Abstract
Study Objectives:
Prior research has linked obstructive sleep apnea (OSA) to varied cognitive deficits. Additionally, OSA in rapid eye movement (REM) versus non-rapid eye movement (NREM) sleep has been shown to be a stronger predictor of outcomes such as hypertension. The present study aimed to investigate whether OSA—as characterized by the apnea-hypopnea index (AHI)—during REM and NREM sleep is associated with performance on a range of cognitive tasks. We also investigated whether the presence/absence of the apolipoprotein E4 allele (APOE4) modifies the associations between AHI during REM and NREM sleep and cognitive performance.
Methods:
A cross-sectional sample of 1,250 observations from 755 community-dwelling adults (mean [standard deviation] age, 62.3 [8.2] years) participating in the Wisconsin Sleep Cohort study was carried out by means of overnight polysomnography, paper-and-pencil cognitive tasks, and genetic data. Linear mixed effects models with repeated measures estimated associations of AHI during REM and NREM sleep with cognitive outcomes, stratified by APOE4 status (carrier versus noncarrier).
Results:
No significant associations were found between REM AHI and cognitive outcomes for either APOE4 carriers and non-carriers. Higher NREM AHI was associated with worse memory retention among APOE4 carriers; among noncarriers of APOE4, higher NREM AHI was associated with worse performance on a test of psychomotor speed, but better performance on two tests of executive function.
Conclusions:
Sleep state-specific (REM, NREM) OSA may be differentially associated with varying dimensions of cognitive deficits in middle-aged to older adults, and such associations are likely to be modified by genetic factors, include APOE polymorphisms.
Citation:
Devita M, Peppard PE, Mesas AE, Mondini S, Rusconi ML, Barnet JH, Hagen EW. Associations between the apnea-hypopnea index during REM and NREM sleep and cognitive functioning in a cohort of middle-aged adults. J Clin Sleep Med. 2019;15(7):965–971.
Keywords: apnea, cognitive functioning, memory, obstructive sleep apnea syndrome, REM sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although there is evidence that a higher apnea-hypopnea index (AHI) may be associated with cognitive impairment, it is not known whether the association varies by the AHI measured during rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. We also investigated whether this association varies according to the presence of the apolipoprotein E4 allele (APOE4).
Study Impact: We found that among APOE4 carriers, higher REM AHI is associated with worse psychomotor speed and higher NREM AHI is associated with worse memory retention. Our study provides the first investigation of the associations between AHI during different sleep states and cognition. Furthermore, our results suggest the possibility that exposure to AHI levels during various sleep states may affect different cognitive domains.
INTRODUCTION
An increasing number of studies have investigated the role that various clinical conditions and pathologies, including obstructive sleep apnea (OSA), have in influencing neuropsychological functioning. OSA is a common sleep disorder characterized by repeated episodes of apneas and hypopneas during sleep, daytime sleepiness, sleep fragmentation, and hypoxia.1,2 There is evidence for an association between OSA and cognitive deficits, although it is not clear which cognitive domains are the most impaired.3 Several review studies suggest that OSA is mainly associated with impaired executive functions and memory, while other domains may be less affected.3,4 However, clinical observations and self-reports of patients with OSA suggest that further research is needed in order to better understand the memory deficits reported.5
To date, the prevalence of cognitive impairments among individuals with OSA is still unclear. A prospective observational study of 49 consecutive patients with OSA showed that 1 in 4 patients had neurocognitive dysfunctions.5 However, establishing the exact prevalence of cognitive deficits can be difficult, since some OSA risk factors are independently associated with abnormalities of brain function and neurocognitive deterioration.5 Moreover, the contribution of OSA to cognitive dysfunction is not established. A recent meta-analysis found no association between OSA and overall cognitive function, but reported substantial variation in the associations found across studies, influenced in particular by study setting.6 These authors suggest that there is likely an association in some older adults and call for further exploration of this relationship.
The apnea-hypopnea index (AHI), the measure typically used to define the presence and severity of OSA, is usually measured across the entire night of sleep, but results from several studies suggest that AHI may differ across rapid eye movement (REM) and non-rapid eye movement (NREM) sleep.7 Associations between AHI during REM and NREM sleep with various outcomes have begun to be investigated. For example, among participants in the Wisconsin Sleep Cohort Study (WSCS), Mokhlesi et al8 investigated the association between NREM and REM OSA and hypertension, finding that the latter is cross-sectionally and longitudinally associated with hypertension. Additionally, Mokhlesi et al9 found that REM OSA is independently associated with incident nondipping of blood pressure.
Because several physiological and cognitive comorbidi-ties are associated with OSA, researchers have started to investigate its genetic and etiological factors.10 There is some evidence of an association between apolipoprotein E4 allele (APOE4) and OSA.11–13 APOE4 is a genetic risk factor for several neurologic disorders, particularly dementia,14 and it has been directly associated with cognitive impairment among individuals without dementia.15 It is not clear whether variability in the APOE gene relates to normal cognitive aging in humans. A few cross-sectional studies have demonstrated that APOE4 is significantly associated with cognitive function in middle-aged and older adults.16 Furthermore, some longitudinal studies show that, among those without dementia, cognitive decline proceeds faster among those with APOE4 than among those without.16–19 Previously published results from the Wisconsin Sleep Cohort Study,10 as well as results from other studies,20,21 provide evidence that individuals with OSA who are APOE4 carriers show worse cognitive performances than non-carrier individuals with OSA. Exposure to intermittent hypoxia, such as occurs in OSA, is associated with oxidative stress and with increased neuronal apoptosis in brain regions involved in learning and memory, as well as in other cognitive functions. APOE4 has been implicated in neurodegenerative disorders, and in vitro studies suggest that one of the functions of APOE4 is to confer protection from oxidant stress-induced neuronal cell loss.22 These mechanisms may be part of the explanation of the influence of APOE4 on cognition.
Many studies show that NREM sleep is mainly involved in consolidating long-term memories.23–25 The fragmentation of NREM sleep has not only been reported to affect memory skills, but it may also contribute to an increased production of amyloid.26 Moreover, although many studies find that OSA is worse during REM sleep,23 other studies report 50% of participants showing a NREM AHI higher than REM AHI.7,27 In the light of these findings and findings that AHI levels during REM and NREM sleep are differently associated with various outcomes, and because REM and NREM sleep may play different roles in various cognitive processes, we investigate in this study whether REM and NREM AHI are differently associated with various cognitive functions.
Building on our previous findings that AHI is more strongly associated with cognitive impairment among APOE4 carriers than noncarriers,10 the aim of this study was to investigate associations between memory and other cognitive impairments and AHI during REM and NREM sleep among individuals in the Wisconsin Sleep Cohort. Our second objective was to evaluate whether APOE4 status modifies these associations between cognition and OSA during REM and NREM sleep. Based on evidence from studies that separately investigated the deleterious effects of fragmentation of sleep on cognition, the importance of NREM sleep in mediating memory consolidation, and the role of APOE4 in these processes,27,28 we hypothesized that higher AHI during NREM sleep would be associated with memory impairment, and that this association would be stronger among APOE4 carriers.
METHODS
Participants and Data Collection
There were 1,545 middle-aged adults enrolled in the WSCS, an epidemiologic study of the natural history, causes and consequences of sleep-disordered breathing.29 Participants completed overnight polysomnography and neuropsychological tests, as well as body habitus measures and questionnaires about lifestyle, health and medications. A subset of the 1,545 participants had available genetic data to identify APOE geno-types as described previously.13 For the present investigation, 1,250 studies from 755 adults, (age range 40–85 years at the time of data collection), who had data on neuropsychological, polysomnography and genetic measures were included. Studies were conducted between September 2000 and August 2015. All study protocols were approved by the University of Wisconsin Health Sciences Institutional Review Board.
Polysomnography
Polysomnography recordings have been described in detail in previous studies from the WSCS.13,30,31 Apneas were defined as a cessation of breathing for at least 10 seconds, while hypopneas were defined as a discernible reduction of breathing effort associated with a 4% or greater reduction in blood oxy-hemoglobin saturation. The AHI was calculated separately for REM and NREM sleep, as the mean number of apneas and hypopneas per hour of sleep.
Neuropsychological Assessment
A neuropsychological paper-and-pencil battery of tests was administered by trained technicians. The overall neuropsycho-logical assessment, which lasted about 45 minutes, is briefly described below.
The Trail Making Test (Part B) is commonly used to assess executive functions, motor speed, and attention. It consists of 25 circles numbered from 1–13 and lettered from A–L. Participants are asked to connect in sequence, as quickly as possible, all the circles alternating, in increasing order, between numbers and letters. The main outcome is seconds required to complete the task.
The Symbol Digit Modalities Test is also used to evaluate executive function, attention and motor skills. In this test, a visual key consisting of 110 paired geometric figures and numbers is provided. Participants are asked to apply a key to supply the proper number that is associated with the specific symbol. The outcome is the number of correct responses in 90 seconds.
The Controlled Oral Word Association Test (COWAT) assesses both executive function and language skills. Participants are asked to name as many words as possible starting with a selected letter of the alphabet. The outcome is the number of words named in 1 minute.
The Digit Cancellation Test evaluates psychomotor speed. Participants are asked to detect and delete, as quickly as possible, the target stimulus among a series of distractors. The outcome is seconds required to complete the task.
The Grooved Pegboard Test evaluates psychomotor speed. In this test, participants are asked to insert, as quickly as possible, 25 grooved pegs into randomly oriented, slotted holes with, alternately, the right and the left hand. The outcome is seconds to complete the task (sum of right and left hands).
The Auditory Verbal Learning Test assesses memory and learning skills. It is composed of several subtests consisting of five presentations of a 15-word list with immediate recall (Learning), one presentation of a second 15-word list to distract, a sixth recall trial of the first list (Retention), a recall of the words from the first list after 30 minutes (Delay), and circling words in a short story (Recognition). Different outcomes are measured for each sub-test. The Learning score consists of the sum of recalled words in trial 1–5; the Retention score is provided by the number of recalled words; the Delay score is the percentage of recalled words and, finally, the Recognition score is represented by the number of words correctly identified.
Statistical Analyses
Participants' sociodemographic and lifestyle characteristics, sleep measures, medical history, use of medications, and presence of APOE4 were described by frequency and percentage for categorical variables or with mean ± standard deviation (SD) for continuous variables. Mean scores of cognitive tests according to AHI levels were also reported.
In order to examine the association between cognitive functioning and AHI, a linear mixed effect model with repeated measures estimation using a compound symmetry covariance structure was fit for each cognitive test (outcome), including both NREM AHI and REM AHI in the models as the main exposure variables. Due to the skewness of the underlying distribution, REM AHI and NREM AHI were log10 transformed for modeling purposes. All models were adjusted by the following potential confounders: use of continuous positive airway pressure in the sleep laboratory, age (years), squared age (years2), sex (male versus female), educational level (in years of completed schooling), body mass index (kg/m2), stroke (no versus yes), hypertension (no versus yes), total sleep time (h/night), percentage of total sleep time in REM sleep, and presence of APOE4 (carrier versus noncarrier). Finally, analyses were stratified by the presence or absence of APOE4 to explore possible interactions between this condition and AHI on the association with cognitive performance.
Two-sided P < .05 was considered statistically significant. Data were analyzed with SAS software, version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
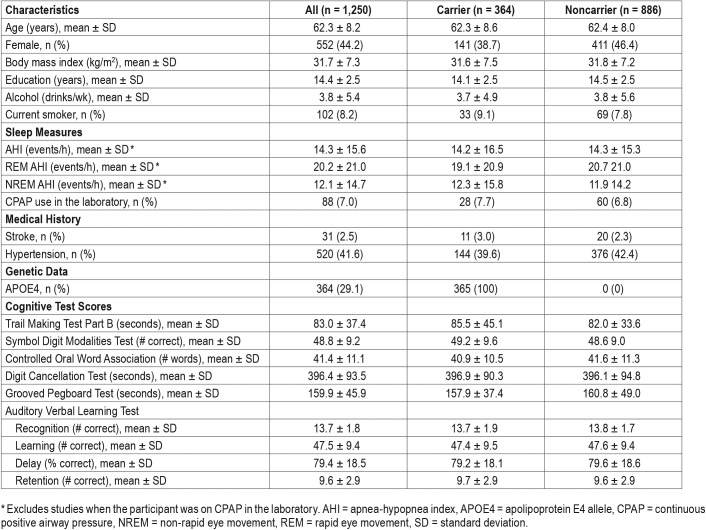
Among the 1,250 studies, the mean (SD) AHI was 14.3 (15.6) events/h. The mean (SD) REM and NREM AHI across studies were 20.2 (21.0) and 12.1 (14.7) events/h, respectively. Selected demographic, neuropsychological and sleep characteristics of the sample are presented in Table 1.
Table 1.
Characteristics of the sample stratified by APOE4 carrier status (n = 1,250 observations from 755 participants).
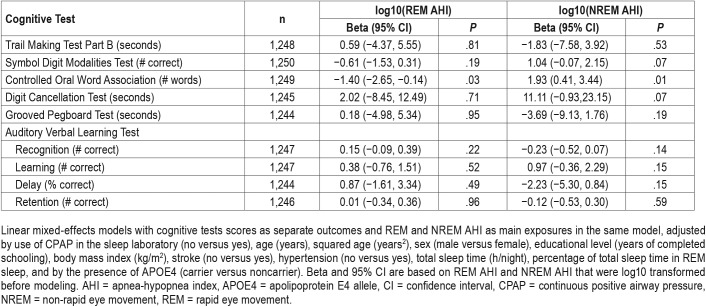
Table 2 shows the estimated association between REM and NREM AHI and each of the cognitive outcomes, adjusted for potential confounders. Findings show that higher AHI during REM sleep is associated with worse executive functions and language skills as evaluated by the COWAT, and higher NREM AHI was associated with slightly better COWAT scores.
Table 2.
Associations between REM and NREM AHI and cognitive performance in adults.
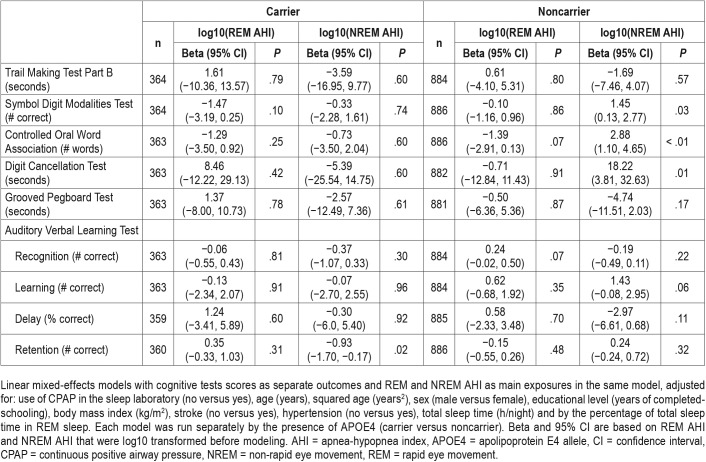
Table 3 shows the results stratified by APOE4 status. Among APOE4 carriers, higher NREM AHI was associated with worse memory retention, as measured by the Auditory Verbal Learning Test. Among APOE4 noncarriers, higher NREM AHI was associated with worse performance on the Digit Cancellation Test, but better performace on the Symbol Digit Modalities Test, and the COWAT. No other signifi-cant associations were found between REM or NREM AHI and any of the cognitive assessments among APOE4 carriers and noncarriers.
Table 3.
Associations between REM and NREM AHI and cognitive performance stratified by APOE4 carrier status.
DISCUSSION
The present study is one of the first to investigate the association between cognitive functioning and AHI during REM and NREM sleep, as well as whether APOE4 status modifies these associations. Stratifying by APOE4 status, we found that higher NREM AHI was associated with worse long-term memory retention skills among APOE4 carriers. Unexpected positive associations between NREM AHI and language abilities and executive function were found among APOE4 noncarriers. Further investigations are needed in order to corroborate or challenge our findings in both expected (more severe sleep-disordered breathing associated with poorer cognitive function) and unexpected directions.
Among APOE4 noncarriers, we found an association between higher NREM AHI and worse performance on the Digit Cancellation Task. Several studies suggest an association between APOE4 and OSA (for a meta-analysis see Small et al32), and previously published results from the WSCS are consistent with these studies.10 In the WSCS, Nikodemova et al20 found that while there was no association between cognitive performance and OSA among APOE4 negative individuals, among APOE4 carriers, those with moderate OSA had worse performance on cognitive tasks.
There is some evidence that the consolidation of long-term memories occurs mainly during NREM sleep.26 NREM sleep has also been found to be more fragmented by apneas and hypopneas,33 which is associated with the deposition of amyloid plaques.24–37 Recent studies have suggested that amyloid accumulation disrupts NREM sleep which may contribute to hippocampal-dependent cognitive decline.35 This would theoretically explain why amyloid deposition is associated with diminished NREM sleep. Furthermore, NREM sleep fragmentation would promote an increased synaptic tone and metabolic activity in the brain that, in turn, may lead to the accumulation of toxic metabolites (namely, the amyloid) in the nervous system, resulting in a subsequent amyloid plaque deposition that further worsens sleep.37
We found that NREM AHI is a predictor of worse memory performance, particularly long memory retention, among APOE4 carriers. Our results are consistent with other studies,35,38,39 and provide additional evidence that there may be brain changes due to elevated AHI during NREM sleep. Our results suggest that encoding processes may not be damaged, as performances in word recognition was not associated with REM or NREM AHI. A selective deficit emerged in retention (ie, the ability of recovering words from memory), suggesting that memory loss, specifically, may be due to the NREM AHI fragmentation. Cosentino et al21 also described a selective deficit for words retrieval in patients with OSA; however, these authors did not analyze the possible differences between REM and NREM sleep. We did not find other significant associations between AHI levels and cognitive outcomes among APOE4; it is possible that this may be explained, in part, by the smaller sample size of the carrier group.
We did not find an association between REM or NREM AHI and tests of recognition, consistent with results from other studies.21 This finding may be consistent with the hippocampal/sub-cortical-frontal dissociation theory. According to this theory, the hippocampus is more involved in encoding and storage processes, while the subcortical-frontal networks are responsible for retrieval.40,41 Our results, that tasks of recognition were not associated with higher AHI, suggest that higher AHI may not be substantially associated with greater amounts of hippocampal memory damage. Conversely, a subcortical-frontal dysfunction—well established in patients with OSA42—is consistent with the retention deficit we see in those with OSA.
CONCLUSIONS
The present study provides the first investigation of the associations between AHI during different sleep states and cognition. Our results showed that among APOE4 carriers, higher NREM AHI is associated with worse performance on a long-term memory task. Further research could explore associations between cognition and AHI levels during different stages of NREM sleep. In particular, stage N3 and N4 sleep, which are characterized by slow wave sleep, could be important for memory consolidation. Our results suggest the possibility that exposure to AHI levels during various sleep states may affect different cognitive domains.
These findings add to our understanding of the associations between AHI levels during REM and NREM sleep and highlight the importance of APOE4 carrier status in understanding these potential relationships. Treatment for OSA to lower AHI levels during each stage of sleep remains an important clinical goal, perhaps especially so for those who carry APOE4.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This research was conducted at the The University of Wisconsin-Madison Department of Population Health Sciences. Data collection for this study was supported by the National Heart, Lung, and Blood Institute (R01HL62252) and the National Center for Research Resources (1UL1RR025011) at the US National Institutes of Health. The authors report no conflicts of interest.
ABBREIVATIONS
- APOE4
apolipoprotein E4 allele
- AHI
apnea-hypopnea index
- COWAT
Controlled Oral Word Association Test
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- REM
rapid eye movement
- SD
standard deviation
- WSCS
Wisconsin Sleep Cohort Study
REFERENCES
- 1.Korson R, Guilleminault C. Obstructive Sleep Apnea Syndrome. In: Chokroverty S, editor. Sleep Disorders Medicine. New York, NY: Springer; 2017. pp. 567–596. [Google Scholar]
- 2.Chokroverty S. Overview of Normal Sleep. In: Chokroverty S, editor. Sleep Disorders Medicine. New York, NY: Springer; 2017. pp. 5–27. [Google Scholar]
- 3.Devita M, Montemurro S, Ramponi S, et al. Obstructive sleep apnea and its controversial effects on cognition. J Clin Exp Neuropsychol. 2017;39(7):659–669. doi: 10.1080/13803395.2016.1253668. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathologie Biologie. 2014;62(5):233–240. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 6.Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis. Neuropsychol Rev. 2017;27(4):389–402. doi: 10.1007/s11065-017-9344-6. [DOI] [PubMed] [Google Scholar]
- 7.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24(1):93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- 8.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70(11):1062–1069. doi: 10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin EK, Patel SR, Redline S, Mignot E, Elston RC, Hallmayer J. Apolipoprotein E and obstructive sleep apnea: evaluating whether a candidate gene explains a linkage peak. Genet Epidemiol. 2006;30(2):101–110. doi: 10.1002/gepi.20127. [DOI] [PubMed] [Google Scholar]
- 11.Uyrum E, Balbay O, Annakkaya AN, Balbay EG, Silan F, Arbak P. The relationship between obstructive sleep apnea syndrome and apolipoprotein E genetic variants. Respiration. 2015;89(3):195–200. doi: 10.1159/000369560. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE ε4 is associated with obstructive sleep apnea/hypopnea the Sleep Heart Health Study. Neurology. 2004;63(4):664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 13.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein eε 4 and sleep-disordered breathing in adults. JAMA. 2001;285(22):2888–2890. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 14.Van Giau V, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63(10):1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 16.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121(1):164–172. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 17.Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46(3):163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- 18.Deary IJ, Whiteman MC, Pattie A, et al. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol Aging. 2004;19(2):367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- 19.Hofer SM, Christensen H, Mackinnon AJ, et al. Change in cognitive functioning associated with apoE genotype in a community sample of older adults. Psychol Aging. 2002;17(2):194–208. [PubMed] [Google Scholar]
- 20.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. Sleep. 2013;36(6):873–880. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino FI, Bosco P, Drago V, et al. The APOE ε4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med. 2008;9(8):831–839. doi: 10.1016/j.sleep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28(11):1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara R, Schröder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE ε4 carriers. Neurology. 2005;65(4):642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 24.Maestri M, Carnicelli L, Tognoni G, et al. Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med. 2015;16(9):1139–1145. doi: 10.1016/j.sleep.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Aricò D, Drago V, Foster PS, Heilman KM, Williamson J, Ferri R. Effects of NREM sleep instability on cognitive processing. Sleep Med. 2010;11(8):791–798. doi: 10.1016/j.sleep.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 28.Stickgold R, Walker MP. Sleep and memory: the ongoing debate. Sleep. 2005;28(10):1225–1227. doi: 10.1093/sleep/28.10.1225. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108(5):246–249. [PMC free article] [PubMed] [Google Scholar]
- 30.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180(8):788–793. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 32.Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 34.Porter VR, Buxton WG, Avidan AY. Sleep, cognition and dementia. Curr Psychiatry Rep. 2015;17(12):97. doi: 10.1007/s11920-015-0631-8. [DOI] [PubMed] [Google Scholar]
- 35.Mander BA, Marks SM, Vogel JW, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucey BP, Bateman RJ. Amyloid-β diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging. 2014;35(Suppl 2):S29–S34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14(5):336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrier J, Viens I, Poirier G, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33(4):758–766. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- 40.Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage. 2006;32(1):104–110. doi: 10.1016/j.neuroimage.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Kramer JH, Schuff N, Reed BR, et al. Hippocampal volume and retention in Alzheimer's disease. J Int Neuropsychol Soc. 2004;10(4):639–643. doi: 10.1017/S1355617704104050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]