Abstract
Background:
Using antibiotics appropriately is critical to slow spread of antibiotic resistance, a major public health problem. Children, especially young children, receive more antibiotics than other age groups. Our objective was to describe antibiotic use in children in the United States (US) and use of azithromycin, which is recommended infrequently for pediatric conditions.
Methods:
We used QuintilesIMS Xponent 2013 data to calculate the number and rate of oral antibiotic prescriptions for children by age (0–2, 3–9 and 10–19 years) and agent. We used log-binomial regression to calculate adjusted prevalence rations (PR) and 95% confidence intervals (CI) to determine if specialty and patient age were associated with azithromycin selection when an antibiotic was prescribed.
Results:
In 2013, 66.8 million antibiotics were prescribed to US children aged ≤19 years (813 antibiotic prescriptions per 1000 children). Amoxicillin and azithromycin were the two most commonly prescribed agents (23.1 million courses, 35% of all antibiotics; 12.2 million, 18%; respectively). Most antibiotics for children were prescribed by pediatricians (39%) and family practitioners (15%). Family practitioners were more likely to select azithromycin when an antibiotic was prescribed in all age groups than pediatricians (for children aged 0–2 years: PR 1.79, 95% CI, 1.78–1.80; 3–9 years: 1.40, 1.40–1.40; and 10–19 years: 1.18, 1.18–1.18).
Conclusion:
Despite infrequent pediatric recommendations, variations in pediatric azithromycin use may suggest inappropriate antibiotic selection. Public health interventions focused on improving antibiotic selection in children as well as reducing antibiotic overuse are needed.
Keywords: Antibiotics, azithromycin, pediatrics, children, antibiotic stewardship
Introduction
Antibiotic resistant pathogens infect an estimated 2 million people and lead to 23,000 deaths annually in the United States (US).1 Antibiotic use drives the spread of antibiotic resistance.2 Young children have the highest rates of antibiotic prescriptions,3,4 and at least 29% of outpatient antibiotics prescribed to children are unnecessary.4 From 2000 to 2010, pediatric outpatient antibiotic prescribing decreased, but the rate of broad-spectrum antibiotic prescribing increased.5,6
In 2010, amoxicillin was the most commonly prescribed medication for children, followed by azithromycin.7 Amoxicillin is narrow-spectrum and is indicated as first-line therapy for common pediatric infections including otitis media, sinusitis, streptococcal pharyngitis, and pneumonia.8–11 Azithromycin by comparison, is broad-spectrum and has more limited first-line recommendations for pediatric conditions, such as atypical pneumonia in school-aged and older children,8 sexually-transmitted diseases,12 and pertussis.13 Macrolide resistance is a concern for Streptococcus pneumoniae14 and Streptococcus pyogenes.10 Among the three most common diagnoses leading to antibiotics in children — acute otitis media, pharyngitis, and sinusitis4 —azithromycin is not recommended for otitis media or sinusitis and is only recommended for streptococcal pharyngitis for patients with penicillin allergy. 9–11,15 Nonetheless, macrolides are commonly prescribed to children for otitis media, pharyngitis, and infections for which antibiotics are never indicated, viral respiratory infections.6 Given prior evidence of overuse of azithromycin in children and to inform the selection of public health interventions for improving antibiotic use in children, we aimed to (1) describe overall outpatient antibiotic use and azithromycin use in US children and (2) examine differences in azithromycin selection by specialty.
Methods
Data sources
We extracted systemic oral antibiotic prescriptions dispensed during 2013 from the QuintilesIMS Xponent database (Danbury, Connecticut). QuintilesIMS Xponent collects data from pharmacies reporting all business to QuintilesIMS weekly. QuintilesIMS captures >80% of prescriptions nationally. QuintilesIMS then reconciles captured prescriptions to wholesale deliveries and projects using a patented method to 100% coverage. The QuintilesIMS projection method produces estimated prescription counts. Using geospatial methods, QuintilesIMS reports that these estimated prescription counts from non-sampled pharmacies are aligned with clinicians whose prescribing behavior is observed for the same product from nearby pharmacies. QuintilesIMS reports routine validation of this method. These data represent all antibiotic prescriptions dispensed in US community and mail-order pharmacies, excluding federal facilities.3 This project was determined to be consistent non-research program evaluation and monitoring by the Human Subjects Advisor in the National Center for Emerging and Zoonotic Infectious Diseases, and thus IRB review was not required.
The number of prescriptions were calculated for children aged ≤19 years by patient age group, sex and prescriber’s state and region. Patients were classified within age categories (0–2, 3–9, and 10–19 years) predefined within the QuintilesIMS Xponent data extract. Antibiotics were classified according to the Uniform System of Classification by QuintilesIMS.16 Antibiotics included agents in the following categories: tetracyclines, cephalosporins, lincosamides, macrolides, narrow-spectrum penicillins, quinolones, trimethoprim-sulfamethoxazole, β-lactams with increased activity (e.g. amoxicillin-clavulanate), urinary anti-infectives (e.g. nitrofurantoin), and others (e.g. metronidazole). Rate calculation was performed using population denominators from the US Census bridging files to determine prescribing rates per 1,000 persons for all antibiotics and for azithromycin by age group, sex, region and state. Additionally, the most commonly prescribed antibiotic agents were identified for all children aged ≤19 years and by age group.
Clinician specialties from the American Medical Association self-designated practice specialties were categorized into family practice, pediatrics, nurse practitioners, physician assistants, and all other specialties (including pediatric subspecialties). The total number of clinicians by specialty was taken from QuintilesIMS Xponent. For analyses based on clinician specialties, the total number of visits by children to clinicians is unknown, so we were unable to calculate visit-based rates for antibiotic prescribing by specialty.
To examine azithromycin selection among children aged ≤19 years old who received any antibiotic prescription, we performed log-binomial regression using the events-trials format.17 The outcome, azithromycin selection, was defined as binary variable (yes/no azithromycin prescription). We investigated four factors: specialty group (pediatrics, family practice, nurse practitioner, physician assistant, others); patient sex (male and female); patient age categories (0–2, 3–9, 10–19 years); and region (Northeast, Midwest, South, West). We created a dataset in the events-trials format,17 with the number of azithromycin prescriptions and number of all antibiotic prescriptions as events and trials, respectively. We did this by stratifying the aggregated QuintilesIMS Xponent data for a combination of specialty group, patient’s sex and age, and region of care to determine the numbers of azithromycin prescribing and all antibiotic prescribing. We computed prevalence ratios (PR) as measures of association between azithromycin selection (dependent/outcome variable) and the factors mentioned above (independent/exposure variable) using log-binomial regression.18 We considered each factor as primary exposure and performed univariate and multivariate regression analyses. Based on previous knowledge and observations, we looked for the presence of interaction between clinician specialty group and patient age. We used the Akaike Information Criterion (AIC) to compare the model with interaction to model without interaction, and selected the model with interaction as the better model as it had the smaller AIC. We then modeled the interaction by stratifying analysis by age group. We adjusted each model from this analysis for sex and region.
In multivariate analyses of the main effects of sex and region, when a factor was the primary exposure, the other factors were considered as potential confounders. For each primary exposure, first, we created a full model using all potential confounders (i.e. the other three factors). Then, we compared this full model to all other possible subsets of potential confounders. Initially, we chose models with PRs of primary exposure (adjusted prevalence ratios) that were within 10% change from the full model. We chose the best model with the adjusted PR that was closest to the estimate from the full model and confidence interval that was more precise17. However, when adjusted estimates from the other subset models were similar but did not result to substantial increase in precision compared to the full model, we considered the full model as the best model. We computed unadjusted and adjusted PR and 95% confidence interval (CI) for these associations. Statistical analyses were performed using PROC GENMOD with SAS version 9.3 (SAS Institute, Cary, NC). We considered α <0.05 as statistically significant.
Results
In 2013, 66.8 million outpatient antibiotic prescriptions were dispensed to children aged ≤19 years, which corresponds to 813 antibiotic prescriptions per 1000 children aged ≤19 years. The highest rate of antibiotic prescriptions was for children aged 0–2 years at 1141 antibiotic prescriptions per 1000 population and decreased with increasing age (Table 1). Children in the South were more likely to receive antibiotics (952 antibiotic prescriptions per 1000 children) versus children in the West (555 prescriptions per 1000 children). The top antibiotic classes prescribed to children were penicillins (24.3 million prescriptions, 36% of all antibiotics), macrolides (12.8 million, 19%), cephalosporins (11.8 million, 18%), β-lactams with increased activity (6.9 million, 10%), and trimethoprim-sulfamethoxazole (4.4 million, 7%) (Supplemental Digital Content table 1). The top outpatient antibiotic agents prescribed to children were amoxicillin (23.1 million prescriptions, 35% of all antibiotics), azithromycin (12.2 million, 18%), amoxicillin-clavulanate (6.9 million, 10%), cefdinir (5.4 million, 8%), and cephalexin (4.5 million, 7%) (Supplemental Digital Content table 2). Amoxicillin accounted for 45% of all antibiotic prescriptions in children 0–2 years old but only 23% of antibiotic prescriptions in children 10–19 years old (Table 2). Azithromycin became a more common antibiotic choice with increasing age, accounting for 15% of antibiotic prescriptions in children aged 0–2 years, 19% of antibiotic prescriptions for children aged 3–9 years, and 20% of antibiotic prescriptions in children aged 10–19.
Table 1.
All antibiotic prescriptions and azithromycin prescriptions according to age group, sex, and region in children aged ≤19 years – United States, 2013.
| Characteristic | All antibiotic prescriptions | Azithromycin prescriptions | ||
|---|---|---|---|---|
| Number (%) in millions |
Rate per 1,000 persons |
Number (%) in millions |
Rate per 1,000 persons |
|
| Age group in years | ||||
| 0–2 | 13.6 (20) | 1,141 | 2.0 (16) | 165 |
| 3–9 | 25.9 (39) | 906 | 4.9 (40) | 172 |
| 10–19 | 27.4 (41) | 655 | 5.3 (44) | 128 |
| Sex | ||||
| Female | 34.7 (52) | 863 | 6.2 (51) | 154 |
| Male | 32.1 (48) | 762 | 6.0 (49) | 144 |
| Region | ||||
| Northeast | 10.9 (16) | 801 | 1.8 (15) | 134 |
| Midwest | 15.2 (23) | 863 | 2.8 (23) | 158 |
| South | 29.7 (44) | 952 | 5.6 (46) | 179 |
| West | 11 (16) | 555 | 2.0 (17) | 103 |
| Total | 66.8 (100) | 813 | 12.2 (100) | 149 |
Table 2.
Top 10 antibiotic agents prescribed to children by age group in number, in millions, and percent of antibiotics prescribed — United States, 2013.
| Antibiotic agent | Number of prescriptions, millions |
Percent of antibiotics prescribed |
|---|---|---|
| Children 0–2 years of age | ||
| Amoxicillin | 6.2 | 45.4 |
| Azithromycin | 2.0 | 14.5 |
| Cefdinir | 1.7 | 12.7 |
| Amoxicillin-clavulanate | 1.7 | 12.4 |
| Trimethoprim-sulfamethoxazole | 0.7 | 5.1 |
| Cephalexin | 0.6 | 4.1 |
| Cefprozil | 0.2 | 1.7 |
| Cefixime | 0.1 | 1.1 |
| Clindamycin | 0.1 | 0.9 |
| Penicillin V | 0.1 | 0.6 |
| Children 3–9 years of age | ||
| Amoxicillin | 10.6 | 40.9 |
| Azithromycin | 4.9 | 19.0 |
| Amoxicillin-clavulanate | 2.8 | 10.9 |
| Cefdinir | 2.6 | 9.9 |
| Cephalexin | 1.7 | 6.5 |
| Trimethoprim-sulfamethoxazole | 1.5 | 5.8 |
| Cefprozil | 0.4 | 1.5 |
| Penicillin V | 0.3 | 1.3 |
| Clindamycin | 0.3 | 1.1 |
| Cefixime | 0.2 | 0.8 |
| Children 10–19 years of age | ||
| Amoxicillin | 6.4 | 23.2 |
| Azithromycin | 5.3 | 19.5 |
| Amoxicillin-clavulanate | 2.4 | 8.7 |
| Cephalexin | 2.3 | 8.3 |
| Trimethoprim-sulfamethoxazole | 2.2 | 7.9 |
| Doxycycline | 2.0 | 7.1 |
| Minocycline | 1.9 | 6.8 |
| Cefdinir | 1.2 | 4.3 |
| Penicillin V | 0.7 | 2.5 |
| Clindamycin | 0.7 | 2.4 |
Overall, 12.2 million azithromycin prescriptions were dispensed to children aged ≤19 years in 2013, 149 azithromycin prescriptions per 1000 population (Table 1). Children aged 3–9 years had the highest rate of azithromycin prescriptions (172 per 1000 population) compared to children aged 0–2 years (165 per 1000 population) and children aged 10–19 years (128 per 1000 population). Regionally, the azithromycin prescription rate was highest in the South (179 per 1000 children aged ≤19 years) and lowest in the West (103 per 1000 children aged ≤19 years). In 2013, states with high overall antibiotic prescribing rates also had high azithromycin prescribing rates (Figure 1, Supplemental Digital Content Figure 1). Kentucky and West Virginia had the highest antibiotic prescribing rates (1341 and 1317 antibiotic prescriptions per 1000 children, respectively) over 3 times the two lowest states, Oregon and Alaska (431 and 324 per 1000 children, respectively). Louisiana and Mississippi had the highest azithromycin prescription rates (303 and 276 azithromycin prescriptions per 1000 children) 4 times higher than Oregon and Alaska (67 and 65 per 1000 children, respectively).
Figure 1.
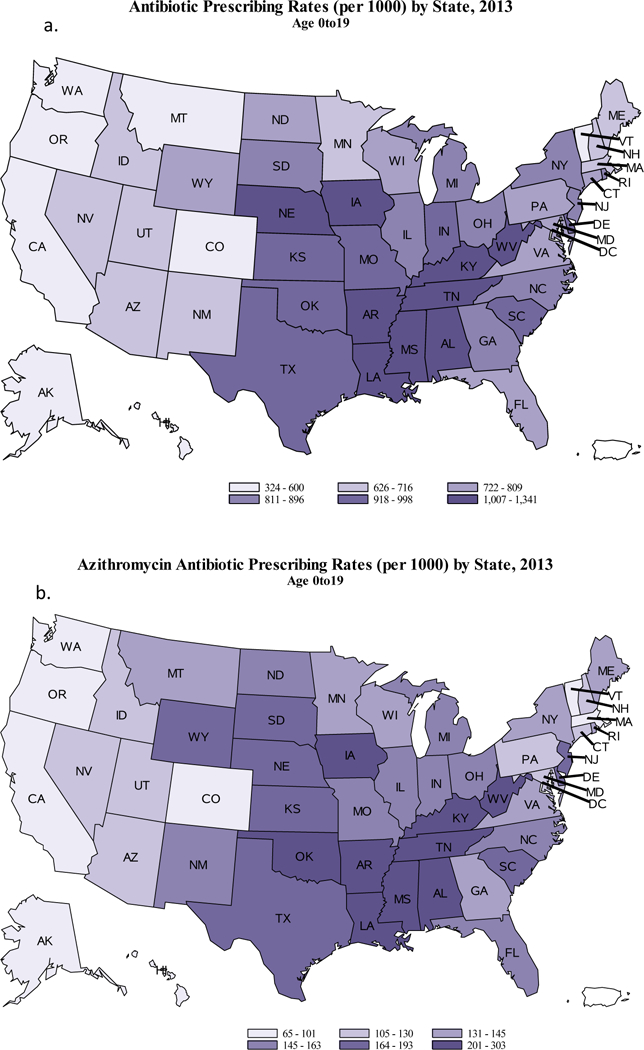
Antibiotic prescribing for all agents (a) and azithromycin prescribing (b) per 1000 children aged ≤19 years by state — United States, 2013.
Pediatricians and family practitioners prescribed the majority of outpatient antibiotics to children in 2013 (pediatricians: 38.6%, family practitioners 15.4%), followed by nurse practitioners (11.4%), physician assistants (8.8%), and emergency medicine (4.8%) (Table 3).
Table 3.
Number in millions and percent of antibiotic prescriptions for children ≤19 years by provider specialty.
| Provider specialty | Number of antibiotic prescriptions in millions |
Percent of antibiotic prescriptions |
|---|---|---|
| Pediatrics | 25.8 | 38.6 |
| Family Practice | 10.3 | 15.4 |
| Nurse Practitioner | 7.6 | 11.4 |
| Physician Assistants | 5.9 | 8.8 |
| Emergency Medicine | 3.2 | 4.8 |
| Dermatology | 2.2 | 3.3 |
| Dentistry | 1.9 | 2.8 |
| Internal Medicine | 1.7 | 2.5 |
| Surgery | 1.3 | 1.9 |
| Otolaryngology | 0.9 | 1.3 |
| Pediatric Subspecialty | 0.6 | 0.9 |
| Obstetrics/Gynecology | 0.5 | 0.7 |
| Internal Medicine/Pediatrics | 0.4 | 0.6 |
| Urology | 0.3 | 0.4 |
| Medical Subspecialty | 0.3 | 0.4 |
| Infectious Diseases | 0.1 | 0.1 |
| Other Specialty | 1.9 | 2.8 |
| Unspecified Specialty | 2.0 | 3.0 |
| All Providers | 66.8 | 100.0 |
In multivariable modeling, interaction was present between specialty and patient age for azithromycin selection, and thus adjusted prevalence ratios for azithromycin selection by specialty were calculated for each age group adjusted for region of care and patient sex. Family practice physicians were more likely than pediatricians to select azithromycin when an antibiotic was prescribed in all age groups (for children aged 0–2 years: PR 1.79, 95% CI, 1.78–1.80; 3–9 years: 1.40, 1.40–1.40; and 10–19 years: 1.18, 1.18–1.18, Table 4). Nurse practitioners and physician assistants were also more likely to select azithromycin than pediatricians in children 0–2 years and 3–9 years of age, but in the 10–19 year old children, nurse practitioners were equally as likely as pediatricians to select azithromycin while physician assistants were less likely to do so. Other specialists were more likely to select azithromycin than pediatricians in children 0–2 years of age but less likely in children 3–9 and 10–19 years of age. Of note, infectious diseases specialists only prescribed 19,600 azithromycin prescriptions to children in 2013.
Table 4.
Adjusted prevalence ratiosa of the association between azithromycin prescribing and specialty group by patient age group among all outpatient antibiotic prescriptions for children — United States, 2013.
|
Specialty group |
0–2 years old | 3–9 years old | 10–19 years old |
|---|---|---|---|
| Adjusted prevalence ratio (95% CI) |
Adjusted prevalence ratio (95% CI) |
Adjusted prevalence ratio (95% CI) |
|
| Pediatrics | Reference | Reference | Reference |
| Family Practice | 1.79 (1.78–1.80) | 1.40 (1.40–1.40) | 1.18(1.18–1.18) |
| Nurse Practitioners | 1.14 (1.14–1.15) | 1.06 (1.06–1.06) | 0.99 (0.99–1.00) |
| Physician Assistants | 1.44 (1.44–1.45) | 1.16 (1.16–1.16) | 0.83 (0.82–0.83) |
| Other specialtiesb | 1.12 (1.12–1.12) | 0.82 (0.82–0.82) | 0.54 (0.54–0.54) |
Adjusted for region of care and patient sex.
The other category includes the following specialties: emergency medicine, dermatology, dentistry, internal medicine, surgery, otolaryngology, pediatric subspecialty, obstetrics/gynecology, internal medicine/pediatrics, urology, medical subspecialty, infectious diseases, all other specialties not otherwise listed, and unspecified specialty.
In multivariate modeling, region and patient sex were independently associated with azithromycin selection, defined as the number of azithromycin prescriptions among all antibiotic prescriptions. However, these associations were small and likely not clinically meaningful. As compared to females, the prevalence ratio of azithromycin selection for males was 1.07 (95% CI 1.07–1.08). For region, with West as the comparison group, the prevalence ratio for the Northeast was 0.93 (95% CI 0.92–0.93); Midwest was 0.97 (95% CI 0.97–0.97) and South was 1.02 (95% CI 1.02–1.03).
Discussion
This analysis shows that in 2013 enough antibiotics were prescribed to give eight out of ten US children an antibiotic. The three-fold variability in rates between the highest and lowest prescribing states may suggest inappropriate antibiotic use among children is more common in certain regions, particularly in the South, similar to patterns seen in adults.3 Despite infrequent recommendations in pediatrics, azithromycin remains the second most commonly prescribed antibiotic in children, accounting for 18% of all antibiotic prescriptions in children. Furthermore, patterns of selection of azithromycin among all antibiotics appear to vary by patient age and clinician specialty, and family practitioners appear to be more likely to select azithromycin than pediatricians for children of all ages when antibiotics were prescribed.
In 2013, amoxicillin remained the most commonly prescribed antibiotic for children, consistent with its first-line treatment recommendation for most common pediatric outpatient infections.9–11 Azithromycin remained the second most commonly prescribed antibiotic for children in 2013. In a previous study, azithromycin was the second most commonly prescribed medicine to children in 2010 and was more commonly prescribed to children than albuterol.8 However, according to national clinical practice guidelines, azithromycin should be used infrequently in children. It is not recommended for acute otitis media, the most common diagnosis leading to antibiotics in children, due to macrolide resistance among Streptococcus pneumoniae.9 However, 13% of children with acute otitis media receive a macrolide.19 In national invasive pneumococcal disease surveillance in 2013, 28% of Streptococcus pneumoniae isolates were macrolide-resistant14, indicating that azithromycin is an inferior choice for acute otitis media. Azithromycin is also not recommended for sinusitis11,15, but macrolides account for 28% of antibiotic prescriptions for pediatric sinusitis.19 Azithromycin is only recommended for streptococcal pharyngitis for patients with penicillin allergy10, while 20% of children with pharyngitis receive a macrolide.19 While up to 10% of patients of all ages report penicillin allergy, likely only 1% of the population has true penicillin allergy, indicating why a thorough evaluation starting with a detailed history is important in cases of suspected penicillin allergy to avoid unnecessarily using a non-first line antibiotic.20 The overuse of macrolides for pharyngitis may be contributing to the high azithromycin prescription rate seen in children 3–9 years of age. Furthermore, macrolide therapy is recommended for the treatment of atypical community-acquired pneumonia, but primarily in school-aged children and adolescents.8 In a large, multi-center study, Mycoplasma pneumoniae, the most common atypical pneumonia pathogen, was identified in 3% of children aged <5 years hospitalized with community-acquired pneumonia.21 Azithromycin is recommended first-line for sexually-transmitted diseases and suspected or confirmed pertussis.12,13
Inappropriate use of azithromycin includes both prescribing it when no antibiotic is necessary and selecting it when another antibiotic would be a more appropriate choice. The 4-fold difference in azithromycin prescribing rates for children between the highest prescribing states and the lowest prescribing states may further suggest inappropriate azithromycin use. Previous studies have shown that among children, broad-spectrum antibiotic prescribing was higher among visits for respiratory conditions that do not warrant antibiotics (e.g., viral upper respiratory infections) and among visits occurring in the South.22 While variations in disease prevalence likely contribute to geographic variation in antibiotic prescribing, it is also possible that cultural differences in antibiotic prescribing practices contribute to the geographic variation in antibiotic use. Previous studies have found that antibiotic prescribing for adults with respiratory conditions not warranting antibiotics is higher in the South than in the West.23
In the global context, the U.S. has historically had higher outpatient antibiotic prescribing rates and macrolide use than most European countries.24 A recent analysis by Youngster and colleagues compared pediatric outpatient antibiotic prescribing rates from 2008–2012 from United Healthcare Group, a large insurance company in the United States, with antibiotic prescribing rates from Italy, South Korea, Spain, Norway, and Germany.25 In that study, among children 0–2 years of age, the U.S. had the third lowest antibiotic prescription rate among the 6 countries.25 However, among children aged 13–18 years, the U.S. had the highest antibiotic prescription rate among the 6 countries. Furthermore, the proportion of antibiotic prescriptions that were second generation macrolides, including azithromycin, was highest among the 6 countries in the United States among children aged 3–5 and 6–12 years and second highest among children aged 0–2 and 13–18 years.25 It is important to note that the proportion of macrolides was higher among children in the study by Youngster than in ours. Our study includes all outpatient antibiotic prescriptions dispensed in U.S. community pharmacies, while the study by Youngster includes children who are insured through working parents. Nonetheless, both studies suggest that macrolide prescribing for U.S. children can be improved.
The differences in antibiotic selection among specialties, particularly between family practice and pediatricians, are intriguing. Pediatricians (excluding pediatric subspecialists) and family practitioners are often engaged in pediatric primary care and may treat similar pediatric conditions among patients of similar ages. However, when adjusted for region and patient sex, family practitioners were more likely than pediatricians to select azithromycin when antibiotics were prescribed in each age group, and the association was strongest in the youngest children (aged 0–2 years). It is also important to note that infectious disease physicians prescribed only 19,600 azithromycin prescriptions to children (accounting for 0.2% of all azithromycin prescriptions to children), which may further indicate that azithromycin should be infrequently used in pediatrics. This pattern suggests there may be differences in antibiotic preferences by clinician specialty. Further studies examining antibiotic selection for children by diagnosis and clinician specialty could help determine if this difference in antibiotic selection is appropriate.
Appropriate antibiotic use is a quality of care issue. All antibiotic use increases the risk of antibiotic resistance and exposes children to other potential harms. Among children, adverse events from antimicrobials lead to 112,000 outpatient clinic visits and 50,000 emergency department visits annually, or 5 visits to clinics or emergency departments for every 1000 antibiotic prescriptions for children.26 Furthermore, it is estimated that among all ages (adults and children) 1 in 2000 outpatient azithromycin prescriptions leads to an emergency department visit for an adverse event, including allergic reactions.27 Another unintended consequence of antibiotic use is Clostridium difficile infections, and the majority of pediatric C. difficile infections are community-associated (i.e., not associated with overnight hospital stays).28 Additionally, antibiotic treatment disrupts the microbiome of children, and evidence is emerging regarding associations between antibiotics and chronic diseases.29 A recent study demonstrated an association between antibiotic exposure during childhood with increased risk of developing juvenile idiopathic arthritis.30 Azithromycin use and macrolide use, in particular, are of concern due to the broad-spectrum of this class with the resulting impact on the microbiota. In a study examining the impact of early-life antibiotic exposure on the intestinal microbiota among Finnish children, macrolide use was associated with altered composition of the microbiota and reduction in microbiota diversity 12 to 24 months after the macrolide course as compared to control children.31 Additionally, in some studies suggesting that antibiotic use in childhood may be linked with increased body mass index, macrolides were associated with more weight gain than other antibiotic classes.31–33 However, evidence is conflicting regarding the association of weight gain and obesity with antibiotic use in early childhood.34,35 Nonetheless, it is important for the health of individual patients and for the public that all antibiotics are used appropriately and only when needed.
The Centers for Disease Control and Prevention have recently released the Core Elements of Outpatient Antibiotic Stewardship, which provide a framework for implementing antibiotic stewardship in outpatient settings.36 Antibiotic stewardship is the effort to measure and improve antibiotic prescribing by clinicians and use by patients.36 Outpatient clinicians and clinic leadership are encouraged to commit to improving antibiotic prescribing, to implement an action for policy or practice aimed at improving antibiotic prescribing, to track and report antibiotic prescribing practices of clinicians, and to educate their patients and their clinicians about appropriate antibiotic use. Effective interventions, such as communications training, audit and feedback, clinical decision support tools, and delayed prescribing as recommended in the Core Elements of Outpatient Antibiotic Stewardship, can be used to address variability in antibiotic prescribing and selection for pediatric patients.36 Additionally, the Centers for Disease Control and Prevention’s Get Smart Know When Antibiotics Work Program (www.cdc.gov/getsmart) focuses on improving antibiotic use in the community and is a source for educational resources and information that can be used by clinicians and be shared with parents when discussing prescribing decisions for children.
Our analysis was subject to at least the following limitations. QuintilesIMS Xponent data are collected as filled-prescription data, which do not lend themselves to optimal use for public health. The data, in the customized extract used in this study, are aggregated with predefined age categories, limiting our ability to examine other age groups. The QuintilesIMS Xponent data lack diagnoses; thus we were unable to assess the appropriateness of the prescribed antibiotics. The data in the extract used in this study also lack dose and duration of antibiotics, which are also important components for assessing appropriate antibiotic use. QuintilesIMS Xponent data are prescription-based, and we are unable to track an individual’s antibiotic use. We assumed for these analyses and in the model that each prescription corresponded to one person, but multiple prescriptions may have been given to the same individual during 2013. For clinicians, we were unable to examine care setting (e.g. primary care, emergency department, or urgent care) which influences the types of condition that clinicians treat and thus antibiotic prescribing patterns. For physician assistants and nurse practitioners, we were unable to determine if they had specialized training or experience in pediatrics or other specialties (e.g. pediatric nurse practitioners) or if they worked with pediatricians, family practitioners or other specialists, as these issues might be expected to impact their antibiotic prescribing and selection habits. Further analyses could examine clinician-level characteristics and diagnostic-level factors associated appropriate use of all antibiotics and azithromycin for children.
In conclusion, variations in overall antibiotic and azithromycin prescribing for children suggests inappropriate use of antibiotics in children. In particular, the common use of azithromycin raises concern about overuse of a broad-spectrum antibiotic that also is an inferior choice for common pediatric infections. Additionally, engagement of all pediatric clinicians, including pediatricians, family practitioners, nurse practitioners, physician assistants and other specialists, is critical to reduce unnecessary antibiotic use and to improve antibiotic selection for children. Our data support that interventions in high-prescribing regions may be needed. In March 2015, the White House released the National Action Plan for Combatting Antibiotic Resistant Bacteria; the plan calls for a 50% reduction in inappropriate antibiotic use in outpatient settings by 2020. While improvements have been seen in prescribing for children, continued efforts to improve antibiotic use and selection in children are needed to meet the national goal, optimize healthcare quality and protect the effectiveness of antibiotics for the future.
Supplementary Material
Acknowledgements:
Funding Source: This work was funded by the Centers for Disease Control and Prevention. The Centers for Disease Control and Prevention was involved in the design of the study; analysis, and interpretation of the data; review of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References:
- 1.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html2013.
- 2.van de Sande-Bruinsma N, Grundmann H, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hicks LA, Bartoces MG, Roberts RM, et al. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 4.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA.. 2016;315(17):1864–1873. [DOI] [PubMed] [Google Scholar]
- 5.Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133(3):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31. [DOI] [PubMed] [Google Scholar]
- 8.Bradley J, Byington C, Shah S, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberthal A, Carroll A, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. [DOI] [PubMed] [Google Scholar]
- 10.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279–1282. [DOI] [PubMed] [Google Scholar]
- 11.Wald E, Applegate K, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262–e280. [DOI] [PubMed] [Google Scholar]
- 12.Workowski K, Bolan G. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari T, Murphy T, Moran J. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep.. 2005;54(RR-14):1–16. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2014. 2014; https://www.cdc.gov/abcs/reports-findings/survreports/spneu14.html. Accessed January 27, 2017.
- 15.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72–e112. [DOI] [PubMed] [Google Scholar]
- 16.IMS. The Uniform System of Classification (USC). http://www.imshealth.com/deployedfiles/ims/Global/Content/Insights/Health%20Services%20Research%20Network/USC_Classiification_Process_2011.pdf. Accessed September 22, 2015.
- 17.Kleinbaum DG, Klein M. Logistic Regression. Third ed New York, NY: Springer; 2010. [Google Scholar]
- 18.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Fleming-Dutra KE, Shapiro DJ, Hyun DY, Hicks LA. Outpatient Antibiotic Use Target-Setting Workgroup. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273. [DOI] [PubMed] [Google Scholar]
- 21.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69(1):234–240. [DOI] [PubMed] [Google Scholar]
- 24.Goossens H, Ferech M, Coenen S, Stephens P. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis. 2007;44(8):1091–1095. [DOI] [PubMed] [Google Scholar]
- 25.Youngster I, Avorn J, Belleudi V, et al. Antibiotic use in children - A cross-national analysis of 6 countries. J Pediatr. 2017;182:239–244.e1. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois FT, Mandl KD, Valim C, Shannon MW. Pediatric adverse drug events in the outpatient setting: an 11-year national analysis. Pediatrics. 2009;124(4):e744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. [DOI] [PubMed] [Google Scholar]
- 28.Wendt J, Cohen J, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics. 2014;133(4):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vangay P, Ward T, Gerber J, Knights D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe. 2015;17(5):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics. 2015;136:e333–e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korpela K, Salonen A, Virta LJ, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–626. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz BS, Pollak J, Bailey-Davis L, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes (Lond). 2016;40:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber JS, Bryan M, Ross RK, et al. Antibiotic exposure during the first 6 months of life and weight gain during childhood. JAMA. 2016;315(12):1258–1265. [DOI] [PubMed] [Google Scholar]
- 35.Scott FI, Horton DB, Mamtani R, et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016;151:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez G, Fleming Dutra K, Roberts R, Hicks L. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(6):1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


