Resource). We performed kidney transplantation approximately 2 weeks after the final daratumumab and plerixafor infusions (Figure 1A). All eight animals swapped kidneys with their paired skin graft donor and underwent simultaneous native bilateral nephrectomy such that kidney transplants were life supporting. Animals received maintenance immunosuppression with tacrolimus (Prograf; Astellas Pharma, Northbrook, IL), mycophenolate mofetil (Cellcept; Genentech, San Francisco, CA), and a methylprednisolone (Solumedrol; Pfizer, New York, NY) taper. Prophylactic ganciclovir (Cytovene; Fresenius Kabi, Lake Zurich, IL) for rhesus cytomegalovirus reactivation was given at 6 mg/kg SQ daily. Real-time PCR was performed to measure rhesus cytomegalovirus.
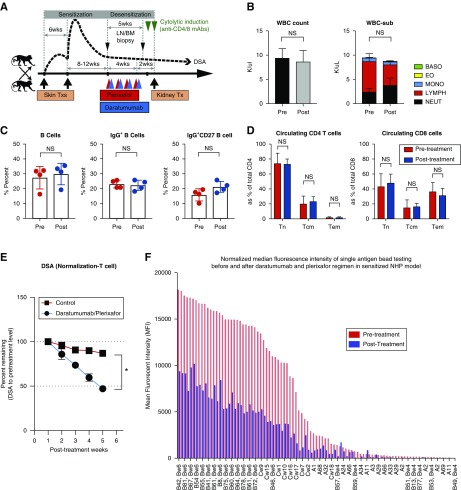
Figure 1.
Desensitization with daratumumab and plerixafor reduces donor-specific alloantibody significantly in sensitized NHPs. (A) Schematic representation of sensitization and desensitization. Maximally MHC-mismatched NHP pairs received two serial skin transplants for sensitization and desensitization treatment or no treatment (control group) before kidney transplantation. (B) The absolute number of peripheral white blood cells and immune cell population. (C) Effect of DPT on peripheral B cells. B cell subpopulations, including CD20+ B cells, IgG+ B cells, and IgG+CD27+ B cells, were not changed. (D) The frequency of naive, central memory, effector memory CD4, and CD8 T cells were not changed with DPT. (E) Desensitization with daratumumab and plerixafor reduces DSA significantly compared with control. DSA levels were measured by T cell flow crossmatch and shown as MFI shift. *P<0.05. (F) Representative normalized MFI of single antigen bead testing before and after DPT. NHP serum samples were evaluated with HLA single antigen bead assay. BASO, basophil; EO, eosinophil; LN/BM, lymph node/bone marrow; LYMPH, lymphocyte; MONO, monocyte; NEUT, neutrophil; Tcm, central memory T cell; Tem, effector memory T cell; Tn, naive T cell; Tx, transplant; WBC, white blood cell.
DSA Monitoring
Flow crossmatch, using donor lymphocytes and recipient serum, assessed alloantibody production as described previously.20,22 Briefly, donor PBMC or splenocytes were incubated with recipient serum, washed, and stained with FITC-labeled anti-monkey IgG (KPL Inc., Gaithersburg, MD), anti-CD20 mAb (2H7) and anti-CD3 mAb (SP34–2) (both BD Bioscience, San Diego, CA), and LIVE/DEAD Fixable Blue staining (Life Technologies, Carlsbad, CA). Mean fluorescence intensity (MFI) of anti-monkey IgG on T cells or B cells was measured on BD LSRFortessa (BD Biosciences, San Jose, CA), analyzed using FlowJo software vX (Tree Star, Ashland, OR), and expressed as MFI change from presensitized time point. NHP serum alloantibody was also measured using a human solid phase Luminex assay that uses single HLA antigen beads (LABScreen Single Antigen; One Lambda, Waltham, MA) to detect crossreactive antibodies. We thawed serum samples collected before and after desensitization from −80°C storage, spun at 14,000 × g for 10 minutes. We tested all samples at a neat (no dilution) or 1:8 dilution with the Luminex (Luminex Corporation, Austin, TX) platform. We analyzed data using HLA Fusion software version 4.3 (One lambda), as provided by the manufacturer.
Immune Cell Monitoring
For monitoring immune cells, cells from blood, lymph nodes, bone marrow, spleen, and graft were stained with the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies, Grand Island, NY) and then the following mAbs against human: CD3, CD4, CD8, CD14, CD20, CD25, CD27, CD28, CD56, CD95, CD159a, CD278 (ICOS), CD279 (PD-1), IgM, IgG, CXCR5, and—after fixation—Ki67 and FoxP3. We collected samples with a BD Fortessa flow cytometer and analyzed them using FlowJo software v9.6. We harvested, fixed, embedded, and stained grafts as previously described.20 All tissue samples were stained with hematoxylin and eosin and periodic acid–Schiff, evaluated blindly by an experienced transplant pathologist (A.B.F.), and scored according to updated Banff criteria.23,24
Histology, Immunohistochemistry, and Quantitative Image Analysis
Allograft tissues were obtained at time of biopsy or necropsy, fixed, and embedded in paraffin. The embedded tissue blocks underwent serial sectioning (5-μm thick) and staining for hematoxylin and eosin and periodic acid–Schiff for routine evaluation and grading for rejection. A trained pathologist (A.B.F.) evaluated histology blindly and scored according to updated Banff criteria.23,24 For germinal center visualization, lymph node biopsies were fixed, embedded, and stained with anti-human Ki67 (clone MM1; Vector, Burlingame, CA) and anti-CD20 (Thermo scientific, Rockford, IL) antibodies. An Aperio ScanScope XT (Aperio Technologies Inc., Vista, CA) acquired all images at ×20. Computer-based software (Aperio Imagescope v11) analyzed and measured scanned images. We traced and quantitated the GC (Germinal Center) area (Ki67+) and B cell follicle area (CD20+). We calculated GC response according to the formula GC area/B cell follicular area expressed as a ratio.
Statistical Analyses
Statistical analyses were performed using Prism software version 7.0 (GraphPad Software, San Diego, CA). Data are presented as mean±SD (error bars). We calculated P values using the two-tailed t test in normally distributed data. For survival analysis, we used the Kaplan–Meier method and log-rank test. P values <0.05 were considered to be statistically significant. For original data related to NHP study, please contact stuart.knechtle@due.edu. For original data related to Créteil study, please contact philippe.grimbert@aphp.fr.
Patients
Two patients from Henri Mondor Hospital (Créteil, France) gave written consent to use daratumumab in this indication. High resolution Luminex assay technology (One Lambda) analyzed circulating anti-HLA antibodies and anti-HLA DSAs. A mean baseline normalized MFI>1000 was classified as a positive result. For each serum sample, we reported the number of antibody subclasses, the sum of MFIs, and the maximum MFI. We analyzed kidney allograft biopsies using the updated Banff 2013 classification.25 We analyzed heart allograft biopsies using the International Society for Heart and Lung Transplantation criteria.26
Results
NHP Experimental Model
Desensitization with Daratumumab (anti-CD38 mAb) and Plerixafor (anti-CXCR4) Reduces Preformed DSA
For depletion of PC populations, we treated sensitized rhesus monkeys weekly with daratumumab and plerixafor for a month (Figure 1A). To maximize depletion, plerixafor was given 11 hours before daratumumab. Because of plerixafor, circulating white blood cell counts fluctuated (Supplemental Figure 1). Whereas overall white blood cell counts normalized, lymphocytes showed a trend toward a proportional decrease (Figure 1B, P=0.06). Because CD38 is expressed in hematopoietic and nonhematopoietic cells, including activated B cell and T cell populations,27–29 we evaluated circulating B and T cell populations, including circulating B cells, IgG+ B cells, and memory B cells (IgG+CD27+CD20+), as well as naive (CD28+CD95−), central memory (CD28+CD95+), and effector memory (CD28−CD95int) subsets of CD4 and CD8 T cells (Figure 1, C and D). We did not observe any significant changes in these populations. Surprisingly, DSA measured via donor T cell flow crossmatch showed significant reduction compared with pretreatment or date-matched untreated controls (Figure 1E). In addition, daratumumab and plerixafor treatment (DPT) profoundly reduced HLA antigen crossreactive antibodies (Figure 1F). A month of DPT diminished DSA levels with no changes in circulating B and T cell populations.
Daratumumab-Plerixafor Combined Treatment Reduces Plasmablasts in Lymph Nodes but Not Follicular Helper T Cells in Sensitized NHP
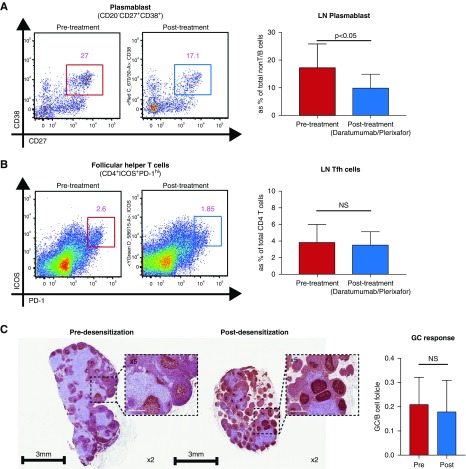
Surprisingly, DPT did not affect circulating B and T cells although DSA declined profoundly. To address the effect of DPT on lymph node B and T cells, we performed lymph node biopsies before the first DPT infusion and a week after the final DPT infusion (Figure 1A). After desensitization, we observed a significant reduction in the frequency of plasmablasts (CD20−CD27+CD38+) (Figure 2A). Notably, the plasmablasts showed higher Ki67 staining after DPT, suggesting that these cells may have repopulated. We also measured the follicular helper T (Tfh) cell population in the lymph node to address whether the reduction of PCs and DSA correlated with Tfh cell reduction. However, DPT did not affect the frequency of Tfh cells (Figure 2B). In accordance with this, clonal B cell expansion (Ki-67+CD20+) which represents germinal center response remained unchanged and CD20+ B cell follicles were not affected after DPT (Figure 2C). Taken together, the data suggest that desensitization with DPT directly affects B cells and PCs independently of Tfh cells.
Figure 2.
Desensitization with daratumumab and plerixafor reduces PC population, but not Tfh cells, in the lymph nodes. (A) Plasmablast population in the lymph nodes after desensitization. Representative flow plot shows the percentage of CD20−CD27+CD38+ cells within the CD3−IgD− population of lymph nodes pre- (blue) and post- (orange) desensitization time points with DPT. (B) Tfh populations in the lymph nodes after desensitization. Representative flow cytometry plots show the percentage of PD1+ICOS+ Tfh cells within the CD4+ T cell population in lymph node pre- (blue) and post- (orange) desensitization with daratumumab and plerixafor. (C) Representative immunohistochemistry shows the Ki67-stained areas within the CD20+ B cell follicles in lymph nodes pre- and postdesensitization with daratumumab and plerixafor. Clonal B cell expansion (Ki67+CD20+) within B cell follicle was not significantly changed after desensitization. Images were adapted from whole slide scan; original magnification ×40 (inset, ×100). GC, Germinal Center.
Delayed AMR after Desensitization with Daratumumab and Plerixafor in the Sensitized Kidney Transplant Model
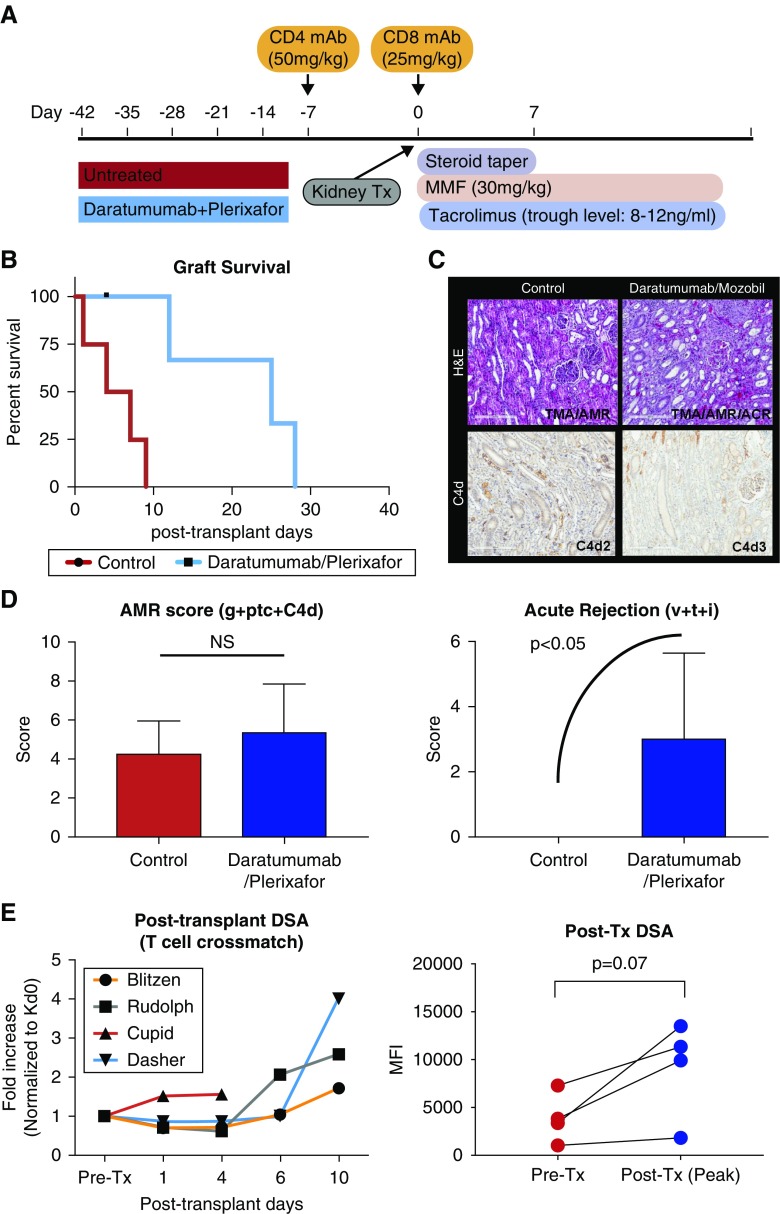
DPT significantly reduced preformed DSA compared with untreated control animals, with more than 50% reduction compared with the pretreatment time point (Figure 1E). After desensitization, animals received kidney transplants from their maximally MAMU mismatched skin donors (Figure 3A). As shown in Figure 3B, sensitized animals without any desensitization showed rapid graft rejection within 10 days (mean survival time=5.0±3.3 days). Animals desensitized with daratumumab and plerixafor showed significantly prolonged graft survival (mean survival time=21.6±8.5 days); however, they uniformly developed rejection within 30 days of transplantation. Surprisingly, animals desensitized with daratumumab and plerixafor exhibited both increased AMR and cell-meditated rejection (Figure 3C). Increased AMR scores and acute rejection scores suggest rebound of both anti–donor T and B cell responses after kidney transplantation (Figure 3D). In parallel, circulating DSA rapidly increased after kidney transplantation, suggesting a short-lived PC/plasmablast depletion (Figure 3E).
Figure 3.
Desensitization with daratumumab and plerixafor significantly prolongs but limits renal allograft survival in sensitized NHPs. (A) Dosing regimen of T cell depletional induction and maintenance immunosuppression for kidney transplantation after desensitization or without desensitization. (B) Percentage graft survival of sensitized NHPs with or without desensitization. Animals treated with DPT showed significantly prolonged graft survival compared with control group (P<0.05). (C) Representative hematoxylin and eosin (top panels) and C4d (bottom panels) staining of kidney allografts from control (left panels) and DPT (right panels) animals at the time of euthanasia. (D) Clustered Banff score related to AMR and TCMR. Animals treated with daratumumab and plerixafor showed elevated AMR score (g+ptc+C4d) and acute rejection (v+t+i) at euthanasia. (E) Post-transplant DSA kinetics. Animals with desensitization showed rapidly increased serum DSA (normalized to pre–renal transplant value=1). Post–kidney transplant peak DSA level showed strong trend of elevation compared with pretransplant time point. Original magnification ×200. ACR, acute cell-mediated rejection; g, glomerulitis; H&E, hematoxylin and eosin; i, interstitial inflammation; MMF, mycophenolate mofetil; ptc, peritubular capilaritis; t, tubulitis; TMA, thrombotic microangiopathy; Tx, transplant; v, intimal arteritis.
Off-Target Effect of Daratumumab on Regulatory Cells
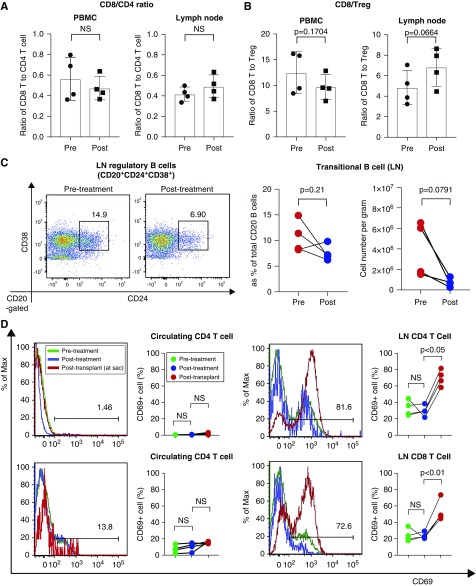
It is reported that daratumumab depletes CD38+ immune regulatory cells, including regulatory T cells (Tregs), regulatory B cells (Bregs), and myeloid-derived suppressor cells, and results in T cell expansion and skewing of the T cell repertoire in multiple myeloma patients.30 Therefore, we assessed CD8+/CD4+ and CD8+/Treg ratios. Overall, animals treated with daratumumab and plerixafor did not show significant increase of memory T cells (Figure 1D) or cytotoxic T cells in peripheral blood and lymph nodes (Figure 4A). However, it is notable that the CD8/Treg ratio showed a trend of increasing in lymph nodes (Figure 4B). Secondly, we evaluated conventional Treg populations pre- and post-treatment. Interestingly, the Treg (CD4+CD25+CD127−) population was not proportionally changed in the peripheral blood or lymph nodes after desensitization and we observed only a trend of reduction in the absolute number of Tregs in the lymph nodes (Supplemental Figure 2). We then assessed transitional B cells because they express CD38 on their surface.31 As shown in Figure 4C, transitional B cells showed a strong trend of reduction in the lymph nodes after DPT. Because rapid peripheral T cell immune deviation was not observed, we evaluated activated T cells after DPT as well as after kidney transplantation in the peripheral blood and lymph nodes. Interestingly, desensitization with daratumumab and plerixafor did not substantially affect T cell activation; however, CD69+ CD4 and CD8 T cells in the lymph nodes significantly increased after kidney transplantation (Figure 4D). This suggests that reduction of regulatory cells (Treg and B reg) by daratumumab and plerixafor did not affect T cells when they were immunologically inert but rather promoted a more vigorous response when challenged or activated. Taken together, targeting CD38 with daratumumab efficiently reduced memory B cell and PC populations but also depleted beneficial regulatory cell populations.
Figure 4.
A possible immune deviation after daratumumab and plerixafor treatment. (A) Ratio of CD8 to CD4 was not changed after desensitization with daratumumab and plerixafor in blood and lymph nodes. (B) Ratio of CD8 cells to Treg cells show a strong increasing trend (P=0.06) in the lymph nodes but not in the blood. (C) Transitional B cell population (CD20+CD24+CD38+) showed a strong trend of reduction (P=0.08). (D) Increased frequencies of activated T (CD69+) cells after kidney transplantation. The results are representative of four monkeys tested individually. LN, lymph node.
Clinical Results
DSA Outcome after Daratumumab in One Refractory Heart and Kidney AMR
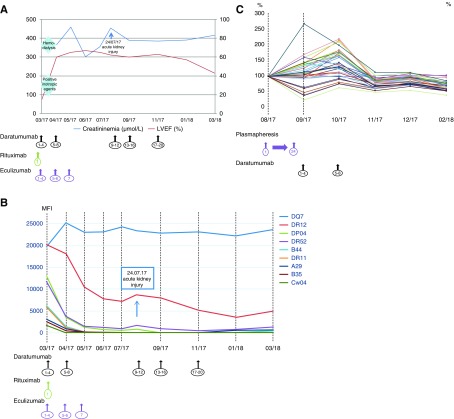
A 32-year-old man, who had heart and kidney engrafted 17 months before due to systemic lupus, presented with severe acute kidney and heart mixed rejection after complete immunosuppressive drug discontinuation (complete clinical description is provided in Supplemental Material). In the kidney allograft biopsy, histologic analysis revealed T cell–mediated rejection (TCMR) and AMR according to the Banff updated classification,24 with PC-predominant infiltration, major interstitial edema, and interstitial hemorrhage (Figure 5, A–F); the heart biopsy revealed TCMR grade 2R, AMR pathologic AMR grade 3, and diffuse PC infiltration (Figure 5, I–K). Nine DSAs were found with four class 1 dnDSAs (two with MFI>6000, one with MFI 3000–6000, and one with MFI 1000–3000; MFI sum 26,554), four class 2 dnDSAs, and one preexisting class 2 DSA (all with MFI>6000; MFI sum 94,476) (Figure 6B). Despite conventional therapy including high-dose steroid pulses, plasmapheresis, anti-thymocyte globulin, rituximab, and high-dose IVIG, the patient developed cardiogenic shock requiring intravenous dobutamine and ARF dependent to dialysis (Figure 6A). In this setting, we prescribed the compassionate use of eight weekly infusions of daratumumab (16 mg/kg each) combined with eculizumab. Outcome of anti-HLA antibodies MFI 3 months after the beginning of daratumumab revealed dramatic decline for eight of nine dnDSAs and MFI<1000 for seven of nine dnDSAs (Figure 6B). Heart allograft function returned to baseline without drugs and serum creatinine was 300 μmol/L (Figure 6A). Kidney allograft biopsy showed significant improvement in acute lesions and the PC infiltrate significantly decreased (Figure 5). Blood-circulating PCs were undetectable. Twenty weeks after the eighth infusion of daratumumab, the patient presented with AKI with recurrent acute PC-rich rejection on kidney biopsy and significant reascension of the MFI of two class 2 anti-HLA DSAs, which is associated with PC reappearance (Figure 6, A and B). Daratumumab was reinitiated weekly (four doses) and then bimonthly for 4 months (Figure 6A). Kidney allograft function improved to serum creatinine 350 μmol/L (Figure 6A). The class 2 anti-HLA DSA MFI decreased gradually and significantly (Figure 6B) and blood-circulating PCs were undetectable.
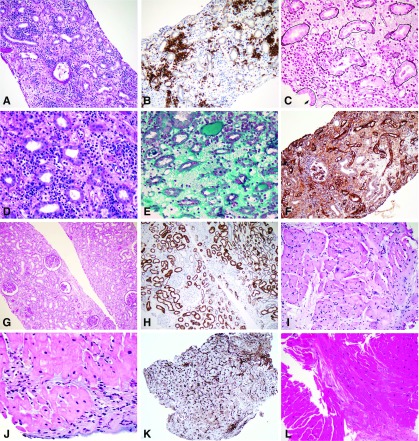
Figure 5.
Histological improvement of heart and kidney acute rejection after daratumumab treatment. (A–F) Kidney allograft biopsy at the time of diagnosis showed (A) interstitial edema and diffuse infiltrate (periodic acid–Schiff stain), made of (B) >10% of PCs (immunohistochemical staining with CD138), (C) with severe tubulitis (methenamine silver-periodic Jones stain Marinozzi), (D) capillaritis (periodic acid–Schiff ), and (E) interstitial hemorrhage (Masson trichrome). (F) Immunohistochemical stain with C4d is diffusely positive on peri-tubular capillaries. (G–H) Kidney allograft biopsy after daratumumab showed significant reduction of the interstitial infiltrate with (G) only mild persistent tubulitis (periodic acid–Schiff ); (H) only scattered PCs are stained with CD138 antibody compared with the first kidney biopsy. (I–K) Heart allograft biopsy at the time of cardiogenic shock showed (I) diffuse edema (hematoxylin eosin saffron [HES] stain), (J) interstitial infiltrate with myocyte damage (HES stain), and (K) diffuse C4d positivity . (L) Heart allograft biopsy after daratumumab showed no significant anomaly (HES stain). Original magnification, ×200 in A; ×200 in B; ×400 in C; ×400 in D; ×400 in E; ×200 in F; ×100 in G; ×200 in H; ×200 in I; ×400 in J; ×100 in K; ×200 in L.
Figure 6.
Significant improvement of AMR and significant decrease of DSA after daratumumab treatment. (A) Heart and kidney function evolution after daratumumab as acute rejection treatment. After the first eight injections of daratumumab, heart and kidney allograft functions improved significantly. Inotropic positive drugs and hemodialysis could be stopped. Twenty weeks after the eighth daratumumab infusion, serum creatinine level increased significantly, and heart allograft function remained stable. Daratumumab was reinitiated and serum creatinine level decreased. (B) DSA evolution after daratumumab as acute rejection treatment. After the first eight injections of daratumumab, DSAs class 1 and class 2 MFI decreased significantly besides DQ7 anti-HLA antibody (class 2). Two DSAs class 2 (DR12 and DR52) remained detectable with MFI>1000. At the time of the AKI episode, three class 2 DSAs were detected with MFI>1000 (DQ7, DR12, and DR52). Of those, two of them (DR12 and DR52) decreased significantly after daratumumab reinfusion. (C) Anti-HLA antibodies evolution after daratumumab as desensitization treatment. After plasmapheresis, anti-HLA antibodies MFI and cPRA increased significantly, both class 1 and class 2. After eight weekly infusions of daratumumab, anti-HLA MFI and cPRA decreased significantly. Maintenance infusion, bimonthly, permitted to decrease MFI and number of significant anti-HLA antibodies.
Anti-HLA Desensitization Using Daratumumab in a Candidate for Heart Transplant
The highly sensitized nature of a 62-year-old woman’s clinical situation confirmed the need for heart transplantation in December 2016 (complete clinical description is provided in Supplemental Material). Initial immunologic evaluation showed that up to 80% of heart allografts from deceased donors were incompatible (calculated PRA [cPRA]) with n=35 class 1 and n=5 class 2 alloantibody specificities. Desensitization included many plasmaphereses, multiple courses of high-dose IVIG, and rituximab. Because of no significant changes in sensitization (cPRA=98% and n=35 class 1 anti-HLA antibodies with MFI>3000) and deterioration of clinical condition, we used daratumumab as desensitization protocol (eight weekly injections of 16 mg/kg each). At the end of the treatment, we observed a significant decrease of cPRA to 62% with n=14 class 1 anti-HLA antibodies with MFI>3000 (Figure 6C) allowing heart transplantation in March 2018, with only two DSAs (one class 1 and one class 2). Heart allograft HLA included two prohibited antigens before daratumumab therapy.
Discussion
This study demonstrates that targeting CD38 with daratumumab significantly reduced anti-HLA antibodies and production of anti-HLA DSAs in both an experimental model and two transplant clinical cases before and after transplant.
Anti-HLA antibodies limit graft access and DSAs, preformed or dnDSAs, are increasingly recognized as the leading cause of early and late kidney allograft failure.4,6 Binding DSAs to antigens expressed on allograft endothelial cells can activate a classic complement pathway, a key pathologic process of AMR phenotypes.4,32 Even without complement activation, some DSAs can induce allograft damage through antibody-dependent cellular cytotoxicity.33,34 This pathogenesis may contribute to transplant glomerulopathy and vasculopathy which feature vascular intimal thickening with smooth muscle cell invasion.35 DSA-mediated rejection increases allograft dysfunction and is generally recalcitrant to the standard therapeutic approaches used for TCMR. To increase transplant access, immunosuppressive therapy combinations have been proposed for incompatible transplants but benefits are unclear, with acute rejection rates after transplant up to 40% and no difference in patient survival compared with dialysis.36 Such treatments allow elimination of circulating anti-HLA antibodies and effectively destroy B lymphocytes expressing CD20 markers; however, they have no effect on antibody-producing PCs.
Daratumumab is a first-in-class human IgG1 mAb that binds CD38-expressing cells with high affinity. Presently, daratumumab is used in cancer studies to promote tumor cell death through diverse mechanisms of action, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and apoptosis induction.15 In a phase 2 trial, daratumumab monotherapy showed encouraging efficacy in heavily pretreated and refractory patients with multiple myeloma with a favorable safety profile.37
Although CD38-targeting antibodies were initially developed to kill malignant PCs, these mAbs may also abrogate the production of autoantibodies in autoimmune disorders or anti-HLA antibodies in transplant recipients before or after transplant, thereby reducing antibody-dependent effector mechanisms. In transplantation, CD38-targeting antibodies provide a therapeutic option for sensitized transplant candidates and refractory AMR. Notably, the ability of daratumumab to induce nonmalignant CD38-PC depletion in the field of auto- or alloimmune-mediated disorders has not yet been evaluated. Daratumumab specifically targets CD38+ cells on the basis of cell surface expression. Furthermore, plerixafor may mobilize PC populations from the bone marrow niche.38,39 Unlike our previous approaches targeting PCs and germinal centers with costimulation blockade and proteasome inhibitor,21,40 we targeted PCs and activated/memory B cells. We hypothesized that we could achieve a more selective and complete desensitization by mobilizing PC populations from the bone marrow and directly target CD38-expressing PCs (as well as memory B cells). As expected, serum DSA levels as well as serum antibodies that crossreact with HLA Ag dropped significantly after DPT (more than 50%; Figure 1). The crossreactivity of anti-MAMU antibodies to HLA Ags is presumably due to the same Bw6 motif present in the Mamu-A1 haplotypes and the Mamu B alleles to HLA.41 The single antigen bead assay provided a better way to compare the rhesus result to a human DSA clinical situation. However, the HLA antigen beads with Bw6 motif (most >10,000 MFI) may be saturated with bound antibody, potentially underestimating the degree of antibody reduction. Diluted serum samples (1:8 dilution) showed a greater diminution compared with neat samples. The circulating B cell population was not significantly affected by the combination of daratumumab and plerixafor over time. However, serial biopsies during desensitization showed a reduction of plasmablasts in the lymph nodes without reducing the Tfh population, suggesting Tfh-independent mechanisms. The level of DSA reduction was comparable, or even superior, to that achieved by combined costimulation blockade–bortezomib.21,40 However, even with profound reduction of DSA before kidney transplantation, all treated animals showed limited durability in regulating the humoral response. Surprisingly, all animals rejected kidney transplants with enhanced acute rejection grade and AMR score, even though they showed pristine graft tissue on early biopsy (Supplemental Figure 3). It is unclear why treating sensitized NHP with daratumumab and plerixafor induces TCMR while effectively lowering DSA levels. We cannot attribute this to low animal numbers because we saw homogeneous outcomes. It is possible that the proximity to the sensitization events and MHC-mismatching status in the NHP model could favor a response. Perhaps if the sensitization had occurred years or months earlier and a well matched transplantation were available, this would be pursued in a human patient. With recent sensitization the cellular rejection may not be as difficult to suppress with conventional immunosuppression, but this is unclear. However, it is clear that, although both agents target PCs, they may also elicit broader immune modulation. Daratumumab could successfully target CD38-expressing memory B cells, plasmablasts, and PCs; however, these are not the only immune cells expressing CD38.27–29 In particular, B cells with regulatory function (Breg or B10 effector cells) are well known to express CD38.42,43 We observed reduction of Bregs and Tregs after DPT treatment with more rapid emergence of activated T cells after kidney transplantation. Krejcik et al.30 made a similar observation with daratumumab treatment in patients with multiple myeloma. They showed depletion of CD38+MM cells, as well as immunosuppressive cells including Treg (CD4+CD25+CD127dimCD38+), Breg (CD19+CD38hiCD24hi), and G-MDSC (CD11b+CD14−HLA−DR−CD15+CD33+), along with T cells skewing toward a phenotype of memory T cells. More recently, it has been shown that CD38 inhibition promoted superior effector function via metabolic reprograming of T cells.44 Furthermore, our observations are similar to the adverse events that occur with general B cell depletion at induction in transplantation. Clatworthy et al.45 showed increased TCMR with rituximab induction compared with noncytolytic induction. B cells with regulatory function may be targeted by both rituximab and daratumumab and contribute to graft rejection in organ transplantation. Whether the daratumumab-induced TCMR can be readily controlled with current or additional immunosuppressive regimens remains to be established.
In contrast to the NHP data, we also observed the potential benefit on daratumumab on anti-HLA antibodies and DSA in two patients, including refractory AMR in a recipient of a combined heart and kidney transplant and a candidate for heart transplantation who was highly refractory and sensitized. We observed in both cases a significant decrease in DSA and anti-HLA antibody MFI after eight injections of daratumumab, associated with a high depletion in peripheral CD38+ PCs, strongly supporting the effectiveness of the molecule to reduce allosensitization intensity. In both cases, plasmapheresis, high doses of IVIG, and rituximab failed to control DSA or PRA levels. MFI decrease after plasmapheresis and high-dose IVIG has already been analyzed in the context of acute AMR and dnDSA.46,47 Mean MFI decrease after plasmapheresis procedure was 25%, but long-term analysis is not currently available.46 High-dose IVIG has no effect on dnDSA MFI.47 Furthermore, DSA MFI rebound has been reported after rituximab induction therapy in recipients of kidney allograft with preformed DSA.48 The efficacy of daratumumab could be enhanced by better control of memory responses, for example by increasing the amount of calcineurin inhibitors immunosuppression. In the AMR-resistant case, both control heart and kidney allografts biopsies (performed 12 weeks postrejection) showed significant intragraft PC depletion. PC-rich rejection has been identified as a morphologically distinct lesion49 that occurs late post-transplantation and can be an independent predictor of poor allograft survival.50 Previous studies have also associated PC-rich rejection with vascular rejection, transplant glomerulopathy, and inadequate immunosuppression due to noncompliance.51 Whether PC infiltrates participate in humoral rejection through local secretion of antibodies is still a challenging question. Our data suggest that daratumumab induces intragraft deletion of CD38+ PCs and could represent a therapeutic option for the management of PC-rich rejection in the context of humoral rejection.
In conclusion, both experimental and preliminary clinical results trends to suggest that daratumumab is a potentially therapeutic strategy to limit DSA production in the setting of allotransplantation. The effect of targeting the PC population with daratumumab could be dependent on contexts such as the level of sensitization, timing of immunologic response, and degree of pathogenesis. However, the potential risk and benefit of daratumumab for treatment of AMR and desensitization should be investigated further to determine the benefit/risk ratio related to concomitant T cell activation.
Disclosures
Dr. Knechtle reports personal fees from Sanofi, outside of the submitted work. Dr. Belhadj reports personal fees from Celgene, personal fees from Takeda, personal fees from Amgen, and personal fees from Janssen, outside of the submitted work. All remaining authors have nothing to disclose.
Funding
This NHP study was supported by U19AI051731 (awarded to Dr. Knechtle) and U19AI131471 (awarded to Dr. Knechtle), part of the NIH NHP Transplantation Tolerance Cooperative Study Group sponsored by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases.
Supplementary Material
Acknowledgments
We appreciate our summer interns, Evelyn Branum (Wake Forest, NC) and Verna Curfman (Gordon College, MA), for collecting data points for this study. We also want to thank Dr. Eileen Chambers (Duke University) for her critical review for this manuscript.
We would like to gratefully acknowledge the Duke Division of Laboratory Animal Resources staff and the expert assistance of Dr. Kyha Williams and Dr. Felicita Smith for animal care. We also would like to acknowledge the Substrate Services Core Research Support for a weekly viral monitoring and histology support by Dr. Mingqing Song.
Anti-CD4 mAb and anti-CD8 mAb used in this study were provided by the NIH Nonhuman Primate Reagent Resource (R24 OD010976, U24 AI126683).
Dr. Kwun, Dr. Matignon, Dr. Knechtle, and Dr. Grimbert designed the study. Dr. Kwun, Dr. Manook, Dr. Guendouz, Dr. Kheav, Dr. Poullot, Dr. Gautreau, Dr. Ezekian, Dr. Bodez, Dr. Damy, Dr. Faivre, Mr. Yoon, and Dr. Belhadj carried out experiments. Dr. Kwun, Dr. Matignon, Dr. Chen, Dr. Bilewski, Dr. Yi, Dr. Farris, Dr. Knechtle, Dr. Park, and Dr. Grimbert analyzed the data. Dr. Poullot and Dr. Matignon made the figures. Dr. Kwun, Dr. Matignon, Dr. Knechtle, and Dr. Grimbert drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018121254/-/DCSupplemental.
Supplemental Figure 1. Longitudinal and transient changes of circulating leukocyte populations during daratumumab and plerixafor treatment.
Supplemental Figure 2. Effect of daratumumab and plerixafor on lymph node Treg cells.
Supplemental Figure 3. No profound prozone but some saturation effect were shown in serial diluted samples.
Supplemental Figure 4. Early renal biopsy after daratumumab and plerixafor treatment.
References
- 1.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K: Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270: 1339–1343, 1993 [PubMed] [Google Scholar]
- 2.Russell JD, Beecroft ML, Ludwin D, Churchill DN: The quality of life in renal transplantation—a prospective study. Transplantation 54: 656–660, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al.: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al.: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, et al.: Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 13: 76–85, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Marchioro TL, Holmes JH, Hermann G, Brittain RS, Stonington OH, et al.: Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery 55: 195–200, 1964 [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan SC, Pescovitz MD: Presensitization: The problem and its management. Clin J Am Soc Nephrol 1: 421–432, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, et al.: Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant 2: 758–760, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Tyan DB, Li VA, Czer L, Trento A, Jordan SC: Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation 57: 553–562, 1994 [PubMed] [Google Scholar]
- 11.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, et al.: Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 70: 887–895, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, et al.: Transplant glomerulopathy: Risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation 86: 681–685, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, et al.: IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 377: 442–453, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Haririan A, Nogueira J, Kukuruga D, Schweitzer E, Hess J, Gurk-Turner C, et al.: Positive cross-match living donor kidney transplantation: Longer-term outcomes. Am J Transplant 9: 536–542, 2009 [DOI] [PubMed] [Google Scholar]
- 15.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al.: Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186: 1840–1848, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Mehta K, Malavasi F: Human CD38 and Related Molecules, Basel, Switzerland, Karger Medical and Scientific Publishers, 2000 [Google Scholar]
- 17.Slifka MK, Antia R, Whitmire JK, Ahmed R: Humoral immunity due to long-lived plasma cells. Immunity 8: 363–372, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Manz RA, Arce S, Cassese G, Hauser AE, Hiepe F, Radbruch A: Humoral immunity and long-lived plasma cells. Curr Opin Immunol 14: 517–521, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai C-H, et al.: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Burghuber CK, Kwun J, Page EJ, Manook M, Gibby AC, Leopardi FV, et al.: Antibody-mediated rejection in sensitized nonhuman primates: Modeling human biology. Am J Transplant 16: 1726–1738, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M, et al.: Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant 19: 724–736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB III, Iwakoshi NN, et al.: Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 14: 59–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al.: Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions [published correction appears in Am J Transplant 15: 2784, 2015]. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, et al.: The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 17: 28–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al.: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al.: Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 24: 1710–1720, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Jackson DG, Bell JI: Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol 144: 2811–2815, 1990 [PubMed] [Google Scholar]
- 28.Malavasi F, Funaro A, Alessio M, DeMonte LB, Ausiello CM, Dianzani U, et al.: CD38: A multi-lineage cell activation molecule with a split personality. Int J Clin Lab Res 22: 73–80, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Mehta K, Shahid U, Malavasi F: Human CD38, a cell-surface protein with multiple functions. FASEB J 10: 1408–1417, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al.: Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128: 384–394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE: Identification and characterization of circulating human transitional B cells. Blood 105: 4390–4398, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stegall MD, Chedid MF, Cornell LD: The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol 8: 670–678, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Wood KJ, Goto R: Mechanisms of rejection: Current perspectives. Transplantation 93: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Legris T, Picard C, Todorova D, Lyonnet L, Laporte C, Dumoulin C, et al.: Antibody-dependent NK cell activation is associated with late kidney allograft dysfunction and the complement-independent alloreactive potential of donor-specific antibodies. Front Immunol 7: 288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schinstock CA, Stegall M, Cosio F: New insights regarding chronic antibody-mediated rejection and its progression to transplant glomerulopathy. Curr Opin Nephrol Hypertens 23: 611–618, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Manook M, Koeser L, Ahmed Z, Robb M, Johnson R, Shaw O, et al.: Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: A matched cohort analysis. Lancet 389: 727–734, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al.: Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387: 1551–1560, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Ma Q, Jones D, Springer TA: The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10: 463–471, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, et al.: Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6: 741–750, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Kwun J, Burghuber C, Manook M, Ezekian B, Park J, Yoon J, et al.: Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv 1: 2115–2119, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colantonio AD, Bimber BN, Neidermyer WJ Jr., Reeves RK, Alter G, Altfeld M, et al.: KIR polymorphisms modulate peptide-dependent binding to an MHC class I ligand with a Bw6 motif. PLoS Pathog 7: e1001316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al.: CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al.: Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117: 530–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, et al. : CD38-NAD(+)Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab 27: 85–100.e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al.: B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med 360: 2683–2685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada C, Ramon DS, Cascalho M, Sung RS, Leichtman AB, Samaniego M, et al.: Efficacy of plasmapheresis on donor-specific antibody reduction by HLA specificity in post-kidney transplant recipients. Transfusion 55: 727–735, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matignon M, Pilon C, Commereuc M, Grondin C, Leibler C, Kofman T, et al.: Intravenous immunoglobulin therapy in kidney transplant recipients with de novo DSA: Results of an observational study. PLoS One 12: e0178572, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KW, Park JB, Cho CW, Lee N, Yoo H, Kim K, et al.: The impact of donor-specific Anti-Human Leukocyte Antigen (HLA) antibody rebound on the risk of antibody mediated rejection in sensitized kidney transplant recipients. Ann Transplant 22: 166–176, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Charney DA, Nadasdy T, Lo AW, Racusen LC: Plasma cell-rich acute renal allograft rejection. Transplantation 68: 791–797, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Desvaux D, Le Gouvello S, Pastural M, Abtahi M, Suberbielle C, Boeri N, et al.: Acute renal allograft rejections with major interstitial oedema and plasma cell-rich infiltrates: High gamma-interferon expression and poor clinical outcome. Nephrol Dial Transplant 19: 933–939, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Gärtner V, Eigentler TK, Viebahn R: Plasma cell-rich rejection processes in renal transplantation: Morphology and prognostic relevance. Transplantation 81: 986–991, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.