Abstract
Study Objectives:
Compare treatment efficacy and objective adherence between the NightBalance sleep position treatment (SPT) device and auto-adjusting positive airway pressure (APAP) in patients with exclusive positional obstructive sleep apnea (ePOSA) defined as a supine apnea-hypopnea index (sAHI) ≥ 2 times the nonsupine AHI (nsAHI) and a nsAHI < 10 events/h.
Methods:
This prospective multicenter randomized crossover trial enrolled treatment naive participants with ePOSA (AHI ≥ 15 events/h and nsAHI < 10 events/h) or (AHI > 10 and < 15 events/h with daytime sleepiness and nsAH < 5 events/h). Polysomnography and objective adherence determination (device data) were performed at the end of each 6-week treatment. Patient device preference was determined at the end of the study.
Results:
A total of 117 participants were randomized (58 SPT first, 59 APAP first). Of these, 112 started treatment with the second device (adherence cohort) and 110 completed the study (AHI cohort). The AHI on SPT was higher (mean ± standard deviation, 7.29 ± 6.8 versus 3.71 ± 5.1 events/h, P < .001). The mean AHI difference (SPT-APAP) was 3.58 events/h with a one sided 95% confidence interval upper bound of 4.96 events/h (< the prestudy noninferiority margin of 5 events/h). The average nightly adherence (all nights) was greater on SPT (345.3 ± 111.22 versus 286.98 ± 128.9 minutes, P < .0001). Participants found the SPT to be more comfortable and easier to use and 53% reported a preference for SPT assuming both devices were equally effective.
Conclusions:
Treatment with SPT resulted in non-inferior treatment efficacy and greater adherence compared to APAP in ePOSA suggesting that SPT is an effective treatment for this group.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: The POSAtive Study: Study for the Treatment of Positional Obstructive Sleep Apnea; Identifier: NCT03061071; URL: https://clinicaltrials.gov/ct2/show/NCT03061071
Citation:
Berry RB, Uhles ML, Abaluck BK, Winslow DH, Schweitzer PK, Gaskins RA Jr, Doekel RC Jr, Emsellem HA. NightBalance sleep position treatment device versus auto-adjusting positive airway pressure for treatment of positional obstructive sleep apnea. J Clin Sleep Med. 2019;15(7):947–956.
Keywords: continuous positive airway pressure, obstructive sleep apnea, position treatment
BRIEF SUMMARY
Current Knowledge/Study Rationale: New treatment options are needed for obstructive sleep apnea. Patients with positional obstructive sleep apnea and a nonsupine apnea-hypopnea index < 10 events/h could potentially be treated with a device preventing supine sleep.
Study Impact: This investigation suggests that the NightBalance sleep position treatment (SPT) device provides a treatment option that compares favorably with auto-adjusting positive airway pressure with respect to reduction in the apnea-hypopnea index and treatment adherence in patients with positional obstructive sleep apnea. Position treatment devices that are comfortable may be more acceptable to patients than positive airway pressure. Objective adherence monitoring capability and physician follow-up are as essential for positioning devices as for positive airway pressure treatment.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder1,2 with important consequences if untreated including daytime sleepiness, an increased risk of cardiovascular morbidity3 and motor vehicle accidents.4 While effective treatments such as positive airway pressure exist, a substantial percentage of patients do not accept or adhere to therapy.5,6 Thus, new treatment alternatives are urgently needed. The importance of sleeping position as a major factor determining the severity of OSA has been recognized for over 30 years.7 With positional OSA (POSA) defined as a supine apnea-hypopnea index (sAHI) at least 2 times the nonsupine AHI (nsAHI), a substantial proportion of patients with OSA exhibit this pattern.8–10 The prevalence of POSA varies between 60% and 75% depending on the definition and the population studied.8–11 In addition, a group of patients with POSA and a nsAHI less than 5 to 10 events/h could be characterized as having exclusive POSA (ePOSA). The prevalence of ePOSA has been estimated to be 25% to 30% and decreases with greater OSA severity and body mass index.9,10 A recent population based study found that ePOSA defined as a nsAHI < 5 events/h was present in 47% of individuals with OSA.10 Thus, positional therapy is potentially an effective treatment for ePOSA which characterizes a substantial portion of patients with OSA.
The treatment efficacy of several devices to prevent sleep in the supine position has been studied.11–13 The devices range from a ball in a pocket on the back of a shirt or a backpack, the so-called tennis ball technique,14–17 bumper cushions held in place by a soft strap around the chest,18,19 and devices that vibrate when the patient turns to the supine position.15,19–25 Discomfort and lack of the ability to provide the clinician with information about adherence and the efficacy of the device in maintaining nonsupine sleep are two problems limiting the use of mechanical positioning devices. A new generation of devices for positional treatment are designed to address these limitations.20,25
The NightBalance sleep position treatment (SPT) device (NightBalance, B.V., a Philips company, The Netherlands) is a small device worn over the center of the chest that vibrates when the wearer is in the supine position. The device is attached by soft comfortable straps and has advanced capabilities including an adaptation period and escalating stimulation if the supine position is maintained. The adherence time and the residual amount of time in the supine position is stored in memory and available for the clinician. The effectiveness of the device has been documented in several clinical trials.15,21–26 However, the relative effectiveness, adherence, and satisfaction compared to the gold standard of positive airway pressure treatment has not been tested in a group of patients with ePOSA. The purpose of this study was to demonstrate noninferiority of the SPT device compared to auto-adjusting positive airway pressure (APAP).
METHODS
The POSAtive Study (NCT03061071) was a prospective, 6-week, randomized crossover trial comparing the SPT to APAP in treatment naïve patients with ePOSA. The coprimary endpoints were (1) noninferiority AHI analysis by polysomnography on each device after 6 weeks of treatment and (2) noninferiority of objective adherence (average nightly minutes of use with zero for nights not used) over the 6 weeks. Patients were recruited from 11 investigational centers in the United States. The study was approved by central or local institutional review boards (IRBs) prior to the start of the patient recruitment. The study was run in accordance with Good Clinical Practices to ensure the rights, safety and well-being of the participants. All participants signed informed consent.
Potential participants were screened for POSA with in-laboratory polysomnography (PSG) which was scored by a central scoring laboratory (Clayton Sleep Institute, Missouri, USA). The main inclusion criteria for POSA included a total night AHI of ≥ 15 events/h, or AHI > 10 and < 15 events/h with the Epworth Sleepiness Scale (ESS)27 score > 10. The supine AHI was required to be at least twice the nsAHI, and the nsAHI < 10 events/h or < 5 events/h in milder participants with a total AHI > 10 and < 15 events/h. Participants were required to have a minimum of 30% sleep time in both supine and non-supine positions during the diagnostic PSG. The minimum sleep time of 30% was chosen to increase the chance that an acceptable amount of non-rapid eye movement (NREM) and rapid eye movement (REM) sleep would be recorded in both positions (supine or nonsupine).
Exclusion criteria consisted of prior surgery to treat OSA (nasal surgery alone not an exclusion), current or past PAP treatment for OSA (CPAP use only during the titration portion of a prior split sleep study not an exclusion), or other current therapy to treat OSA. Other exclusions included unstable or severe medical conditions as well as treatment with medications that at the discretion of the local primary investigator were felt to potentially impair sleep quality, increase daytime sleepiness, or adversely affect the participant's ability to safely complete the study.
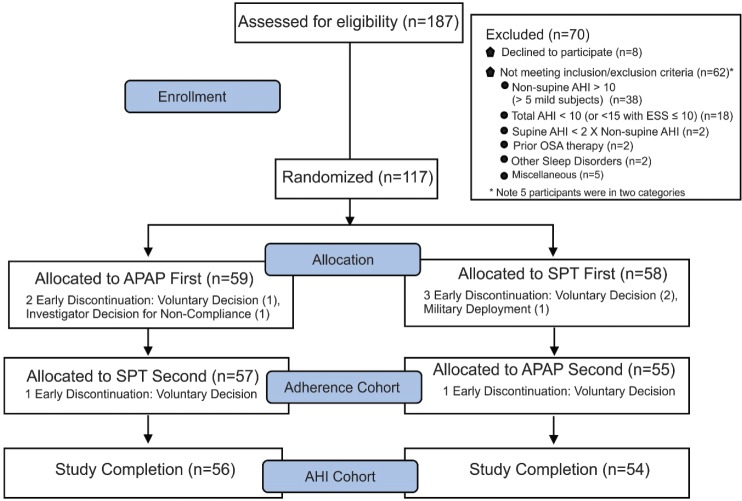
The use of nocturnal supplemental oxygen, severe claustrophobia, or shoulder, neck or back complaints that would restrict sleeping position or usage of APAP or SPT were also exclusions. A total of 187 participants (62% male, 20 to 77 years of age) were enrolled and 117 participants were randomized (Figure 1). Of those not meeting inclusion/exclusion criteria (Figure 1), the main reasons for screen failure included nsAHI > 10 events/h (or > 5 events/h in mild participants; n = 38) and a total AHI < 15 events/h with ESS score ≤ 10 or total AHI < 5 events/h if ESS score > 10 (n = 18).
Figure 1. Flow chart.
A flow chart showing the number of participants at each step of the study as well as the adherence and AHI primary endpoint cohorts. AHI = apnea-hypopnea index, APAP = auto-adjusting positive airway pressure, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea, SPT = sleep position treatment.
Fifty-eight participants were randomized to the SPT first, and 59 to APAP first. A total of five participants withdrew in the first 6 weeks (three started with SPT, two started with APAP). A total of 112 participants crossed over to the second device, and two participants withdrew after crossover (one on SPT and one on APAP). All 112 were included in the adherence primary endpoint analysis with 0 minutes of use imputed for each night of non-use up to 6 weeks. A total of 110 participants completed the study and were analyzed for the AHI primary endpoint.
Baseline measures consisted of basic demographics, ESS,27 the Functional Outcomes of Sleep Questionnaire (FOSQ),28 and the Short Form Health Survey (SF-36).29 Once deemed eligible to participate, participants were randomized 1:1 block design, stratified by site and AHI severity, (EDC system, IBM Clinical Connect, Armonk, New York, USA) to treatment order. Participants were trained on the device randomly selected as the first treatment and commenced a 6-week home use period. The participants also kept a daily sleep diary recording their estimate of the previous night's total sleep time.
The 6-week clinic visit consisted of collection of the current device 6-week objective adherence information, an in-laboratory PSG with the current device, and repeat assessments of ESS, FOSQ, and SF-36. Following completion of the PSG night and assessments, the participant was trained on use of the device randomly selected as the second treatment and proceeded with a 6-week home use period. All follow-up visits and procedures were the same as the first 6 weeks. All data were monitored and verified prior to database lock by protocol trained monitors. At the final study visit, participants completed a device satisfaction/preference survey.
Polysomnography
Full-night PSG tests were recorded following the recommendations of The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (AASM Scoring Manual)30 and included two electooculograms; a chin electromyogram; two frontal, two central, and two occipital EEG channels; an electrocardiogram; two lateral anterior tibialis electromyogram channels; nasal pressure and oral-nasal thermal flow (SPT device) or positive airway pressure flow (APAP device); thoracic and abdominal effort; and pulse oximetry. Body position was noted via technologist documentation and confirmed via time synchronized video. The recorded data was converted to European Data Format (EDF) and sent to a central scoring site (Clayton Sleep Institute, Missouri, USA) for analysis. The baseline and two treatment studies for each participant were scored by the same experienced registered polysomnography technologist. Recordings were scored according to AASM Scoring Manual30 using the recommended hypopnea definition (30% reduction in airflow [nasal pressure or PAP flow] associated with a ≥ 3% arterial oxygen desaturation or arousal). An awakening was defined as an arousal that lasted 15 seconds or longer.
Sleep Position Treatment
The SPT device is a rechargeable battery-operated device worn around the chest in an elasticized torso band and contains a digital accelerometer that continuously monitors a patient's sleep position. When using the device, if a patient turns to the supine position, it will react with a soft vibration that will continue until the patient returns to a nonsupine position. Initiation of patient therapy begins with an adaptation program intended to customize the vibrational stimuli to the patient's needs and gives the patient the opportunity to gradually adjust to wearing and being treated by the device. After SPT treatment initiation participants had scheduled follow-up phone calls at 1 day, 1 week, and 4 weeks to intervene for treatment issues. Sleep technologists were available at any time by telephone to answer participant questions. The SPT records the time the device is turned on as well as duration of use (temperature sensor). Adherence was determined at a 2-week clinic visit and the participant encouraged to maintain or improve (as indicated by the adherence data) nightly use for the entire duration of sleep.
APAP Treatment
Patients were set up on an APAP device (Dreamstation Auto, Philips Respironics, Murrysville, Pennsylvania, USA) with pressure range settings of 4 to 20 cmH2O, and a mask as tolerated. Mask fitting and device education were performed at the start of treatment. The standard masks used were the Wisp (nasal), Nuance (nasal pillows) and Amara View (full face) all manufactured by Philips Respironics. If these masks were not satisfactory, the individual sites could try other mask options of their choice. Sleep technologists were available by telephone at any time to intervene for participant issues. Scheduled telephone calls at 1 day, 1 week, and 4 weeks were made to answer participant questions. Mask changes were allowed as indicated for participant comfort. APAP adherence was determined at a two-week clinic visit and the participant encouraged to maintain or improve (as indicated by the adherence data) nightly use for the entire duration of sleep.
Statistical Analysis
The primary objective of the study was to demonstrate non-inferiority of treatment with the SPT as compared to APAP. Both primary endpoints (AHI and adherence) must be passed to declare SPT not inferior to APAP in treating this population. For the adherence primary endpoint, the noninferiority (SPT-APAP) margin was 30 minutes. For the AHI primary endpoint, a noninferiority margin of 5 events/h was used. The noninferiority delta values were selected to represent the minimum clinically meaningful difference. Assuming both APAP and SPT performed similarly with a one-sided alpha of .05 and with a standard deviation of up to 71 minutes for adherence and 11 events/h for AHI, a sample size of 100 participants had at least 90% power (calculated using PASS 14, NCSS Software, Kaysville, Utah, USA) to demonstrate noninferiority.
A mixed model with indicator variables for treatment, period and sequence (carry-over) fixed effects31 was fit to the data where the parameter of interest was available for both treatment periods, and participant was a random effect nested under sequence. Participants with incomplete data were excluded from these analyses. For the primary analyses, the one-sided 95% confidence bound was used for testing noninferiority as specified previously. Additional crossover analyses were performed using this same approach for secondary endpoints including PSG and questionnaire data. No adjustments were made for multiple testing, an alpha of .05 was considered statistically significant, and no imputations were performed for missing data aside from adherence. Carry-over effects were not statistically significant. Paired proportions were compared using the McNemar test and unpaired proportions using Fisher exact test.
As a secondary analysis, the AHI values at baseline and on the two treatments were compared for each of three conditions (sleep, NREM sleep, and REM sleep) using the analysis of variance for repeated measures. Individual pairwise comparisons were made using the Bonferonni correction. A preliminary analysis revealed no effect of treatment order. Analysis of device preference was performed with a binomial analysis of device preference compared to a 50% proportion (no preference). All analyses were conducted using SAS 9.4 (Cary, North Carolina, USA) or MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Baseline Patient Characteristics
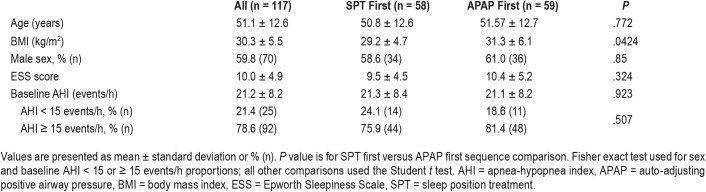
Fifty-eight participants were randomized to SPT first and 59 to APAP first. The participants were middle aged, mildly obese, and had, on average, moderate OSA. As shown in Table 1 the participants in each sequence of treatment had similar characteristics. The APAP first group had a slightly higher body mass index but the difference was not clinically important. Patients with all severities of sleep apnea were recruited. The AHI cohort included mild (AHI 5 to < 15 events/h, n = 17), moderate (AHI 15 to 30 events/h, n = 77), and severe (AHI > 30 events/h, n = 16) participants.
Table 1.
Baseline patient characteristics.
Sleep Architecture and Device Efficacy
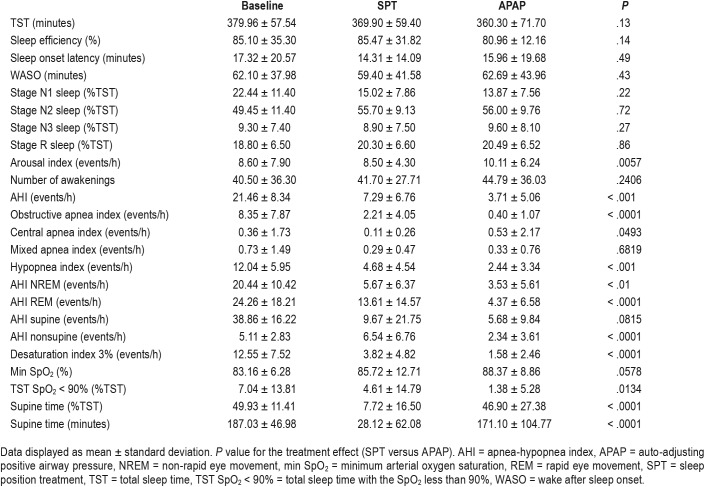
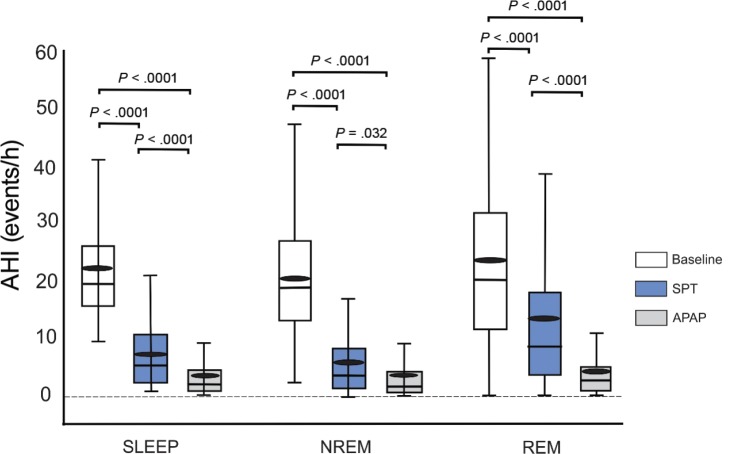
The results of PSG after 6 weeks of device use are shown in Table 2. There was no treatment effect (SPT versus APAP) for total sleep time, sleep efficiency, sleep latency, wake after sleep onset, or the duration of sleep stages as a percentage of total sleep time. The arousal index was lower on SPT, although the difference was small. The treatment AHI on SPT was greater than on APAP (mean 7.29 versus 3.71 events/h, Figure 2). However, the mean difference between the AHI on the SPT and APAP (3.58 events/h with a one sided 95% confidence interval upper bound of 4.96 events/h) was within our prestudy noninferiority difference (AHI [SPT-APAP] < 5 events/h). The number of participants with an AHI < 5 events/h was 54 on SPT and 88 on APAP (P < .001). The corresponding numbers of participants with an AHI < 10 events/h were 79 on SPT and 99 on APAP (P < .002). The AHI during NREM and REM sleep was also significantly greater on SPT than APAP with a larger difference during REM sleep (Figure 2).
Table 2.
Polysomnographic data (n = 110).
Figure 2. Baseline and treatment AHI values for sleep, NREM sleep and REM sleep.
The box plot shows AHI values—median (horizontal line), interquartile range (rectangle), and the range of values excluding outlier values (whiskers) at baseline and on SPT and APAP treatment. The small black ellipses are the means. For sleep, NREM sleep, and REM sleep both treatments were significantly lower than baseline. The AHI for APAP was also lower than for SPT. The mean (SPT-APAP) AHI difference for participants was 3.58 events/h. However, the upper bound of the one sided 95% confidence interval of the mean difference was 4.96 events/h and within the prestudy noninferiority difference hypothesis of 5 events/h. AHI = apnea-hypopnea index, APAP = auto-adjusting positive airway pressure, NREM = non-rapid eye movement, REM = rapid eye movement, SPT = sleep position treatment.
Analysis of the type of respiratory events showed higher obstructive apnea and hypopnea indices and a slightly lower central apnea index on SPT compared to APAP. The AHI in the nonsupine positions was lower on APAP. The supine AHI was slightly lower on APAP but this difference was not statistically significant. As expected the minutes of supine sleep and % supine sleep (% total sleep time) were much lower on SPT treatment (Table 2). The desaturation index was lower on APAP, but the values were low on both treatments. The minimum SpO2 was slightly but not significantly higher on APAP. The percentage of total sleep time with an arterial oxygen saturation less than 90% was smaller on APAP. The heart rate and blood pressure measured on the night of the polysomnography did not differ between SPT and APAP nights (Table S1 in the supplemental material).
As the study group included participants with mild, moderate, and severe AHI, the relative efficacy of SPT in varying severity of sleep apnea is of clinical interest. A post hoc analysis comparing the differences in AHI between SPT and APAP treatments with respect to OSA severity group (Figure 3) using the analysis of variance showed no significant difference between groups. The fractions of participants with a greater than 50% decrease in the AHI AND an AHI < 10 events/h on SPT in the mild, moderate, and severe diagnostic AHI groups were 12/17, 51/77, and 10/16, respectively. There was no signifi-cant difference between the fractions for the different severities (P = .68 by chi-square analysis).
Figure 3. Apnea-hypopnea index differences by severity.
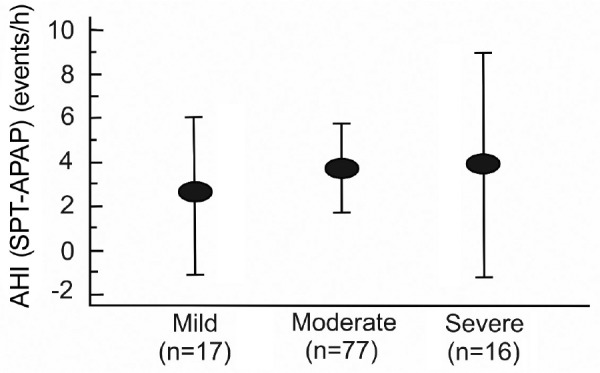
The mean AHI difference between SPT and APAP treatments for mild (AHI 5 to < 15 events/h), moderate (AHI 15–30 events/h), and severe (AHI > 30 events/h) categories of severity. The error bars are the 95% confidence intervals of the means. The mean group AHI differences were not different across OSA severity (P = .4235). AHI = apneahypopnea index, APAP = auto-adjusting positive airway pressure, OSA = obstructive sleep apnea, SPT = sleep position treatment.
Sleepiness and Quality of Life Outcomes
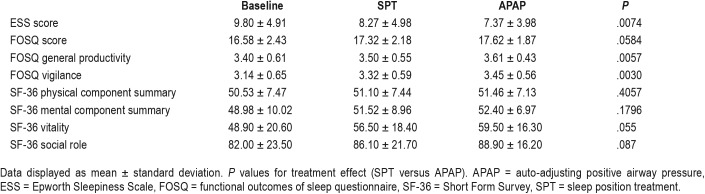
Outcomes of treatment are displayed in Table 3. The mean ESS score at baseline was just within the normal range suggesting that a substantial fraction of the study cohort was composed of individuals who were not clinically sleepy. Although participants with mild OSA were required to have an ESS score > 10, those with AHI ≥ 15 events/h were not required to be sleepy. There was a small decrease in the ESS on both treatments with the mean ESS lower on APAP than SPT. However, the difference between treatments was small (less than 1) and unlikely to be clinically important. Because only 49 of 110 participants had a baseline ESS score > 10, a post hoc analysis was performed on this sleepy sub-cohort using the analysis of variance for repeated measures with the Bonferroni correction for pairwise comparisons. In the sleepy cohort, the baseline ESS score was 14.4 ± 2.8, the APAP ESS score was 9.5 ± 4.2, and the SPT ESS score was 11.5 ± 4.9. The APAP and SPT ESS values were significantly less than baseline (P ≤ .0001) and the APAP ESS score was significantly less than the SPT ESS score (P = .0034). In addition, 31 participants on APAP and 21 on SPT dropped the ESS score to 10 or less (31/49 versus 21/49, P = .04 by chi-square analysis).
Table 3.
Sleepiness and quality of life outcomes (n = 110).
The total FOSQ score (higher is better) was minimally increased on treatment with either device. There was slightly more improvement of the total FOSQ score on APAP compared to SPT. The posttreatment FOSQ components (general productivity and vigilance) were significantly higher on APAP but the differences were small. The vitality component of the SF-36 was slightly higher on APAP but this difference was not statistically significant. The physical component summary and the mental component summary did not differ between SPT and APAP. None of the other SF-36 components were statistically different between SPT and APAP (Table S2 in the supplemental material).
Objective Adherence, Adverse Events, and Patient Preference
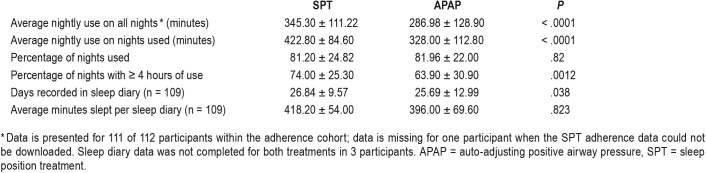
Nightly adherence (Table 4 and Figure 4) defined as the average nightly device use (including 0 minutes for nights not used) was significantly higher on SPT than APAP (345.3 versus 286.9 minutes). The mean SPT-APAP adherence difference was 58.9 minutes with a 95% confidence one sided lower bound of 36.6 minutes. That is, the lower bound for SPT adherence was 36.6 minutes greater than APAP adherence. In contrast, the non-inferiority hypothesis required SPT adherence to be no more than 30 minutes lower than that for APAP. In addition, average nightly use (nights used) and the percentage of nights with use ≥ 4 hours were also both significantly better with the SPT than APAP. The percentage of nights each device was used did not differ. As noted above, participants also completed a sleep diary to provide participant reported sleep duration. Of interest the reported sleep duration on SPT was similar to the average objective device use (nights used).
Table 4.
Device adherence and sleep diary data (n = 111).*
Figure 4. Treatment adherence.
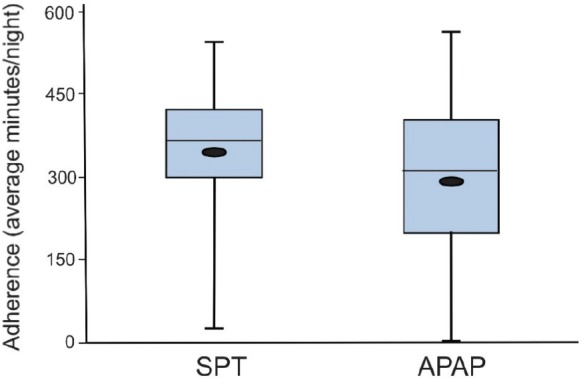
A box plot showing median, interquartile range, and range of night adherence in minutes per night for the SPT and APAP treatments. The small black ellipses are the means. The SPT adherence (all nights) over the six-week treatment period was significantly longer than for APAP (P < .001). APAP = auto-adjusting positive airway pressure, SPT = sleep position treatment.
A total of 29 device-related adverse events (AEs) were reported in 24 (20.5%) participants and are listed in detail in Table S3 in the supplemental material. All device-related AEs resolved without complication. The APAP-related AEs (n = 24) were noted in 22 participants and included nasal, facial, and ear irritation and dry throat/mouth. The SPT-related AEs in 5 participants included back, shoulder or neck pain (n = 4) and abdominal skin irritation (n = 11) and were all classified as mild.
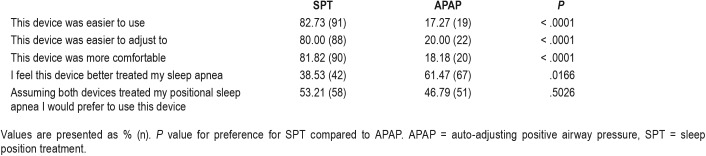
Patient treatment preference was determined at the end of the study and the results are shown in Table 5. The patients found the SPT device easier to use, easier to adjust to and more comfortable. However, patients felt the APAP device treated their sleep apnea better. The patient preference of a device for long-term treatment (assuming both were effective) did not differ between the two devices.
Table 5.
Patient preference (n = 110).
DISCUSSION
The major findings of this study are that the treatment AHI on SPT was noninferior to that on APAP in patients with exclusive positional sleep apnea (nsAHI < 10 events/h) and that objective adherence to treatment (average nightly use for all nights) was significantly better with SPT. Patients felt that the SPT was easier to use, easier to adjust to, and more comfortable. Although patients did express more confidence in the ability of APAP to treat their positional sleep apnea, roughly half would still choose to continue treatment with SPT if available. Of note, this study included a substantial number of the participants with moderate to severe POSA.
While it has long been appreciated that position control has potential as a treatment option for POSA, use of this therapeutic option has been limited by the characteristics of the available devices. Comfort has been a major issue both with respect to patient acceptance and long-term adherence.11,16 Lack of capability for the objective monitoring of adherence has also limited physician enthusiasm for the available devices. Recently positional devices have become available that are more comfortable and have objective adherence monitoring capability.15,20,21–26 The SPT is one such device. However, treatment efficacy and adherence with the SPT device had not been previously compared with positive airway pressure. In addition, the ability of the SPT device to treat patients with moderate to severe OSA has not been well studied. The current investigation was designed to compare SPT and APAP in patients with POSA over a wide spectrum of OSA severity. While the treatment AHI was lower on APAP than SPT, the relative effectiveness of SPT and APAP was similar across the categories of AHI severity (Figure 3).
In the current study, APAP resulted is a significantly lower AHI compared to SPT. However, the difference between APAP and the SPT device was within our a priori noninferiority hypothesis of 5 events/h. Approximately 84% of our study AHI cohort included patients with an AHI ≥ 15 events/h (77/110 moderate, 16/110 severe). Ha et al13 evaluated 3 studies comparing positional treatment with CPAP and found a mean AHI difference of 4.28 events/h. Jokic et al17 used a crossover design with 2-week treatment periods and found a mean AHI of 3.4 events/h on CPAP versus 9.5 events/h on positional treatment in 13 participants with a mean baseline AHI of 17.9 events/h. These results are similar to those found in the current study. The study by Permut and coworkers18 compared one night of CPAP to one night of treatment with a positional device in a group of patients with POSA and a nonsupine AHI < 5 events/h. The median AHI values were 11, 2, and 0 events/h on baseline, position treatment, or CPAP respectively. In that study position treatment, reduced the AHI to less than 5 events/h in 92% of patients. Our study group had more severe OSA (mean AHI 21 events/h) and a nonsupine AHI < 10 events/h (< 5 events/h only in milder patients). The fact that our patient group was more severe may explain why our average AHI values on both SPT and APAP treatments were higher and the AHI was reduced to less than 10 events/h in 79/110 patients by the SPT device.
Our inclusion criteria of a nsAHI < 10 events/h during the qualifying PSG did not result in a total AHI < 10 events/h in some participants on SPT. The average supine sleep time on SPT was only about 28 minutes, therefore the main factor determining the total night AHI was the nsAHI on SPT. During non-supine sleep, the AHI during REM sleep is usually greater than during NREM sleep.32 In the current study the nsAHI on SPT during REM sleep was over twice that during NREM sleep. A greater amount of nonsupine REM sleep on SPT during the treatment PSG compared to the qualifying study might explain a higher total AHI in some participants.
The percentage of nights with > 4 hours of use in the current study was 74% on SPT and 63.9% on APAP. A study of SPT adherence over 6 months by van Maanen et al23 found use ≥ 4 hours of 64% in patients with available data over the study period. The APAP adherence found in our study is typical of a number of previous PAP adherence studies. For example, a multicenter investigation comparing various PAP modalities33 found an average nightly use over 3 months on APAP of 4.64 hours (278 minutes) which is very similar to the average value (all nights) of 286 minutes in the current study. These comparisons show that our adherence results are consistent with those of prior studies of the SPT and APAP.
The reason for higher adherence on SPT than APAP treatment could not be determined by our study. Better adherence could have been due to patient perception that the device was easier to use and more comfortable than APAP. The SPT treatment program consists of a 3-phase algorithm to optimize adaptation to the device. Phase 1 (nights 1 and 2) measures the patient's supine sleep percentage without any vibrational feedback. Phase 2 (nights 3 through 9) will gradually decrease the time allowed sleeping in the supine position and customize the vibrational stimuli required to keep the patient in a nonsupine position. In phase 3, the treatment phase (nights 10 +) the device will provide vibrational feedback every time the patient moves to the supine position following a “sleep in” mode to allow the individual to fall asleep naturally on their back if they choose. If the wearer pushes a pause button, the SPT will also stop treatment for 5 minutes. Although there was higher SPT adherence, a majority of participants felt that APAP treated their OSA better and roughly 50% would choose APAP for long-term treatment. In a study by Jokic et al17 comparing CPAP and position therapy (ball in back pack) only 4 of 13 patients preferred positional treatment. While the current study did not directly compare types of position devices, it seems likely that patients might prefer a less cumbersome position treatment device. Our study did not explore the reasons for treatment preference or the reason a majority of the participants felt better treated on APAP. It seems likely that many of the study participants were aware that PAP is considered the treatment of choice for OSA from discussions with the physician initiating the evaluation for sleep apnea. Thus, pretreatment perceptions might have influenced their attitude about which treatment they felt was more effective. It is also possible that some patients prefer to sleep supine for some portion of the night or that residual snoring persisted in the non-supine positions on SPT. In addition, some participants may have noted a greater improvement in symptom on APAP. In any case, having an opportunity to try both treatments, around 50% of participants would choose SPT for long term treatment. This suggests that SPT would be acceptable to a substantial proportion of patients with POSA.
The current study has some limitations that must be considered. Because of different flow signals (nasal pressure, oro-nasal thermal airflow on SPT, PAP flow signal on APAP), the technologists scoring sleep studies were not blind to the type of device being used. To reduce variability, the same technologist scored both APAP and SPT treatment studies for a given participant. In addition, a central scoring location was used to reduce variability in scoring. Another limitation is that each device was only used for 6 weeks. It is possible that a longer duration study would reveal different results. Published data suggests that the adherence to both PAP and SPT treatments decreases over time.5,6,21 To our knowledge a study systematically comparing the durability of PAP and position treatment adherence has not been performed. A study by de Rutier et al21 investigated the durability of treatment effects of the SPT and oral appliance therapy over 12 months in patients with positional sleep apnea. An intention to treat analysis found the percentages of nights with > 4 hours use to be 57.4% on SPT and 47.8% on the oral appliance. Ongoing adherence monitoring would be as important for SPT treatment as for PAP and oral appliance therapy. Future long-term studies of SPT treatment are needed to assess the durability of adherence.
Clinical outcomes other than reduction in AHI and adherence are important in evaluating a treatment. In the current study there were small decreases in ESS score on both treatments with a greater decrease on APAP. In one previous study of the SPT device the median ESS score decreased from 9 to 7 at 3 months.21 Another study found a decrease in ESS score from 11 to 8 after 1 month of treatment.23 In general, patients with POSA have a lower AHI and less daytime sleepiness. A post hoc, analysis of patients with a ESS score > 10 in our study found a significant improvement (statistically and clinically) on SPT treatment, although the effect was smaller than with APAP treatment. A recent investigation estimated that the minimum clinically important difference in the ESS score after treatment to be −2 to −3.34 The mean drop in the ESS score in the sleepy group on SPT was 2.9 suggesting that the decrease was clinically significant (although less than APAP). Future studies of the SPT in an a priori sleepy cohort are needed to better assess the impact of SPT treatment on daytime sleepiness.
In summary, the current investigation suggests that the SPT can provide an effective treatment option for patients with POSA and a nsAHI < 10 events/h. The SPT reduced the AHI in all categories of OSA severity. In this study the SPT treatment AHI was noninferior to that of APAP and the nightly adherence longer. Patients found the SPT more comfortable and easier to use with about 50% of patients choosing SPT over APAP for long term treatment.
DISCLOSURE STATEMENT
All authors reviewed and approved the manuscript. This study was funded by NightBalance, B.V., a Philips company. Richard B. Berry reports research grants from Philips Respironics and ResMed; Paula Schweitzer reports research funding from Apnimed, Inc., Avadel/Flamel Pharmaceuticals, Balance Therapeutics, Inc., Harmony Biosciences, LLC, and Jazz Pharmaceuticals; Helene A. Emsellem reports advisory board fees from Jazz Pharmaceutical, Harmony Bioscience, Vanda Pharmaceuticals, and research funding from Jazz Pharmaceuticals, Vanda Pharmaceuticals, NightBalance, Harmony Bioscience, Eisai Pharmaceuticals, Flamel/Avadel Pharmaceuticals, Idorsia Pharmaceuticals, Balance Pharmaceutical, Novartis Pharmaceuticals and Philips Respironics. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Andrea L. Brown, MS, ABio Clinical Research Partners, Midlothian, VA. Study investigators: Brian K. Abaluck, MD, Paoli Hospital Sleep Center, Paoli, PA; J. Todd Arnedt, PhD, University of Michigan, Ann Arbor, MI; Richard B. Berry, MD, University of Florida, Gainesville, FL; Richard K. Bogan, MD, Bogan Sleep Consultants, Columbia, SC; Robert C. Doekel, Jr, MD, Sleep Disorders Center of Alabama, Birmingham, AL; Helene A. Emsellem, MD, The Center for Sleep & Wake Disorders, Chevy Chase, MD; Raymond A. Gaskins, Jr, MD, Med One Sleep, Fayetteville, NC; Mark H. Gotfried, MD, Pulmonary Associates, Phoenix, AZ; Mark J. Muehlbach, MD, Clayton Sleep Institute, Maplewood, MO; Paula K. Schweitzer, PhD, St. Luke's Hospital, Chesterfield, MO; David H. Winslow, MD, Norton Clinical Research Group, Louisville, KY.
ABBREVIATIONS
- AHI
apnea hypopnea index
- APAP
auto-adjusting positive airway pressure
- ePOSA
exclusive positional obstructive sleep apnea
- nsAHI
non-supine AHI
- POSA
positional obstructive sleep apnea
- sAHI
supine AHI
- SPT
sleep position treatment
- TST
total sleep time
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohli P, Balachandran JS, Malhotra A. Obstructive sleep apnea and the risk for cardiovascular disease. Curr Atheroscler Rep. 2011;13(2):138–146. doi: 10.1007/s11883-011-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tregear S, Reston J, Schoelles K, et al. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581. [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen AR, Eriksen F, Hansen RW, et al. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One. 2017;12(12):e0189614. doi: 10.1371/journal.pone.0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 8.Oksenberg A, Silverberg DS, Arons E, et al. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 9.Mador MJ, Kufel TJ, Magalang UJ, et al. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128(4):2130–2137. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 10.Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med. 2018;48:157–162. doi: 10.1016/j.sleep.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Omobomi O, Quan SF. Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath. 2018;22(2):297–304. doi: 10.1007/s11325-017-1561-y. [DOI] [PubMed] [Google Scholar]
- 12.Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E. Positional modification techniques for supine obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:107–115. doi: 10.1016/j.smrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014;18(1):19–24. doi: 10.1016/j.smrv.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope. 2006;116(11):1995–20014. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 15.Eijsvogel MM, Ubbink R, Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11(2):139–147. doi: 10.5664/jcsm.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5(5):428–430. [PMC free article] [PubMed] [Google Scholar]
- 17.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 18.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries GE, Hoekema A, Doff MH, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med. 2015;11(2):131–137. doi: 10.5664/jcsm.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levendowski DJ, Seagraves S, Popovic D, et al. Assessment of a neck based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863–871. doi: 10.5664/jcsm.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ruiter MHT, Benoist LBL, de Vries N, de Lange J. Durability of treatment effects of the Sleep Position Trainer versus oral appliance therapy in positional OSA: 12-month follow-up of a randomized controlled trial. Sleep Breath. 2018;22(2):441–445. doi: 10.1007/s11325-017-1568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoist L, de Ruiter M, de Lange J, de Vries N. A randomized, controlled trial of positional therapy versus oral appliance therapy for position-dependent sleep apnea. Sleep Med. 2017;34:109–117. doi: 10.1016/j.sleep.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 23.van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21(3):322–329. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 24.van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014;37(7):1209–1215. doi: 10.5665/sleep.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pépin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13(6):813–824. doi: 10.5664/jcsm.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyers J, Dieltjens M, Kastoer C, et al. Evaluation of a trial period with a sleep position trainer in patients with positional sleep apnea. J Clin Sleep Med. 2018;14(4):575–583. doi: 10.5664/jcsm.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 29.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(6890):1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry RB, Albertario CL, Harding SM, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2018. Version 2.5. [Google Scholar]
- 31.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276–281. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oksenberg A, Arons E, Nasser K, Vander T, Radwan H. REM-related obstructive sleep apnea: the effect of body po sition. J Clin Sleep Med. 2010;6(4):343–348. [PMC free article] [PubMed] [Google Scholar]
- 33.Kushida CA, Berry RB, Blau A, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34(8):1083–1092. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.