Abstract
Purpose.
The purpose of this study was to translate our in vitro therapy approach to an in vivo model. Increased glutamine uptake is known to drive cancer cell proliferation, making tumor cells glutamine-dependent. Studying lymph-node aspirates containing malignant lung tumor cells showed a strong correlation between glutamine consumption and glutathione (GSH) excretion. Subsequent experiments with A549 and H460 lung tumor cell lines provided additional evidence for glutamine's role in driving synthesis and excretion of GSH. Using stable-isotope-labeled glutamine as a tracer metabolite, we demonstrated that the glutamate group in GSH is directly derived from glutamine, linking glutamine utilization intimately to GSH syntheses.
Materials and Methods.
To understand the possible mechanistic link between glutamine consumption and GSH excretion, we studied GSH metabolism in more detail. Inhibition of glutaminase (GLS) with BPTES, a GLS-specific inhibitor, effectively abolished GSH synthesis and excretion. Since our previous work, several novel GLS inhibitors became available and we report herein effects of CB-839 in A427, H460 and A549 lung tumor cells and human lung tumor xenografts in mice.
Results.
Inhibition of GLS markedly reduced cell viability, producing ED50 values for inhibition of colony formation of 9, 27 and 217 nM in A427, A549 and H460, respectively. Inhibition of GLS is accompanied by ~30% increased response to radiation, suggesting an important role of glutamine-derived GSH in protecting tumor cells against radiation-induce injury. In subsequent mouse xenografts, short-term CB-839 treatments reduced serum GSH by >50% and increased response to radiotherapy of H460-derived tumor xenografts by 30%.
Conclusion.
The results support the proposed mechanistic link between GLS activity and glutathione synthesis and suggest that GLS inhibitors are effective radiosensitizers.
Introduction
Lung cancer is one of the leading causes of cancer-related deaths in the US, with an estimated 222,500 new cases and an estimated 155,870 deaths in 2017 (American Cancer Society 2017; Siegel et al. 2017). Despite immense research efforts, the overall 5-year survival rate (all stages combined) of <17% remains poor compared with other cancers. The poor survival of lung cancer patients is attributed to the fact that approximately 70% of patients are diagnosed at an advanced stage (II,III or IV), because they do not exhibit any symptoms during the early stages of tumor development (Morgensztern et al. 2010; Devarakonda et al. 2013). For these patients, the advanced stage and presence of metastases precludes complete surgical resection, and treatment relies solely on thoracic radiation, chemotherapy, immunotherapy or a combination of them. In the past, treatment of advanced lung cancer followed a straightforward algorithm of platinum-based combination therapy or third-generation cytotoxic drugs, irrespective of histopathology subtypes (Johnson et al. 1990; Breathnach et al. 2001; Hennessy et al. 2003).
More recently, treatment efficacies have improved due to patient pre-selection based on histopathology subtypes and identification of specific driver mutations (Ausborn et al. 2012). Considering a patient’s tumor biology in therapy selection (personalized medicine) is transforming the diagnosis and treatment of lung cancer (Langer et al. 2010; Kim & Pandya 2013; Saito et al. 2018). Further, metabolic deregulation is a hallmark of cancer, as tumors exhibit an increased demand for nutrients and macromolecules to fuel their rapid proliferation (Hanahan & Weinberg 2011; Hosios et al. 2016). Significant improvements in lung cancer treatment are being made by targeting biochemical pathways essential for tumor growth (Song et al. 2018). For example, studying lymph-node aspirates that contained malignant lung tumor cells suggested a mechanistic link between glutamine consumption and GSH excretion (Sappington et al. 2017). In fact, we and others demonstrated that lung tumors require large amounts of glutamine to drive GSH synthesis (Hensley et al. 2013; Sappington et al. 2016). Inhibiting glutaminase (GLS) in lung tumor cells reduced GSH synthesis and resulted in increased sensitivity to ionizing radiation (Sappington et al. 2016).
The first step in glutaminolysis, mediated by mitochondrial GLS, is the enzymatic conversion of glutamine to ammonia and glutamate (van den Heuvel et al. 2012). Mammalian cells contain 2 genes that encode GLS: kidney-type (GLS1) and liver-type (GLS2) enzymes (Xiang et al. 2015; Momcilovic et al. 2017). GLS is overexpressed in various human tumors and has been shown to be positively regulated by oncogenes such as Myc (Dang 2016). Consistent with the observed dependence of cancer cell lines on glutamine metabolism, pharmacological inhibition of GLS offers the potential to target glutamine-dependent tumors. Glutamine, the most abundant amino acid in circulation, is known to play an essential role in providing cancer cells with biosynthetic intermediates required to support proliferation and survival. Specifically, glutaminolysis provides proliferating cancer cells with a source of nitrogen for amino acid and nucleotide synthesis, and a carbon building block to fuel the tricarboxylic acid (TCA) cycle (Mohamed et al. 2014). GLS inhibitors such as UPGL00004 (Huang et al. 2018), 968 (Yuan et al. 2016), CB-839 (Gross et al. 2014a), BPTES (Sappington et al. 2016), 6-diazo-5-oxo-L-norleucine (Rahman et al. 1985) and ebselen (Thomas et al. 2013) effectively inhibit GLS1 and reduce tumor growth in vitro and in vivo, suggesting that GLS is a suitable target for cancer therapy (Seltzer et al. 2010; Le et al. 2012; Gross et al. 2014a; Xiang et al. 2015).
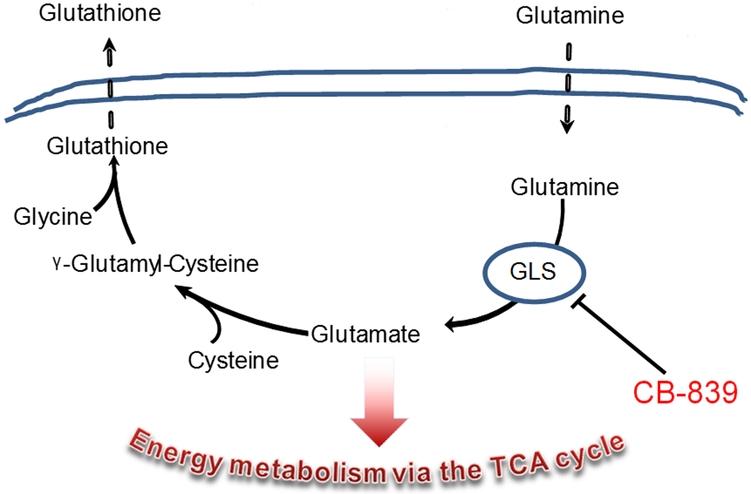
In addition to providing building blocks for cell growth, glutamine metabolism plays a critical role in maintaining cellular redox homeostasis, as glutamate is a precursor for GSH (Figure 1). GSH is the most abundant endogenous antioxidant and protects cells and tissues against oxidative stress such as that induced by radiation therapy. Inhibition of GLS by BPTES led to GSH depletion and increased response of A549 and H460 lung tumor cells to radiation treatment (Mukundan et al. 1999; Sappington et al. 2016). Surprising to many was the finding was that lung tumor cells seem to excrete micromolar amounts of GSH in clinical specimens (Sappington et al. 2017) and in cell culture (Sappington et al. 2016). Glutathione concentrations have previously been reported to be significantly higher in lung tumor tissues (20.8±9.4 nmol/mg protein) compared with normal lung tissue (11.6±3.0 nmol/mg protein, P<0.05), suggesting active GSH synthesis (Blair et al. 1997).
Figure 1.
Scheme for CB-839 effect on glutamine derived GSH syntheses and excretion.
The standard of care for lung cancer patients includes a combination of radiation therapy (RT) with surgery, chemotherapy or immunotherapy (Featherstone et al. 2007). Many approaches have been exploited to establish effective and low-toxicity radiosensitizers, compounds that increase the amount of cell killing in response to radiation therapy, and their utilization has become a highly desirable aspect in combination therapy (Dings et al. 2005; Amano et al. 2007; Dings et al. 2007; Koonce et al. 2015). Most radiosensitizers are small molecules that inhibit the repair of radiation-induced DNA damage and directly or indirectly increase oxidative stress or related mechanisms (Wang et al. 2018). Antioxidants such as GSH directly counteract these mechanisms, and GSH depletion is expected to enhance RT outcome (Bamatraf et al. 1998). Here we report the efficacy of CB-839 for GSH depletion and the subsequent increased response to radiation therapy in vitro and in vivo.
Material and Methods
Cell Culture
Tumor cell lines were from American Type Culture Collection. H460, A427 and A549 human lung carcinoma cell lines were grown in RPMI 1640 (Corning) with 10% fetus bovine serum (Atlanta Biological) and 100 units/ml penicillin/streptomycin in an incubator with a 5% CO2 atmosphere and maintained by sub-culturing every 3-4 days.
Clonogenic Assay
Effects of CB-839 on clonogenic viability and subsequent radiation sensitivity were determined as described previously (Sappington et al. 2016). In brief, H460, A427 and A549 cells (for several treatment groups) were seeded in 6-well plates containing 3 ml of standard complete medium, and allowed to attach for 24 hours. For the dose response of CB-839 on clonogenic viability, cells were treated with various concentrations of CB-839 ranging from 0.0001 to 100 μM in DMSO. In parallel, cells were grown in standard media or media containing 1% DMSO (Veh). In all treatments, DMSO concentrations was 1%. After 24 hours, cells were placed in complete RPMI medium and allowed to form colonies for a minimum of 6 doubling times (~8 days).
For the radiation response experiments, cells were seeded as described above. After 24 hours, plates were divided into treatment groups and grown in standard medium (control), glutamine-free medium (gln-), medium containing 1 μM CB-839 or medium containing only DMSO (veh). After 24 hours, cells were subsequently irradiated using a Faxitron X-ray Generating System (CP-160, Faxitron X-Ray Corp.). Single-doses of 4 or 8 Gray (Gy) were delivered at a dose rate of 1 Gy/min (150 kVp and 6.6 mA). Cells were then placed in complete RPMI medium and allowed to form colonies as described above.
For both experiments, the surviving colonies were stained and the colonies of >50 cells were counted on a stereomicroscope. Plating efficiency (PE) of cells after each treatment were determined and normalized to that of untreated control cells after each treatment, and the surviving fractions were expressed as a ratio of treated PE over untreated PE.
Metabolite quantitation by LC/MS
To confirm the effect of CB-839 on the activity of GLS, metabolites were extracted and analyzed as described previously by our group (Sappington et al. 2016). In brief, cells were cultured in 96-well plates at 3000 cells/well in 200 μl complete media with and without CB-839. Metabolites were extracted from 25 μl media or 25 μl mouse plasma by the addition of 200 μl 50% methanol/0.2% formic acid to the frozen cells in the culture flasks. Solvents were removed using a SpeedVac and pellets were reconstituted in 250 μl 50% methanol/0.2% formic acid. Proteins were precipitated by the addition of 1050 μL acetonitrile/0.2% formic acid, incubated on ice for 30 min and centrifuged for 10 min at 13000 g. Supernatants were transferred to new vials, solvents were removed in a SpeedVac and the concentrated metabolites were stored at −80 °C until analysis. For analyses, samples were reconstituted with 100 μL 50% methanol/0.2% formic acid and analyzed by LC-MS/MS (Agilent, 1290 Infinity LC coupled to an Agilent 6490 triple quadrupole mass analyzer). Individual metabolites were monitored with the multiple reactions monitoring (MRM) mode, monitoring their specific ion transitions as described previously (Sappington et al. 2016). Quantitation of the individual metabolites was based on external calibration curves that were generated with each set of samples.
Human Xenografts Model in Mouse
To demonstrate in vivo efficacy, H460-cell-derived tumors were established subcutaneously on the hind flank of 20 male nude mice. When tumors reached a size of >100mm3 (day 12) mice were divided into four groups: control, CB-839, 18 Gy radiation and CB-839 plus 18 Gy radiation. In a follow-up experiment, H460 cell-derived tumors were established subcutaneously on the hind flank of 50 female nude mice. When tumors reached a size of >100mm3 (day 12), mice were divided into ten groups: control, CB-839, and 2, 4, 8 and 12 Gy radiation with and without CB-839. For both experiments, CB-839 was given as three oral gavages of 200mg/kg body weight, 28, 16 and 4 hours prior to blood draw from the tail vein and subsequent exposure to the corresponding dose of radiation. For these initial experiments we chose to administer CB-839 as recommended 2 × 200 mg/kg body weight the day before and 4 hours prior to administration of a single dose of radiation. This dosing regimen was chosen to increase dosage response at a time when serum CB-839 concentration was expected to peak (Gross et al. 2014b). Serum was analyzed for reduced and oxidized GSH by LC-MS. Tumor sizes were measured using a metric caliper. For radiation treatments mice were anesthetized with 2.5% isoflurane and placed supine on a mouse bed. The radiation was applied using a standard x-ray beam generator and the body was shielded using a custom designed 1/8 inch lead shield that reduces exposure by over 90% and the tumor-bearing hind leg was irradiated using a Faxitron cabinet x-ray system operating at 160kV.
Data Analysis
Longitudinal tumor-size data from each mouse were normalized to their Day-0 values. The resulting relative tumor volumes were log-transformed to stabilize variance and reduce right-skewing, then analyzed via mixed-models repeated-measures ANOVA. The post hoc analysis consisted of pairwise comparisons among treatment groups within each time point. Each comparison was 2-sided and employed an unadjusted alpha=0.05 significance level in order not to inflate Type II error in this modestly powered study, despite the multiple comparisons.
Results and Discussion
Inhibition of GLS1 in H460 and A549 cells by BPTES has been shown to reduce glutamine-dependent GSH synthesis, and the reduction in GSH led to increased response to radiation therapy (Sappington et al. 2016). In contrast to the first generation of GLS inhibitors which are glutamine analogues, screening a library of chemical compounds revealed that BPTES and CB-839 were effective GLS inhibitors that cause the formation of a stable but inactive GLS tetramer (Robinson et al. 2007). Since its discovery, many other GLS inhibitors have been reported and studied in combination with other drugs. To the best of our knowledge, the potential for synergy of a GLS inhibitor with radiation therapy in vivo remains unexplored. Therefore, building on our previous work with BPTES in vitro, we set out to determine if GLS inhibition in fact reduces GSH synthesis in vivo and subsequently improves response to radiation therapy.
Unfortunately, BPTES’s weak solubility of 0.144 μg/mL and subsequent poor bioavailability made it less favorable for in vivo studies (Shukla et al. 2012). To overcome this limitation, Elgogary et al. demonstrate the improved pharmacokinetics and efficacy of BPTES bound to nanoparticles compared with free BPTES (Elgogary et al. 2016). More recently, several other GLS inhibitors have been reported, demonstrating an interest in GLS inhibition as a cancer-drug target. The emergence of novel GLS inhibitors prompted us to investigate CB-839, which is currently in phase I and phase II clinical trials ( NCT02071862) and considered the best-in-class GLS inhibitor (Katt et al. 2017; Thompson et al. 2017; Momcilovic et al. 2018). CB-839 in combination with paclitaxel largely increased sensitivity to paclitaxel in xenograft models of triple-negative breast cancer (Gross et al. 2014a), and in combination with metformin against pancreatic cancer (Elgogary et al. 2016). CB-839 also synergized with the proteasome inhibitor carfilzomib against myeloma (Thompson et al. 2017) and showed efficacy in combination with erlotinib on epidermal growth-factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) (Momcilovic et al. 2017).
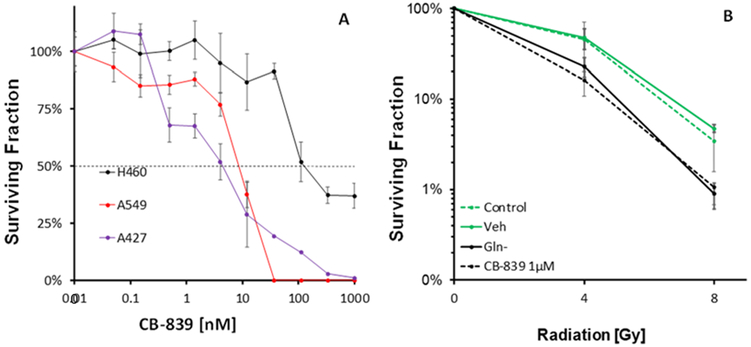
We first established the in vitro dose response for CB-839 in lung tumor cells. The CB-839 ED50s for inhibition of colony formation were 9.1, 27.0 and 217 nM for A427, A549 and H460, respectively. These ED50 compare favorably to the respective BPTES ED50 of 1,000 and 4,200 nM against A549 and H460, thus demonstrating the markedly greater potency of CB-839 compared to BPTES (Sappington et al. 2016). Treating H460 cells with 1 μM CB-839 elicited an increased response to radiation that was essentially identical to culturing in glutamine-free medium (Figure 2), suggesting that CB-839 completely blocked glutamine utilization at that concentration. Compared to our previous studies with 10 μM BPTES, CB-839 was as effective at a 10-fold lower concentration. Future studies are underway with lower CB-839 concentrations to determine the minimum concentration needed to block glutamine utilization in lung tumor cells.
Figure 2.
Effect of GLS inhibition by CB-839 on colony formation (A) in H460, A427 and A549 lung tumor cells, (B) in response to radiation in H460 cells. Shown are mean ± SD of three independent experiments.
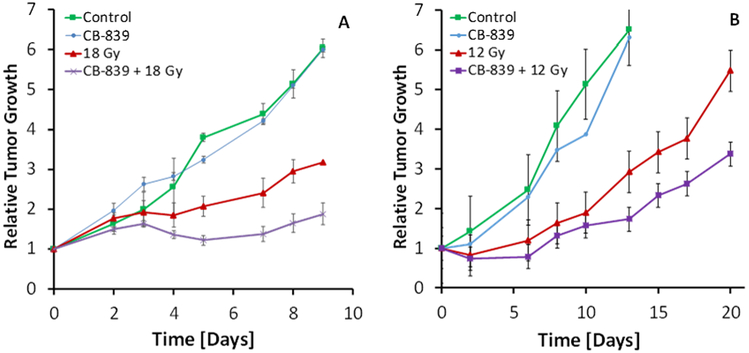
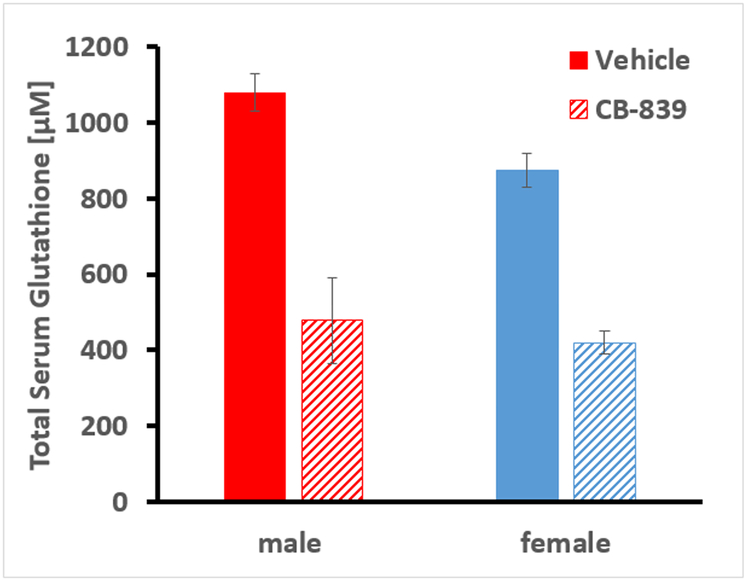
We then explored the efficacy of the CB-839 with and without a single dose of radiation in a mouse xenograft model of human lung tumors. H460 lung tumors were established on the hind flank of male and female nude mice and treated with CB-839, radiation, both or neither. In the first round of xenografts, using male mice, the radiation dose of 18 Gy was chosen based on common clinical practice (Nagata et al. 2011; Timmerman et al. 2018). The results in the male mice suggest that 18 Gy is quite sufficient and for the subsequent round of xerographs with female several doses of radiation, ranging from 2, 4, 8 and 12 Gy were studied, because the goal is to reduce radiation doses to avoid adverse side effects. In both male and female mice, the combination treatments reduced tumor growth by 15%–30% (Figure 3a & b and Supplemental Figure S1), providing strong evidence for efficacy of the combination therapy. The single radiation dose was administered 4 hours after the last dose of CB-839, the time by which CB-839 reaches peak serum concentrations as reported previously (Gross et al. 2014b). The CB-839 treatments produce a 50% reduction of serum GSH concentrations (Figure 4) confirming the predicted drug effect on GSH synthesis. The short-term CB-839 dosing alone does not affect tumor growth, which was expected since CB-839 is recommended for daily BID dosing up to 30 days.
Figure 3.
Response of H460 xenografts in mouse to radiation with and without CB-839 treatment in male (A) and female (B) nude mice. Male and female mice were irradiated to 18 and 12 Gy, respectively. CB-839 was given by oral gavage 28, 16 and 4 hours prior to radiation at 200 mg/kg. Shown are the mean and SD from 3 male and 5 female mice per group, respectively.
Figure 4.
Effect of CB-839 on total serum glutathione in male and female nude mice after 3 doses of oral gavage of CB-839. Shown are the mean and SD from 3 male and 5 female mice per group, respectively.
There were several limitations in this study that should be addressed in future studies. The numbers of animals per group was low and a larger group sizes may allow us to observe statistically significant differences earlier in the growth delay assessment process. Effects of CB-839 on GSH concentrations in tumor and non-tumor tissues prior to radiation would also increase our ability to interpret our results and add depth to the understanding of manipulating this pathway. Third, the hypoxic status of the tumor microenvironment should be assessed. In addition, varied dosing regimens of CB-839 plus radiation would help to further elucidate the efficacy of CB-893 given before, during or after a single dose or multiple low dose radiation fractions. Lastly, the benefits of the combination of glutaminase inhibitor with radiation should be evaluated in an orthotopic lung tumor model in an immune competent mouse model.
Together, the in vivo and in vitro experiments demonstrate that the metabolic pathway identified in lymph node aspirates of lung cancer patients is a suitable therapy target. Further, our data show that CB-839 dosing can be significantly reduced when given in combination with a single dose of radiation, a regimen that fits well with the current trend toward low fraction number, high dose stereotactic radiotherapy for lung cancer.
Supplementary Material
Acknowledgments
Support has been provided in part by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grants UL1TR000039 and KL2TR000063, the Arkansas Bioscience Institute, and the Envoys, an advocacy group of the UAMS Cancer Institute Foundation. Further, we are grateful to Calithera Biosciences for providing CB-839.
Footnotes
Declaration of Interest
Drs. Boysen and Griffin are actively developing GLS inhibitors as radio sensitizers and have filed patent applications related to the same.
References
- Amano M, Suzuki M, Andoh S, Monzen H, Terai K, Williams B, Song CW, Mayo KH, Hasegawa T, Dings RPM, Griffin RJ. 2007. Antiangiogenesis therapy using a novel angiogenesis inhibitor, anginex, following radiation causes tumor growth delay. Int J Clin Oncol [Internet]. 12:42–7. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. 2017. Cancer Facts & Figures.
- Ausborn NL, Le QT, Bradley JD, Choy H, Dicker AP, Saha D, Simko J, Story MD, Torossian A, Lu B. 2012. Molecular profiling to optimize treatment in non-small cell lung cancer: A review of potential molecular targets for radiation therapy by the translational research program of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 83. [DOI] [PubMed] [Google Scholar]
- Bamatraf MMM, O’Neill P, Rao BSM. 1998. Redox dependence of the rate of interaction of hydroxyl radical adducts of DNA nucleobases with oxidants: Consequences for DNA strand breakage. J Am Chem Soc. 120:11852–11857. [Google Scholar]
- Blair SL, Heerdt P, Sachar S, Abolhoda A, Hochwald S, Cheng H, Burt M. 1997. Glutathione metabolism in patients with non-small cell lung cancers. Cancer Res [Internet]. 57:152–5. [PubMed] [Google Scholar]
- Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR, O’Connell M, Shepherd FA, Johnson BE. 2001. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol [Internet]. 19:1734–42. [DOI] [PubMed] [Google Scholar]
- Dang CV. 2016. A Time for MYC: Metabolism and Therapy. Cold Spring Harb Symp Quant Biol. 81:79–83. [DOI] [PubMed] [Google Scholar]
- Devarakonda S, Morgensztern D, Govindan R. 2013. Molecularly targeted therapies in locally advanced non-small-cell lung cancer. Clin Lung Cancer [Internet]. 14:467–72. [DOI] [PubMed] [Google Scholar]
- Dings RPM, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, Griffin RJ. 2007. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res [Internet]. 13:3395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dings RPM, Williams BW, Song CW, Griffioen AW, Mayo KH, Griffin RJ. 2005. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J cancer [Internet]. 115:312–9. [DOI] [PubMed] [Google Scholar]
- Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, Fu J, Chen B, Xia S, Liu Y, et al. 2016. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci [Internet]. 113:E5328–E5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone C, Colley A, Tucker K, Kirk J, Barton MB. 2007. Estimating the referral rate for cancer genetic assessment from a systematic review of the evidence. Br J Cancer [Internet]. 96:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, et al. 2014a. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther [Internet]. 13:890–901. [DOI] [PubMed] [Google Scholar]
- Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, et al. 2014b. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 13:890–901. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell [Internet]. 144:646–74. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Hanrahan EO, Breathnach OS. 2003. Chemotherapy options for the elderly patient with advanced non-small cell lung cancer. Oncologist [Internet]. 8:270–7. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, Deberardinis RJ. 2013. Review series Glutamine and cancer : cell biology, physiology, and clinical opportunities. J Clin Invest. 123:3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel APJ, Jing J, Wooster RF, Bachman KE. 2012. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther [Internet]. 13:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosios AM, Hecht VC, Danai L V., Johnson MO, Rathmell JC, Steinhauser ML, Manalis SR, Vander Heiden MG. 2016. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev Cell [Internet]. 36:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Stalnecker C, Zhang C, McDermott LA, Iyer P, O’Neill J, Reimer S, Cerione RA, Katt WP. 2018. Characterization of the interactions of potent allosteric inhibitors with glutaminase C, a key enzyme in cancer cell glutamine metabolism. J Biol Chem. 293:3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BE, Grayson J, Makuch RW, Linnoila RI, Anderson MJ, Cohen MH, Glatstein E, Minna JD, Ihde DC. 1990. Ten-year survival of patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. J Clin Oncol [Internet]. 8:396–401. [DOI] [PubMed] [Google Scholar]
- Katt WP, Lukey MJ, Cerione RA. 2017. A tale of two glutaminases: homologous enzymes with distinct roles in tumorigenesis. Future Med Chem [Internet]. 9:223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Pandya KJ. 2013. Advances in personalized therapy for lung cancer. Expert Opin Med Diagn [Internet]. 7:475–85. [DOI] [PubMed] [Google Scholar]
- Koonce NA, Quick CM, Hardee ME, Jamshidi-Parsian A, Dent JA, Paciotti GF, Nedosekin D, Dings RPM, Griffin RJ. 2015. Combination of Gold Nanoparticle-Conjugated Tumor Necrosis Factor-α and Radiation Therapy Results in a Synergistic Antitumor Response in Murine Carcinoma Models. Int J Radiat Oncol Biol Phys [Internet]. 93:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer CJ, Besse B, Gualberto A, Brambilla E, Soria J-C. 2010. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol [Internet]. 28:5311–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21079145 [DOI] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. 2012. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in b cells. Cell Metab [Internet]. 15:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A, Deng X, Khuri FR, Owonikoko TK. 2014. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 15:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Braas D, Go J, Graeber TG, Parlati F, Demo S, Li R, et al. 2018. The GSK3 Signaling Axis Regulates Adaptive Glutamine Metabolism in Lung Squamous Cell Carcinoma. Cancer Cell [Internet]. [cited 2018 Jul 16]; 33:905–921.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Magyar C, Braas D, Graeber T, Jackson NJ, Czernin J, Emberley E, et al. 2017. Targeted Inhibition of EGFR and Glutaminase Induces Metabolic Crisis in EGFR Mutant Lung Cancer. Cell Rep [Internet]. 18:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgensztern D, Ng SH, Gao F, Govindan R. 2010. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol [Internet]. 5:29–33. [DOI] [PubMed] [Google Scholar]
- Mukundan H, Bahadur AK, Kumar A, Sardana S, Naik SL, Ray A, Sharma BK. 1999. Glutathione level and its relation to radiation therapy in patients with cancer of uterine cervix. Indian J Exp Biol [Internet]. 37:859–64. [PubMed] [Google Scholar]
- Nagata Y, Wulf J, Lax I, Timmerman R, Zimmermann F, Stojkovski I, Jeremic B. 2011. Stereotactic radiotherapy of primary lung cancer and other targets: results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys. 79:660–9. [DOI] [PubMed] [Google Scholar]
- Rahman A, Smith FP, Luc PT, Woolley P V. 1985. Phase I study and clinical pharmacology of 6-diazo-5-oxo-L-norleucine (DON). Invest New Drugs [Internet]. 3:369–74. [DOI] [PubMed] [Google Scholar]
- Robinson MM, McBryant SJ, Tsukamoto T, Rojas C, Ferraris DV., Hamilton SK, Hansen JC, Curthoys NP. 2007. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem J. 406:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Suzuki H, Kono K, Takenoshita S, Kohno T. 2018. Treatment of lung adenocarcinoma by molecular-targeted therapy and immunotherapy. Surg Today [Internet]. 48:1–8. [DOI] [PubMed] [Google Scholar]
- Sappington DR, Helms SA, Siegel ER, Penney RB, Jeffus SK, Bartter TT, Bartter TT, Boysen G. 2017. Diagnosis of lung tumor types based on metabolomic profiles in lymph node aspirates. Cancer Treat Reseach Commun. Vol 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington DR, Siegel ER, Hiatt G, Desai A, Penney RB, Jamshidi-Parsian A, Griffin RJ, Boysen G. 2016. Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A549 and H460 lung cancer cell lines. Biochim Biophys Acta - Gen Subj. 1860:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV., Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, et al. 2010. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 70:8981–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K, Ferraris DV, Thomas AG, Stathis M, Duvall B, Delahanty G, Alt J, Rais R, Rojas C, Gao P, et al. 2012. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem [Internet]. 55:10551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Miller KD, Ahmedin J. 2017. Cáncer Statistics. Ca Cáncer J. 67:7–30. [DOI] [PubMed] [Google Scholar]
- Song M, Kim S-H, Im CY, Hwang H-J. 2018. Recent Development of Small Molecule Glutaminase Inhibitors. Curr Top Med Chem. 18:432–443. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Rojas C, Tanega C, Shen M, Simeonov A, Boxer MB, Auld DS, Ferraris DV., Tsukamoto T, Slusher BS. 2013. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem Biophys Res Commun [Internet]. 438:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RM, Dytfeld D, Reyes L, Robinson RM, Smith B, Manevich Y, Jakubowiak A, Komarnicki M. 2017. Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. 8:35863–35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG, et al. 2018. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Mu X, He H, Zhang X-D. 2018. Cancer Radiosensitizers. Trends Pharmacol Sci [Internet]. 39:24–48. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, Hsieh AL, Gouw AM, Thomas AG, Gao P, et al. 2015. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 125:2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Sheng X, Clark LH, Zhang L, Guo H, Jones HM, Willson AK, Gehrig PA, Zhou C, Bae-Jump VL. 2016. Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am J Transl Res. 8:4265–4277. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.