Abstract
Objectives:
To estimate the pooled relative risk of incident acute myocardial infraction (AMI) among HIV-infected adults compared to HIV-uninfected controls and explore the contribution of traditional and HIV-related risk factors.
Background:
Understanding AMI risk and associated risk factors in HIV-infected populations has the potential to inform clinical management and prevention strategies.
Methods:
We systematically identified cohort studies of HIV-infected or HIV-infected and matched uninfected adults reporting AMI incidence rates published up to January 1, 2017. Random-effects meta-analysis models were used to estimate the aggregate relative risk of AMI by HIV status. Subgroup analysis and meta-regression were used to explore factors affecting risk.
Results:
16 studies (N=1,619,690, median age 38.5 years, 78.9% male, mean follow-up of 6.5 years) were included. In pooled analyses of HIV-infected and matched uninfected cohorts (n=5), HIV-infected individuals had higher AMI incidence rates (absolute risk difference=2.2 cases per 1000 persons per year) and twice the risk of AMI (RR=1.96 [1.5, 2.6]) compared with matched HIV-uninfected controls. In a multivariate meta-regression, each additional percentage point in the proportion of male participants (OR=1.20 [1.14, 1.27]) and each additional percentage point in the prevalence of hypertension (OR=1.19 [1.12, 1.27]), dyslipidemia (OR=1.09 [1.07, 1.11]), and smoking (OR=1.09 [1.05, 1.13]) were independently associated with increased AMI risk in HIV-infected adults.
Conclusions and Relevance:
Chronic HIV infection is associated with a two-fold higher AMI risk. Traditional risk factors such as hypertension, dyslipidemia, and smoking are significant contributors to AMI risk among HIV-infected adults and should be aggressively targeted in routine HIV care.
Introduction
The advent and widespread rollout of antiretroviral therapy (ART) over the past several decades has resulted in dramatic reductions in AIDS-related deaths and a rise in life expectancy (1-8). In this setting, cardiovascular disease (CVD) has emerged as a leading comorbidity and cause of death among HIV-infected adults around the world (9). CVD also poses a greater burden on HIV-infected than HIV-uninfected populations, with the HIV population facing higher mortality rates of acute myocardial infarction (AMI) and prevalence of related risk factors (4, 10). This excess in AMI risk has been linked to the double burden of highly prevalent traditional (e.g., smoking, hypertension, diabetes) and HIV-specific risk factors (e.g., inflammation, immune suppression, viremia) the HIV population faces (1, 6, 11, 12). Gains in life expectancy in the HIV population may be threatened if CVD risk is not optimally managed (13).
The existence of several cohort studies exploring CVD risk in HIV-infected populations has made possible the conduction of meta-analyses, which are helpful evidence summaries to inform clinical practice. For instance, meta-analyses have estimated the HIV population has about a two-fold greater CVD risk than their uninfected counterparts (14, 15). Meta-analyses have also shown that both HIV-specific risk factors (such as immunosuppression and viral load) and vascular risk factors contribute to increased CVD risk in the HIV population (14-16). However, no previous meta-analysis has explored the relative contribution of traditional and HIV-specific risk factors, which limits our ability to design cost-effective preventive interventions for the HIV population.
To address this gap, we conducted a comprehensive systematic review and meta-analysis of longitudinal cohort studies and estimated the pooled incidence rates and relative risk of AMI in HIV-infected compared to HIV-uninfected populations. We also examined the contribution of traditional and HIV-related risk factors to AMI risk. Understanding the presence of and contributors to AMI risk in HIV-infected populations is important to inform clinical CVD management.
Methods
Search Strategy and Study Selection
We systematically searched the PubMed and Embase databases for articles published in English prior to January 1, 2017 and containing one or more MeSH terms or keywords for HIV infection (“hiv infections,” “AIDS,” “HIV/AIDS,” “anti-retroviral agents”) and cardiovascular outcomes of interest (“myocardial ischemia,” “myocardial infarction,” “cardiovascular disease,” “cardiovascular events,” “stroke,” “cardiovascular mortality” or “cardiovascular death”). We followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines in the conduction and reporting of this meta-analysis (17).
To be included, studies had to use a longitudinal study design, include HIV-infected adults over 18 years of age with or without an uninfected comparator group, and report incidence rates of AMI. Where multiple publications from a single cohort were available, the most current publication that met the inclusion criteria was selected. Articles including only individuals with comorbid infection by hepatitis B virus, hepatitis C virus, tuberculosis, or Chagas disease were excluded as these conditions independently confer elevated cardiac risk. Populations for whom only non-atherosclerotic cardiac outcomes were reported (including heart failure, dilated cardiomyopathy, and infectious cardiomyopathy) were excluded as this was outside the scope of our current study. Titles and abstracts were independently screened for inclusion by two authors (SGR and HCG) and disagreements were resolved by a third author (MKA).
Data Extraction and Outcomes
Baseline and outcome data, along with relevant study- and participant-level characteristics were extracted and entered into a standardized data extraction form. The outcome of interest was incidence of AMI, defined and adjudicated by each study, with most performing chart review and/or endpoint adjudication by external reviewers. HIV infection was defined in most cohorts as receiving HIV care or chart diagnosis of HIV infection.
Outcome data were extracted for crude AMI event rates and total person-years of follow-up with associated confidence intervals. Data on the prevalence of traditional cardiovascular risk factors at baseline (smoking, dyslipidemia, hypertension, and diabetes), and HIV-related risk factors (exposure to anti-retroviral therapy and prior AIDS diagnosis) were extracted where these were reported.
Study Quality Assessment
The Newcastle–Ottawa Scale (18) for cohort studies was used to assess the quality of included studies. The scale consists of three domains (participant selection, comparability of cohorts, and outcome assessment) evaluated through eight questions. For the selection domain, we assessed whether the exposed cohort was representative of the study intended population (1 star) or not representative (0 stars); whether HIV infection was ascertained via medical records (1 star), structured interviews (1 star), written self-report (0 stars), or not described (0 stars); and whether studies demonstrated AMI was not present at study start (yes=1 star, no=0 stars). Selection of the non-exposed cohort (i.e. HIV-uninfected) was not used as studies with only HIV-infected persons were also included. For the same reason, we adapted the comparability of cohorts item to assess whether age (yes=1 star, no=0 stars), and other AMI risk factors (yes=1 star, no=0 stars) were controlled for in the design/analyses. For outcome assessment, we examined how AMI was determined (clinical tests/medical records=1 star, no description=0 stars), the length of follow-up (≥3 years=1 star, <3 years=0 stars), and the proportion of participants lost at follow-up (0–30%=1 star, >30% or not reported=0 stars). Each study was given a maximum of one star for each question within the selection and outcome domains; a maximum of two stars were given for comparability. We summed the number of stars earned by each study and categorized them as high (5–8 stars) or low quality (1–4 stars).
Data Synthesis and Statistical Analysis
Among studies with HIV-infected and matched HIV-uninfected cohorts, we pooled AMI incidence rates and estimated the absolute risk difference between HIV-infected and uninfected individuals. To account for heterogeneity between studies, a random-effects meta-analysis model was used to estimate the pooled relative risk (RR) for an AMI event and the corresponding 95% confidence intervals (CI), with HIV-uninfected individuals as reference. A RR with an associated 95% CI that did not contain 1 was considered statistically significant.
Using data from all cohort studies, we grouped HIV-infected participant groups and HIV-uninfected participant groups together and conducted a random effects meta-analysis to estimate the odds of having an AMI event, using HIV-uninfected participants as reference. We then performed subgroup analyses to examine the likelihood of having an AMI event according to participant demographic characteristics (age, sex, and race), AMI risk factor prevalence (hypertension, smoking, dyslipidemia, and diabetes), and HIV-related risk factor prevalence (percentage of participants on any antiretroviral therapy and AIDS prevalence). Our definitions of subgroups were based on the distributions reported in the included articles; for instance, based on reported diabetes prevalence (mean 10.8%, median 9%, range 3% to 25%), a 10% prevalence cutoff was selected for subgroup analysis. Finally, multivariate meta-regressions were used to explore the contribution of different risk factors to AMI risk heterogeneity in HIV-infected adults.
Between-study heterogeneity was assessed by computing I², where I²>75% indicated significant heterogeneity. Publication bias was assessed using Egger’s test and by visually exploring funnel plots. Finally, we conducted sensitivity analyses among high-quality studies and obtained a pooled estimate for this group of studies. We used the random-effects meta-analysis package (19) in R programming language (version 3.2.1) to fit the models described.
Results
From the 2,117 titles screened, 108 were selected for full text review and 16 were included in the meta-analysis (Figure 1). Five studies included HIV-infected and matched uninfected cohorts (6, 10, 20-22), while 11 studies only reported on HIV-infected cohorts only (3, 23-32). Duration of study follow-up ranged from 1.8 years to 6.3 years. About half of the studies were conducted in North America (44%) and a third in Europe (31%). Characteristics of included studies are presented in Online Table 1.
Figure 1.
Study selection flow diagram.
The 16 studies included 248,145 HIV-infected participants (median age 40 years, 81% male, 47% white) and 1,371,545 HIV-uninfected participants (median age 42 years, 77% male, 50% white). Risk factor baseline prevalence and AMI incidence rates reported in each study by HIV status are presented in Table 1. The average prevalence of hypertension in HIV-infected and uninfected individuals (19% vs. 15%, respectively), smoking (46% vs. 49%, respectively), dyslipidemia (22% vs. 18%, respectively) and diabetes (6% vs. 7%, respectively) did not significantly differ (p> 0.05). The average prevalence of AIDS reported across studies was 27%, while the average proportion of HIV-infected participants with exposure to ART was 67%.
Table 1.
Baseline risk factor prevalence and AMI incidence rates by HIV status among included cohort studies (n=16)
| Author (year) | Smoker (%) | Dyslipidemia (%) |
Hypertension (%) |
Diabetes (%) |
AIDS (%) |
ART (%) |
IR per 1000 PY (95% CI) |
|---|---|---|---|---|---|---|---|
| HIV-infected arms | |||||||
| Althoff (2015) | 68.0 | 34.0 | 22.0 | 14.0 | 25.0 | 45.0 | 2.0 (1.8, 2.3) |
| Bedimo (2011) | 29.0 | 26.0 | 38.0 | 13.0 | 75.0 | 3.7 (2.3, 4.2) | |
| Durand (2011) | 38.1 | 23.9 | 6.6 | 31.1 | 76.2 | 3.9 (3.3, 4.6) | |
| Hasse 2011 | 23.8 | 12.7 | 56.3 | 4.1 | 23.2 | 85.0 | 2.4 (1.9, 3.2) |
| Holmberg (2002) | 56.5 | 27.9 | 11 | 4.5 | 1.19 (0.8, 1.8) | ||
| Rasmussen (2015) | 65.7 | 3.5 | 2.9 | 21.7 | 77.2 | 5.2 (4.3, 6.4) | |
| Sabin (2013) | 54.8 | 38.4 | 9.2 | 2.9 | 23.2 | 3.2 (3.0, 3.4) | |
| Silverberg (2014) | 43.3 | 5.0 | 7.3 | 2.9 | 39.4 | 46.2 | 2.8 (2.5, 3.2) |
| Triant (2007) | 23.3 | 21.2 | 11.5 | 11.1 (9.6, 12.7) | |||
| Escaut (2003) | 36.1 | 85.5 | 5.2 (3.0, 8.4) | ||||
| Rickerts (2000) | 40 | 25.7 | 1.8 (1.2, 2.5) | ||||
| Lang (2010) | 1.2 (1.1, 1.4) | ||||||
| Kwong (2006) | 40.3 | 2.01 | 1.08 | 100 | 1.2 (1.0, 1.5) | ||
| Brothers (2009) | 2.1 (0.8, 5.7) | ||||||
| Brouwer (2014) | 5.9 (4.3, 8.2) | ||||||
| Ribaudo (2011) | 38.0 | 12.0 | 18.0 | 4.0 | 19.0 | 2.1 (1.5, 2.8) | |
| HIV-uninfected arms | |||||||
| Althoff (2015) | 65.0 | 38.0 | 32.0 | 21.0 | 1.3 (1.2, 1.4) | ||
| Durand (2011) | 12.3 | 15.1 | 4.7 | 0.0 | 0.0 | 2.2 (1.9, 2.5) | |
| Rasmussen (2015) | 53.9 | 3.0 | 1.3 | 0.0 | 0.0 | 2.0 (1.7, 2.4) | |
| Triant (2007) | 17.6 | 15.9 | 6.6 | 0.0 | 0.0 | 7.0 (6.9, 7.1) |
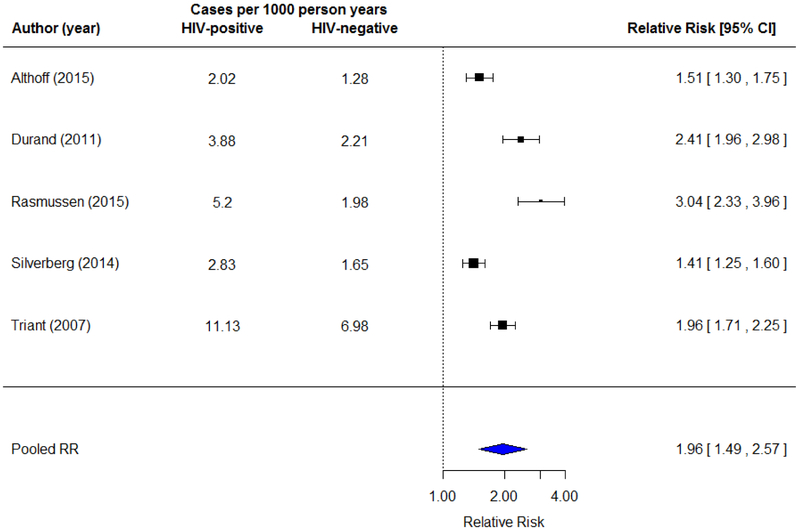
In HIV-infected and matched uninfected cohorts (n=5), the average AMI incidence rate was 5.0 cases per 1000 person years (95% CI=4.3, 5.8) in HIV-infected individuals, and 2.8 cases per 1000 person years (95% CI=2.6, 3.0) in HIV-uninfected individuals (absolute risk difference=2.2 per 1000 person years). The random effects meta-analysis (Figure 2) showed HIV-infected participants have a two-fold higher risk of AMI compared to HIV-uninfected participants (RR= 1.96 [1.48, 2.57], I2=92.7%).
Figure 2.
Random effects meta-analysis showing AMI incidence rates and relative risk in HIV-infected individuals compared to matched HIV-uninfected individuals (n=5). Square markers indicate risk ratios for AMI comparing HIV-infected and uninfected adults with error bars demonstrating 95% confidence intervals.
We grouped HIV-infected participants across the 16 studies included and compared AMI risk against that of HIV-uninfected participants included in 5 matched cohorts. HIV-infected participants had 87% greater odds of having an AMI event (OR=1.87 [1.42, 2.47]) than HIV-uninfected participants. In unadjusted subgroup analyses exploring AMI risk in HIV-infected compared to uninfected individuals (Table 2), significantly greater odds for having an AMI event were observed in studies where >50% participants were exposed to any antiretroviral therapy (OR=2.66 [2.25, 3.13]) compared to studies where <50% of participants were exposed (OR=1.46 [1.32, 1.61]). Concerning traditional risk factors, significantly greater odds for having an AMI event were observed in studies with <80% male participants (OR=2.44 [1.99, 2.97]), than in studies with ≥80% male participants (OR=1.38 [1.11, 1.71]).
Table 2.
Subgroup analysis comparing AMI risk between HIV-infected and HIV-uninfected (reference) participants by demographic characteristics and risk factor prevalence.
| Variable | N | AMI OR [95% CI] |
|---|---|---|
| Age | ||
| <38 | 4 | 1.32 [0.65, 2.69] |
| ≥38 | 9 | 1.92 [1.19, 3.10] |
| Gender | ||
| <80% male | 6 | 2.44 [1.99, 2.97] |
| ≥80% male | 9 | 1.38 [1.11, 1.71] |
| Race | ||
| <50% white | 3 | 1.55 [1.06, 2.28] |
| ≥50% white | 5 | 1.67 [1.27, 2.19] |
| Hypertension | ||
| <20% | 6 | 1.75 [1.10, 2.79] |
| ≥20% | 5 | 1.96 [1.32, 2.92] |
| Smoking | ||
| <50% | 6 | 1.51 [0.87, 2.61] |
| ≥50% | 4 | 2.11 [1.34, 3.33] |
| Dyslipidemia | ||
| <20% | 4 | 0.92 [0.42, 2.01] |
| ≥20% | 5 | 2.27 [1.22, 4.23] |
| Diabetes | ||
| <10% | 3 | 1.86 [1.26, 2.77] |
| ≥10% | 8 | 1.90 [1.18, 3.06] |
| AIDS | ||
| <30% | 5 | 2.07 [1.23, 3.49] |
| ≥30% | 3 | 1.81 [1.05, 3.11] |
| ARTa | ||
| <50% | 3 | 1.46 [1.32, 1.61] |
| ≥50% | 6 | 2.66 [2.25, 3.13] |
| Overall | 16 | 1.87 [1.42, 2.47] |
Subgroup analysis with all HIV-infected participants in the 16 included studies compared against all HIV-uninfected participants from the 5 matched cohort studies. Eleven studies only contributed HIV-infected participants while five studies contributed HIV-infected and HIV-uninfected participants.
N=studies included in the subgroup
AMI= acute myocardial infraction; OR=odds ratio with HIV-uninfected participants as reference
Proportion of participants on antiretroviral therapy (ART)
In a multivariate meta-regression (n=7), participant median age, percentage of male participants, and prevalence of smoking, hypertension, diabetes, and dyslipidemia were associated with increased AMI risk. Each additional percentage point in the proportion of male participants (OR=1.20 [1.14, 1.27]) and each additional percentage point in the prevalence of hypertension (OR=1.19 [1.12, 1.27]), dyslipidemia (OR=1.09 [1.07, 1.11]), and smoking (OR=1.09 [1.05, 1.13]) were associated with 9–20% greater AMI risk. Conversely, each additional year in median age (OR=0.60 [0.50, 0.72]) and diabetes prevalence percentage point (OR=0.78 [0.73, 0.83]) associated with 40% and 22% lower AMI risk, respectively.
Regarding quality assessment, 9 studies were classified as high quality and 7 studies as low quality (Online Table 2). A sensitivity analysis including only high-quality studies showed HIV-infected participants had 91% higher odds than HIV-uninfected participants of having an AMI event (OR=1.91 [1.44, 2.52]), which is similar to that observed when all studies are included. Low-quality studies did not show a significant increased risk (OR=1.01 [0.45, 2.29]).
The Egger test suggested publication bias was present (z=−3·6, p<0·0001) in HIV-infected and uninfected matched cohort studies (n=5). A visual examination of funnel plots among all studies confirmed that smaller studies with null associations were less likely to be published than studies with positive higher-magnitude associations (Online Figure 1).
Discussion
In this comprehensive systematic review and meta-analysis, we examined AMI risk in HIV-infected compared to HIV-uninfected populations and assessed the contribution of traditional and HIV-specific risk factors to excess AMI risk. We found HIV-infected adults have a two-fold higher AMI risk compared to matched HIV-uninfected controls, with an absolute risk difference of 2.2 cases per 1000 persons per year. AMI risk was higher in HIV-infected individuals in every cohort included, despite differences in location, year of publication, and demographic variations between cohorts. Traditional risk factors, namely hypertension, smoking, and dyslipidemia, emerged as significant contributors to AMI risk in HIV-infected individuals. Overall, our findings confirm and expand the evidence base showing HIV infection confers increased CVD risk and add to calls to integrate aggressive CVD risk management in routine HIV care.
Our results align with evidence from other meta-analyses. For instance, a meta-analysis exploring CVD risk in HIV-infected adults found a 61% increased risk of composite CVD outcomes compared to HIV-uninfected adults, while a two-fold increased risk was observed among those exposed to antiretroviral therapy (14). Similarly, another meta-analysis reports a 60% greater AMI risk among HIV-infected compared to uninfected controls, and that antiretroviral therapy use contributes to increased CVD risk (15). We found a two-fold increase in AMI risk among HIV-infected groups compared to matched uninfected groups; this allows us to speculate that excess risk cannot be solely explained by differences in demographic characteristics and risk factor prevalence but may be linked to HIV-related factors and amplified by traditional risk factors.
Regarding risk factors for AMI, we found increases in the prevalence of hypertension associated with a 20% increased AMI risk, while increases in the prevalence of hyperlipidemia and smoking associated with a 9% increased risk each. These findings align with those from the VACS cohort study showing that traditional risk factors contribute to increased AMI risk (1). Further, data from seven cohorts contributing to the NA-ACCORD study showed that eliminating smoking and hypertension in HIV-infected adults would avert 38% and 41% of AMI, respectively (33). Since HIV-infected adults have been found to have higher rates of smoking, dyslipidemia, diabetes, and hypertension than the general population (10, 34), aggressive management of these risk factors is needed to reduce AMI risk in the HIV population. Studies are needed to determine what specific treatment targets would be most beneficial for the HIV population.
We also found higher age and higher diabetes prevalence associated with lower AMI risk among HIV-infected participants. While this may be driven by the younger age of most HIV-infected cohorts, other potential explanations include lower competing risks, healthcare use and smoking rates in the uninfected group that were not accounted for in our analysis (35). This finding may also be a reflection of the more rapid progression to clinically significant disease in the setting of HIV. The inverse association between diabetes prevalence and AMI risk could indicate that HIV-infected individuals with diabetes are benefiting from diabetes treatment and potentially from other CVD prevention strategies they receive. Overall, these findings suggest that aggressive CVD prevention efforts should be integrated in routine HIV care.
Subgroup analyses showed greater AMI risk in studies where ≥50% of participants had been exposed to any form of antiretroviral therapy. Though this suggest antiretroviral therapy has an impact on AMI risk, we were not able to parse out this association. We could not explore the link between specific antiretroviral therapy classes and AMI risk due to incomplete reporting in included studies, while other meta-analyses have implicated protease inhibitors and abacavir use in AMI risk (16). For the same reason, we could not explore the effect of older and newer drugs, an important aspect to explore given the change in therapies used in the early and current antiretroviral therapy eras (36). Indeed some therapies used in the included studies are no longer first line HIV therapies used in HIV care. It is therefore difficult to make valid conclusions about the link between antiretroviral therapy use and AMI risk in the present analysis.
This meta-analysis has limitations. CVD risk factor data was reported in half of the included studies and only at study entry: thus, the time varying nature of risk factors was not accounted for and MI risk may have been under- or over-estimated in our analyses. HIV-related variables, including mean CD4-cell counts, viral loads, exposure to specific antiretroviral therapy classes, and duration of HIV infection were inconsistently reported and could not be included in our analysis. For the same reason, we were not able to explore the effects of specific antiretroviral therapy classes on MI risk or the effects of old vs. new drugs in our analyses. Though we were unable to differentiate between Type 1 and Type 2 AMI, most included studies used a strong adjudication protocol (with chart diagnoses, EKG testing and biomarker confirmation) that likely included mostly Type 1 AMI. Finally, we noted a high level of clinical heterogeneity in our study attributable to substantial variability in demographics of participants followed in the included cohorts; this was partially accounted for in subgroup analyses.
Firmly establishing—and quantifying—an association between HIV infection and CVD events contributes to epidemiological research that may help clarify the mechanism of this association. Future studies should examine the association of AMI with individual markers of HIV infection, including CD4 cell count and viral load, as well as known mediators of risk. Therapeutic goals for the management of hyperlipidemia, hypertension and diabetes in the HIV-infected population have not been studied, though ongoing research into the role of statin therapy in preventing CVD events has potential to inform clinical management in this arena. Since the receipt of CVD risk factor treatment among HIV-infected people is often inadequate (37), opportunities for actionable change in HIV care are numerous.
Clinical Perspectives
As HIV-infected individuals are living longer because of effective antiretroviral therapy, they now face an increased risk of morbidity and death caused by CVD. It is increasingly clear that appropriate HIV care requires not only chronic viral suppression, but also early recognition and management of cardiovascular risk factors. We found HIV-infected adults have two times the risk of AMI compared to HIV-uninfected individuals and that traditional risk factors play a pivotal role in this increased risk. Our findings underscore the importance of introducing aggressive management of traditional CVD risk factors such as hypertension, hyperlipidemia, diabetes, and smoking early in the care of HIV-infected individuals. Unless CVD risk is effectively managed in the HIV population, the gains in life expectancy conferred by antiretroviral therapies may be lost.
Supplementary Material
Acknowledgments
Sources of Funding
Drs. KMVN and MKA were partially supported by the Georgia Center for Diabetes Translation Research (P30-DK-111024) funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Drs. WSA and CDR were partially supported by the Emory Center for AIDS Research (P30AI050409) funded by the National Institute of Allergy and Infectious Diseases.
Footnotes
Disclosures
All authors of this manuscript declare no competing interests.
References
- 1.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. [DOI] [PubMed] [Google Scholar]
- 3.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 4.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reekie J, Kowalska JD, Karpov I, Rockstroh J, Karlsson A, Rakhmanova A, et al. Regional differences in AIDS and non-AIDS related mortality in HIV-positive individuals across Europe and Argentina: the EuroSIDA study. PLoS One. 2012;7(7):e41673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65(2):160–6. [DOI] [PubMed] [Google Scholar]
- 7.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205 Suppl 3:S355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(4):435–47. [DOI] [PubMed] [Google Scholar]
- 12.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55(5):615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colasanti J, Galaviz KI, Christina Mehta C, Palar K, Schneider MF, Tien P, et al. Room for Improvement: The HIV-Diabetes Care Continuum Over 15 Years in the Women’s Interagency HIV Study. Open Forum Infect Dis. 2018;5(6):ofy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–68. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: A systematic review of the literature and meta-analysis. PLoS One. 2017;12(5):e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8(3):e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed on February 22, 2017. [Google Scholar]
- 19.Viechtbauer W Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36(3):1–48. [Google Scholar]
- 20.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(4):627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57(3):245–53. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(9):1415–23. [DOI] [PubMed] [Google Scholar]
- 23.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(1):84–91. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360(9347):1747–8. [DOI] [PubMed] [Google Scholar]
- 25.Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. Aids. 2013;27(17):2735–48. [DOI] [PubMed] [Google Scholar]
- 26.Escaut L, Monsuez JJ, Chironi G, Merad M, Teicher E, Smadja D, et al. Coronary artery disease in HIV infected patients. Intensive Care Med. 2003;29(6):969–73. [DOI] [PubMed] [Google Scholar]
- 27.Rickerts V, Brodt H, Staszewski S, Stille W. Incidence of myocardial infarctions in HIV-infected patients between 1983 and 1998: the Frankfurt HIV-cohort study. Eur J Med Res. 2000;5(8):329–33. [PubMed] [Google Scholar]
- 28.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. Aids. 2010;24(8):1228–30. [DOI] [PubMed] [Google Scholar]
- 29.Kwong GP, Ghani AC, Rode RA, Bartley LM, Cowling BJ, da Silva B, et al. Comparison of the risks of atherosclerotic events versus death from other causes associated with antiretroviral use. Aids. 2006;20(15):1941–50. [DOI] [PubMed] [Google Scholar]
- 30.Brothers CH, Hernandez JE, Cutrell AG, Curtis L, Ait-Khaled M, Bowlin SJ, et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKline-sponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr. 2009;51(1):20–8. [DOI] [PubMed] [Google Scholar]
- 31.Brouwer ES, Napravnik S, Eron JJ Jr., Stalzer B, Floris-Moore M, Simpson RJ Jr., et al. Effects of combination antiretroviral therapies on the risk of myocardial infarction among HIV patients. Epidemiology. 2014;25(3):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribaudo HJ, Benson CA, Zheng Y, Koletar SL, Collier AC, Lok JJ, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(7):929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althoff KN, Palella FJ, Gebo K, Gange SJ, Rabkin C, Thorne JE, et al. Impact of smoking, hypertension and cholesterol on myocardial infarction in HIV+ adults. Conference on Retroviruses and Opportunistic Infections (CROI); February 13–16; Seattle, Washington2017. [Google Scholar]
- 34.Vachiat A, McCutcheon K, Tsabedze N, Zachariah D, Manga P. HIV and Ischemic Heart Disease. Journal of the American College of Cardiology. 2017;69(1):73–82. [DOI] [PubMed] [Google Scholar]
- 35.Althoff K, Gange S. A critical epidemiological review of cardiovascular disease risk in HIV-infected adults: the importance of the HIV-uninfected comparison group, confounding, and competing risks. HIV Medicine. 2013;14(3):191–2. [DOI] [PubMed] [Google Scholar]
- 36.Freiberg Matthew S, So‐Armah K. HIV and Cardiovascular Disease: We Need a Mechanism, and We Need a Plan. Journal of the American Heart Association. 2016;5(3):e003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.[GeSIDA/National AIDS Plan: Consensus document on antiretroviral therapy in adults infected by the human immunodeficiency virus (Updated January 2014)]. Enferm Infecc Microbiol Clin. 2014;32(7):446.e1–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.