Abstract
Context
Permanent childhood hearing loss (PCHL) can affect speech, language, and wider outcomes. Adverse effects are mitigated through universal newborn hearing screening (UNHS) and early intervention.
Objective
We undertook a systematic review and meta-analysis to estimate prevalence of UNHS-detected PCHL (bilateral loss ≥26 dB HL) and its variation by admission to neonatal intensive care unit (NICU). A secondary objective was to report UNHS programme performance (PROSPERO: CRD42016051267).
Data sources
Multiple electronic databases were interrogated in January 2017, with further reports identified from article citations and unpublished literature (November 2017).
Study selection
UNHS reports from very highly-developed (VHD) countries with relevant prevalence and performance data; no language or date restrictions.
Data extraction
Three reviewers independently extracted data and assessed quality.
Results
We identified 41 eligible reports from 32 study populations (1799863 screened infants) in 6195 non-duplicate references. Pooled UNHS-detected PCHL prevalence was 1.1 per 1000 screened children (95% confidence interval [CI]: 0.9, 1.3; I2 = 89.2%). This was 6.9 times (95% CI: 3.8, 12.5) higher among those admitted to NICU. Smaller studies were significantly associated with higher prevalences (Egger’s test: p = 0.02). Sensitivity and specificity ranged from 89–100% and 92–100% respectively, positive predictive values from 2–84%, with all negative predictive values 100%.
Limitations
Results are generalisable to VHD countries only. Estimates and inferences were limited by available data.
Conclusions
In VHD countries, 1 per 1000 screened newborns require referral to clinical services for PCHL. Prevalence is higher in those admitted to NICU. Improved reporting would support further examination of screen performance and child demographics.
Introduction
Universal newborn hearing screening (UNHS) programmes enable prompt detection and intervention for early-onset permanent childhood hearing loss (PCHL). This facilitates improved speech and language development, as well as better health, educational and social outcomes [1, 2]. Most very highly-developed (VHD) countries [3] have now implemented UNHS programmes, defined as universal screening by age 6 months with otoacoustic emissions (OAE) tests, auditory brainstem response (ABR) tests, or both, followed by diagnostic referral where indicated [4].
Implemented programmes vary in size, programme quality, performance, and reported prevalence of PCHL. The latter may be attributable to differences in type, timing, and frequency of test procedures, referral criteria, diagnostic case definition, and management of at-risk children (usually defined according to Joint Committee on Infant Hearing [JCIH] criteria [1] or by neonatal intensive care unit [NICU] admission) [4–8]. Methods of follow-up to ascertain PCHL among those with negative screening results (i.e. children that are not referred to diagnostic testing) can also play an important role (for example, targeted surveillance or subsequent screening).
Previous reviews of UNHS programmes have evaluated screening test methods or quality, and timing of intervention after PCHL diagnosis [9–11]. There has been little quantitative synthesis of PCHL prevalence or analysis of heterogeneity, particularly relating to demographic and individual differences in the populations screened. Additionally, performance of entire UNHS programme pathways as oppose to single tests (relating to measures of accuracy including sensitivity, specificity, negative predictive value, and positive predictive value) have not been systematically examined. Addressing these research gaps will inform screening policy and improve service planning for children with PCHL, by enabling evaluation of UNHS programmes in respect to key indicators and benchmarks. Estimating the prevalence of PCHL detected through UNHS also permits examination of secular trends and heterogeneity in terms of population and UNHS programme characteristics. This provides vital information on UNHS programme performance and the effectiveness of PCHL prevention efforts.
We carried out a systematic review and meta-analysis to estimate the prevalence of PCHL (defined as bilateral PCHL ≥26 dB HL confirmed by diagnostic tests) detected through UNHS in VHD countries. Our secondary objectives were to examine how detected PCHL prevalence varies between studies and by demographic characteristics, as well as to estimate UNHS programme performance.
Methods
The review was carried out in accordance with the registered PROSPERO protocol (CRD42016051267) and reported following MOOSE (S1 Table) and PRISMA (S2 Table) guidelines.
Search strategy
To identify eligible studies, in January 2017 one reviewer (EB) interrogated electronic databases (PubMed, Medline(OvidSP), EMBASE, CINAHL, and the Cochrane Library) and reviewed the first 100 Google Scholar search results. Further reports were identified from citations of included papers and unpublished literature (November 2017). Text-word searches, along with MeSH terms or Subject Headings, were used to construct database searches. Key text-words related to: hearing loss, hearing impairment, deafness, epidemiology, incidence, prevalence, and newborn, neonatal, child, infant, etc. (S1 File). There were no date or language restrictions; all published reports were considered for inclusion if there was an English abstract. Searches of unpublished literature included relevant screening programme reports in any language, whether or not they had English abstracts.
PCHL definition
The review case definition for PCHL was bilateral PCHL ≥26 dB HL. This reflects the minimum severity of PCHL defined by WHO that is expected to require long-term active management [12] and excludes temporary conditions. Although milder (15–26 dB HL) and unilateral PCHL can also impact on outcomes [13], these were not included as they are not always detected, or systematically reported, by UNHS programmes. Acquired, progressive or late-onset conditions were not included as UNHS does not capture these.
Inclusion and exclusion criteria
We included reports of programmes from VHD countries as defined by the United Nations Development Programme (UNDP), such as the United Kingdom, United States, and Germany, [3], as these are similar to each other in terms of UNHS provision, access to health care, and child health and socioeconomic conditions. Studies were included if an English abstract was available (not applicable to unpublished reports), UNHS was in place during the study, and the total number of children with UNHS-detected PCHL fitting the review case definition was reported, as well as the total number considered for, or undergoing, UNHS.
Studies were excluded if any inclusion criteria were not met, the minimum threshold for PCHL exceeded 61 dB HL, they were an ineligible study or article type (review without a systematic search strategy, comment piece, letter, or editorial), there was evidence of ascertainment bias, or all participants were aged over 1 year by study start (as UNHS should occur before age 6 months). As the aim was to estimate population-based prevalence, studies involving a selective sample of children considered to be at high-risk of PCHL were excluded, whilst those including only children at low-risk of PCHL were included.
Article selection, data extraction, and quality evaluation
One reviewer (EB) screened titles and abstracts of all identified reports against the inclusion and exclusion criteria. A second reviewer (RK or CD) each reviewed a random 10% sample, with inter-rater concordance assessed by calculation of the unweighted kappa statistic; discrepancies were resolved by discussion. EB screened full reports against inclusion and exclusion criteria; uncertainties were discussed with RK and CD.
Two reviewers (EB with either RK or CD) independently extracted data for each included study using a form piloted on several studies before use (S2 File). Each study was classified into one of four geographical regions based on included countries: Asia, Europe, North America, and Australia. This form included questions regarding quality based on the JCIH guidelines [1], Newcastle-Ottawa scale [14], STARD [15], and QUADAS-2 [16] criteria. Studies were scored against eight quality criteria, with a maximum of nine points available (Table 1), as well as by the original QUADAS-2 criteria. We resolved discrepancies by discussion. Study data were collected and managed using REDCap (Research Electronic Data Capture), a web-based application for data collection, hosted at University College London [17].
Table 1. Quality scoring criteria.
| Factor | Score | Scoring |
|---|---|---|
| PCHL definition | 0 | Not clearly defined—missing information on >1 feature (laterality, anatomy and severity of study target condition) |
| 1 | Mostly defined—missing information on 1 feature | |
| 2 | Clearly defined—specified all features | |
| Other concerns | 0 | Further concern identified |
| 1 | No other concerns identified | |
| Clear protocol | 0 | Not clearly defined–missing information on one or more features (which tests and number of stages) |
| 1 | Clearly defined | |
| Clear at-risk protocol | 0 | Not clearly defined (how at-risk infants, as defined by the study, were managed) |
| 1 | Clearly defined | |
| Sample bias | 0 | Any identified concerns |
| 1 | No identified concerns | |
| UNHS coverage | 0 | <95% or unclear (# receiving ≥1 UNHS tests) / (target population) |
| 1 | ≥95% | |
| UNHS follow-up | 0 | <70% or unclear (# not lost to follow-up by end of diagnostic tests) / (# failing first stage of UNHS) |
| 1 | ≥70% | |
| Overall follow-up | 0 | No follow-up after completion of relevant screening tests (screen negatives) or diagnostic testing (screen positives) |
| 1 | Yes, some form of follow-up reported |
PCHL: permanent childhood hearing loss; UNHS: universal newborn hearing screening.
Statistical analysis plan
We included each study sample in analyses only once, thus multiple reports from single or overlapping study populations were combined where possible; when indicated, the report providing most detail was used with related papers included as additional references.
We evaluated the characteristics of included studies. We calculated, for each study, the prevalence and 95% confidence intervals (CI) of children with PCHL detected via UNHS and fitting the review case definition in the screened population (defined as the number of children receiving one or more screening tests). Pooled PCHL prevalence and 95% CIs were calculated using the Freeman-Tukey double arc-sine transformation of proportions and Wilson (Score) method [18]. Random-effects models using the Der Simonian and Laird method [19] were fitted to account for the expected heterogeneity in the screened populations and screening methods used. Heterogeneity was evaluated with the I2 statistic (proportion of observed variance not explained by chance) [20] and explored by stratifying prevalence calculations by study characteristics. Sensitivity analyses were carried out excluding outliers and studies of low quality. Funnel plots and Egger’s test were used to assess small-sample bias, along with a sensitivity analysis excluding studies with fewer than 7832 children (based on minimum sample size required to accurately detect a prevalence of 1 per 1000 children within 95% CIs and a precision of 0.0005) [21]. Significance tests were conducted at 5% level.
Screening programme performance was assessed using available data on programme outcomes, namely number of children with screen positive and negative results, and with (true positives, false negatives) or without (false positives, true negatives) confirmed diagnoses. Screen positives comprised all children referred to diagnostic testing, and screen negatives all those not referred, regardless of attendance at diagnostic testing and attrition before the point of diagnostic referral. True positives were defined as all screen-positive children diagnosed with PCHL fitting the review case definition, whilst false positives were all screen-positive children that did not have PCHL fitting the review case definition. We only calculated negative predictive value (NPV), sensitivity and specificity for studies with follow-up to ascertain false negatives (excluding unscreened children with PCHL, plus late-onset, acquired and progressive PCHL from the false negative number where possible). Positive predictive value (PPV) calculation was not limited by follow-up. Quantitative pooling of performance estimates was not undertaken due to methodological differences between studies, such as tests used or diagnostic referral criteria for testing.
PCHL prevalence by reported demographic and individual characteristics and in those with or without NICU admission were explored and calculated where data were available. Analyses were performed using STATA 15 (Stata Corp, College Station, TX).
Results
Selection of eligible studies
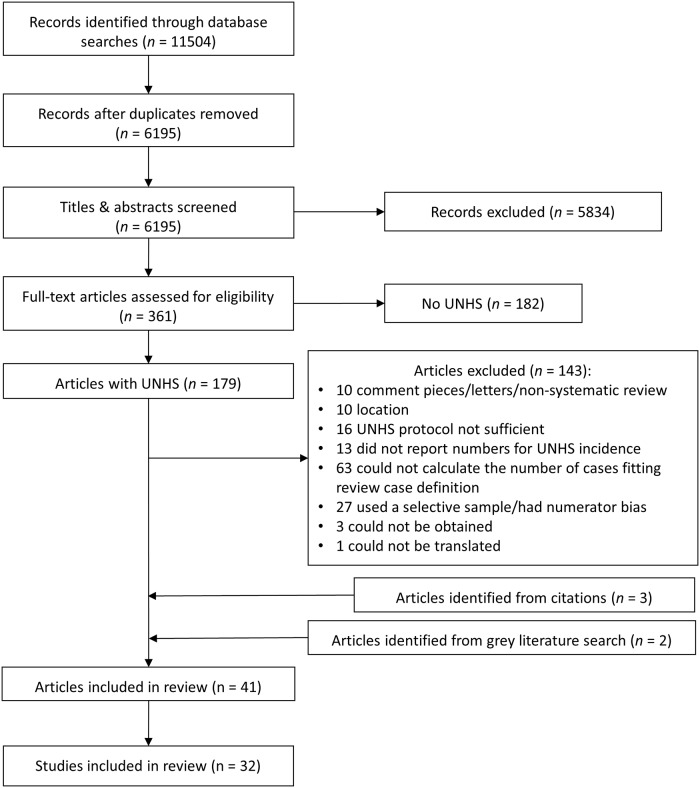
The literature search identified 6195 non-duplicate records, of which 5834 were excluded at the title and abstract screen. A further 325 records were excluded after screening full texts. Five relevant articles were identified from searches of the grey literature and references of included papers. This resulted in 41 articles for inclusion in the meta-analysis, reporting on 32 separate study populations (Fig 1). Two systematic reviews [9, 10] were included in the total number of articles, however these were not included in data extraction as they contained no additional data (all reviewed studies were already included as individual studies).
Fig 1. PRISMA flow diagram.
The summary unweighted inter-rater kappa statistic for abstract inclusion were 0.8 for EB with both RK and CD, implying good agreement. Reviewers agreed the final list of eligible studies by consensus, therefore inter-rater differences were not assessed.
Study characteristics
The characteristics of the included studies are described in Table 2, separately for those with and without follow-up after screening and diagnostic testing was completed. Over 70% of included studies were from Europe (n = 23, 72%), with the remainder from North America (n = 4, 13%), Australia (n = 3, 9%), or Asia (n = 2, 6%). Seven studies aimed to include out-of- hospital births (i.e. were ‘population-based’), whilst 12 involved a single hospital, and 13 multiple hospitals or birth centres. The Wessex study involved four hospitals that alternated UNHS and non-UNHS screening periods; only data reported from UNHS screening periods are considered here.
Table 2. Included study characteristics.
| Study authors | Location | Setting | Period of UNHS | UNHS protocol | Reported PCHL meeting review case definition | Study population, n (% screened) |
PCHL diagnoses via UNHS, n | Follow-up after UNHS and diagnostic testing |
|---|---|---|---|---|---|---|---|---|
| Studies with follow-up | ||||||||
| Almenar Latorre [22] | Spain | 1 H | Unclear | OAE & ABR | Bilateral >40 dB HLa | 1532 (n/a)e | 4 | Parent questionnaire at age 1 year (n = 825, 53.8%, followed up) |
| Antoni [23] | France | 4 H | 2005–2010 | ABR | Bilateral >35 dB HLa | 27885 (96.0) | 30 | Children with PCHL diagnoses via UNHS had audiology records checked (mean follow-up length = 34 months, range 0–75 months) |
| Berninger [24] | Sweden | 2 H | 1999–2004 | OAE & ABR | Bilateral SNHL, CHL & mixed >30 dB HLb | 31092 (n/a)e | 57 | Clinician report of further cases and acoustic amplification referral database checked up to age 10 years |
| Calcutt [25] | Australia | P—1 region | 2009–2011 | ABR | Bilateral SNHL >40 dB HLb | 185205 (94.9) | 121 | Targeted surveillance screen at 9–12 months (2606 attended of 4361 referred) and audiology records checked up to age 4 years |
| Calevo [26] | Italy | 13 H/BC | 2002–2004 | OAE & ABR | Bilateral >40 dB HLa,b,c | 32502 (99.2) | 20 | PCHL registry and audiology records checked up to age 2 years |
| Cao-Nguyen [27] | Switzerland | 1 H | 2000–2004 | OAE & ABR | Bilateral >40 dB HLa,d | 17535 (n/a)e | 24 | Audiology records checked to unclear age (excluding late-onset PCHL) |
| De Capua [28] | Italy | 3 H | 1998–2007 | OAE | Bilateral SNHL, CHL & mixed >30 dB HLb | 21125 (93.3) | 24 | Audiology records checked up to age 1 year |
| Ng [29] | Hong Kong | 1 H | 1999–1999 | OAE | Bilateral ≥40 dB HLa,b | 1076 (98.9) | 3 | Parent interviews at 18 and 36 months (n = 1020; 95.9% followed up) |
| O'Connor [30] | Ireland | 6 H | 2011–2012 | OAE & ABR | Bilateral SNHL, CHL & mixed >40 dB HLb,c | 11763 (99.8) | 12 | Targeted surveillance screen up to age 1 year |
| Watkin [31–33] | UK | P—1 region | 1992–2002 | OAE | Bilateral ≥40 dB HLa,b,c | 35668 (94.9) | 32 | Audiology records checked up to age 12 years (excluding late-onset PCHL) |
| Wessex [34–36] | UK | 4 H—only periods with UNHS included | 1993–1996 | OAE & ABR | Bilateral ≥40 dB HLa | 25609 (83.1) | 22 | Active follow-up and audiology, healthcare and educational records checked up to age 9 years (excluding late-onset PCHL) |
| Studies without follow-up | ||||||||
| Adelola [37] | Ireland | 2 H | 2000–2007 | OAE & ABR | Bilateral >40 dB HLa,b | 26281 (97.9) | 19 | |
| Aidan [38] | France | 1 H | 1995–1997 | OAE | Bilateral SNHL >40 dB HLb | 1727 (82.3) | 2 | |
| Bailey [39] | Australia | 5 H–excluding some NICU infants | 2000–2001 | OAE & ABR | Bilateral >35 dB HLa,b | 13214 (96.2) | 5 | |
| Caluraud [40] | France | 14 H/BC | 1999–2011 | OAE & ABR | Bilateral >35 dB HLa,d | 101916 (99.8) | 142 | |
| Fornoff [41] | USA | P—Statewide | 2003–2004 | OAE & ABR | Bilateral SNHL, CHL & mixed ≥30 dB HLb | 335412 (98.0) | 160 | |
| Ghirri [42] | Italy | 1 H | 2005–2009 | OAE & ABR | Bilateral >40 dB HLa,b,c | 8113 (n/a)e | 21 | |
| Gonzalez de Aledo Linos [43, 44] | Spain | 2 H | 2001–2003 | OAE | Bilateral SNHL >40 dB HLb | 8836 (98.4) | 11 | |
| Guastini [45] | Italy | 1 H | 2006–2009 | OAE & ABR | Bilateral >40 dB HLa,b,d | 8671 (n/a)e | 2 | |
| Habib [46] | Saudi Arabia | 1 H—excluding children with JCIH 1994 risk factors | 1996–2004 | OAE | Bilateral SNHL ≥26 dB HLb | 11986 (n/a)e | 20 | |
| Magnani [47] | Italy | 1 H | 2010–2013 | OAE & ABR | Bilateral SNHL >40 dB HLb,c | 11624 (99.7) | 26 | |
| Martinez [48] | Spain | 1 H | 2001–2002 | OAE & ABR | Bilateral >35 dB HLa,b | 1277 (94.2) | 9 | |
| Mason [49] | USA | 1 H | 1992–1997 | ABR | Bilateral SNHL, CHL & mixed >35 dB HLb | 10773 (98.2) | 16 | |
| Mehl [50] | USA | 57 H | 1992–1999; only 1999 included | ABR | Bilateral SNHL, CHL & mixed ≥35 dB HLb | 63590 (87.0) | 63 | |
| Metzger [51] | Switzerland | 1 H—excluding preterm births | 2005–2010 | OAE | Bilateral ≥40 dB HLa | 12080 (n/a) | 15 | |
| NSW [52] | Australia | P– 1 region | 2003–2009 | ABR | Bilateral >40 dB HLa,b | 284694 (99.0) | 283 | |
| Rohlfs [53] | Germany | 14 H/BC | 2002–2006 | OAE & ABR | Bilateral ≥41 dB HLa,b,c | 65466 (92.8) | 73 | |
| Uilenburg [54] | Netherlands | P—3 regions, excluding NICU infants | 1999–2000 | OAE | Bilateral SNHL ≥40 dB HLb | 3336 (94.0) | 1 | |
| Uus [55] | UK | 23 H | 2001–2004 | OAE & ABR | Bilateral SNHL, CHL & mixed ≥40 dB HL | 169487 (n/a)e | 169 | |
| Van der Ploeg [56] | Netherlands | P—entire country, excluding NICU infants | 2002–2009 (excluding 2007) | OAE & ABR | Bilateral ≥40 dB HLa,b | 552820 (n/a) | 427 | |
| Van Kerschaver [6, 57, 58] | Belgium | P—1 region | 1999–2008 | ABR | Bilateral SNHL, CHL & mixed >40 dB HLb | 628337 (95.9) | 646 | |
| White [59] | USA | 1 H—random sample | 1990–1991 | OAE & ABR | Bilateral SNHL, CHL & mixed >25 dB HLb | 1850 (n/a)e | 6 | |
aAnatomy of HL (CHL, SNHL, mixed) not specified.
bUnilateral loss also reported in study, but excluded in meta-analysis PCHL count.
cPCHL <40 dB HL also reported but <26 dB HL and 26–40 dB HL PCHL could not be separated.
dDiagnostic threshold unclear; assumed to be the same as the screening test threshold.
eUnclear study population considered; number given is those receiving ≥1 UNHS test.
ABR: auditory brainstem response; BC: birth clinic; CHL: conductive HL; dB HL: decibels hearing level; H: hospital; n/a: not applicable: NICU: neonatal intensive care unit; OAE: otoacoustic emissions; P: population-based (including out of hospital births); PCHL: permanent childhood hearing loss; SNHL: sensorineural HL; UNHS: universal newborn hearing screening.
Median study duration was 3 years (25th percentile [Q1]: 2, 75th percentile [Q3]: 6), with included studies covering births between 1990 and 2014. Results from 1999 for the Mehl study [50] are included as the numbers screened and diagnosed were unclear for other years.
Study population ranged between 1076 and 628337 (median 25945, Q1: 11198, Q3: 83691) children for the 24 studies with this information available. Median study population recruitment or screening coverage was 96.2% (Q1: 94.2, Q3: 98.9%) for 21 studies specifying both the total study population and the number screened, with a total of 1799863 screened children in the included studies.
UNHS screen protocol types were broadly grouped to: OAE-only (8 studies), ABR-only (6 studies), or OAE and ABR (18 studies), based on the screening tests used in the main study protocol (S3 Table).
PCHL diagnoses
Between 1 and 646 children were diagnosed with PCHL fitting the review case definition via UNHS in the individual studies. Most studies did not report follow-up after completion of UNHS and diagnostic testing (n = 21). Of the 11 studies with any follow-up, nine attempted to follow-up the entire study population, one only reported targeted surveillance results for screen negative children with risk factors (O’Connor) [30] and one study only followed up true positive children (Antoni) [23]. Thus there were 10 studies with follow-up of screen negative children. Methods of follow-up for screen negatives involved checking for PCHL diagnoses via local clinicians, audiology databases, registers or other records (n = 5), interviewing parents (n = 2), targeted surveillance (n = 2; with one study also assessing audiology records), and active testing along with checking for further diagnoses from multiple health, education and audiology databases and staff involved in management of children with PCHL (n = 1) (Table 2).
Study quality
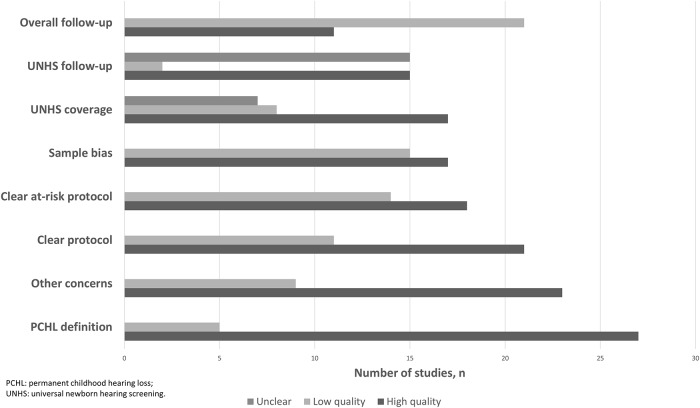
The majority of studies were of high quality for the following criteria: clarity of PCHL case definition, protocol details, clear description for management of at-risk children, lack of sample bias, high UNHS coverage (≥95%), and absence of other identified concerns. Lower or more variable quality was seen for UNHS follow-up, as 15 studies did not clearly state the number of children lost to follow-up during the UNHS and diagnostic testing stages. Additionally, few studies reported any follow-up after screening and diagnostic testing was completed (Table 3; Fig 2). Those with follow-up usually relied on passive methods, as described above, and rarely reported whether screen-negative children with later diagnoses were failures of detection (false negatives) or had late-onset, acquired or progressive PCHL. Results of QUADAS-2 scoring (relating specifically to risk of bias) are presented in S3 File.
Table 3. UNHS meta-analysis study quality scoring results.
| Study | PCHL definition | Other concerns | Clear protocol | Clear at-risk protocol | Sample bias | UNHS coverage | UNHS follow-up | Overall follow-up |
|---|---|---|---|---|---|---|---|---|
| De Capua | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| O'Connor | 2 | 1 | 1 | 1 | 1 | 1 | n/a | 1 |
| Calcutt | 2 | 1 | 1 | 1 | 0 | 1 | n/a | 1 |
| Calevo | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Magnani | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Mason | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Ng | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Caluraud | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Guastini | 1 | 1 | 1 | 1 | 1 | n/a | 1 | 0 |
| Uilenburg | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Uus | 2 | 1 | 1 | 1 | 1 | n/a | n/a | 0 |
| White | 2 | 1 | 1 | 1 | 0 | n/a | 1 | 0 |
| Almenar Latorre | 1 | 0 | 1 | 1 | 0 | n/a | 1 | 1 |
| Gonzalez de Aledo Linos | 1 | 1 | 1 | 0 | 1 | 1 | n/a | 0 |
| Habib | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| NSW | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Van der Ploeg | 1 | 1 | 1 | 1 | 0 | 1 | n/a | 0 |
| Watkin | 1 | 1 | 0 | 0 | 1 | 1 | n/a | 1 |
| Wessex | 1 | 1 | 1 | 1 | 0 | 0 | n/a | 1 |
| Adelola | 1 | 0 | 0 | 1 | 1 | 1 | n/a | 0 |
| Antoni | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Bailey | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| Cao-Nguyen | 0 | 1 | 0 | 0 | 1 | 1 | n/a | 1 |
| Ghirri | 1 | 1 | 1 | 1 | 0 | n/a | n/a | 0 |
| Metzger | 1 | 1 | 1 | 0 | 0 | n/a | 1 | 0 |
| Berninger | 2 | 0 | 0 | 0 | 0 | n/a | n/a | 1 |
| Fornoff | 1 | 0 | 0 | 0 | 1 | 1 | n/a | 0 |
| Martinez | 1 | 1 | 0 | 0 | 1 | 0 | n/a | 0 |
| Mehl | 2 | 0 | 0 | 0 | 1 | 0 | n/a | 0 |
| Rohlfs | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Van Kerschaver | 1 | 0 | 0 | 0 | 1 | 1 | n/a | 0 |
| Aidan | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total high quality |
11 high, 16 medium |
23 | 21 | 18 | 17 | 17 | 15 | 11 |
| Total low quality | 5 | 9 | 11 | 14 | 15 | 8 | 2 | 21 |
| Unclear | n/a | n/a | n/a | n/a | n/a | 7 | 15 | n/a |
n/a indicates unclear quality. PCHL: permanent childhood hearing loss; UNHS: universal newborn hearing screening.
Fig 2. Study quality indicators.
Prevalence
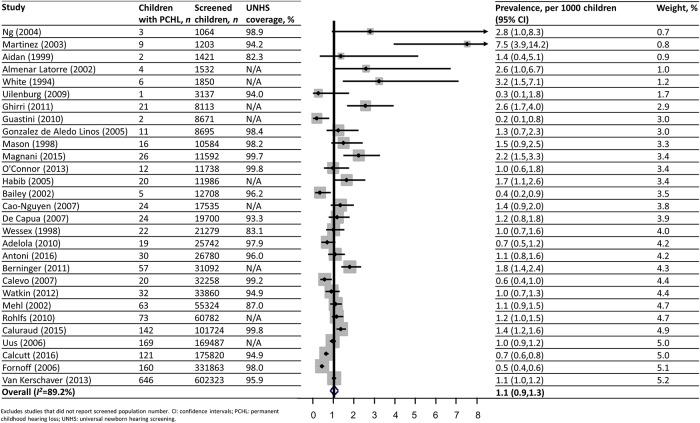
Pooled prevalence of UNHS-detected PCHL in the screened population was 1.1 (95% CI: 0.9, 1.3) per 1000 children (I2 = 89.2%) (Fig 3). Sensitivity analyses excluding studies with outlying prevalences or of low quality[48], or those with identified sample bias [22–25, 35, 38, 39, 42, 46, 49, 53, 54, 59] did not alter prevalence estimates. Similarly, prevalence estimates using the study population, rather than screened population, as the denominator did not affect estimates (prevalence: 0.9, 95% CI: 0.8, 1.1 per 1000 children).
Fig 3. UNHS-detected PCHL prevalence in the screened population.
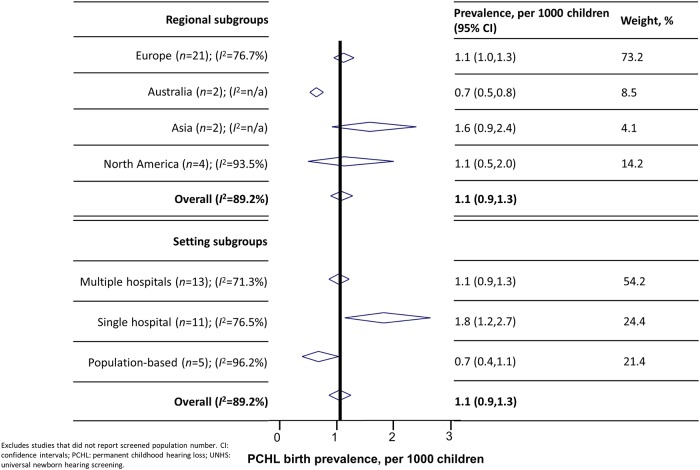
Funnel plot and Egger’s test results indicated that studies with smaller study populations produced significantly larger prevalence estimates (Egger’s test p = 0.02). Studies based on single hospitals had significantly smaller study populations and correspondingly higher PCHL prevalence estimates than those based in multiple hospitals or population-based (heterogeneity between settings, p = 0.003) (Fig 4). This association remained after exclusion of outliers [48], and was not due to significant differences in measured quality indicators (including studies with sample bias, such as exclusion of high-risk children) or study characteristics. Excluding studies with small study populations did not modify pooled prevalence estimates significantly.
Fig 4. UNHS-detected PCHL prevalence in the screened population by regional and setting subgroups.
Analyses of between-region heterogeneity revealed that prevalence estimates were significantly smaller for studies carried out in Australia (heterogeneity between regions, p<0.001) compared with other regions (Fig 4). Study characteristics or quality were similar between regions with the exception of screening protocol type (Australian studies were significantly more likely to use ABR than other protocols). Prevalence estimates did not vary significantly by screening protocol type, year started, study duration, PCHL case definition, individual quality indicators. Lack of comparability between UNHS programme protocols (for example ages of testing, number of tests, specific equipment, referral criteria) precluded investigation of associations between these factors and PCHL prevalence.
Differences in prevalence by demographic and individual characteristics
Ethnic group of children with PCHL or of the study population was not reported in any study. Sex distribution was reported for children with PCHL fitting the review case definition in only two studies (44 and 57% female in the individual studies) [24, 55], however this was not reported for the entire study population.
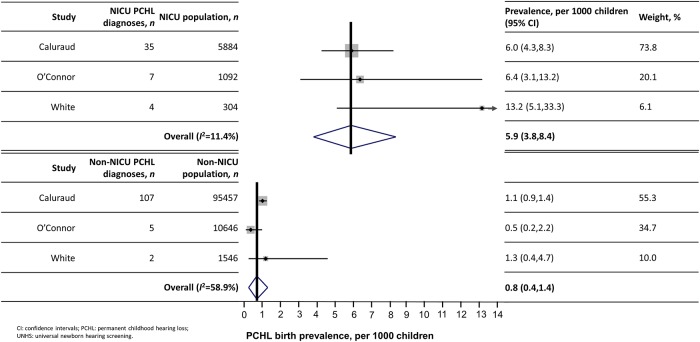
In three studies [30, 40, 59], information was provided on UNHS-detected PCHL prevalence by NICU admission status. Pooled PCHL prevalence was 5.9 (95% CI: 3.8, 8.4) per 1000 screened children admitted to NICU, compared with 0.8 (95% CI: 0.4, 1.4) per 1000 not admitted: a PCHL prevalence rate ratio of 6.9 (95% CI: 3.8, 12.5) (Fig 5).
Fig 5. UNHS-detected PCHL prevalence in NICU versus non-NICU populations.
Screening programme performance
Screening programme coverage and yield is presented in Table 2 and Fig 3. In the 25 studies with available information, PPV ranged from 2% to 84% (Table 4). A median of 93% (Q1: 86%, Q3: 100%) of screen positive children attended diagnostic testing in 21 studies reporting this information.
Table 4. Positive predictive values for UNHS studies.
| Study authors | Criteria for diagnostic referral | Referral of bilateral only (Bi), or both unilateral and bilateral (Bo), failure? | SP, n | TP, n | PPV, % 95% CI |
|---|---|---|---|---|---|
| OAE only | |||||
| Uilenburg | OAE failure | Bo | 69 | 1 | 1.5 0.3–7.8 |
| De Capua | OAE failure or presence of risk factors | Bo | 1536 | 24 | 1.6 1.1–2.3 |
| González de Aledo Linos | OAE failure or presence of risk factors | Bo | 342 | 11 | 3.2 1.8–5.7 |
| Metzger | OAE failure | Bi | 253 | 15 | 5.9 3.6–9.6 |
| Habib | OAE failure | Bo | 300 | 20 | 6.7 4.4–10.1 |
| Ng | OAE failure at 40 dB HL | Bo | 37 | 3 | 8.1 2.8–21.3 |
| Aidan | OAE failure | NS | 9 | 2 | 22.2 6.3–54.7 |
| ABR only | |||||
| Mehl | ABR failure (or OAE in few hospitals) | Bo | 1283 | 63 | 4.9 3.9–6.2 |
| Calcutt | ABR failure or presence of risk factors | Bo | 1633 | 121 | 7.4 6.2–8.8 |
| Antoni | ABR failure at 30 dB HL (modified in 2009 to only bilateral failures) | Bo | 226 | 30 | 13.3 9.5–18.3 |
| Van Kerschaver | ABR failure at 35 dB HL | Bo | 2316 | 646 | 27.9 26.1–29.8 |
| OAE & ABR | |||||
| O'Connor | ABR failure | Bo | 525 | 12 | 2.3 1.3–4.0 |
| White | ABR failure at 30 dB HL | Bo | 115 | 6 | 5.2 2.4–10.9 |
| Wessex | ABR failure at 35 dB HL (modified Oct 1994 to only bilateral) | Bo | 392 | 22 | 5.6 3.7–8.4 |
| Fornoff | Failure of last screening test (OAE or ABR) | Bo | 2135 | 160 | 7.5 6.5–8.7 |
| Magnani | ABR failure | Bo | 241 | 26 | 10.8 7.5–15.3 |
| Martínez | OAE failure or presence of risk factors | Bo | 69 | 9 | 13.0 7.0–23.0 |
| Adelola | ABR failure | Bo | 92 | 19 | 20.7 13.6–30.0 |
| Bailey | ABR failure at 35 dB HL | Bo | 23 | 5 | 21.7 9.7–41.9 |
| Ghirri | ABR failure | Bo | 84 | 21 | 25.0 17.0–35.2 |
| Guastini | ABR failure at 40 dB HL | Bo | 6 | 2 | 33.3 9.7–70.0 |
| Rohlfs | ABR failure at 35 dB HL | Bo | 217 | 73 | 33.6 27.7–40.2 |
| Almenar Latorre | ABR failure at 40 dB HL | Bo | 11 | 4 | 36.4 15.2–64.6 |
| Calevo | ABR failure | Bo | 41 | 20 | 48.8 34.3–63.5 |
| Caluraud | ABR failure at 35 dB HL | Bo | 170 | 142 | 83.5 77.2–88.4 |
ABR: auditory brainstem responses test; NS: not stated; OAE: otoacoustic emissions test; PPV: positive predictive value; SP: screen positives; TP: true positives; UNHS: universal newborn hearing screening.
The Wessex study was deemed to have highest quality follow-up after UNHS and diagnostic testing, with active and passive follow-up to age 9 years, using multiple sources and excluding acquired, late-onset, or progressive PCHL. Sensitivity and specificity for this study were 92% (95% CI: 74%, 98%) and 98% (95% CI: 98%, 98%), respectively. NPV was 100% (95% CI: 100%, 100%). The remaining nine studies used less robust methods to follow-up screen negatives, as described earlier, however they reported similar screen performance measures to the Wessex study; overall NPV was 100% in the seven studies with available information, sensitivity 89–100% (for eight studies) and specificity 92–100% (for seven studies) (Table 5). Only 4 studies reported loss to follow-up of children in the screen negative group; this was ≤2%.
Table 5. Negative predictive value, sensitivity, and specificity for studies with follow-up.
| Study authors | SP, n | TP, n | SN, n | FN, n | NPV, % 95% CI |
Sensitivity, % 95% CI |
Specificity, % 95% CI |
|---|---|---|---|---|---|---|---|
| Almenar Latorre | 11 | 4 | 1521 | 0 | 100.0 99.9–100.0 |
100.0 51.0–100.0 |
99.5 99.6–99.8 |
| Berninger | n/a | 57 | n/a | 0 | n/a | 100.0 93.7–100.0 |
n/a |
| Calcutt | 1633 | 121 | 174187 | 10 | 100.0 100.0–100.0 |
92.4 86.5–95.8 |
99.1 99.1–99.2 |
| Calevo | 41 | 20 | 32217 | 0 | 100.0 100.0–100.0 |
100.0 83.9–100.0 |
99.9 99.9–100.0 |
| Cao-Nguyen | n/a | 24 | n/a | 2 | n/a | 92.3 75.9–97.9 |
n/a |
| De Capua | 1536 | 24 | 18164 | 1 | 100.0 100.0–100.0 |
96.0 80.5–99.3 |
92.3 91.9–92.7 |
| Ng | 37 | 3 | 1027 | 0 | 100.0 99.6–100.0 |
100.0 43.9–100.0 |
96.8 95.6–97.7 |
| O'Connor | 525 | 12 | 11213 | 1 | 100.0 100.0–100.0 |
92.3 66.7–98.6 |
95.6 95.2–96.0 |
| Watkin | n/a | 32 | n/a | 4 | n/a | 88.9 74.7–95.6 |
n/a |
| Wessex | 392 | 22 | 20887 | 2 | 100.0 100.0–100.0 |
91.7 74.2–97.7 |
98.3 98.1–98.4 |
CI: confidence intervals; FN: false negatives; n/a: not given or could not be calculated; NPV: negative predictive value: SN: screen negatives; SP: screen positives; TP: true positive
Discussion
Key findings
We estimate that UNHS programmes in VHD countries identify PCHL in 1 out of every 1000 children screened. This was lower in studies carried out in Australia and higher in studies carried out in single hospitals, which included smaller study populations relative to population-based studies or those based on several hospitals. The highest prevalence was found in infants admitted to NICU, a group known to be at higher risk of PCHL, compared with those who were not. No studies reported ethnic group and only two studies reported sex, precluding estimation of sex- or ethnic-specific pooled prevalences.
Analysis of screening programme performance demonstrated good population coverage with high detection rates, however PPV varied widely across studies. Although NPV, sensitivity and specificity appeared high, these could only be estimated from studies with follow-up of screen negatives, which had marked variation in methods for, and completeness of, follow-up.
Strengths and limitations
Strengths of this systematic review and meta-analysis include prospective publication of our protocol on PROSPERO, the systematic search strategy employed, as well as the inclusion of unpublished literature and articles in all languages, which reduced likelihood of inclusion bias. Independent article selection, data extraction, and quality assessment by multiple reviewers reduced risk of bias or error. Finally, we used robust statistical methods, including random-effects models, to calculate prevalence, screening programme performance and to examine heterogeneity.
Our searches were not restricted by language and included unpublished literature, including UNHS programme reports and evaluations, however we cannot exclude the possibility that we failed to identify relevant unpublished evidence. Our findings are generalisable to VHD countries only, reflecting the selection criteria employed, and we cannot assume they apply to other settings. Our analyses were constrained by the limited reporting of demographic and individual characteristics including on sex, ethnic group and age at diagnosis, loss to follow-up of those with screen positive results, as well as lack of reporting on follow-up (active or passive) with which to identify children diagnosed at older ages. Later PCHL diagnoses may reflect failures of screening, diagnosis, or management, or variation in natural history resulting in PCHL of progressive or later onset. Few studies employed high-quality active ascertainment of later diagnoses limiting the studies available for estimating NPV, sensitivity, and specificity. We were unable to assess bias due to selective attrition in screen performance estimates as this was rarely reported; however the consistently reported high attendance at diagnostic testing reduced the likelihood of bias in PPV estimates.
Interpretation
Our pooled estimate of PCHL prevalence of around 1 per 1000 children is consistent with that reported from existing studies of congenital PCHL prevalence [60] prior to UNHS, suggesting that UNHS detects most newborns with early-onset PCHL. We did not detect differences in UNHS-detected PCHL prevalence by study date or target PCHL definition. There was also no association between screening protocol type and detected prevalence. This is consistent with at least one previous study comparing an OAE-only versus ABR-only protocol [61] and indicates that reported variation in referral rates by protocol type has little impact on detected prevalence [62]. Protocol type may influence programme performance and cost-effectiveness, however, further research is required to explore this. We found a higher prevalence of PCHL in studies reporting the experience of single hospitals, in comparison to those based on multiple hospitals or whole populations. The single hospital studies tended to have smaller samples than the other setting types. The difference in prevalence by settings or sample size was not clearly attributable to the measured quality indicators, including risk of sample bias, or other study characteristics. It may be that unmeasured differences in the study populations explain this finding, for instance differences in the NICU population size and characteristics.
Demographic factors, such as sex and ethnicity, which may provide insights into potential causal mechanisms, were largely not reported. This restricted exploration of variations in prevalence by these characteristics, and might have resulted in the large I2 value. In particular, we were unable to examine whether ethnic variation explained regional variations in prevalence. For example, Martínez et al suggested that the high prevalence in their study may reflect the high proportion of children of Roma ethnic origin in their study population [48]. Although there is no evidence that children of Roma ethnicity are at higher risk of PCHL, two studies have suggested other ethnic differences in hearing loss [63, 64]. The small number of studies in regions other than Europe may have reduced statistical power to detect regional differences in prevalence.
Prevalence of PCHL in babies admitted to NICU was almost seven times higher than for those not admitted. This is consistent with our previous finding based on a UK-wide cohort study whereby NICU or special care baby unit admission was associated with 6.3 (95% CI: 2.3, 17.6) times higher risk of PCHL, and neonatal illness without NICU admission with 2.6 (95% CI: 1.2, 6.0) times higher risk, than children with no neonatal illness [65]. The association between NICU and high PCHL risk may result from the underlying cause of PCHL, for example craniofacial anomalies or other syndromal pathologies, as well as exposure to ototoxic antibiotics, prolonged mechanical ventilation and asphyxia, hyperbilirubinaemia, and high noise levels in NICU [1, 4, 66, 67].
UNHS programme screening, diagnostic, and follow-up protocols varied greatly across studies. We therefore did not combine PPV estimates due to differences in screening equipment, testing protocol (including choice of stages and number of repeats), tester training, referral criteria and age at testing [68–71]. NPV, sensitivity and specificity are also affected by these factors, as well as by the strategies employed to ascertain children with later PCHL diagnoses and false negatives. PPV varied widely between studies whilst NPV, sensitivity and specificity estimates were reasonably consistent where they could be calculated. This reinforces findings from a previous review suggesting that guidelines to ensure standardised, high quality public reporting of UNHS programme performance are required [10]. Further evaluation, using adequate follow-up to detect false negatives, is justified to inform improvements in the quality and performance of screening programmes. Adequate follow-up should also involve assessing hearing threshold changes in children diagnosed with PCHL, as these can fluctuate or normalise over time [23, 24], reflecting the difficulty in conclusively diagnosing PCHL in very young children [72].
Conclusions
We estimate that in VHD countries, audiological and other services will be required for around 1 per 1000 children with PCHL following UNHS screening, however this may vary depending on the proportion of children admitted to NICU, in whom PCHL prevalence is much greater. Future research to investigate differences in PCHL prevalence by sex and ethnicity is required, and to compare the performance of different screening protocols to identify those that are most effective. Both will depend on the quality of data collection and reporting, including attrition, and implementation of active follow-up measures to ascertain false negatives.
Supporting information
(DOC)
(DOC)
(DOCX)
(DOCX)
(PDF)
(DOCX)
Acknowledgments
This research was supported by the National Institute for Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR or the UK Department of Health.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
EB was funded by an Economic and Social Research Council studentship (grant number: ES/J500185/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.JCIH. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. 10.1542/peds.2007-2333 . [DOI] [PubMed] [Google Scholar]
- 2.Pimperton H, Blythe H, Kreppner J, Mahon M, Peacock JL, Stevenson J, et al. The impact of universal newborn hearing screening on long-term literacy outcomes: a prospective cohort study. Archives of disease in childhood. 2016;101(1):9–15. 10.1136/archdischild-2014-307516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Development Programme. Human Development Report. 2015.
- 4.Wroblewska-Seniuk KE, Dabrowski P, Szyfter W, Mazela J. Universal newborn hearing screening: methods and results, obstacles, and benefits. Pediatr Res. 2017;81(3):415–22. Epub 2016/11/20. 10.1038/pr.2016.250 . [DOI] [PubMed] [Google Scholar]
- 5.White KR, Forsman I, Eichwald J, Munoz K, editors. The evolution of early hearing detection and intervention programs in the United States. Seminars in perinatology; 2010: Elsevier. [DOI] [PubMed]
- 6.Van Kerschaver E, Boudewyns AN, Declau F, Van de Heyning PH, Wuyts FL. Socio-demographic determinants of hearing impairment studied in 103 835 term babies. The European Journal of Public Health. 2013;23(1):55–60. 10.1093/eurpub/cks010 [DOI] [PubMed] [Google Scholar]
- 7.Van Kerschaver E. An integrated Public Health Programme for the prevention of hearing impairment 10 years of general AABR-screening for newborns in Flanders: Universiteit Antwerpen (Belgium); 2012.
- 8.Public Health England. Newborn hearing screening: programme overview. https://www.gov.uk/guidance/newborn-hearing-screening-programme-overview2013.
- 9.Papacharalampous GX, Nikolopoulos TP, Davilis DI, Xenellis IE, Korres SG. Universal newborn hearing screening, a revolutionary diagnosis of deafness: real benefits and limitations. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology—Head and Neck Surgery. 2011;268(10):1399–406. Epub 2011/06/24. 10.1007/s00405-011-1672-1 . [DOI] [PubMed] [Google Scholar]
- 10.Mincarone P, Leo CG, Sabina S, Costantini D, Cozzolino F, Wong JB, et al. Evaluating reporting and process quality of publications on UNHS: A systematic review of programmes. BMC Pediatrics. 2015;15 (1) (no pagination)(86). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde ML. Newborn hearing screening programs: overview. The Journal of otolaryngology. 2005;34 Suppl 2:S70–8. Epub 2005/08/04. . [PubMed] [Google Scholar]
- 12.WHO. Classification of hearing impairment 2013. http://www.who.int/pbd/deafness/hearing_impairment_grades/
- 13.Fitzpatrick EM, Durieux-Smith A, Whittingham J. Clinical practice for children with mild bilateral and unilateral hearing loss. Ear & Hearing (01960202). 2010;31(3):392–400. 10.1097/AUD.0b013e3181cdb2b9 Language: English. Entry Date: 20110318. Revision Date: 20150711. Publication Type: Journal Article. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. oxford. asp; 2011. [Google Scholar]
- 15.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology. 2015;277(3):826–32. 10.1148/radiol.2015151516 [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72(1):39 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Archives of orofacial Sciences. 2006;1:9–14. [Google Scholar]
- 22.Almenar Latorre A, Tapia Toca MC, Fernandez Perez C, Moro Serrano M. [A combined neonatal hearing screening protocol]. An Esp Pediatr. 2002;57(1):55–9. Epub 2002/07/26. . [PubMed] [Google Scholar]
- 23.Antoni M, Rouillon I, Denoyelle F, Garabedian EN, Loundon N. Newborn hearing screening: Prevalence and medical and paramedical treatment of bilateral hearing loss in a neonatal series in the Ile-de-France region of France. European annals of otorhinolaryngology, head and neck diseases. 2016;133(2):95–9. Epub 2015/11/02. 10.1016/j.anorl.2015.10.001 . [DOI] [PubMed] [Google Scholar]
- 24.Berninger E, Westling B. Outcome of a universal newborn hearing-screening programme based on multiple transient-evoked otoacoustic emissions and clinical brainstem response audiometry. Acta oto-laryngologica. 2011;131(7):728–39. 10.3109/00016489.2011.554440 . Language: English. Entry Date: 20110627. Revision Date: 20150711. Publication Type: Journal Article. [DOI] [PubMed] [Google Scholar]
- 25.Calcutt TL, Dornan D, Beswick R, Tudehope DI. Newborn hearing screening in Queensland 2009–2011: Comparison of hearing screening and diagnostic audiological assessment between term and preterm infants. Journal of paediatrics and child health. 2016;52(11):995–1003. 10.1111/jpc.13281 [DOI] [PubMed] [Google Scholar]
- 26.Calevo M, Mezzano P, Zullino E, Padovani P, Serra G. Ligurian experience on neonatal hearing screening: clinical and epidemiological aspects. Acta paediatrica. 2007;96(11):1592–9. 10.1111/j.1651-2227.2007.00475.x [DOI] [PubMed] [Google Scholar]
- 27.Cao-Nguyen M-H, Kos M-I, Guyot J-P. Benefits and costs of universal hearing screening programme. International journal of pediatric otorhinolaryngology. 2007;71(10):1591–5. 10.1016/j.ijporl.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 28.De Capua B, Costantini D, Martufi C, Latini G, Gentile M, De Felice C. Universal neonatal hearing screening: the Siena (Italy) experience on 19,700 newborns. Early Hum Dev. 2007;83(9):601–6. Epub 2007/02/20. 10.1016/j.earlhumdev.2007.01.001 . [DOI] [PubMed] [Google Scholar]
- 29.Ng PK, Hui Y, Lam BCC, Goh WHS, Yeung CY. Feasibility of implementing a universal neonatal hearing screening programme using distortion product otoacoustic emission detection at a university hospital in Hong Kong. Hong Kong Med. 2004;10(1):6–13. [PubMed] [Google Scholar]
- 30.O’Connor A, O’Sullivan PG, Behan L, Norman G, Murphy B. Initial results from the newborn hearing screening programme in Ireland. Ir J Med Sci. 2013;182(4):551–6. 10.1007/s11845-013-0924-z . [DOI] [PubMed] [Google Scholar]
- 31.Watkin P, Baldwin M. The longitudinal follow up of a universal neonatal hearing screen: the implications for confirming deafness in childhood. International journal of audiology. 2012;51(7):519–28. 10.3109/14992027.2012.673237 . [DOI] [PubMed] [Google Scholar]
- 32.Watkin PM. Outcomes of neonatal screening for hearing loss by otoacoustic emission. Archives of disease in childhood Fetal and neonatal edition. 1996;75(3):F158–68. 10.1136/fn.75.3.f158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkin PM, Baldwin M. Identifying deafness in early childhood: requirements after the newborn hearing screen. Arch Dis Child. 2011;96(1):62–6. 10.1136/adc.2010.185819 . [DOI] [PubMed] [Google Scholar]
- 34.Kennedy CR. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment: coverage, positive predictive value, effect on mothers and incremental yield. Wessex Universal Neonatal Screening Trial Group. Acta paediatrica (Oslo, Norway: 1992) Supplement. 1999;88(432):73–5. Epub 2000/01/08. . [DOI] [PubMed] [Google Scholar]
- 35.Wessex Universal Neonatal Hearing Screening Trial Group. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. The Lancet. 1998;352(9145):1957–64. 10.1016/s0140-6736(98)06359-4 [DOI] [PubMed] [Google Scholar]
- 36.Kennedy C, McCann D, Campbell MJ, Kimm L, Thornton R. Universal newborn screening for permanent childhood hearing impairment: an 8-year follow-up of a controlled trial. The Lancet. 2005;366(9486):660–2. 10.1016/s0140-6736(05)67138-3 [DOI] [PubMed] [Google Scholar]
- 37.Adelola OA, Papanikolaou V, Gormley P, Lang J, Keogh IJ. Newborn hearing screening: a regional example for national care. Irish medical journal. 2010;103(5):146–9. . [PubMed] [Google Scholar]
- 38.Aidan D, Avan P, Bonfils P. Auditory screening in neonates by means of transient evoked otoacoustic emissions: a report of 2,842 recordings. The Annals of otology, rhinology, and laryngology. 1999;108(6):525–31. Epub 1999/06/23. 10.1177/000348949910800601 . [DOI] [PubMed] [Google Scholar]
- 39.Bailey HD, Bower C, Krishnaswamy J, Coates HL. Newborn hearing screening in Western Australia. The Medical journal of Australia. 2002;177(4):180–5. . [DOI] [PubMed] [Google Scholar]
- 40.Caluraud S, Marcolla-Bouchetemble A, de Barros A, Moreau-Lenoir F, de Sevin E, Rerolle S, et al. Newborn hearing screening: analysis and outcomes after 100,000 births in Upper-Normandy French region. Int J Pediatr Otorhinolaryngol. 2015;79(6):829–33. Epub 2015/04/19. 10.1016/j.ijporl.2015.03.012 . [DOI] [PubMed] [Google Scholar]
- 41.Fornoff JE, Li G., Bazil C., and Tanner G. Universal Newborn Hearing Screening in Illinois 2003–2004. Springfield, IL: Illinois Department of Public Health; 2006. [Google Scholar]
- 42.Ghirri P, Liumbruno A, Lunardi S, Forli F, Boldrini A, Baggiani A, et al. Universal neonatal audiological screening: experience of the University Hospital of Pisa. Italian journal of pediatrics. 2011;37:16 Epub 2011/04/13. 10.1186/1824-7288-37-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez de Aledo Linos A, Bonilla Miera C, Morales Angulo C, Gomez Da Casa F, Barrasa Benito J. [Universal newborn hearing screening in Cantabria (Spain): results of the first two years]. Anales de pediatria (Barcelona, Spain: 2003). 2005;62(2):135–40. Epub 2005/02/11. . [DOI] [PubMed] [Google Scholar]
- 44.Morales AC, González dALA, Bonilla MC, Mazón GA, Santiuste AF, Barrasa BJ, et al. [Program of hearing loss early detection in newborn infants in Cantabria. Results of the first year of activities]. Acta otorrinolaringologica espanola. 2002;54(7):475–82. [DOI] [PubMed] [Google Scholar]
- 45.Guastini L, Mora R, Dellepiane M, Santomauro V, Mora M, Rocca A, et al. Evaluation of an automated auditory brainstem response in a multi-stage infant hearing screening. European Archives of Oto-Rhino-Laryngology. 2010;267(8):1199–205. 10.1007/s00405-010-1209-z [DOI] [PubMed] [Google Scholar]
- 46.Habib HS, Abdelgaffar H. Neonatal hearing screening with transient evoked otoacoustic emissions in Western Saudi Arabia. International journal of pediatric otorhinolaryngology. 2005;69(6):839–42. 10.1016/j.ijporl.2005.01.018 [DOI] [PubMed] [Google Scholar]
- 47.Magnani C, Bacchi G, Borghini AM, Del Monte D, Fava G, Occasio AM, et al. Universal newborn hearing screening: The experience of the University Hospital of Parma. Acta Biomedica. 2015;86(3):273–7. [PubMed] [Google Scholar]
- 48.Martinez R, Benito JI, Condado MA, Morais D, Fernandez Calvo JL. [Results of one year’s application of a universal protocol for the early detection of hearing loss in neonates]. Acta otorrinolaringologica espanola. 2003;54(5):309–15. Epub 2003/08/15. . [DOI] [PubMed] [Google Scholar]
- 49.Mason JA, Herrmann KR. Universal infant hearing screening by automated auditory brainstem response measurement. Pediatrics. 1998;101(2):221–8. 10.1542/peds.101.2.221 . [DOI] [PubMed] [Google Scholar]
- 50.Mehl AL, Thomson V. The Colorado newborn hearing screening project, 1992–1999: on the threshold of effective population-based universal newborn hearing screening. Pediatrics. 2002;109(1):e7–e. 10.1542/peds.109.1.e7 [DOI] [PubMed] [Google Scholar]
- 51.Metzger D, Pezier TF, Veraguth D. Evaluation of universal newborn hearing screening in Switzerland 2012 and follow-up data for Zurich. Swiss medical weekly. 2013;143:w13905 Epub 2013/12/18. 10.4414/smw.2013.13905 . [DOI] [PubMed] [Google Scholar]
- 52.NSW. Evaluation of the statewide infant screening–hearing (SWISH) program. In: Health Do, editor. 2011.
- 53.Rohlfs AK, Wiesner T, Drews H, Muller F, Breitfuss A, Schiller R, et al. Interdisciplinary approach to design, performance, and quality management in a multicenter newborn hearing screening project. Discussion of the results of newborn hearing screening in Hamburg (part II). European journal of pediatrics. 2010;169(12):1453–63. Epub 2010/06/15. 10.1007/s00431-010-1229-0 . [DOI] [PubMed] [Google Scholar]
- 54.Uilenburg N, Kauffman-de Boer M, van der Ploeg K, Oudesluys-Murphy AM, Verkerk P. An implementation study of neonatal hearing screening in the Netherlands. International journal of audiology. 2009;48(3):108–16. 10.1080/14992020802448992 [DOI] [PubMed] [Google Scholar]
- 55.Uus K, Bamford J. Effectiveness of population-based newborn hearing screening in England: ages of interventions and profile of cases. Pediatrics. 2006;117(5):e887–e93. 10.1542/peds.2005-1064 [DOI] [PubMed] [Google Scholar]
- 56.Van der Ploeg C, Uilenburg N, Kauffman-de Boer M, Oudesluys-Murphy A, Verkerk P. Newborn hearing screening in youth health care in the Netherlands: National results of implementation and follow-up. International journal of audiology. 2012;51(8):584–90. 10.3109/14992027.2012.684402 [DOI] [PubMed] [Google Scholar]
- 57.Van Kerschaver E. Universal neonatal hearing screening in Flanders reveals socio-demographic risk factors for hearing impairment. B-ent. 2013;Suppl 21:3–8. Epub 2014/01/05. . [PubMed] [Google Scholar]
- 58.Van Kerschaver E, Boudewyns A, Stappaerts L, Wuyts F, Van de Heyning P. Organisation of a universal newborn hearing screening programme in Flanders. B-ent. 2006;3(4):185–90. [PubMed] [Google Scholar]
- 59.White KR, Vohr BR, Maxon AB, Behrens TR, McPherson MG, Mauk GW. Screening all newborns for hearing loss using transient evoked otoacoustic emissions. International journal of pediatric otorhinolaryngology. 1994;29(3):203–17. [DOI] [PubMed] [Google Scholar]
- 60.Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent Region, 1985–1993. Br J Audiol. 1997;31(6):409–46. . [DOI] [PubMed] [Google Scholar]
- 61.Benito-Orejas J, Ramirez B, Morais D, Almaraz A, Fernández-Calvo J. Comparison of two-step transient evoked otoacoustic emissions (TEOAE) and automated auditory brainstem response (AABR) for universal newborn hearing screening programs. International journal of pediatric otorhinolaryngology. 2008;72(8):1193–201. 10.1016/j.ijporl.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 62.Vohr BR, Oh W, Stewart EJ, Bentkover JD, Gabbard S, Lemons J, et al. Comparison of costs and referral rates of 3 universal newborn hearing screening protocols. The Journal of pediatrics. 2001;139(2):238–44. 10.1067/mpd.2001.115971 [DOI] [PubMed] [Google Scholar]
- 63.Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. European journal of public health. 2013;23(1):146–52. Epub 2011/12/27. 10.1093/eurpub/ckr176 . [DOI] [PubMed] [Google Scholar]
- 64.Sutton GJ, Rowe SJ. Risk factors for childhood sensorineural hearing loss in the Oxford region. Br J Audiol. 1997;31(1):39–54. . [DOI] [PubMed] [Google Scholar]
- 65.Butcher E, Dezateux C, Knowles RL. Risk factors for permanent childhood hearing impairment. Archives of disease in childhood. 2018:archdischild-2018-315866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vohr BR, Widen JE, Cone-Wesson B, Sininger YS, Gorga MP, Folsom RC, et al. Identification of neonatal hearing impairment: characteristics of infants in the neonatal intensive care unit and well-baby nursery. Ear & Hearing (01960202). 2000;21(5):373–82. Language: English. Entry Date: 20010608. Revision Date: 20150820. Publication Type: Journal Article. [DOI] [PubMed] [Google Scholar]
- 67.Coenraad S, Goedegebure A, van Goudoever JB, Hoeve LJ. Risk factors for sensorineural hearing loss in NICU infants compared to normal hearing NICU controls. Int J Pediatr Otorhinolaryngol. 2010;74(9):999–1002. Epub 2010/06/18. 10.1016/j.ijporl.2010.05.024 . [DOI] [PubMed] [Google Scholar]
- 68.Leeflang MM, Bossuyt PM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. Journal of clinical epidemiology. 2009;62(1):5–12. 10.1016/j.jclinepi.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 69.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Statistics in medicine. 2002;21(11):1525–37. 10.1002/sim.1185 [DOI] [PubMed] [Google Scholar]
- 70.Davis A, Bamford J, Stevens J. Performance of neonatal and infant hearing screens: sensitivity and specificity. Br J Audiol. 2001;35(1):3–15. Epub 2001/04/21. . [DOI] [PubMed] [Google Scholar]
- 71.Psarommatis I, Voudouris C, Kapetanakis I, Athanasiadi F, Douros K. Recovery of Abnormal ABR in Neonates and Infants at Risk of Hearing Loss. International journal of otolaryngology. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gravel JS, Seewald R, editors. Potential pitfalls in the audiological assessment of infants and young children. A sound foundation through early amplification: Conference proceedings from the second international conference; 2001.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOCX)
(DOCX)
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.