Abstract
Introduction:
We aimed to identify noninvasive imaging parameters that can serve as biomarkers for the integrity of the spinal cord, which is paramount to neurological function. Diffusion tensor imaging (DTI) indices are sensitive to axonal and myelin damage, and have strong potential to serve as such biomarkers. However, averaging DTI indices over large regions-of-interest (ROIs), a common approach to analyzing the images of injured spinal cord, leads to loss of subject-specific information. We investigated if DTI-tractography-driven, subject-specific demarcation approach can yield measures that are more specific to impairment.
Methods:
In 18 individuals with chronic spinal cord injury (SCI), subject-specific demarcation of the injury region was performed using DTI tractography; which yielded three regions-relative-to-injury (RRI; regions superior to, at, and below injury epicenter). DTI indices averaged over each RRI were correlated with measures of residual motor and sensory function, obtained using the International Standard of Neurological Classification for Spinal Cord Injury (ISNCSCI).
Results:
Total ISNCSCI score (ISNCSCI-tot; sum of ISNCSCI motor and sensory scores) was significantly (p<0.05) correlated with fractional anisotropy and axial and radial diffusivities. ISNCSCI-tot showed strongest correlation with indices measured from the region inferior to the injury epicenter (IRRI), the degree of which exceeded that of those measured from the entire cervical cord – suggesting contribution from Wallerian degeneration.
Conclusion:
DTI tractography-driven subject-specific injury demarcation approach provided measures that were more specific to impairment. Notably, DTI indices obtained from the IRRI region showed the highest specificity to impairment, demonstrating their strong potential as biomarkers for the SCI severity.
Keywords: Spinal cord injury, Diffusion tensor imaging, Subject specific analysis, Injury region demarcation
1. Introduction
Diffusion tensor imaging (DTI) [1, 2]-derived indices of injured spinal cord are sensitive to myelin and axonal damage [3–10] and report on the integrity of the cord at a molecular level that is useful for prognosis of recovery [11–14]. However, averaging DTI indices over large regions of interest (ROIs), a common approach to analyzing the images of injured spinal cord, can lead to a loss of subject-specific information. Therefore, accurate image segmentation of the spinal cord and placement of more biochemically homogeneous ROI around injury epicenter may maximize the potential of DTI indices as noninvasive imaging biomarkers for the severity of SCI.
Methods for spinal cord segmentation can be broadly categorized as manual, automated, and semi-automated. Although most time-consuming, the manual segmentation [11, 15–17] is highly accurate. However, application of the fully automated methods [18–21] in clinical environment is complicated by the small size of the spinal cord that often leads to low-resolution images that are suboptimal for fully automated segmentation. In semi-automated methods, compromise is made between the accuracy of the manual method and the efficiency of the fully automated method – by first performing automated segmentation of the entire spinal cord, followed by manual segmentation of the spinal cord columns [21, 22].
After segmentation, ROIs must be placed along the longitudinal axis of spinal cord. In one conventional approach, an entire neurological region (e.g., cervical region) is defined as an ROI [21, 23]. Examples of other approaches include obtaining finer-grained ROIs by further dividing the neurological regions into arbitrary sub-regions (e.g., upper, middle, and lower cervical regions) [11] and single vertebra levels (e.g., C1,…,C8) [14]. In these approaches, the placement of ROIs is entirely anatomically determined – i.e., ROIs are placed at the same anatomically defined regions for all participants, irrespective of their varying levels or size of lesion. Such methods have been successfully used to observe robust correlations between clinical measures of function and DTI indices [11, 14, 21, 23]. However, those methods are also potentially less sensitive to pathological changes, due to extensive averaging of DTI index values over regions of healthy spinal cord. Such regional averaging can be minimized by placing the ROIs in regions that are subject- and injury-region-specific, reflecting the varying levels and sizes of lesion in each individual [17, 24, 25].
In this study, we analyzed DTI data in individuals with chronic SCI by first delineating the spinal cord and its columns using a robust semi-automated segmentation method [24, 26] that utilizes DTI fiber tractography [14, 27, 28], and then adapting a subject-specific ROI placement approach to demarcate individual injury regions. We hypothesized that DTI indices obtained using the DTI-tractography-driven, subject-specific ROI placement approach would better correlate with residual sensorimotor function, compared to those obtained using the non-subject-specific, anatomically-determined ROI placement approach.
2. Material and Methods
2.1. Participants
Initially, 19 individuals with chronic cervical SCI gave informed written consent to participate in the study, which was approved by the Johns Hopkins Medicine Institutional Review Board. Data from one individual was eventually excluded from further data analysis, as described in details below. Of the 19 individuals, 15 had traumatic injuries and four had non-traumatic (specifically, transverse myelitis; TM) injuries. In individuals with TM, a one-year follow-up chart review was performed to exclude those who subsequently developed neurological conditions that are unrelated to SCI. Subsequently, one individual with TM who was later diagnosed with recurrent disseminated encephalopathy was identified and excluded from further data analysis. The remaining cohort of 18 study participants (20–66 years, mean:47, M/F ratio:14/4; Table 1) consisted of 15 individuals with traumatic injuries and three individuals with TM.
Table 1.
Demographics of the individuals with chronic spinal cord injury (SCI)†
| No. | Age (yr) | Time since injury (yr) | Injury level | AIS Grade | ISNCSCI Motor | ISNCSCI Sensory | ISNCSCI Total | Sex | Cause |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 4 | C2 | A | 0 | 13 | 13 | F | Traumatic |
| 2 | 38 | 1 | C4 | A | 14 | 21 | 35 | F | TM |
| 3 | 50 | 24 | C4 | A | 21 | 14 | 35 | M | Traumatic |
| 4 | 26 | 5 | C2 | B | 1 | 12 | 13 | M | Traumatic |
| 5 | 52 | 34 | C5 | B | 34 | 65 | 99 | M | Traumatic |
| 6 | 62 | 1 | C4 | C | 6 | 24 | 30 | M | Traumatic |
| 7 | 53 | 11 | C6 | C | 34 | 33 | 67 | M | TM |
| 8 | 60 | 4 | C3 | C | 55 | 51 | 106 | M | Traumatic |
| 9 | 33 | 15 | C2 | C | 51 | 60 | 111 | M | Traumatic |
| 10 | 28 | 3 | C3 | C | 56 | 60 | 116 | F | Traumatic |
| 11 | 20 | 3 | C4 | C | 23 | 96 | 119 | F | TM |
| 12 | 62 | 2 | C4 | D | 69 | 28 | 97 | M | Traumatic |
| 13 | 54 | 32 | C2 | D | 97 | 27 | 124 | M | Traumatic |
| 14 | 60 | 2 | C5 | D | 68 | 95 | 163 | M | Traumatic |
| 15 | 66 | 3 | C3 | D | 89 | 87 | 176 | M | Traumatic |
| 16 | 43 | 30 | C5 | D | 92 | 112 | 204 | M | Traumatic |
| 17 | 39 | 1 | C5 | D | 97 | 108 | 205 | M | Traumatic |
| 18 | 65 | 2 | C2 | D | 100 | 110 | 210 | M | Traumatic |
[yr: year; AIS: American Spinal Injury Association Impairment Scale; ISNCSCI: International Standard of Neurological Classification for Spinal Cord Injury; ISNCSCI-tot: total ISNCSCI score; M: male, F: female, TM: transverse myelitis]
Of the 19 individuals with chronic cervical SCI who initially provided informed written consent to participate in the study, data from one individual with TM was excluded from further data analysis after a one-year follow-up chart review.
Previously acquired spinal cord images from 10 healthy individuals (21–49 years, mean:33, M/F ratio:6/4) [24] were used as a control dataset.
2.2. Spinal cord injury classification
The International Standard of Neurological Classification for Spinal Cord Injury (ISNCSCI) scoring system, developed by the American Spinal Injury Association (ASIA), was used to determine the level and severity of SCI [29, 30], and the classification of SCI was provided by the ASIA Impairment Scale (AIS) grade system. The evaluations consisted of testing of five arm and leg muscles to assess residual motor functions, and light touch and pinprick examinations of 28 sensory dermatomes to assess residual sensory functions.
Four ISNCSCI metrics were tabulated: injury level (C2-C6), total motor score (sum of upper and lower extremity motor scores; max: 100), total sensory score (sum of left and right light touch sensory scores; max: 112), and the ISNCSCI-tot score (sum of total motor score and total sensory score; max: 212). For the sensory score, the light touch examination scores were arbitrarily selected for use in the study, as scores from the light touch and pinprick sensory examinations were highly correlated (R2=0.89).
2.3. Image acquisition
The image acquisition protocol and analysis pipeline used in this study were previously described in detail [24], and are summarized here.
All participants (including both the healthy control and SCI patient cohorts) were scanned on a Philips 3 Tesla scanner, using a 16-channel neurovascular coil. In order to increase the directional resolution of the DTI dataset while minimizing the effect of motion, three DTI scans were acquired and entered into the tensor calculation as separate entities (i.e. no pre-calculation averaging was performed): multi-slice pulsed gradient spin echo sequence, b=0 and 500 s/mm2, 16 diffusion weighted directions that sample a prolate tensor, TR/TE=6300/63 ms, SENSE factor=2, 96×96×40 volume matrix, 1.5×1.5×3 mm3 resolution (axial sections of 3 mm thickness; zero-filled to 0.57×0.57×3 mm3), and matrix size=256×256×40. Field of view was chosen to span the length of the cervical spinal cord. Smith et al [26] and Landman et al [31, 32] optimized the above-mentioned imaging parameters used in this study.
Studies have shown that magnetization transfer imaging (MTI) can be used to obtain high contrast and resolution images that can assist semi-automated segmentation of the spinal cord [6, 24, 26]. For the purpose of this study, we specifically exploited the high contrast and resolution of the MT-weighted images to aid the manual drawing of ROIs within individual spinal cord columns. Note that the MT-weighted images were utilized for ROI placement purpose only, and further analysis of the MT parameters was not performed. MT-weighted images were acquired using a three-dimensional spoiled gradient-echo sequence with multi-shot EPI readout: EPI factor=3, TR/TE=102/13 ms, α=9°, and SENSE-factor=2, 368×276×40 image volume matrix, 0.61×0.69×3 mm3 resolution. MT weighting was achieved using a 24 ms, 5 lobed, sinc-shaped saturation pulse with peak amplitude of 8.5 µT at offset frequency of 1.5 kHz.
Total image acquisition time, including survey, SENSE reference, sagittal T2-weighted (T2-w), STIR (short tau inversion recovery), three DTI, and two MTI scans, was 28 minutes.
2.4. Image processing and analysis
2.4.1. Image registration and diffusion tensor estimation
CATNAP (Coregistration, Adjustment, and Tensor-solving, a Nicely Automated Program, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA) [31] was used to perform volume-wise coregistration as well as the estimation of DTI-derived indices (i.e., fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD))[31]. Specifically, each diffusion-weighted image was registered to an initial b=0 s/mm2 (b0; non-diffusion-weighted image) volume using a six degrees of freedom rigid-body registration, and the DTI-derived indices were estimated using a multivariate log-linear fitting method. Also, the diffusion gradient tables were updated to account for any rotation prior to the diffusion tensor estimation.
Registration of the MT dataset to b0 volume was performed in two steps. First, the MT dataset was registered to the b0 volume using a three-dimensional, six degrees of freedom, rigid body transformation [33]. For added accuracy, this was followed by an additional two-dimensional registration, using a three degrees of freedom rigid body transformation that involved two in-plane translations and one rotation. The detailed description of this registration process, as well as the process’ degree of reproducibility, can be found in Smith et al [26].
2.4.2. Diffusion fiber tractography of spinal cord columns and creation of column profiles
Diffusion fiber tractography was performed using DTIStudio [34]. MT-images were used to manually place ROIs in the left and right lateral, dorsal, and ventral spinal cord columns, example of which is shown in Figure 1. The manual ROI placement was performed on every third axial section along the entire cervical cord in order to yield seed regions for the tractography. FA threshold of 0.2 and a maximum tract turning angle of 60° were used as the stopping criterion, and spurious fibers were manually excluded. Finally, column profiles [26, 35] spanning the vertebral levels C2 and C6 were created for each DTI index. Detailed description of the data processing pipeline and the method’s degree of reproducibility can be found in Smith et al [26].
Figure 1. Placement of seed regions in magnetization transfer (MT) images for diffusion fiber tractography.

Examples of seed region placements are shown for images obtained from a healthy control (a, c) and an individual with spinal cord injury (SCI) (b, d). (a and b) Seed regions for the right lateral (red), left lateral (yellow), dorsal (green), and ventral (blue) spinal columns were placed within the MT-images. (c and d) Diffusion tensor imaging (DTI) fiber tractography was performed using the seed regions. White bracket in (d) highlights the individual’s injury epicenter.
[V: ventral; D: dorsal; R: right; L: left; S: superior; I: inferior, C2-C5: cervical levels 2–5].
Previous study has shown that typical neck length of individuals is approximately 75 mm [26], corresponding to 25, 3 mm thick image sections. Using this information, each column profile was normalized to 25 equally-distanced points that span the length of the cervical cord.
2.4.3. Demarcation of regions relative to injury
For each SCI patient, three regions-relative-to-injury (RRI) were identified (Figure 2). First, epicenter RRI (ERRI) was manually identified using each individual’s sagittal T2-w and axial MT images. Superior RRI (SRRI) was then defined as the region located above the superior edge of the ERRI, up to approximately the length of one vertebral level (~15 mm). Similarly, inferior RRI (IRRI) was defined as the region located below the inferior edge of the ERRI, up to approximately the length of one vertebral level.
Figure 2. Identification of regions-relative-to-injury (RRI).
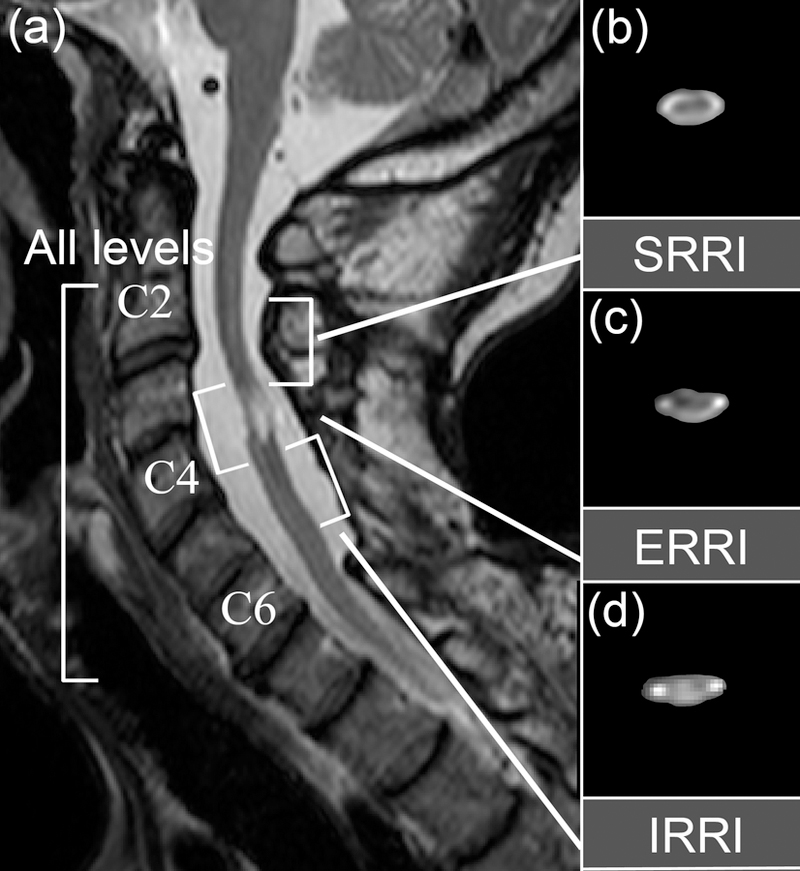
(a) Sagittal T2-weighted (T2-w) images were used to demarcate subject-specific injury regions. Three regions-relative-to-injury – regions superior to injury epicenter (SRRI; (b)), at injury epicenter (ERRI; (c)), and inferior to injury epicenter (IRRI; (d)) – were identified, where ERRI was defined as the injury epicenter, and SRRI and IRRI were defined as one vertebral level above and below the ERRI region. The entire length of the cervical spinal cord spanning the vertebral levels C2 to C6 was defined as the all levels (AL) region. Representative axial views of fractional anisotropy (FA) maps for each corresponding RRI regions are shown in (b-d), without diffusion direction color-coding.
Next, locations of the RRIs within the normalized column profile were identified, and DTI index measures for each spinal cord column (left, right, dorsal, and ventral columns), within each RRI region (SRRI, ERRI, and IRRI) were obtained. In instances where severe spinal cord atrophy yielded non-measurable DTI values at the site of injury or the extent of injury site expanded beyond the C2 to C6 vertebral levels, ROIs were treated as having missing data points.
2.4.4. Statistical analysis
Statistical analysis was performed using Matlab (The Mathworks, MA, USA).
A two-way analysis of variance (ANOVA) was performed to identify significant sources of variation on DTI-derived indices. The result was Bonferroni-corrected, and any corrected p-values larger than one were set to one.
Results of the ANOVA analysis were used to eventually conclude that spinal cord columns did not have significant effects on either the DTI indices or the degree of correlation between the DTI indices and ISNCSCI scores, as later described in the Results section. Based on this result, for each DTI-derived index, all following data analyses were performed using only the single ‘whole cord profile’ (obtained by averaging the individual column profiles of the four spinal cord columns) instead of using separate cord profiles for each spinal cord columns. Similarly, as we are no longer separating ventrolateral motor columns from dorsal sensory columns by using the whole cord profile, ISNCSCI-tot score was selected as the behavioral outcome measure of choice.
The effect of spinal cord RRI on the degree of correlations between diffusion measurements (derived from the whole cord profiles of DTI index values) and residual sensorimotor function (obtained from ISNCSCI) was assessed using a series of linear regression analyses. Specifically, a set of four linear regression analyses was performed for each DTI-derived index, where ISNCSCI-tot was defined as a common independent variable, and four separate dependent variables were defined as DTI index values obtained from: 1) the entire length of the cervical spinal cord (between vertebral levels C2-C6, here on referred to as ‘all levels’; AL); 2) SRRI; 3) ERRI; and 4) IRRI regions. Bonferroni correction was used to account for multiple comparisons across different RRI regions.
In order to investigate if motor or the sensory ISNCSCI score had more explanatory power for DTI-derived indices, a stepwise regression analysis was performed. Specifically, the motor and sensory scores were considered as separate potential independent variables, and their effects on each SRRI- and IRRI-region DTI index were tested. And finally, stepwise regression analyses were performed to evaluate whether age and/or time since injury (TSI) had significant effects on DTI indices. TSI, age, and ISNCSCI-tot scores were considered as potential independent variables, and we tested for their effects on each IRRI-region DTI index. Correction for multiple comparisons was not performed for the two stepwise regression analyses.
3. Results
3.1. Demarcation of regions relative to injury
In an effort to increase the potential of DTI indices to serve as imaging biomarkers, we aimed to identify biochemically homogeneous ROIs that are more specific to the damage of the spinal cord, and two such classes of ROIs were initially investigated: a) one class of ROI was identified using the described spinal cord column segmentation approach with DTI fiber tractography, and constituted of individual spinal cord columns (right and left lateral, dorsal, and ventral columns; Figure 1). b) The other class of ROI was identified using subject-specific injury region demarcation approach, and constituted of spinal cord RRIs (SRRI, ERRI, and IRRI regions; Figure 2). The effect of spinal cord columns and RRI on FA, AD, and RD was tested using two-way ANOVA. This analysis revealed that significant effect (p<0.05) of spinal cord RRI existed for all DTI indices (Table 2), indicating that DTI index values of different RRI regions were different from each other. The analysis also revealed that there was no significant effect of spinal cord columns on DTI indices (i.e., different spinal cord columns did not have significantly different DTI index values). Therefore, the relationship between DTI indices obtained from individual spinal cord columns and ISNCSCI scores was further investigated using a series of post-hoc linear regression analyses, and no significant correlation between DTI indices from different spinal cord columns and ISNCSCI scores was observed (i.e., no significant lateral effects was observed; results not shown).
Table 2. Effect of spinal cord column and spinal cord region-relative-to-injury (RRI) on diffusion tensor imaging (DTI) indices.
The effect of spinal cord column (right and left lateral, dorsal, and ventral columns) and RRI region (regions superior to, at, and inferior to injury epicenter) on fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) was tested using a two-way analysis of variance (ANOVA). Spinal cord RRI was identified as the main source of variation for all three DTI indices. In other words, DTI index values measured from each RRI region were significantly different from each other. Conversely, different spinal cord columns did not have significantly different DTI index values.
| DTI indices | Source of variation | |
|---|---|---|
|
Spinal Cord Columns (p-value) |
Spinal Cord RRI (p-value) |
|
| FA | 0.258 | 0.004* |
| AD | 0.539 | 0.002* |
| RD | 0.781 | 0.002* |
Statistically significant (p< 0.05; corrected)
FA, AD, and RD values obtained by averaging over each ROI (i.e., AL, SRRI, ERRI, and IRRI regions) of the whole cervical cord profiles are summarized in Table 3 (note that hereon, an ROI from which a specific DTI measures is obtained will be expressed as a subscript of the DTI index – e.g. FA measurement obtained from the AL region is expressed as FAAL, etc.). Lack of injury in healthy spinal cords renders the injury region demarcation and the identification of RRI regions inapplicable. Therefore in healthy spinal cord, we only considered DTI indices from the AL region. For the AL region, significant decrease in FAAL (p<0.05), and increase in RDAL (p<0.05) were observed in chronic SCI individuals compared to the healthy individuals – indicating severe damage in the injured cords.
Table 3. Mean and standard deviation measurement of each DTI index.
In healthy individuals, only the DTI indices from the all levels (AL) region were obtained, and the measurements were then compared with the corresponding AL-region DTI index values from the individuals with SCI. Significant decrease in FAAL† and increase in RDAL indicate severe damage in injured cords.
| Mean ± SD | |||||
|---|---|---|---|---|---|
| Healthy individuals | Individuals with SCI | ||||
| AL | AL | SRRI | ERRI | IRRI | |
| FA | 0.72±0.04 | 0.47±0.09* | 0.51±0.10 | 0.46±0.10 | 0.43±0.13 |
| AD (µm2/ms) | 2.04±0.14 | 2.22±0.28 | 2.13±0.38 | 2.12±0.31 | 2.47±0.48 |
| RD (µm2/ms) | 0.53±0.09 | 1.15±0.29* | 1.01±0.35 | 1.10±0.30 | 1.37±0.54 |
Statistically significant (p <0.05; corrected); [SD: standard deviation]
Note that hereon, an ROI from which a specific DTI measures is obtained will be expressed as a subscript of the DTI index – e.g. FA measurement obtained from the AL region is expressed as FAAL, etc.
3.2. Regional dependence of associations between impairment and diffusion indices
Next, we wanted to determine if there are correlations between DTI indices from each ROI and ISNCSCI-tot scores. For this, we utilized linear regression analysis. Table 4 lists the adjusted R2 values, slopes with the corresponding p-values, and intercept of the estimated regression lines for all three DTI indices. The slope and intercept values describe the existence of the correlations between variables, while R2 values describe the strength of the correlations. Supplementary Figure S1 visualizes the linear regression analyses results of FA. Results showed that a significant relationship exist between FAAL and ISNCSCI-tot (p=0.013). The slope was positive, indicating that as impairment became more severe (smaller ISNCSCI-tot), FAAL values decreased. While this positive linear trend between FA and ISNCSCI-tot was preserved when FAAL was further divided into subject-specific regions of FASRRI, FAERRI, and FAIRRI, the correlation was significant only for FAIRRI (p=0.002). Also, the adjusted R2 value for the FAIRRI (0.576) was also larger than that for the FAAL (0.389). Finally, this tendency of tighter correlation between ISNCSCI-tot and IRRI-region DTI indices, compared to the DTI indices from other ROIs, was also true for AD (p=0.042) and RD (p=0.009).
Table 4. Spatial dependence of the correlation between DTI indices and total ISNCSCI scores.
Stronger correlation (i.e., larger R2 value) is observed between total ISNCSCI scores (ISNCSCI-tot) and DTI indices obtained from the IRRI-region, compared to that between the ISNCSCI-tot and DTI indices obtained from SRRI-, ERRI-, and AL-regions – indicating spatial dependence of the correlation between DTI indices and total ISNCSCI scores.
| RRI | Adjusted R2 | Slope [p-val] | Intercept |
|---|---|---|---|
| FASRRI | 0.061 | 0.001 [0.666] | 0.452 |
| FAERRI | 0.230 | 0.001 [0.102] | 0.370 |
| FAIRRI | 0.576 | 0.002 [0.002*] | 0.236 |
| FAAL | 0.389 | 0.001 [0.013*] | 0.370 |
| ADSRRI | −0.051 | −0.001 [1.000] | 2.195 |
| ADERRI | −0.008 | −0.001 [1.000] | 2.240 |
| ADIRRI | 0.339 | −0.005 [0.042*] | 3.026 |
| ADAL | 0.339 | −0.001 [0.752] | 2.365 |
| RDSRRI | 0.009 | −0.001 [1.000] | 1.165 |
| RDERRI | 0.272 | −0.003 [0.062] | 1.381 |
| RDIRRI | 0.466 | −0.006 [0.009*] | 2.091 |
| RDAL | 0.343 | −0.003 [0.025*] | 1.448 |
Statistically significant: p <0.05, corrected
We hypothesized that the observed stronger correlation of the IRRI region-derived DTI index values with ISNCSCI-tot may indicate directional contribution from Wallerian degeneration, and that the use of subject-specifically derived DTI indices as a biomarker for the severity of SCI would be affirmed if the degree of individual residual motor or sensory function of patients with SCI could be explained specifically by DTI indices obtained from either the IRRI or SRRI region. To investigate this possibility, we used a stepwise regression analysis. Our data, summarized in Table 5, indicates that the motor score had significant explanatory power for FAIRRI (p<0.001), ADIRRI (p<0.01), and RDIRRI (p<0.01), while neither motor nor sensory scores had significant explanatory power for SRRI-region DTI indices. In addition, we used a stepwise regression analysis to investigate if other factors such as age or TSI could explain changes in the IRRI region-derived DTI indices. Our data, summarized in Table 6, confirms that the level of neurological function assessed by ISNCSCI-tot had significant explanatory power for all three DTI indices (FAIRRI (p<0.001); ADIRRI (p<0.05); RDIRRI (p<0.001)), while age had significant explanatory power for RDIRRI only (p<0.05). TSI had no significant explanatory for any IRRI-region DTI indices. These results confirm that DTI indices measured from the IRRI region, particularly FAIRRI and ADIRRI, are indeed more suitable to serve as noninvasive biomarkers for the severity of SCI and structural improvements in the injured spinal cord independent of age and TSI.
Table 5. Effect of ISNCSCI motor score and ISNCSCI sensory score on DTI indices obtained from SRRI- and IRRI-regions.
Effect of ISNCSCI motor score and sensory score on SRRI- and IRRI-region DTI indices was investigated using stepwise regression analyses. Motor score had significant explanatory power for IRRI region-derived DTI indices (FAIRRI, ADIRRI, and RDIRRI), while neither motor scores nor sensory scores had significant explanatory power for SRRI region-derived DTI indices (FASRRI, ADSRRI, and RDSRRI).
| FASRRI | ADSRRI | RDSRRI | ||||
|---|---|---|---|---|---|---|
| Motor | Sensory | Motor | Sensory | Motor | Sensory | |
| Coefficients | 1.30E-03 | 4.58E-04 | −6.18E-04 | −4.47E-04 | −1.40E-03 | −1.40E-03 |
| Standard error | 6.35E-04 | 6.78E-04 | 1.50E-03 | 2.60E-03 | 1.30E-03 | 2.40E-03 |
| P-value | 5.31E-02 | 5.09E-01 | 6.77E-01 | 8.67E-01 | 2.97E-01 | 5.83E-01 |
| FAIRRI | ADIRRI | RDIRRI | ||||
| Motor | Sensory | Motor | Sensory | Motor | Sensory | |
| Coefficients | 3.00E-03 | 9.94E-04 | −4.80E-03 | −9.28E-04 | −6.20E-03 | 2.10E-03 |
| Standard error | 6.79E-04 | 7.90E-04 | 1.60E-03 | 7.00E-03 | 1.60E-03 | 7.10E-03 |
| P-value | < 0.001* | 2.30E-01 | < 0.01* | 8.96E-01 | < 0.01* | 7.76E-01 |
Statistically significant (p<0.05; not corrected)
Table 6. Effect of time since injury and age.
Stepwise regression analyses were performed to determine the optimal multi-linear model for each indices of interest – specifically, FAIRRI, ADIRRI, and RDIRRI. Time since injury (TSI), age, and ISNCSCI-tot score were considered as potential independent variables and their explanatory power on each DTI index value were tested for significance. ISNCSCI-tot had significant explanatory power for all three DTI indices, while age had significant explanatory power for RD only. TSI had no significant explanatory for all three DTI indices.
| FAIRRI | ADIRRI | RDIRRI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TSI | Age | ISNCSCI total | TSI | Age | ISNCSCI total | TSI | Age | ISNCSCI total | |
| Coefficients | 0.000 | −0.003 | 0.002 | 0.007 | 0.014 | −0.005 | 0.004 | 0.015 | −0.007 |
| Standard error | 0.002 | 0.001 | 0.000 | 0.009 | 0.007 | 0.002 | 0.008 | 0.007 | 0.002 |
| P-value | 0.887 | 0.067 | < 0.001* | 0.439 | 0.061 | < 0.05* | 0.600 | < 0.05* | < 0.001* |
Statistically significant (p < 0.05; not corrected)
4. Discussion
DTI tractography can be used to perform semi-automated segmentation of the spinal cord in ways that are effective and reproducible [26], as well as clinically applicable [24]. In this study, we investigated the usage of DTI indices as biomarkers for spinal cord integrity by introducing a subject-specific injury demarcation approach to a previously established segmentation method, to define a more biochemically homogeneous ROIs around the injury epicenter.
4.1. Subject-specific demarcation of injury regions
Two classes of ROIs – specifically, spinal cord columns (right and left lateral, dorsal, and ventral columns) and spinal cord RRI (SRRI, ERRI, and IRRI regions) – were initially identified. In particular, spinal cord RRIs were identified using a subject-specific ROI placement approach to demarcate individual injury regions, which yielded ROIs that are focal and biochemically homogeneous. Such subject-specific approach can reduce partial voluming of DTI index values within ROIs and enable us to probe the tissues more specifically in relation to SCI. This is supported by the observation that while the spinal cord columns did not have significant effects on either the DTI indices or the degree of correlation between DTI indices and ISNCSCI scores, the spinal cord RRI showed significant effects on all three DTI indices (Table 2) – which suggests that spinal cord RRI is more sensitive to injury severity.
4.2. Regional dependence of associations between impairment and diffusion indices
The mean DTI values measured from the entire length of the cervical cord (e.g., FAAL, ADAL, and RDAL) (Table 3) that represents DTI index values obtained using the conventional non-regions specific approach, were within the range of values reported in previous studies, for healthy controls [26, 36–39] and chronic SCI patients [14, 23]. Studies have shown that axonal and myelin damage that occurs following SCI is associated with decreased FA and increased RD values [11–14]. The significant changes in FAAL and RDAL observed in individuals with chronic SCI (Table 3) are also in agreement with the previous findings. Similarly, the observed significant correlation between functional impairment and the values of the DTI indices is in accordance with previous studies [11, 14, 17, 23], where lower FA and higher AD and RD values are reported to be correlated with worse ISNCSCI scores.
Notably, we observed that compared to FAAL, FAIRRI showed stronger correlation with ISNCSCI-tot, which is in accordance with a previous study [17] – indicating that subject-specific regional measures of FA, and in particular, those measured from IRRI-region, may be more specific to impairment. A similar trend of stronger correlations between ISNCSCI-tot and other IRRI-region DTI indices, specifically RDIRRI and ADIRRI, was also observed (Table 4).
Inclusion of the TM patient cohort was intentional, as we expected the proposed method to be applicable to SCI of different etiologies. However, in order to address the question of whether the inclusion of the TM patient data could have introduced any bias to the result, additional analyses were performed on the subset of data comprised exclusively of traumatic SCI individuals, as shown in the Supplemental Materials (Table S1–S3). The data showed that the results were consistent, irrespective of the inclusion/exclusion of the data from the TM patient cohort – affirming that the proposed method is applicable to SCI of different etiologies.
4.3. Wallerian degeneration
Wallerian degeneration describes a secondary anterograde degeneration of axons and myelin sheaths that occurs in regions distal to the primary injury, and identification and characterization of Wallerian degeneration is important for the comprehensive assessment and prognosis of SCI. In spinal cord, Wallerian degeneration is characterized by directionality, as the cord consists of longitudinally arranged major descending motor fibers and ascending sensory fibers – leading to involvement of the mainly ventrolateral motor columns inferior to the primary injury region, and dorsal columns superior to the primary injury region [23, 25, 40, 41]. The directionality observed in the current study (i.e., the high correlation between functional impairment and IRRI-region DTI indices) may therefore indicate that water diffusion measurements in the IRRI-region reflect the effects of Wallerian degeneration that are specific to the severity of injury. This is supported in part by the observation that the motor scores displayed significant explanatory power for all three IRRI-region DTI indices (Table 5). However, neither motor nor sensory scores had significant explanatory power for SRRI-region DTI indices (Table 5).
Conventionally, descending motor and ascending sensory columns are defined as ventrolateral and dorsal spinal cord columns, respectively. While extensively adapted in SCI studies by others [23, 42] and this study, the definition greatly simplifies the complex structure of the spinal cord. Most noticeably, the above definition of motor and sensory columns disregards the existence of sensory fibers that ascend laterally, such as the spinocerebellar and spinothalamic fibers [43, 44]. Thus, one explanation for the observed lack of correlation of sensory scores with SRRI-region DTI indices, compared to that with IRRI-region DTI indices, may be due to the more distributed nature of the ascending sensory fibers in the spinal cord, which is not fully accounted for in this study. This may also explain the observed lack of significant effect of spinal cord columns on DTI indices (Table 2).
Finally, ISNCSCI sensory scores are ordinal and non-linear, with each dermatome tested and scored as absent (0), abnormal (1), or normal (2). This introduces subjectivity and rater-variability into the scoring system and may lower the direct correspondence between objective MRI markers and subjective clinical ones [45, 46]. Alternative measures of residual sensory function with higher sensitivity exist [45, 47, 48], but the methods are often time-consuming and impractical for clinical use. Nonetheless, sensory testing methods with high sensitivity may be used to better understand the relationship between the SRRI-region DTI indices and residual sensory function in individuals with SCI.
4.4. Study Limitations
Recruitment of individuals with SCI for MRI studies – which require participants to lie still for extended time – is challenging, due to their often severe sensorimotor impairments. This greatly limited our ability to recruit a homogeneous cohort of SCI patients – of similar TSI, age, and injury level. Here, we briefly discuss the effects of these potential confounds.
First, previous studies have shown that TSI and age affect the values of DTI indices [38, 49–51]. In order to investigate whether TSI and age had significant effect on DTI indices, we therefore performed a series of stepwise regression analyses. The results showed that ISNCSCI-tot had significant explanatory power (p<0.05; not corrected) on FAIRRI, ADIRRI, and RDIRRI, while TSI and age did not, as shown in Table 6. One exception was the relationship between age and RDIRRI, which was significantly correlated. This observation is consistent with previous findings that RD is more sensitive to the effects of aging, compared to other DTI indices [50, 52]. It is, however, also possible that the effect sizes of TSI and age were too small to be observed in this small-sampled group study. This is supported in part by the decreased amount of correlations (but still in the same direction) between DTI index and ISNCSCI scores observed in the data analysis results of a sub-group that include only the patients with a traumatic SCI (Table S1–S3).
Secondly, previous studies have shown that FA values decrease 10–15% along the length of the cervical cord (in C2 to C6 direction), possibly modulated by the varying ratio of gray to white matter in different spinal segmental levels [39]. Therefore, we also investigated the possible effects of such underlying decrease of baseline FA values, by weighting the FAIRRI values to reflect the varying injury level in SCI patients (refer to Supplemental Text and Figure S2 for further description). Figure S2 shows that when adjusted to reflect the varying injury level in SCI patients, the strength of the correlation between FAIRRI and ISNCSCI-tot becomes stronger. This result, while suggesting that the segmental-level-dependent changes in FA may exist in individuals with SCI, also shows compared to the conventional approach that the proposed subject-specific injury demarcation approach can provide robust outcome measures that are more specific to impairments despite possible confound,. A possible explanation of this observation is that by no longer diluting the effect of the injury (e.g., 30–40% FA decrease at and around the injury epicenter) across the entire cervical region, the benefit of the subject-specific demarcation approach outweighs the possible confounding effects.
The small size of the study cohort (n=18) limits our ability to utilize the above mentioned confounds (i.e., TSI, age, and level of injury) to derive a multivariate model that better describes the association between the behavioral and DTI outcome measures. A future multi-center study with a larger cohort could help create a more accurate description of the relationship by enabling the inclusion of TSI, age, as well as the level of injury as covariates in the model.
The small size of the spinal cord was an inherent limitation of this study, leading to increased vulnerability to partial volume effect and motion artifact [53]. At cervical level, the anterior-posterior and transverse diameters of the cervical cord are reported as ca. 8.7–14 mm [54, 55]. This may have contributed to the observed lack of significant effect of spinal cord column on DTI indices (Table 2). One approach for addressing this limitation is to acquire the spinal cord images at higher magnetic field strengths. Our study was performed at 3T, which provided images with higher SNR and spatial resolution compared to those expected to be provided by 1.5T magnet, commonly used for clinical diagnostic purposes. As DTI is inherently low in SNR, such benefits of higher field strengths are especially important for DTI, and we expect that the sensitivity and the accuracy of DTI in probing spinal cords microstructures would be even more enhanced in higher field strength (e.g. 7T). However, tradeoffs also exist – that at higher field strengths, chemical shift, susceptibility, as well as DTI-derived indices flow/motion artifacts are enhanced – and caution is warranted when analyzing images acquired at higher fields. Another approach would be to acquire higher resolution images using motion-artifact reducing techniques, such as respiratory and cardiac gating. However, these techniques often require longer scan times that are clinically infeasible for individuals with paralysis. There is also the issue of image misregistration, which is exacerbated by the small cord size. In this study, we used a robust registration scheme utilizing a publically available CATNAP program [31, 56] to minimize the effects of misregistration. Future studies may also benefit from more advanced MR acquisition methods such as accelerated parallel imaging techniques [57, 58].
Finally, CATNAP was also used to perform the tensor estimation, by utilizing a multivariate log-linear fitting method. During this process, each DW image and its corresponding vector from the gradient table were entered as unique entries, and no averaging of DW images was performed. The multivariate log-linear fitting method is a simple, yet widely adapted tensor estimation method that has previously been shown to reproducibly estimate DTI-derived indices [31, 59, 60]. It is however, a well-known fact that the use of different fitting algorithms such as weighted linear and nonlinear least squares can systematically influence the estimation of DTI-derived indices [61]. Consequently, other more robust fitting methods have been proposed over the years to address the issue [61–64]. An interesting area of future research would be to evaluate how different tensor fitting methods impact the precision and accuracy of the DTI-derived indices from impaired spinal cords, and whether more robust fitting methods will yield DTI measures that are even more specific to impairment.
5. Conclusion
DTI allows for noninvasive assessment of the severity and level of SCI, as well as objective and efficient semi-automated segmentation of the spinal cord. We investigated whether subject-specific demarcation of the injury region, performed using DTI fiber tractography, yields diffusion measures that are more specific to impairment. Results showed that the proposed approach preserved information specific to each individual’s injury, and the resulting subject-specific RRIs were a large source of variation for DTI indices. Importantly, IRRI-region DTI indices demonstrated the strongest correlation with impairment, indicating that DTI index measurements from IRRI region have a strong potential to serve as biomarkers for the severity of SCI. Indeed, this correlation suggests that diffusion measures in this region may be more sensitive to Wallerian degeneration in the descending ventrolateral motor columns. We therefore conclude that regional analysis of water diffusion using subject-specific injury demarcation is more specific to impairment, and potentially can have a clinical application in diagnosis of SCI severity and in measuring anatomical improvements in response to therapeutic interventions.
Supplementary Material
Acknowledgements
The authors thank Ms. Terri Brawner, Ms. Kathleen Kahl, Ms. Ivana Kusevic, and Mr. Joseph S. Gillen for experimental assistance. This work was supported in part by grants from the Craig H. Neilsen Foundation (338419), DOD (W81XWH-08-1-0192), and NIH (P41 EB015909).
Footnotes
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Dr. Choe reports grants from Craig H. Neilsen Foundation (338419), U. S. Department of Defense (W81XWH-08-1-0192), and National Institute of Biomedical Imaging and Bioengineering (P41 EB015909) during the conduct of the study. Dr. Pekar serves as Manager of the F.M. Kirby Research Center, which receives research support from Philips Healthcare, which makes the MRI scanner used for this study. Dr. van Zijl is a paid lecturer for Philips and the inventor of technology that is licensed to Philips. This arrangement has been approved by Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254 [DOI] [PubMed] [Google Scholar]
- 2.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648 [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15:435–455 [DOI] [PubMed] [Google Scholar]
- 4.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436 [DOI] [PubMed] [Google Scholar]
- 5.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140 [DOI] [PubMed] [Google Scholar]
- 6.Smith SA, Golay X, Fatemi A, Jones CK, Raymond GV, Moser HW, van Zijl PC (2005) Magnetization transfer weighted imaging in the upper cervical spinal cord using cerebrospinal fluid as intersubject normalization reference (MTCSF imaging). Magn Reson Med 54:201–206 [DOI] [PubMed] [Google Scholar]
- 7.Farrell JA, Zhang J, Jones MV, Deboy CA, Hoffman PN, Landman BA, Smith SA, Reich DS, Calabresi PA, van Zijl PC (2010) Q-Space and Conventional Diffusion Imaging of Axon and Myelin Damage in the Rat Spinal Cord After Axotomy. Magn Reson Med 63:1323–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landman BA, Farrell JA, Smith SA, Reich DS, Calabresi PA, van Zijl PC (2010) Complex geometric models of diffusion and relaxation in healthy and damaged white matter. NMR Biomed 23:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levesque IR, Giacomini PS, Narayanan S, Ribeiro LT, Sled JG, Arnold DL, Pike GB (2010) Quantitative magnetization transfer and myelin water imaging of the evolution of acute multiple sclerosis lesions. Magn Reson Med 63:633–640 [DOI] [PubMed] [Google Scholar]
- 10.Choe AS, Stepniewska I, Colvin DC, Ding Z, Anderson AW (2012) Validation of diffusion tensor MRI in the central nervous system using light microscopy: quantitative comparison of fiber properties. NMR Biomed 25:900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, Gullapalli RP (2011) Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma 28:1881–1892 [DOI] [PubMed] [Google Scholar]
- 12.Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL (2011) Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55:1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed FB, Hunter LN, Barakat N, Liu CS, Sair H, Samdani AF, Betz RR, Faro SH, Gaughan J, Mulcahey MJ (2011) Diffusion tensor imaging of the pediatric spinal cord at 1.5T: preliminary results. AJNR Am J Neuroradiol 32:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S (2012) Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 29:1556–1566 [DOI] [PubMed] [Google Scholar]
- 15.Lundell H, Barthelemy D, Skimminge A, Dyrby TB, Biering-Sorensen F, Nielsen JB (2011) Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord 49:70–75 [DOI] [PubMed] [Google Scholar]
- 16.Qian W, Chan Q, Mak H, Zhang Z, Anthony MP, Yau KK, Khong PL, Chan KH, Kim M (2011) Quantitative assessment of the cervical spinal cord damage in neuromyelitis optica using diffusion tensor imaging at 3 Tesla. J Magn Reson Imaging 33:1312–1320 [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Shin MJ, Chang JH, Lee CH, Shin YI, Shin YB, Ko HY (2015) Correlation of diffusion tensor imaging and phase-contrast MR with clinical parameters of cervical spinal cord injuries. Spinal Cord 53:608–614 [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Carass A, Oh J, Nair G, Pham DL, Reich DS, Prince JL (2013) Automatic magnetic resonance spinal cord segmentation with topology constraints for variable fields of view. Neuroimage 83:1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asman AJ, Smith SA, Reich DS, Landman BA (2013) Robust GM/WM segmentation of the spinal cord with iterative non-local statistical fusion. Med Image Comput Comput Assist Interv 16:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taso M, Le Troter A, Sdika M, Cohen-Adad J, Arnoux PJ, Guye M, Ranjeva JP, Callot V (2015) A reliable spatially normalized template of the human spinal cord - Applications to automated white matter/gray matter segmentation and tensor-based morphometry (TBM) mapping of gray matter alterations occurring with age. Neuroimage 117:20–28 [DOI] [PubMed] [Google Scholar]
- 21.Samson RS, Ciccarelli O, Kachramanoglou C, Brightman L, Lutti A, Thomas DL, Weiskopf N, Wheeler-Kingshott CA (2013) Tissue- and column-specific measurements from multi-parameter mapping of the human cervical spinal cord at 3 T. NMR Biomed 26:1823–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh J, Saidha S, Chen M, Smith SA, Prince J, Jones C, Diener-West M, van Zijl PC, Reich DS, Calabresi PA (2013) Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology 80:540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, Benali H (2011) Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55:1024–1033 [DOI] [PubMed] [Google Scholar]
- 24.Choe AS, Belegu V, Yoshida S, Joel S, Sadowsky CL, Smith SA, van Zijl PC, Pekar JJ, McDonald JW (2013) Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Front Hum Neurosci 7:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Adad J, Leblond H, Delivet-Mongrain H, Martinez M, Benali H, Rossignol S (2011) Wallerian degeneration after spinal cord lesions in cats detected with diffusion tensor imaging. Neuroimage 57:1068–1076 [DOI] [PubMed] [Google Scholar]
- 26.Smith SA, Jones CK, Gifford A, Belegu V, Chodkowski B, Farrell JA, Landman BA, Reich DS, Calabresi PA, McDonald JW, van Zijl PC (2010) Reproducibility of tract-specific magnetization transfer and diffusion tensor imaging in the cervical spinal cord at 3 tesla. NMR Biomed 23:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21:426–433 [DOI] [PubMed] [Google Scholar]
- 28.Kerkovsky M, Bednarik J, Dusek L, Sprlakova-Pukova A, Urbanek I, Mechl M, Valek V, Kadanka Z (2012) Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 37:48–56 [DOI] [PubMed] [Google Scholar]
- 29.Marino RJ, Ditunno JF Jr, Donovan WH, Maynard F Jr (1999) Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil 80:1391–1396 [DOI] [PubMed] [Google Scholar]
- 30.Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W (1997) International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 35:266–274 [DOI] [PubMed] [Google Scholar]
- 31.Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S (2007) Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage 36:1123–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landman BA, Farrell JA, Huang H, Prince JL, Mori S (2008) Diffusion tensor imaging at low SNR: nonmonotonic behaviors of tensor contrasts. Magn Reson Imaging 26:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes F, Vandermeulen D, Suetens P (1999) Comparative evaluation of multiresolution optimization strategies for multimodality image registration by maximization of mutual information. Med Image Anal 3:373–386 [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116 [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Adad J, El Mendili MM, Morizot-Koutlidis R, Lehericy S, Meininger V, Blancho S, Rossignol S, Benali H, Pradat PF (2013) Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph Lateral Scler Frontotemporal Degener 14:30–38 [DOI] [PubMed] [Google Scholar]
- 36.Clark CA, Werring DJ (2002) Diffusion tensor imaging in spinal cord: methods and applications - a review. NMR Biomed 15:578–586 [DOI] [PubMed] [Google Scholar]
- 37.Ducreux D, Fillard P, Facon D, Ozanne A, Lepeintre JF, Renoux J, Tadie M, Lasjaunias P (2007) Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: current and future indications. Neuroimaging Clin N Am 17:137–147 [DOI] [PubMed] [Google Scholar]
- 38.Mamata H, Jolesz FA, Maier SE (2005) Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 22:38–43 [DOI] [PubMed] [Google Scholar]
- 39.Wheeler-Kingshott CA, Hickman SJ, Parker GJ, Ciccarelli O, Symms MR, Miller DH, Barker GJ (2002) Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage 16:93–102 [DOI] [PubMed] [Google Scholar]
- 40.Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, Noth J, Schwab ME, Brook GA (2005) Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain 128:356–364 [DOI] [PubMed] [Google Scholar]
- 41.Qian J, Herrera JJ, Narayana PA (2010) Neuronal and axonal degeneration in experimental spinal cord injury: in vivo proton magnetic resonance spectroscopy and histology. J Neurotrauma 27:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Shimony JS, Klawiter EC, Snyder AZ, Trinkaus K, Naismith RT, Benzinger TL, Cross AH, Song SK (2013) Improved in vivo diffusion tensor imaging of human cervical spinal cord. Neuroimage 67:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willis WD Jr (2007) The somatosensory system, with emphasis on structures important for pain. Brain Res Rev 55:297–313 [DOI] [PubMed] [Google Scholar]
- 44.Rub U, Schultz C, Del Tredici K, Gierga K, Reifenberger G, de Vos RA, Seifried C, Braak H, Auburger G (2003) Anatomically based guidelines for systematic investigation of the central somatosensory system and their application to a spinocerebellar ataxia type 2 (SCA2) patient. Neuropathol Appl Neurobiol 29:418–433 [DOI] [PubMed] [Google Scholar]
- 45.Hayes KC, Wolfe DL, Hsieh JT, Potter PJ, Krassioukov A, Durham CE (2002) Clinical and electrophysiologic correlates of quantitative sensory testing in patients with incomplete spinal cord injury. Arch Phys Med Rehabil 83:1612–1619 [DOI] [PubMed] [Google Scholar]
- 46.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A, International Campaign for Cures of Spinal Cord Injury Paralysis (2007) Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45:206–221 [DOI] [PubMed] [Google Scholar]
- 47.Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS (2003) Sensory function in spinal cord injury patients with and without central pain. Brain 126:57–70 [DOI] [PubMed] [Google Scholar]
- 48.Ellaway PH, Catley M (2013) Reliability of the electrical perceptual threshold and Semmes-Weinstein monofilament tests of cutaneous sensibility. Spinal Cord 51:120–125 [DOI] [PubMed] [Google Scholar]
- 49.Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM (2013) Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Res 1512:22–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R (2009) Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46:530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy MR, Wozniak JR, Muetzel RL, Mueller BA, Chiou HH, Pantekoek K, Lim KO (2009) White matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc 15:130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H (2008) Changes in white matter microstructure during adolescence. Neuroimage 39:52–61 [DOI] [PubMed] [Google Scholar]
- 53.Kharbanda HS, Alsop DC, Anderson AW, Filardo G, Hackney DB (2006) Effects of cord motion on diffusion imaging of the spinal cord. Magn Reson Med 56:334–339 [DOI] [PubMed] [Google Scholar]
- 54.Sherman JL, Nassaux PY, Citrin CM (1990) Measurements of the normal cervical spinal cord on MR imaging. AJNR Am J Neuroradiol 11:369–372 [PMC free article] [PubMed] [Google Scholar]
- 55.Sigmund EE, Suero GA, Hu C, McGorty K, Sodickson DK, Wiggins GC, Helpern JA (2011) High-resolution human cervical spinal cord imaging at 7 T. NMR Biomed 25:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, Mori S (2007) Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J Magn Reson Imaging 26:756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen-Adad J, Mareyam A, Keil B, Polimeni JR, Wald LL (2011) 32-channel RF coil optimized for brain and cervical spinal cord at 3 T. Magn Reson Med 66:1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuchiya K, Fujikawa A, Suzuki Y (2005) Diffusion tractography of the cervical spinal cord by using parallel imaging. AJNR Am J Neuroradiol 26:398–400 [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar M, Gupta RK, Saksena S, Behari S, Malik GK, Kureel SN, Pandey CM, Rathore RK (2010) A diffusion tensor imaging study of deep gray and white matter brain maturation differences between patients with spina bifida cystica and healthy controls. J Clin Neurosci 17:879–885 [DOI] [PubMed] [Google Scholar]
- 60.Saksena S, Husain N, Malik GK, Trivedi R, Sarma M, Rathore RS, Pandey CM, Gupta RK (2008) Comparative evaluation of the cerebral and cerebellar white matter development in pediatric age group using quantitative diffusion tensor imaging. Cerebellum 7:392–400 [DOI] [PubMed] [Google Scholar]
- 61.Lanzafame S, Giannelli M, Garaci F, Floris R, Duggento A, Guerrisi M, Toschi N (2016) Differences in Gaussian diffusion tensor imaging and non-Gaussian diffusion kurtosis imaging model-based estimates of diffusion tensor invariants in the human brain. Med Phys 43:2464. [DOI] [PubMed] [Google Scholar]
- 62.Veraart J, Van Hecke W, Sijbers J (2011) Constrained maximum likelihood estimation of the diffusion kurtosis tensor using a Rician noise model. Magn Reson Med 66:678–686 [DOI] [PubMed] [Google Scholar]
- 63.Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B (2013) Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage 81:335–346 [DOI] [PubMed] [Google Scholar]
- 64.Koay CG, Chang LC, Carew JD, Pierpaoli C, Basser PJ (2006) A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J Magn Reson 182:115–125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


