Abstract
Study Objectives:
Sleep-disordered breathing (SDB) may significantly impact the course of medical illness in hospitalized children. Polysomnography (PSG) is the gold standard for establishing diagnosis of SDB, but its availability is limited. The aim of this study was to explore the feasibility and utility of level III portable sleep studies in hospitalized children with SDB.
Methods:
A retrospective study was conducted at a tertiary hospital over the preceding 2 years in hospitalized children < 18 years who had undergone a level III sleep study using the Nox T3 system. The information obtained included demographic data, comorbidities, indication for admission and sleep study, time interval between the study ordered and done, adequacy of technical data from sleep study, study diagnosis, and subsequent management interventions for SDB.
Results:
A total of 51 hospitalized children had these studies; 32 were female and mean age was 4.3 years. Approximately 90% of children had significant comorbidities, including neurological and craniofacial abnormalities. The majority (80%) of studies were conducted within 24 hours of the time requested and 92.1% studies had technically adequate data for analysis. Thirty-nine (76.5%) children were identified with SDB; all but one patient underwent therapy for SDB during that same hospitalization, including supplemental oxygen (48.7%), positive airway pressure therapy (23%), surgical intervention (38.2%) or caffeine (10.2%). Twelve percent of children had more than one intervention done.
Conclusions:
The level III portable sleep study is readily available, sufficient to diagnose SDB, and help to provide appropriate medical and/or surgical therapies in hospitalized children with complex medical conditions.
Citation:
Singh G, Hardin K, Bang H, Nandalike K. The feasibility and utility of level III portable sleep studies in the pediatric inpatient setting. J Clin Sleep Med. 2019;15(7):985–990.
Keywords: high risk comorbidities, inpatient, obstructive sleep apnea, level III portable sleep studies, pediatric, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep breathing problems are increasingly recognized in pediatric inpatient settings, but proper diagnosis and management is challenging given limited resources. Our study looks at the feasibility and utility of level III portable sleep studies in optimal management of pediatric sleep breathing disorders in children, especially children with complex medical conditions, in the inpatient setting.
Study Impact: Our study shows that the level III studies are feasible in the inpatient setting and the results lead to appropriate and timely intervention in children with complex medial conditions.
INTRODUCTION
Sleep-disordered breathing (SDB) is a general term for breathing difficulties occurring during sleep. Obstructive sleep apnea (OSA) is one of the common forms of SDB and results in recurrent episodes of partial or complete upper airway obstruction during sleep causing disruption in normal oxygenation and ventilation, and sleep architecture.1 The reported prevalence of OSA in general pediatric population is estimated between 1% to 5%.2–7 Additionally, the prevalence of SDB is reported much higher in children with high risk comorbidities like neurological impairment, craniofacial disorders, lung disease, neuro-muscular disorders, and obesity.6,8–15
SDB is a major contributor to morbidity and increased health care utilization in children.16,17 Undiagnosed SDB can have adverse effect on neurocognitive growth and behavior resulting in impaired attention and learning in children.6,18,19 Children with untreated SDB are at risk of blood pressure dysregulation secondary to elevated sympathetic activity associated with respiratory events, which may contribute to endothelial activation and left ventricular remodeling, increasing risk for long term cardiovascular complications.6,20–22 SDB is also a significant risk factor lower respiratory tract infection and respiratory compromise in children.23 Untreated SDB may contribute to increased health care utilization with increased intensive care unit admissions, prolonged hospital length of stay and increased risk of readmission especially in children with complex medical conditions.
Polysomnography (PSG) is considered the gold standard for diagnosis of SDB in children.2 PSG provides comprehensive and objective information on all sleep related parameters including sleep architecture, cardiac and respiratory patterns and gas exchange. Diagnosis of SDB is often delayed in children with high risk comorbidities due to lack of resources, availability, or technical capability in outpatient sleep laboratories leading to potential delay in management of SDB. In addition, PSG is cumbersome, costly, and not readily available in the inpatient hospital environment.
Level III sleep studies, commonly known as home sleep studies, are limited channel sleep studies, that are shown to be accessible and convenient and considered reliable for diagnosis of SDB in selected adult patient groups.24 Though the current data do not support the use of in home level III studies in children,25 utilizing portable level III devices during hospitalization can provide an opportunity to expedite diagnosis and management of SDB, thus reducing morbidity and health care cost in these children. There is increasing interest in using portable devices in the inpatient pediatric setting but the data is limited in this population.26 The aim of the study is to evaluate the feasibility of level III portable studies for diagnosing SDB in hospitalized pediatric patients and to assess its utility in changing the inpatient management.
METHODS
Study Design and Study Population
This descriptive retrospective study was conducted at UC Davis Children's Hospital over the preceding 2 years in hospitalized children less than 18 years. All children who had an inpatient level III portable sleep study performed in the pediatric intensive care unit (PICU), neonatal intensive care unit (NICU), or general pediatric floor between October 2014 and October 2016 were included in the analysis. The study was approved by the UC Davis institutional review board (Project 869492-1).
Level III Portable Sleep Study
The sleep studies were ordered by the primary physicians whenever there is a suspicion for SDB in hospitalized children, in consultation with the sleep physicians. The sleep physician (KN) individually assessed the patients, reviewed the orders and approved the level III studies when considered appropriate. The study was ordered after a reasonable waiting period for patients hospitalized with respiratory insufficiency. All studies were initiated at the patient's bedside by a registered polysomnographic technician (RPSGT) or sleep physician using the portable device, Nox T3 (Carefusion, Germany). Nox T3 portable respiratory sleep monitor has been previously validated in adults for diagnosing SDB.27 The following parameters were monitored: airflow with nasal pressure transducer airflow (PTAF), respiratory effort with abdominal and thoracic respiratory inductance plethysmography (RIP) belts, body position with piezo belts, body movement with actigraphy, oxygen saturations with peripheral pulse oximetry, and snoring with microphone. Studies were conducted overnight, between the hours of 6:00 pm and 6:00 am. The studies were not supervised by the RPSGT but the bedside nurse was involved to ensure the positions of the sensors were maintained at night. The bedside nurse was also provided with sleep laboratory contact number and advised to contact the laboratory for any questions regarding the study or the equipment. Informed consent was obtained from the parents prior to conducting the study. Standard infection control precautions were followed for the sleep study and cleaning the device to reduce the risk of cross contamination.
The device was subsequently removed, data loaded to a secured designated computer with Nox T3 software, and scored by the RPSGT according to the American Academy of Sleep Medicine rules for respiratory events in children and interpreted by a sleep physician (KN) on the same day.28 Apnea-hypopnea index (AHI) was defined as the number of obstructive and central apneas and hypopneas per hour. Obstructive apnea was defined as > 90% reduction in airflow with continued respiratory effort for 2 breaths, central apnea as > 90% reduction in airflow and absence of respiratory effort for two breaths associated with 3% reduction in oxygen saturation or apnea duration of 20 seconds or more and obstructive hypopnea as 30% reduction in flow with continued respiratory effort followed by 3% reduction oxygen saturation. SDB was defined as mild (AHI 2 to < 5 events/h), moderate (AHI 5–10 events/h) and severe (AHI > 10 events/h) based on the severity of respiratory events. OSA was defined as obstructive AHI ≥ 2 events/h. Central sleep apnea (CSA) was defined as central apnea index ≥ 5 events/h.
Chart Review and Data Collection
All patient data was obtained from the electronic medical record only, which was de-identified and stored on a secured sever. Data was collected on patient characteristics, sleep study variables and patient outcomes.
Patient Characteristics
Data was collected on age, sex, body mass index, any oxygen or medication use prior to sleep study, high risk comorbid conditions including morbid obesity, prematurity, neurological abnormalities, neuromuscular conditions, craniofacial abnormalities, lung disease or congenital heart disease, indication for hospitalization and for sleep study, hospital unit where the study was performed and hospital length of stay.
Sleep Study Variables
Data was collected on the time interval between the sleep study order and the actual study date, the reasons for delay in the study, technical acceptability of the data and sleep study results. The minimal recording time for level III studies is not well established but we considered individual recordings to be acceptable if a minimum of 4 hours of pulse oximetry, nasal PTAF and respiratory effort by RIP plethysmography belts was recorded.26,29 Sleep study results included AHI and stratification into central and obstructive apneas, baseline oxygen and oxygen saturation nadir, longest period of apnea/hypopnea, heart rate (mean) and total duration of recording.
Patient Outcomes
Data was collected on the patient outcomes including any medical or surgical interventions performed on any of the patients.
Statistical Analysis
Standard descriptive statistics were used to analyze the data from the study. Statistical analysis was conducted using STATA version 10. Characteristics were expressed as mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables.
RESULTS
During the study period between October 2014 to October 2016, 51 hospitalized children had level III sleep studies performed. All children were included in the study.
Patient Characteristics
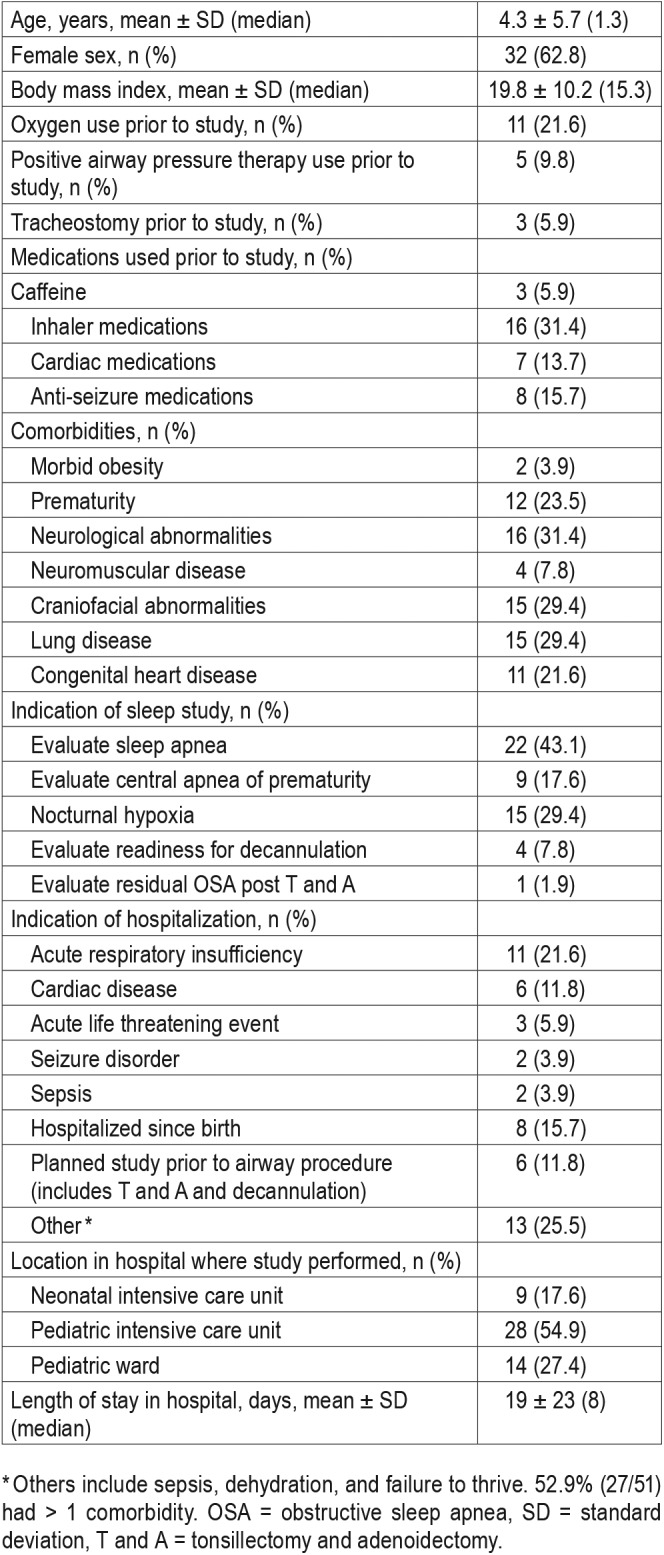
Among these patients, 32 were female and mean age was 4.3 ± 5.7 years. Forty-six (90%) patients had significant comorbidities and 27 (52.9%) of them had more than one comorbid condition. Forty-five (88%) patients were hospitalized for unrelated causes, including acute respiratory insufficiency (11 [21.6%]), hospitalized since birth related to prematurity or congenital disorders (8 [15.7%]), or related to cardiac disease (6 [11.8%]). Only 6 (11.8%) patients had planned hospitalization for inpatient sleep study for the purpose of surgical planning of tonsillectomy and adenoidectomy (T and A) or tracheostomy tube decannulation. Eleven (21.6%) patients were on supplemental oxygen prior to the sleep study. The majority, 28 (54.9%), of these studies were performed in the PICU. The most common indication for the study was evaluation of sleep apnea 22 (43.1%) (Table 1).
Table 1.
Baseline characteristics of cohort (n = 51).
Sleep Study Variables
Only 6 (11.8%) patients had a delay of more than 24 hours after the order was placed to undergoing the sleep study. This was due to lack of technician availability in 3 of these cases and patient not being medically ready in the remainder of the cases. Only 4 (7.8%) studies were considered non diagnostic with 3 of them due to inadequate recoding of pulse oximetry.
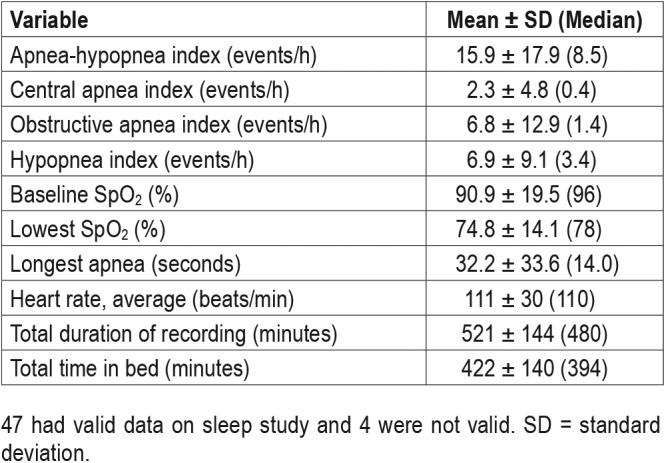
Of the 47 (92.1%) technically adequate studies, 39 (82.9%) studies identified SDB with 26 (55.3%) demonstrating OSA, 3 (6.4%) with CSA, 10 (21.3%) combined OSA with CSA, and 8 (14%) revealed no diagnostic abnormality. Twenty one (44.7%) patients had severe SDB, 10 (21.3%) had moderate SDB and 8 (17%) patients had mild SDB. The average AHI was 15.9 ± 17.9 events/h. Further details of the sleep study results are listed in Table 2.
Table 2.
Summary of valid data from portable studies (n = 47).
Patient Outcomes
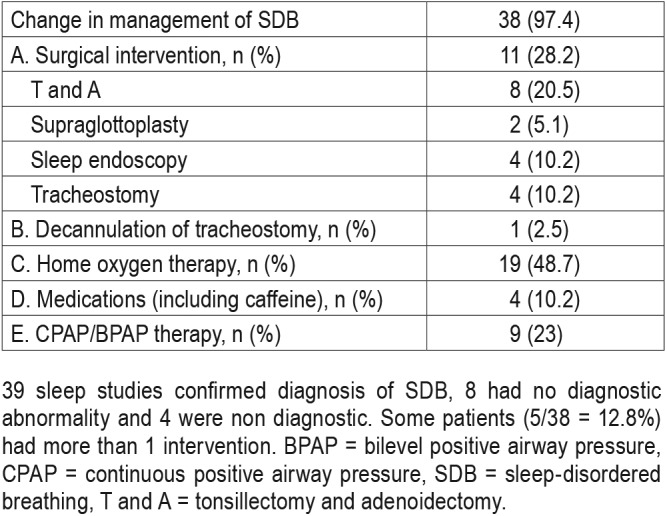
Among the 39 patients identified with SDB, 38 had some treatment intervention for SDB during same hospitalization.
There were 28 studies that were performed in the PICU, of which 2 studies were invalid, 2 studies were normal, and 24 studies were abnormal. One patient underwent decannulation due to normal study and 3 underwent tracheostomy for severe SDB. Five patients underwent T and A, 2 patients had supraglottoplasty, 7 patients were started on positive airway pressure (PAP) therapy, 12 patients were started on supplemental oxygen, one patient was started on caffeine, and 3 patients had more than one intervention done.
A total of 9 studies were performed in the NICU, of which 1 study was invalid and all the valid studies were abnormal. One patient underwent tracheostomy for severe SDB, 1 patient underwent T and A, 1 patient was started on PAP therapy, 5 patients were started on supplemental oxygen, 2 were started on caffeine, and 2 patients had more than one intervention done.
There were 14 studies that were performed on pediatric floor, of which 1 study was invalid, 6 studies were normal, and 7 studies were abnormal. Six patients underwent intervention due to abnormal study results. The interventions included T and A for 2 patients, PAP therapy for 1 patient, supplemental oxygen for 2 patients and caffeine for 1 patient. The patient with an abnormal result had severe SDB but preferred outpatient intervention (Table 3).
Table 3.
Change in management after portable study confirmed SDB (n = 39).
DISCUSSION
Our retrospective chart analysis study shows that level III portable studies are feasible in the pediatric inpatient setting and the results lead to appropriate and timely intervention in hospitalized children with significant comorbid conditions. Majority of our studies (88%) were done within 24 hours of ordering the study and 92% of the studies were technically adequate. These studies were done mostly unattended with minimum nursing supervision during the study.
There is limited evidence in current literature regarding the use of level III studies for inpatient evaluation of SDB, especially in children.26 Our study results are similar to the study results by Povitz et al,24 a retrospective chart analysis study assessing the utility of limited studies in an adult inpatient setting, where they showed 87% technical success in obtaining adequate level III inpatient studies. About 64% of their patients were diagnosed with new onset SDB and 42% were started on PAP therapy during the hospitalization. In our study, 92% of the studies were technically adequate, 82% of the children got diagnosed with new onset SDB that led to a change in medical or surgical management in most (97%), and 12.8% of patients underwent more than one intervention. To our knowledge, ours is the first study to assess the feasibility and utility of level III studies in the pediatric inpatient setting.
Level III home sleep studies are used as an alternative to in-laboratory studies in adults with high pretest probability for moderate to severe OSA but without significant comorbidities.30 The level III studies have been shown to have a reasonably good sensitivity and specificity compared to in-laboratory studies, even in children with high pretest probability for moderate to severe OSA.31 However the technical failure rate is reported to be between zero and 25%; younger age group (children less than 5 years) and poor nasal flow signal are reported to be the most common reasons for technical failure.31,32 In our study, only 4 (7.8%) studies were technically inadequate, and the lower failure rate is likely secondary to the presence of nursing supervision during the study. The level III studies can have a better application in the hospital setting for the same reason. In addition, level III studies are readily available and less expensive compared to in-laboratory studies making them a reasonable alternative to standard PSG in the inpatient setting.
About 90% of our patient population had significant comorbid conditions, and most of these children were adequately treated for SDB during the same hospitalization. The recent guidelines by American Academy of Sleep Medicine28 and American Academy of Pediatrics2 are mainly focused on OSA in uncomplicated pediatric patients and does not provide clear guidelines on children with complex comorbidities or inpatient sleep studies. In children with complex comorbidi-ties requiring frequent hospitalizations, scheduling an outpatient sleep study after discharge may be challenging. Usually there is longer wait time and the outpatient studies are resource consuming compared to level III inpatient studies. A timely intervention for management of SDB may reduce risk of read-mission and outpatient clinic visits. Although we did not study post-discharge health care utilization, this should be investigated in future studies which may further support utilization of level III studies compared to outpatient PSG after discharge in children with high comorbidity burden.
The results of this study may have been influenced by several limitations. First, most of the children had a high comorbidity burden, so results may not be representative of general pediatric population. However, it is noteworthy that technically adequate data was obtained even in these children. A second limitation was that the sleep studies were not attended by a sleep technician; more than two-third of these children were monitored in critical care units with continuous cardiorespiratory monitoring by nursing staff. Additionally, a simultaneous or follow-up outpatient PSG was not performed for comparison and validation of results of initial unattended study. Also, end tidal carbon dioxide was not measured and is considered an essential component of pediatric sleep testing.25,33 Therefore, we may not have recognized alveolar hypoventilation, which is particularly important in children with neuromuscular disease. Future studies should incorporate end tidal carbon dioxide into portable level III devices for appropriate measurement of alveolar hypoventilation.
CONCLUSIONS
In conclusion, we found that the level III portable studies are readily available and easy to apply in the inpatient setting. Adequate data can be obtained in a monitored setting like pediatric inpatient units and the study results help to provide appropriate medical and/or surgical therapies in hospitalized children with complex medical conditions.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This research work was performed at UC Davis Medical Center. Heejung Bang's work is partly supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CSA
central sleep apnea
- CHF
congestive heart failure
- NICU
neonatal intensive care unit
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PICU
pediatric intensive care unit
- PSG
polysomnography
- PTAF
pressure transducer airflow
- RPSGT
registered polysomnographic technician
- RIP
respiratory inductance plethysmography
- SDB
sleep-disordered breathing
- T and A
tonsillectomy and adenoidectomy
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Marcus CL, Brooks LJ, Ward SD, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8-to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti L, Rana S, Lospalluti ML, et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy. Chest. 2001;120(6):1930–1935. doi: 10.1378/chest.120.6.1930. [DOI] [PubMed] [Google Scholar]
- 6.Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a short primer. In: Nevšímalová S, Bruni O, editors. Sleep Disorders in Children. Switzerland: Springer, Cham; 2017. pp. 185–226. [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker M, Scott B, Johnson RF, Mitchell RB. Predictors of obstructive sleep apnea severity in adolescents. JAMA Otolaryngol Head Neck Surg. 2017;143(5):494–499. doi: 10.1001/jamaoto.2016.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein NA, Aronin C, Kantrowitz B, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50(11):1128–1136. doi: 10.1002/ppul.23120. [DOI] [PubMed] [Google Scholar]
- 11.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SLD. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88(1):132–139. [PubMed] [Google Scholar]
- 12.Arens R, Muzumdar H. Sleep, sleep disordered breathing, and nocturnal hypoventilation in children with neuromuscular diseases. Paediatr Respir Rev. 2010;11(1):24–30. doi: 10.1016/j.prrv.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Saleh S, Riekstins A, Forrest CR, Philips JH, Gibbons J, Narang I. Sleep-related disordered breathing in children with syndromic craniosynostosis. J Craniomaxillofac Surg. 2011;39(3):153–157. doi: 10.1016/j.jcms.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Anderson ICW, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48(5):614–618. doi: 10.1597/10-100. [DOI] [PubMed] [Google Scholar]
- 15.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88(1):75–78. doi: 10.1136/adc.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2007;175(1):55–61. doi: 10.1164/rccm.200604-577OC. [DOI] [PubMed] [Google Scholar]
- 17.Jennum P, Ibsen R, Kjellberg J. Morbidity and mortality in children with obstructive sleep apnoea: a controlled national study. Thorax. 2013;68(10):949–954. doi: 10.1136/thoraxjnl-2012-202561. [DOI] [PubMed] [Google Scholar]
- 18.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44(5):417–422. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- 19.Hunter SJ, Gozal D, Smith DL, Philby MF, Kaylegian J, Kheirandish-Gozal L. Effect of sleep-disordered breathing severity on cognitive performance measures in a large community cohort of young school-aged children. Am J Respir Crit Care Med. 2016;194(6):739–747. doi: 10.1164/rccm.201510-2099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter LM, Yiallourou SR, Vlahandonis A, et al. Impaired blood pressure control in children with obstructive sleep apnea. Sleep Med. 2013;14(9):858–866. doi: 10.1016/j.sleep.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. doi: 10.1016/j.smrv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Kheirandish-Gozal L, Etzioni T, Bhattacharjee R, et al. Obstructive sleep apnea in children is associated with severity-dependent deterioration in overnight endothelial function. Sleep Med. 2013;14(6):526–531. doi: 10.1016/j.sleep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Goldbart AD, Tal A, Givon-Lavi N, Bar-Ziv J, Dagan R, Greenberg D. Sleep-disordered breathing is a risk factor for community-acquired alveolar pneumonia in early childhood. Chest. 2012;141(5):1210–1215. doi: 10.1378/chest.11-1998. [DOI] [PubMed] [Google Scholar]
- 24.Povitz M, Kimoff RJ. Use of a level 3 portable monitor for the diagnosis and management of sleep-disordered breathing in an inpatient tertiary care setting. Can Respir J. 2014;21(2):96–100. doi: 10.1155/2014/214943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk V, Baughn J, D'Andrea L, et al. American Academy of Sleep Medicine position paper for the use of a home sleep apnea test for the diagnosis of OSA in children. J Clin Sleep Med. 2017;13(10):1199–1203. doi: 10.5664/jcsm.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013;77(12):1960–1964. doi: 10.1016/j.ijporl.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Cairns A, Wickwire E, Schaefer E, Nyanjom D. A pilot validation study for the NOX T3TM portable monitor for the detection of OSA. Sleep Breath. 2014;18(3):609–614. doi: 10.1007/s11325-013-0924-2. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus CL, Traylor J, Biggs SN. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10(8):913–918. doi: 10.5664/jcsm.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collop NA, Anderson WM, Boehleke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 31.Tan H, Kheirandish-Gozal L, Gozal D. Pediatric home sleep apnea testing, slowly getting there. Chest. 2015;148(6):1382–1395. doi: 10.1378/chest.15-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scalzitti N, Hansen S, Maturo S, Lospinoso J, O'Connor P. Comparison of home sleep apnea testing versus laboratory polysomnography for the diagnosis of obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2017;100:44–51. doi: 10.1016/j.ijporl.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Tan HL, Gozal D, Ramirez HM, et al. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep. 2014;37(2):255–260. doi: 10.5665/sleep.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]


