Abstract
Study Objectives:
Periodic limb movements (PLMs) have been associated with increased risk of stroke, but there is currently scarce research exploring this relationship in the setting of sickle cell disease (SCD). The aim of this study was to explore whether increased PLMs in children with SCD are associated with increased risk of cerebrovascular disease and to determine if there are any clinical or laboratory differences between children with SCD with elevated periodic limb movement index (PLMI) versus those with normal PLMI.
Methods:
This study is a comprehensive review of medical records of 129 children with SCD (aged ≤ 18 years) who had undergone polysomnography for evaluation of sleep-disordered breathing.
Results:
Elevated PLMI (PLMI > 5 events/h) was present in 42% (54/129) of children with SCD. Children with elevated PLMI were found to have higher percentage of hemoglobin S, lower total iron, higher arousal index and tendency toward elevated transcranial Doppler velocity (P = .063, odds ratio = 3.9, 95% CI 0.93–16.22). While association between elevated PLMI and isolated cerebrovascular stenosis (P = .050, odds ratio 5.6, 95% CI 1.0–31.10) trended toward significance, there was significantly greater proportion of children with elevated PLMI who had cerebrovascular stenosis with Moyamoya disease (P = .046) as demonstrated by magnetic resonance imaging (MRI).
Conclusions:
The prevalence of elevated PLMI in children with SCD was higher than in previously published data. Elevated PLMI was significantly associated with greater rates of cerebrovascular disease as detected by MRI.
Citation:
Lin J, Morrone K, Manwani D, Chernin R, Silver EJ, Shifteh K, Sin S, Arens R, Graw-Panzer K. Association between periodic limb movements in sleep and cerebrovascular changes in children with sickle cell disease. J Clin Sleep Med. 2019;15(7):1011–1019.
Keywords: periodic limb movements in sleep, sickle cell disease, children, cerebrovascular disease, magnetic resonance imaging, transcranial doppler, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: Children with sickle cell disease (SCD) have a higher frequency of periodic limb movements (PLMs) compared to healthy children. Recent studies noted that PLMs may contribute to adverse cardiovascular and cerebrovascular outcomes in children with SCD who are already at risk for stroke, but there is scarce research investigating this.
Study Impact: In our retrospective study, elevated periodic limb movement index (PLMI) was significantly associated with higher rates of cerebrovascular stenosis with Moyamoya disease and a trend toward significance for isolated cerebrovascular stenosis. Our findings could suggest that elevated PLMI may be associated with earlier signs of cerebrovascular compromise in children with SCD and could serve as a marker in screening for cerebrovascular disease, but further investigation with prospective studies is needed.
INTRODUCTION
Sickle cell disease (SCD) is one of the most common and severe inherited blood disorders worldwide, affecting an estimated 90,000–100,000 Americans.1,2 SCD is an autosomal recessive disease characterized by a single point mutation in the beta-globin gene resulting in a mutated hemoglobin, HbS.3 The polymerization of the deoxygenated HbS within the erythrocyte results in two major pathophysiological processes: micro-vascular vaso-occlusion with ischemia-perfusion injury and chronic hemolytic anemia with subsequent vasculopathy.2,3
Several complications of SCD contribute to the morbidity and mortality of its patients, including: acute vaso-occlusive pain episodes, infections, stroke, acute chest syndrome, cardiovascular disease and renal damage.2 Acute stroke and chronic cerebral ischemia are among the most debilitating sequelae in SCD with the risk of stroke being highest during the first decade of life, especially between the ages of 2 and 5 years old.4–7 Transcranial Doppler (TCD) ultrasonography and magnetic resonance imaging (MRI)/magnetic resonance imaging of the arteries (MRA) have been used to evaluate for cerebral vasculopathy in children with SCD.8–11 TCD can help identify changes in blood flow within cerebral vessels that might be suggestive of stenosis or infarct.2,8–11 TCD has served as a screening tool in children starting at age 2 years, and those with elevated TCD velocities are often started on chronic blood transfusion therapy which can help reduce the risk of stroke by 90%.2,8–11 Research with MRI has also revealed that up to 20% of children with SCD have silent brain infarcts which may not be clinically apparent in the acute setting, but are associated with poor school performance and neurocognitive impairment.2,4
Sleep-related disorders including nocturnal hypoxemia and obstructive sleep apnea (OSA) have also been linked to adverse disease outcomes in SCD.12–15 More recently, periodic limb movements (PLMs) were found to be more common in children with SCD.16 PLMs are repetitive, highly stereotyped movements of the arms or legs occurring during sleep, which may lead to transient arousals and sleep fragmentation. In one retrospective study of children with SCD from a referred population, the prevalence of elevated PLM index (PLMI ≥ 5 events/h) was 26%, compared to the general population of healthy normal children with estimated rates of 1.2% to 8.0%.16–19 PLMs have been associated with nocturnal hypertension in children, as well as transient fluctuations in cerebral hemodynamics in adults.20,21 Adults with restless legs syndrome (with associated PLMs) and existing stroke risk factors have also shown a trend toward higher microvascular lesion load on brain MRI.22 In the setting of increased cardiac workload caused by severe anemia in SCD, it has been suggested that PLMs may contribute to adverse cardiovascular and cerebrovascular outcomes including stroke in children with SCD who are already at risk, but there is currently a scarcity of research investigating this relationship.16
The primary purpose of this study was to perform a retrospective analysis in a tertiary care center of children with SCD with evidence of PLMs, and to evaluate if the presence of PLMs was associated with increased risk for elevated TCD velocities and abnormal MRI/MRA findings as compared to those without PLMs. A secondary aim was to describe and compare the clinical and laboratory characteristics of our sample population with elevated PLMI versus those with normal PLMI.
METHODS
Participants
This was a retrospective chart review of children with SCD referred to the Sleep Disorders Center at The Children's Hospital at Montefiore (CHAM) for evaluation of sleep-disordered breathing between October 1, 2005 and February 1, 2016. All children with SCD were followed by a single hematology practice at CHAM. Inclusion criteria included all children (age ≤ 18 years) with diagnosis of sickle cell disease who had polysomnography (PSG) performed. Children were excluded from the study if they were started on chronic transfusion therapy prior to PSG. The study was approved by the Institutional Review Board at Albert Einstein College of Medicine.
Procedure
Chart Review
Data was obtained from electronic medical records, including demographic and clinical information as well as laboratory results gathered from previous hospitalizations and clinic visits. The laboratory data was reported as the mean of test values 1 year before and after PSG to avoid reporting acute fluctuations in values.
Polysomnography
Regarding PSG testing, participants were studied overnight in the Sleep Disorders Center at CHAM in a quiet, darkened room with an ambient temperature of 24°C. Participants remained asleep supine under natural sleep conditions. The following variables were recorded and stored on a computerized PSG acquisition and analysis system (XLTEK, Oakville, Ontario, Canada): chest and abdominal wall movement by respiratory inductance plethysmography (Respitrace Systems; Ambulatory Monitoring Inc., Ardsley, New York); heart rate by ECG; inspired and expired end-tidal CO2 tension by capnography (Capnogard 1265; Novametrix, Wallingford, Connecticut); airflow was monitored at the nose by nasal pressure (Pro-Tech, Mukilteo, Washington) and 3-pronged thermistor, (Nihon Kohden, Tokyo, Japan). Arterial oxygen saturation was assessed by pulse oximetry (Masimo, Irvine, California). In addition, we obtained the following electroencephalogram derivations (F4-M1, C4-M1, O2-M1, with back up electrodes at F3, C3 and O1 and M2, submental and tibial electromyograms, and continuous infra-red video digital recording.
Sleep study data was retrospectively obtained. The studies were scored using the American Academy of Sleep Medicine pediatric scoring criteria relevant during each respective time period.23,24 Obstructive apnea was considered as cessation of airflow at the nose and mouth measured by thermal sensor associated with out-of-phase movement of the rib cage and abdomen lasting two breathing cycles.23 Hypopnea was defined as decrease of 30% or more in the amplitude of the nasal pressure transducer associated with a fall in 3% or more of basal oxygen saturation or with an arousal.23 OSA was defined as having relevant clinical signs/symptoms with obstructive apnea index > 1 event/h and/or apnea-hypopnea index (AHI) ≥ 2 events/h.23 Hypoventilation was defined as 25% or more of total sleep time with end tidal CO2 > 50 mmHg.23 PLMs were defined as a series of ≥ 4 consecutive leg movements with electromyography voltage ≥ 8 μV above resting baseline, and involved leg movements lasting 0.5 to 10 seconds in duration and recurring every 5 to 90 seconds.23 A PLMI > 5 events/h during sleep was considered abnormal.19 For patients with multiple sleep studies performed, the most recent PSG was selected for analysis.
Transcranial Doppler
Regarding TCD testing, the Doppler imaging of the arteries of the brain was performed by trained technicians from the Montefiore Neurovascular laboratory while adhering to the STOP study criteria for performance and interpretation of these studies.9 TCD studies were normally ordered by the Pediatric Hematology team at CHAM as part of general guidelines for surveillance for all children with SCD (HbSS and HbS-beta thalassemia genotypes) starting at age 2 years to screen for risk of stroke, with more frequent TCD studies performed in children who presented with persistent findings of elevated TCD velocities. The TCD results were recorded and interpreted by the operating technician, and later reviewed by a Pediatric Hematology attending. Based on STOP study guidelines for TCD screening protocol,9 the highest time-averaged mean blood velocity was recorded in the middle cerebral artery (MCA), the distal internal carotid artery (ICA) and its bifurcation, the anterior cerebral artery (ACA) and posterior cerebral artery. The basilar artery velocity was also recorded using the sub-occipital approach. All TCD studies were classified based on the highest time-averaged mean blood velocity in the ICA or MCA according to STOP criteria, and then interpreted as: normal (< 170 cm/s), conditional (170–199 cm/s), or abnormal (200 cm/s or higher).9 Studies where readings were unable to be obtained in the ICA and MCA bilaterally were classified as inadequate, unless one region of vasculature had clearly abnormal findings.9 For patients with multiple TCDs, we deferred to the most abnormal TCD in categorizing results for each patient.
Magnetic Resonance Imaging
MRI of the brain without contrast and MRA of the head without contrast were performed in the Radiology Department of Montefiore Medical Center. MRI and MRA studies for children with SCD were typically obtained by the Pediatric Hematology team at CHAM to screen for silent infarcts and vasculopathy respectively, when there were clinical findings of poor school performance or history of persistently elevated TCD velocities. All MRI results were interpreted by a physician from the Radiology Department of the Montefiore Medical Center. Positive results of the brain MRI/MRA included findings of new brain infarct and cerebral vessel stenosis11 such as: lacunar infarct, watershed ischemia, and anterior vascular narrowing (of the ICA, MCA or ACA). For patients with multiple MRIs, we deferred to the most abnormal MRI in categorizing results for each patient. Later, the MRI/MRA scans were independently reviewed by a neuroradiologist blinded to the results to ensure consistency of findings reported in this study.
Statistical Analysis
Descriptive statistics included mean, standard deviation and percentage of variables. We compared groups of children with normal versus elevated PLMI (> 5 events/h)23,24 using Pearson chi-square and ANOVA to determine if there were differences between groups in risk for elevated TCD velocities and abnormal MRI/MRA findings, or in clinical and laboratory characteristics. With the exception of demographics data and PSG results, we then reanalyzed all other outcomes comparing normal and elevated PLMI groups to control for the diagnosis of OSA using logistic regression for categorical variables and two-way ANOVA for continuous variables. Adjusted odds ratio (OR) and mean values are reported. Significance was set at P ≤ .05.
RESULTS
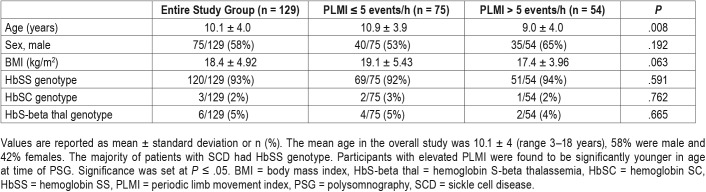
A total of 145 participants with SCD had completed a PSG; 16 were excluded because chronic transfusion therapy was started before PSG. A total of 129 patient records were reviewed, of which 122 participants had TCD and 71 had MRI brain scans with 58 also having had MRA brain scans (Figure 1). The mean age in the overall study was 10.1 ± 4 years (range 3–18), 58% were male and 42% females. Of 129 children, 42% had elevated PLMI. The majority of patients with SCD had hemoglobin SS (HbSS) genotype (93%) with the remainder having hemoglobin SC (HbSC, 2%) or hemoglobin HbS-beta thalassemia (HbS-beta thal, 5%) genotypes.
Figure 1. Flowchart of study design.
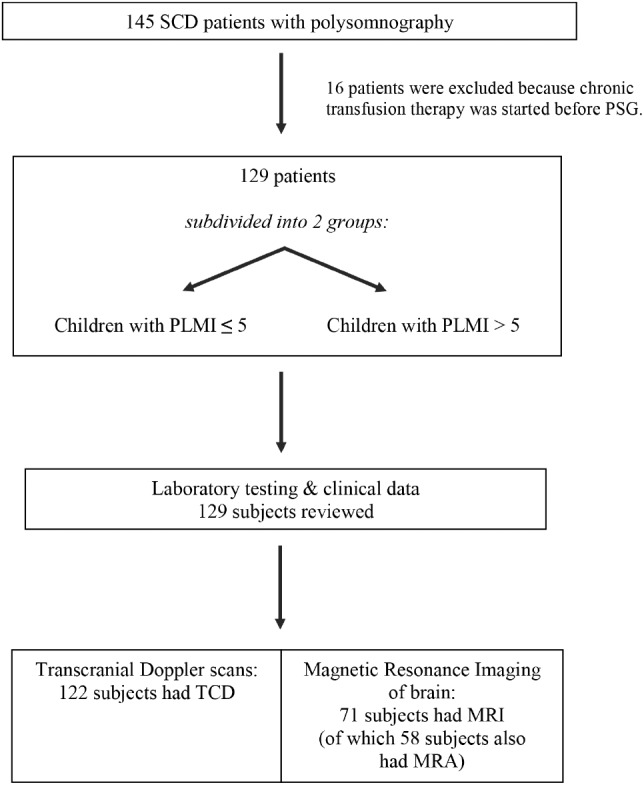
Of the 145 children with SCD who underwent PSG, 129 participants met inclusion criteria and were subdivided into 2 groups: children with PLMI ≤ 5 events/h and those with PLMI > 5 events/h. MRA = magnetic resonance imaging of arteries, MRI = magnetic resonance imaging, PLMI = periodic limb movement index, SCD = sickle cell disease, TCD = transcranial Doppler.
In comparing groups of children with normal PLMI versus elevated PLMI, we found no significant differences in sex or sickle cell genotypes. Children with elevated PLMI were significantly younger (P = .008) and tended to have lower body mass index (P = .063) at time of PSG (Table 1).
Table 1.
Patient demographics.
Laboratory Results
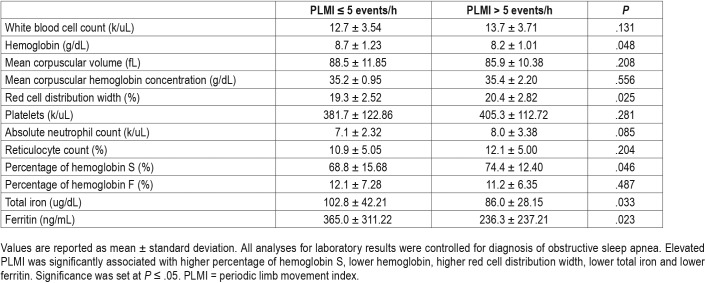
Elevated PLMI was significantly associated with higher percentage of hemoglobin S (HbS%), lower hemoglobin, higher red cell distribution width (RDW), lower ferritin and lower total iron (Table 2) with all analyses for laboratory results controlling for diagnosis of OSA. There were no significant differences between elevated PLMI and the other blood count indices, percentage of hemoglobin F (HbF%), total iron binding capacity, bilirubin (total and direct) or lactate dehydrogenase. Furthermore, when we evaluated the relationship between elevated PLMI and HbS% in association with HbF% less than or greater than 15, the results did not show statistical significance.
Table 2.
Laboratory results.
Clinical Characteristics
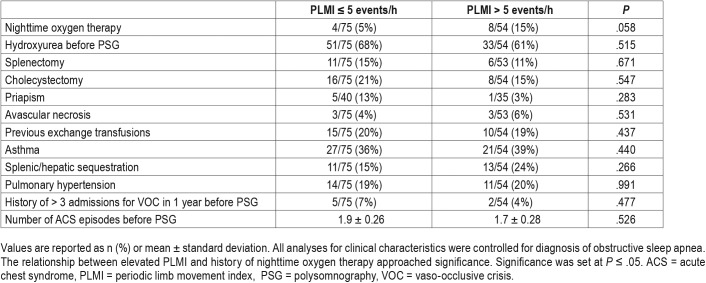
While controlling for diagnosis of OSA within all clinical characteristics, the relationship between elevated PLMI and history of nighttime oxygen therapy was approaching significance (P = .058, OR 3.5, 95% CI 0.96–12.54) (Table 3). Elevated PLMI was not significantly associated with history of pulmonary hypertension, number of acute chest syndrome episodes before PSG, hydroxyurea treatment before PSG, splenic/hepatic sequestration, splenectomy, cholecystectomy, priapism, avascular necrosis, previous exchange transfusions, asthma, continuous positive airway pressure/bilevel positive airway pressure (CPAP/BPAP) treatment or attention deficit hyper-activity disorder. No statistical significance was found in the relationship between elevated PLMI and number of patients with greater than three admissions for vaso-occlusive crises in the year prior to PSG.
Table 3.
Clinical characteristics.
Polysomnography Results
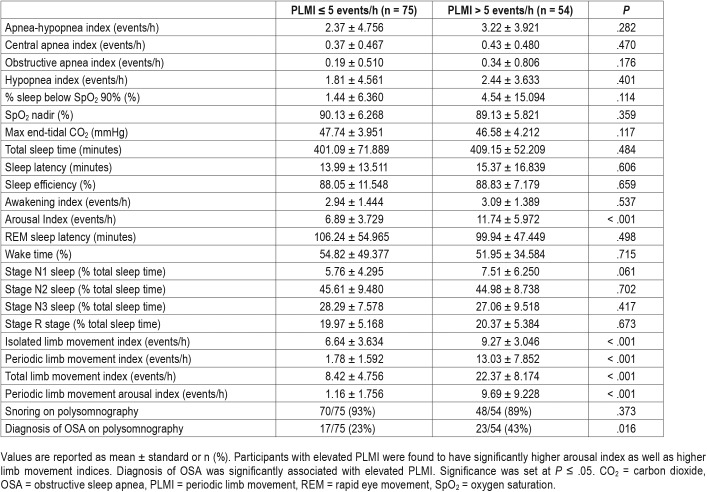
All patients had PSG performed; 116 participants had PSG performed on room air, 9 had oxygen titration studies, and 4 had CPAP/BPAP titration studies. Elevated PLMI was present in 42% (54/129) of children with SCD. Participants with elevated PLMI were found to have higher arousal index as well as higher limb movement indices. Elevated PLMI was not significantly associated with any respiratory events, oxygenation, ventilation or snoring (Table 4). Despite the diagnosis of OSA being significantly associated with elevated PLMI, our participants with elevated PLMI had slightly higher (but not significant) AHI. In an effort to address this discrepancy, we reviewed the entire data set and found that both groups (elevated PLMI versus normal PLMI) had mostly similar distributions for AHI means, with the exception of one outlier with an abnormally high AHI. Due to concern that this outlier may have skewed the results of our data analyses, we reanalyzed the PSG results excluding the 1 outlier, and found that elevated PLMI became significantly associated with higher AHI (P = .027) and higher hypopnea index (P = .038).
Table 4.
Polysomnography results.
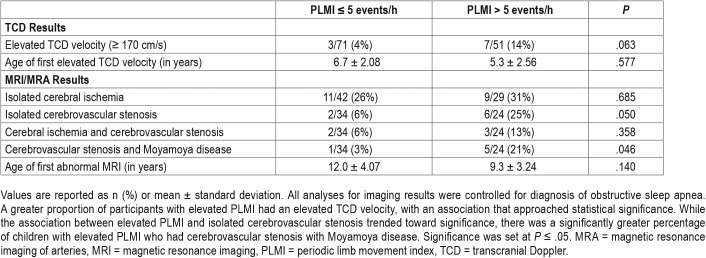
TCD Results
While controlling for diagnosis of OSA in all analyses for imaging results, a greater proportion of participants with elevated PLMI had an elevated TCD velocity, with an association approaching statistical significance (P = .063, OR 3.9, 95% CI 0.93–16.22) (Table 5). All patients with elevated TCD velocity had conditional TCD values (within the range of 170–199 cm/s). The age of first elevated TCD velocity tended to be lower in children with elevated PLMI as compared to those with normal PLMI, but this value was not statistically significant. Interestingly, if the PLMI cutoff was raised so that elevated PLMI equaled PLMI ≥ 10 events/h, the association between elevated PLMI and elevated TCD velocity reached significance (P = .043, OR 3.961, 95% CI 1.047–14.987) with all analyses controlling for diagnosis of OSA.
Table 5.
Imaging results.
MRI Results
While the association between elevated PLMI and isolated cerebrovascular stenosis (P = .050, OR 5.6, 95% CI 1.0–31.10) trended toward significance, there was a significantly greater percentage of children with elevated PLMI who had cerebrovascular stenosis with Moyamoya disease (P = .046, OR 9.8, 95% CI 1.0–92.94) (Table 5) with all analyses controlling for diagnosis of OSA. Of those with Moyamoya disease, the majority had a component of basal Moyamoya disease (Figure 2). While controlling for diagnosis of OSA in all imaging results, elevated PLMI was not significantly associated with isolated cerebral ischemia. The age of first abnormal MRI tended to be lower in children with elevated PLMI as compared to those with normal PLMI, but this difference was not statistically significant.
Figure 2. Brain imaging.
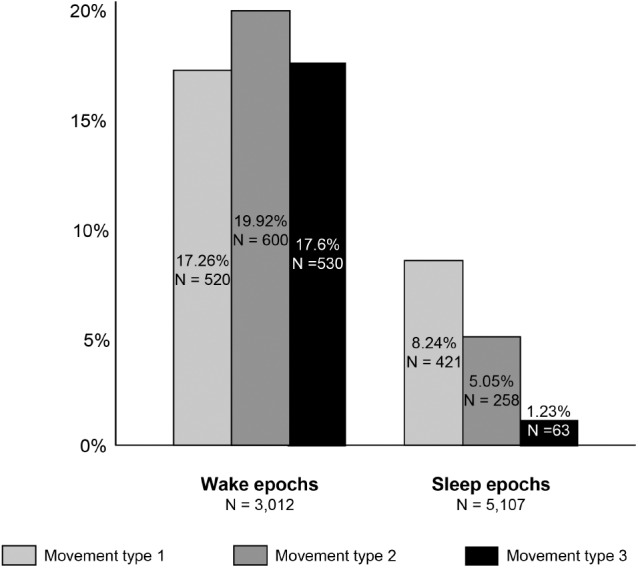
Axial T2 brain magnetic resonance imaging (A) and intracranial magnetic resonance imaging of arteries (B,C) show decreased flow in A1 segment of anterior cerebral artery (arrowhead) and a collateral network of medial lenticulostriate arteries (known as basal Moyamoya seen with arrows).
To further account for any confounding effect of OSA on PLMI, we performed additional subgroup analysis excluding the 40 patients with diagnosis of OSA, and still found a significant association between children with elevated PLMI and cerebrovascular stenosis with Moyamoya disease (P = .035). Moreover, when the PLMI threshold was raised so that elevated PLMI equaled PLMI ≥ 8 events/h, elevated PLMI became significantly associated with both isolated cerebrovascular stenosis (P = .018, OR 8.229, 95% CI 1.441–46.984) and cerebrovascular stenosis with Moyamoya disease (P = .021, OR 14.508, 95% CI 1.507–139.670), with all analyses controlling for diagnosis of OSA.
DISCUSSION
In this retrospective review of children with SCD referred for PSG, we found that the prevalence of elevated PLMI was 42%, which is greater than previously published rates of 26%.18 This finding is in accordance with prior research showing that children with SCD have considerably higher frequency of PLMs compared to healthy children without SCD.16–19 Our study also examined a larger sample size of 129 children with SCD compared to those in prior studies of similar participants,16 thereby providing us with a greater opportunity to investigate the effects of PLMs on such a pediatric population. In light of recent research showing that PLMs are associated with higher risk of developing cardiovascular and cerebrovascular diseases,20–22 it was particularly important for us to study the clinical implications of elevated PLMI in children with SCD who are already at risk for stroke.
In comparing the clinical and laboratory characteristics of our sample population with elevated PLMI versus those with normal PLMI, we noted a greater proportion of participants with elevated PLMI had higher percentage of HbS which is of unclear significance. Although HbS% may contribute to the pathology of SCD, it does not alone reflect severity of SCD since there are several other factors involved in the polymerization of deoxygenated HbS including the degree of cell deoxygenation, pH and HbF%.25 Our study also showed that patients with elevated PLMI were significantly more likely to have a history of OSA and tended to have more frequent clinical history of nighttime oxygen therapy (with P value approaching significance). This was an interesting finding given previous research with patients with SCD that showed that nocturnal hypoxemia was associated with painful sickle cell crises and potentially increased risk of stroke, seizures and transient ischemic attacks.12,13,15 Moreover, we examined many other variables related to clinical complications of SCD including history of splenic/hepatic sequestration, priapism and vaso-occlusive admissions before PSG, but they did not reveal any statistically significant association with elevated PLMI.
Additional laboratory analysis revealed that a significantly greater percentage of our participants with elevated PLMI had lower ferritin, lower hemoglobin and lower total iron levels with increased RDW, which is consistent with previous studies that have shown an association between iron deficiency and PLM disorder. Although children with progressive complications of SCD may eventually require frequent blood transfusions contributing to iron overload,2 the children with elevated PLMI in our study had significantly lower ferritin levels. Given the findings of lower total iron levels in the setting of relatively normal ferritin values for these children, there could also be some degree of anemia of inflammation contributing to iron-restricted erythropoiesis. Regarding the relationship between iron and PLMs, the pathophysiology of PLMs is thought be related to abnormalities involving insufficient central dopamine with iron serving as a cofactor for a rate-limiting enzyme (tyrosine hydroxylase) needed in dopamine synthesis.26 Recently, PLMs have also been associated with low regional brain iron levels rather than low peripheral iron availability as seen in studies examining CSF and MRI brain imaging.26–28 Both low brain iron stores as well as possible differences in control of iron metabolism in SCD have been proposed as mechanisms for iron dysregulation in the pathogenesis of PLMs in SCD.16
In recent years, there has been growing interest in evaluating periodic limb movements in sleep in relation to hyper-tension, heart disease and stroke.22 In our study, children with elevated PLMI have significantly higher arousal index as well as higher limb movement parameters which were as expected, but equivocal awakening index. Other reports have shown that PLMs could occur with EEG micro-arousals.29–31 and that micro-arousals in the absence of frank awakening may cause significant morbidity with adverse neurocognitive outcomes and cardiovascular disease.16,31 This effect of micro-arousals is particularly relevant to the pediatric SCD population who is already at greater risk for neurocognitive deficits from acute and often silent strokes.2,4,32,33 PLMs might also have an effect on the cardiovascular system through periodic increases in heart rate and blood pressure that may result in sustained adrenergic surges causing persistent elevation in blood pressures not only during sleep but also during wakefulness.31
Moreover, research has shown that the sympathetic overactivity associated with PLMs in the case of patients with restless legs syndrome (RLS) may predispose to heart disease and stroke either directly or indirectly via atherosclerotic plaque formation and rupture, especially in the setting of daytime hypertension.22 Although studies are limited, it has been reported that PLMs in the setting of RLS and traditional stroke risk factors may lead to increased risk for silent stroke as determined by microvascular lesion load.22 This is especially important to recognize in children with SCD in whom the prevalence of silent infarct is high with associated lower intelligence quotient and poor academic performance.33 The reverse may also be true with anecdotal evidence showing acute clinical stroke in the basal ganglia, internal capsule or corona radiata leading to RLS/PLMs.22 PLMs in sleep have also been associated with sympathetic bursts and changes in cerebral hemodynamics detected on near infrared spectroscopy, which may contribute to increased risk of stroke in at-risk populations such as those with SCD.16,20 In addition, given the known association between PLMs and OSA, it is important to note that OSA itself is an independent risk factor for stroke.34
With regards to imaging studies, we found that children with elevated PLMI tended to have elevated TCD velocities (with P approaching significance). Although elevated PLMI was not significantly associated with infarct on brain MRI/MRA scans, it was significantly associated with higher rates of cerebrovascular stenosis with Moyamoya disease and showed a strong trend toward significance for isolated cerebrovascular stenosis. These findings could suggest that elevated PLMI may be associated with earlier signs of cerebrovascular compromise before progression to infarction in children with SCD. Of interest, Moyamoya disease was present in 6/58 (10%) children with SCD. Moyamoya disease with its associated development of collateral vessels in the setting of progressively worsening cerebrovascular stenosis has become increasingly recognized as an important cause of stroke in children.35,36 We also found that participants with elevated PLMI were significantly younger, which may reflect a trend toward greater occurrence of cerebrovascular disease at a younger age and is consistent with prior research that has shown approximately 11% of patients with SCD having clinically apparent strokes before the age of 20.7
Our MRI/MRA findings support the importance of continuing to consider MRI as a modality in assessing for cerebrovascular disease especially given the high prevalence of silent stroke in children with SCD.2,4 Although MRI/MRA studies are accompanied by need for sedation in the pediatric population with potential risk associated with general anesthesia, the clinical yield of performing such imaging may be high for children with SCD who are already at greater risk for stroke. Presence of elevated PLMI on PSG therefore has the potential to serve as a noninvasive tool in assessing for risk of cerebral vasculopathy in children with SCD, and may provide more information to assist in deciding the need for brain MRI/MRA in these children in addition to current indications for imaging. Further investigation is still necessary to determine causality and evaluate whether PLMI can be used as a marker to identify patients at increased risk for cerebrovascular stenosis with or without Moyamoya disease.
This study had some limitations. It was a retrospective review of medical records, making it difficult to control for all of the variables involved. The participants in our SCD patient population were referred for PSG for assessment of sleep-disordered breathing, so there was inherent selection bias with potential for overestimating the prevalence of elevated PLMI in the children with SCD. However, other previously published retrospective and prospective studies of PLMs in patients with SCD have shown similarly higher prevalence rates for PLMI.16,18 There was also no clinical information on symptoms of RLS or PLMD available for our study. Although not all patients in our study underwent brain imaging, we did observe changes in clinical practice over time with more brain MRI/MRA studies being done in the past few years, possibly due to more concerns to evaluate for silent infarcts. Since not all patients had brain MRI/MRA or TCD studies, this limited the power of our study. Furthermore, in the setting of our retrospective study, the associations found between elevated PLMI and cerebrovascular disease do not necessarily confer causation, and further investigation is needed to evaluate causality.
CONCLUSIONS
In this retrospective study of a referred patient population, we were able to demonstrate a high prevalence of elevated PLMI in children with SCD compared to previously published data. Children with elevated PLMI tended to have elevated TCD velocities with an association that was approaching significance. Elevated PLMI was significantly associated with higher rates of cerebrovascular stenosis with Moyamoya disease and showed a strong trend toward significance for isolated cerebrovascular stenosis, but not for isolated cerebral ischemia. These findings could suggest that elevated PLMI may be associated with earlier signs of cerebrovascular compromise in children with SCD and could serve as a marker in screening for cerebrovascular disease, but further investigation is needed to fully delineate this in prospective studies.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at The Children's Hospital at Montefiore, Albert Einstein College of Medicine, in Bronx, New York. The authors report no conflicts of interest.
ABBREVIATIONS
- ACA
anterior cerebral artery
- AHI
apnea-hypopnea index
- BPAP
bilevel positive airway pressure
- CHAM
Children's Hospital at Montefiore
- CPAP
continuous positive airway pressure
- HbS-beta-thal
hemoglobin S-beta thalassemia genotype
- HbF%
hemoglobin F
- HbS%
hemoglobin S
- HbSC
hemoglobin SC genotype
- HbSS
hemoglobin SS genotype
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MRA
magnetic resonance imaging of the arteries
- MRI
magnetic resonance imaging
- OR
odds ratio
- OSA
obstructive sleep apnea
- PLM
periodic limb movement
- PLMI
periodic limb movement index
- PSG
polysomnography
- RDW
red cell distribution width
- RLS
restless legs syndrome
- SCD
sickle cell disease
- TCD
transcranial Doppler
REFERENCES
- 1.Centers for Disease Control and Prevention website. Data & Statistics on Sickle Cell Disease. [Accessed May 10, 2018]. https://www.cdc.gov/ncbddd/sicklecell/data.html.
- 2.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59(13):1123–1133. doi: 10.1016/j.jacc.2011.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravorty S, Williams TN. Sickle cell disease: a neglected chronic disease of increasing global health importance. Arch Dis Child. 2015;100(1):48–53. doi: 10.1136/archdischild-2013-303773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005;353(26):2743–2745. doi: 10.1056/NEJMp058274. [DOI] [PubMed] [Google Scholar]
- 6.Platt OS. Prevention and management of stroke in sickle cell anemia. Hematology Am Soc Hematol Educ Program. 2006:54–57. doi: 10.1182/asheducation-2006.1.54. [DOI] [PubMed] [Google Scholar]
- 7.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114(25):5117–5125. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]
- 8.Adams RJ. Stroke prevention and treatment in sickle cell disease. Arch Neurol. 2001;58(4):565–568. doi: 10.1001/archneur.58.4.565. [DOI] [PubMed] [Google Scholar]
- 9.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr. 2010;157(3):479–484. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliegman RN, Stanton BF, St. Geme JW 3rd, Schor NF, Behrman RE, editors. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- 11.Sheehan VA, Hansbury EN, Smeltzer MP, Fortner G, McCarville MB, Aygun B. Transcranial Doppler velocity and brain MRI/MRA changes in children with sickle cell anemia on chronic transfusions to prevent primary stroke. Pediatr Blood Cancer. 2013;60(9):1499–1502. doi: 10.1002/pbc.24569. [DOI] [PubMed] [Google Scholar]
- 12.Hankins JS, Verevkina NI, Smeltzer MP, Wu S, Aygun B, Clarke DF. Assessment of sleep-related disorders in children with sickle cell disease. Hemoglobin. 2014;38(4):244–251. doi: 10.3109/03630269.2014.919941. [DOI] [PubMed] [Google Scholar]
- 13.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 14.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659–665. doi: 10.1097/MPH.0b013e31817eb7ef. [DOI] [PubMed] [Google Scholar]
- 15.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357(9269):1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 16.Rogers VE, Marcus CL, Jawad AF, et al. Periodic limb movements and disrupted sleep in children with sickle cell disease. Sleep. 2011;34(7):899–908. doi: 10.5665/SLEEP.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus CL, Traylor J, Gallagher PR, et al. Prevalence of periodic limb movements during sleep in normal children. Sleep. 2014;37(8):1349–1352. doi: 10.5665/sleep.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6(4):374–381. [PMC free article] [PubMed] [Google Scholar]
- 19.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 20.Pizza F, Biallas M, Wolf M, Valko PO, Bassetti CL. Periodic leg movements during sleep and cerebral hemodynamic changes detected by NIRS. Clin Neurophysiol. 2009;120(7):1329–1334. doi: 10.1016/j.clinph.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Wing YK, Zhang J, Ho CK, Au CT, Li AM. Periodic limb movement during sleep is associated with nocturnal hypertension in children. Sleep. 2010;33(6):759–765. doi: 10.1093/sleep/33.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 26.Haba-Rubio J, Staner L, Petiau C, Erb G, Schunck T, Macher JP. Restless legs syndrome and low brain iron levels in patients with haemochromatosis. J Neurol Neurosurg Psychiatry. 2005;76(7):1009–1010. doi: 10.1136/jnnp.2003.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earley CJ, P BB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7(5):458–461. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 29.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68(15):1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 30.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic limb movements during sleep in healthy subjects. Sleep Med. 2013;14(6):555–561. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Nannapaneni S, Ramar K. Periodic limb movements during sleep and their effect on the cardiovascular system: is there a final answer? Sleep Med. 2014;15(4):379–384. doi: 10.1016/j.sleep.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Kassim AA, Galadanci NA, Pruthi S, DeBraun MR. How I treat and manage strokes in sickle cell disease. Blood. 2015;125(22):3401–3410. doi: 10.1182/blood-2014-09-551564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBraun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell disease. N Engl J Med. 2014;371(8):699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coelho FM, Georgsson H, Narayansingh M, Swartz RH, Murray BJ. Higher prevalence of periodic limb movements of sleep in patients with history of stroke. J Clin Sleep Med. 2010;6(5):428–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahimi DM, Tamargo RJ, Ahn ES. Moyamoya disease in children. Childs Nerv Syst. 2010;26(10):1297–1308. doi: 10.1007/s00381-010-1209-8. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7(11):1056–1066. doi: 10.1016/S1474-4422(08)70240-0. [DOI] [PubMed] [Google Scholar]