Abstract
Background: Resting energy expenditure (REE) is a valuable measure in clinical management of obesity and other chronic illnesses. Gold standard methods for measuring REE (e.g., Douglas bags and metabolic cart) are too expensive and cumbersome for an outpatient clinical setting. The purpose of this study was to determine the accuracy of a handheld indirect calorimeter (HHIC) and prediction equations (PEs) for measurement of REE in youth with and without obesity.
Methods: Fifty-three children and adolescents (12.8 ± 4.3 years, 50.9% female) had REE measured first with a MedGem™ HHIC for 10 minutes, followed by a reference indirect calorimeter system (ParvoMedics TrueOne 2400™) with hood canopy and dilution pump for 30 minutes. REE was also estimated using nine PEs as follows: Henry-1, Henry-2, Schofield, World Health Organization, Molnar, Muller, Herrmann, Schmelzle, and Harris–Benedict. Concordance correlation coefficients and Bland–Altman analyses were used for comparisons among PEs, MedGem HHIC, and metabolic cart.
Results: The observed correlation between the HHIC and the reference system was rc = 0.89 with a mean bias of 2.27 ± 3.41 kcal/(kg·d) (9.1% ± 14.7%). Regarding PE, Molnar had the highest agreement with the reference system [rc = 0.93, bias of 2.17 ± 2.04 kcal/(kg·d); 9.8% ± 8.1%], followed by Harris–Benedict (rc = 0.89; 13.8% ± 8.9%), Henry-2 (rc = 0.89; 15% ± 7.6%), and Henry-1 (rc = 0.86; 16.7% ± 7.3%). All PEs were less accurate for children with overweight/obesity.
Conclusions: Compared to PE, the HHIC provided more accurate REE estimates for children across the age and BMI spectrum, although positive bias was present throughout. Difference in positive bias between the HHIC and the Molnar equation may be clinically significant for youth with overweight/obesity.
Keywords: children and adolescents, handheld calorimeter, prediction equations, resting energy expenditure
Introduction
Measures of resting metabolic demand and caloric needs have become an increasingly valuable tool in clinical management of patients with obesity,1–4 obesity related comorbidities,5 pulmonary diseases,6,7 and other chronic diseases.8–11 Total daily energy expenditure (TDEE) consists of basal metabolic rate, thermogenic effect of food, and energy expenditure associated with physical activity.9
Basal metabolic rate, defined as energy expenditure while fasting and in a state of complete rest immediately after waking, accounts for 60%–75% of TDEE, while resting energy expenditure (REE), defined as the energy required by the body to maintain physiological homeostasis while fasting,9,12 accounts for 50%–70% of TDEE. In a clinical setting, measurement of REE is preferred over measurement of basal metabolic rate due to the ease of data collection and the small differences in contribution to TDEE.9,11 Unfortunately, the highest quality methods for measuring REE (e.g., Douglas bags with an expiratory gas analyzer, open or closed-circuit indirect calorimeters, and/or direct whole body calorimeters) are large and cumbersome, require a skilled technician with expertise in the field, and are not cost-effective nor practical tools for clinical settings.13
Although validated prediction equations (PEs) based on age, sex, and body weight offer a low cost, low skill alternative to direct assessment of REE, there are over 200 equations for estimating REE and they often lack accuracy.9,11 Some evidence suggests that PEs only account for 52%–89% of the variability associated with REE,13,14 may require measures of fat free mass, and are often developed and validated in normal weight and healthy individuals who are not always generalizable to the pediatric patient population.
Small, portable handheld calorimeters, such as the MedGem™ device, offer a third option for clinical measurement of REE. The MedGem is an indirect calorimeter that measures oxygen consumption (VO2) in resting conditions and estimates REE using a modified Weir equation. This type of device is more cost-effective than gas analyzers or direct calorimetry devices, collects data quickly, and requires little training. Although the device has been validated in children, adolescents, and adults,4,9,13 some studies have questioned its accuracy for youth, particularly those with overweight or obesity.15,16
The objective of this investigation was to determine the accuracy of a handheld indirect calorimeter (HHIC) vs. age-specific PEs for measurement of relative REE in youth with and without obesity and compare agreement with REE measured by indirect calorimetry metabolic cart system. We hypothesize that the HHIC will have greater accuracy in measuring relative REE than the age-specific PEs.
Methods
Design and Subjects
In this single center, prospective, cross-sectional comparative investigation, we recruited 53 youth, aged 6–21 years, from The Children's Mercy Hospital (CMH) outpatient clinics and pediatric clinical research unit. The study was approved by the CMH Institutional Review Board. Written informed consent and assent were collected from parents and children before participation in research-related activities.
Male and female subjects, considered medically stable, with normal respiratory function, met BMI criteria for normal (10–84th percentile for age), overweight (85–94th percentile), or obese (≥95th percentile) weight status. Subjects were asked to fast for at least 8 hours and refrain from strenuous physical activity or exercise from when they woke before arriving to their morning study visit. Study visits took place between 7:30 am and 9:30 am. As subjects arrived for the study visit, height (cm) was measured on a wall mounted stadiometer; weight (kg) was measured on a ScaleTronix digital scale. Blood pressure and oxygen saturations were collected using a GE ProCare 100–400 series oscillometric sphygmomanometer. Subjects were then placed in supine position on a standard hospital bed, required to rest, without movement, for a minimum of 10 minutes before the start of the MedGem testing.
While resting, proper technique and instructions for using both the metabolic cart (reference measure) and HHIC REE methods were discussed with both subjects and parents. To avoid fatigue from prolonged testing time, the 10-minute handheld calorimeter measurement was performed first, followed by the 30-minute metabolic cart measurement. Both devices were calibrated before measurement. Depending on testing tolerance, subjects were provided a 20–60 minute (mean 42 ± 18) rest period between the two measures to minimize the chance of physical fatigue impacting the results of subsequent measurements.
Subjects were permitted to use the restroom between measurements but were required to rest, without movement, for a minimum of 10 minutes before the metabolic cart testing. All testing was performed with room lights on to help prevent subjects from falling asleep during testing, in room ambient temperature set to 21°C–23°C. During testing, all subjects watched the same excerpt from a children's film to minimize external distraction, inhibit restlessness, and standardize mental stimulation.
Reference Measure
The TrueOne 2400™ (ParvoMedics, Salt Lake City, UT) metabolic cart system with hood canopy and dilution pump was used as the reference measure.17,18 Before data collection, the Parvo system was calibrated according to the manufacturer's recommendations. As the subject lay supine, a clear hood with a plastic canopy was placed around the subject's head. Subjects were required to remain immobile, without sleeping, throughout the 30-minute sampling process.
The first 5 minutes of data collection were excluded from the analysis, and the 20-minute period with the least amount of variability was used for analysis. Parents/guardians were instructed to refrain from interacting with the subject while the hood and canopy were on. The Parvo system measures oxygen concentration with a paramagnetic analyzer and measures carbon dioxide with an infrared, single beam, single wave-length analyzer. Gas flow is measured with a pneumotachometer with correction for lower flow rates.19,20 This system uses a mixing chamber to overcome intrabreath fluctuations in oxygen and carbon dioxide.
Handheld Indirect Calorimeter
The MedGem HHIC (Microlife USA, Inc., Clearwater, FL) was used to estimate REE. The MedGem is a stand-alone, portable, indirect calorimeter that displays an estimate of REE in kcal/day, based on a subject's measured oxygen uptake over the course of 10 minutes.4 Before data collection, the MedGem device was calibrated according to the manufacturer's recommendations. Although the device starts to collect inspiratory and expiratory oxygen concentration data once the first breath is detected, data from the first 2 minutes of collection are automatically excluded, allowing the REE to be calculated with 8 minutes of data collection.
Subjects were instructed to maintain a supine position with the MedGem analyzer resting on their chest, with one arm supporting the device while the other arm rested by their side. Data collection was performed using techniques described by McDoniel.4 Briefly, oxygen concentration is measured using a proprietary fluorescent-quenching sensor, detecting the deactivation of ruthenium fluorescence in the presence of oxygen. The degree of ruthenium fluorescence suppression, or quenching, is directly proportional to the concentration of oxygen. Ventilatory rate and direction is determined through the difference in the transmission time between the sending and receiving transducer.4 VO2 in mL/min is automatically entered into a preprogrammed modified Weir equation with a fixed respiratory quotient (RQ) of 0.85 representing mixed-macronutrient metabolism.21,22
Prediction Equations
REE was estimated using the following eight PEs developed for children, adolescents, and adults: Henry-1,14 Henry-2,23 Schofield,24 World Health Organization (WHO/FAO),25 Molnar,2 Muller,26 Herrmann,27 and Schmelzle.28 The Harris–Benedict29 equation was also included in the analysis due to its frequent use in clinical settings. Equations were excluded if they require a value representing fat free mass, which is not a variable commonly collected in outpatient clinical settings.12 Since commonly used PEs developed for children and adolescents were tested, only subjects <18 years old were included in the PE analysis (n = 46).
REE Relative to Body Weight
Outcome data from the MedGem device, PEs, and Parvo reference system were calculated in absolute terms (kcal/day). Each subject's absolute REE was divided by their weight in kg and is presented in relative terms as kcal/(kg·d). This approach was selected due to: (1) similar patterns of change in relative REE and basal metabolic rate relative to lean body mass with growth27,30–32; and (2) the regular use of macronutrient and caloric recommendations relative to kg of body weight in medical nutrition therapy for metabolic and other chronic disease.
Statistical Analysis
Independent sample t-tests and chi-square analysis were used to describe the sample. Agreement between REE estimates from the MedGem device and PE vs. the Parvo reference system was calculated by computing the concordance correlation coefficient for each comparison.33 In this comparison, the 45-degree line through the origin of a plot of one set of measurements vs. another indicates perfect agreement between measurements; the concordance correlation coefficient is a measure of fit about the 45-degree line, attenuated by constant and/or proportional bias (i.e., a propensity of one device or calculation to over- or underestimate relative to another).
We further examined agreement between the MedGem device and the Molnar equation by plotting their estimates against the Parvo reference system, along with the 45-degree line of perfect agreement, and generating a Bland–Altman plot for each comparison. The Bland–Altman plots include 95% prediction limits for a newly sampled difference, computed using the standard Gaussian-based formula; these define an interval within which ∼95% of observed differences between measurements are expected to fall. Results are presented for the total patient population (n = 53), as well as subpopulations with normal weight status (BMI <85th percentile for age; n = 36) and overweight/obese weight status (BMI ≥85th percentile for age; n = 17).34
Results
Descriptive statistics is summarized in Table 1. Briefly, the sample had a mean age of 12.8 years, 51% of the sample was female, and 68% of the sample was non-Hispanic white. Compared to children without obesity, children with overweight/obesity were older (mean 14.7 vs. 11.9 years) and 76.5% female. Males had higher REE compared to females, and REE was higher in participants without obesity, irrespective of REE measurement method.
Table 1.
Descriptive Statistics
| Total sample (n = 53) | Males (n = 26) | Females (n = 27) | Normal weight (n = 36) | Overweight/obese (n = 17) | Sex, p-value | Weight status, p-value | |
|---|---|---|---|---|---|---|---|
| Descriptive characteristics | |||||||
| Age (years) | 12.8 ± 4.3 | 11.7 ± 4.2 | 13.9 ± 4.1 | 11.9 ± 4.2 | 14.7 ± 3.8 | 0.06 | 0.03 |
| Sex | 49.1% | 50.9% | 38.9% F | 76.5% F | 0.01 | ||
| Weight (kg) | 55.4 ± 25.8 | 49.3 ± 27.3 | 61.2 ± 23.4 | 43.8 ± 18.4 | 79.8 ± 22.4 | 0.10 | <0.01 |
| Ethnicity | 67.9% | 76.9% | 59.3% | 77.8 | 47.1 | 0.17 | 0.03 |
| White | 32.1% | 23.1% | 40.7% | 22.2 | 52.9 | ||
| Non-white | |||||||
| Direct measures of REE [kcal/(kg·d)] | |||||||
| Parvo system | 25.8 ± 8.1 | 29.5 ± 7.7 | 22.1 ± 6.8 | 29.0 ± 7.7 | 18.9 ± 2.8 | <0.01 | <0.01 |
| MedGem™ | 28.0 ± 9.0 | 31.6 ± 8.6 | 24.6 ± 8.2 | 31.7 ± 8.4 | 20.2 ± 3.8 | <0.01 | <0.01 |
| PEs [kcal/(kg·d)] | |||||||
| Henry-1 | 31.1 ± 8.6 | 34.7 ± 8.1 | 27.2 ± 7.6 | 34.8 ± 7.7 | 22.7 ± 2.8 | <0.01 | <0.01 |
| Henry-2 | 30.6 ± 8.1 | 34.2 ± 6.8 | 26.6 ± 7.5 | 34.1 ± 6.9 | 22.6 ± 3.1 | <0.01 | <0.01 |
| Schofield | 31.1 ± 8.7 | 35.5 ± 7.6 | 26.3 ± 7.3 | 35.0 ± 7.3 | 22.1 ± 3.3 | <0.01 | <0.01 |
| WHO | 31.5 ± 8.4 | 35.2 ± 7.7 | 27.4 ± 7.4 | 35.1 ± 7.4 | 23.3 ± 2.3 | <0.01 | <0.01 |
| Molnar | 29.2 ± 7.7 | 32.1 ± 6.5 | 26.0 ± 7.7 | 32.4 ± 6.8 | 21.8 ± 3.1 | <0.01 | <0.01 |
| Muller | 31.3 ± 9.5 | 35.2 ± 9.1 | 27.0 ± 8.1 | 35.6 ± 8.1 | 21.4 ± 3.0 | <0.01 | <0.01 |
| Herrmann | 28.6 ± 9.7 | 29.6 ± 10.9 | 27.5 ± 8.4 | 32.0 ± 8.9 | 20.7 ± 6.5 | 0.470 | <0.01 |
| Schmelzle | 35.2 ± 18.3 | 39.9 ± 19.3 | 30.0 ± 15.9 | 41.3 ± 18.2 | 21.2 ± 7.6 | 0.07 | <0.01 |
| Harris–Benedict | 30.2 ± 7.7 | 32.8 ± 6.2 | 27.4 ± 8.3 | 33.6 ± 6.5 | 22.3 ± 2.9 | 0.02 | <0.01 |
Results are presented as percent or mean ± standard deviation. REE data are reported as kcal/(kg·d). Chi-square analysis was used for comparison between sex and weight status and ethnicity. Two sample t-tests were used for all other analyses. PE analysis only included subjects who were ≤18 years old (n = 46).
F, female; PE, prediction equation; REE, resting energy expenditure; WHO, World Health Organization.
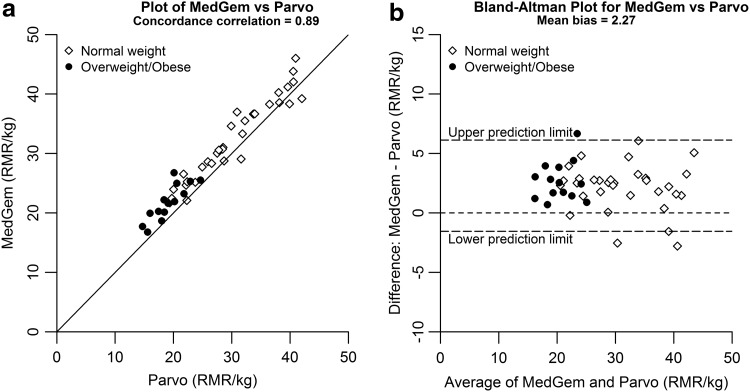
Concordance correlations between the Parvo reference system and the MedGem were strong (rc = 0.89) (Fig. 1A), with the MedGem tending to overestimate REE compared to the reference system [mean bias 9.1% ± 14.7%, 2.3 ± 3.4 kcal/(kg·d)] (Fig. 1B). When analyzed by weight status, the mean overestimation biases were greater for subjects with normal weight [10% ± 14.9%, 2.7 ± 3.6 kcal/(kg·d)] vs. subjects with overweight/obesity [7.3% ± 14.7%, 1.3 ± 2.6 kcal/(kg·d)].
Figure 1.
(a) Plot of MedGem™ vs. Parvo. (b) Bland–Altman plot for MedGem vs. Parvo. RMR, resting metabolic rate.
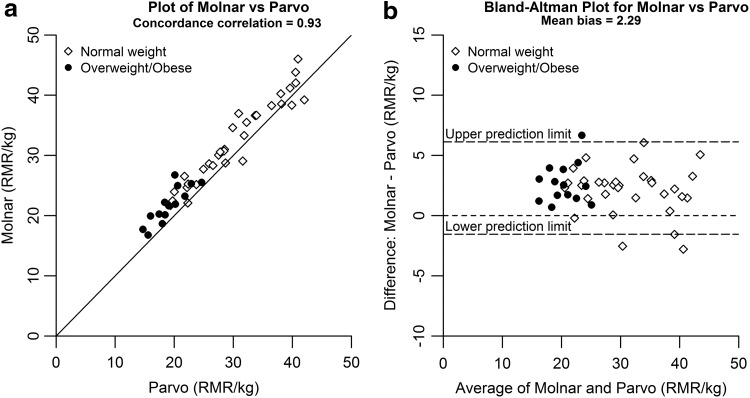
Concordance correlations between the Parvo system and REE PEs varied, ranging from rc = 0.51 to 0.93 (Table 2). The Molnar equation demonstrated the strongest agreement with the Parvo reference measure (rc = 0.93) (Fig. 2A), with slight overestimation compared to the reference [mean bias 9.8% ± 8.1%, 2.3 ± 1.9 kcal/(kg·d)] (Fig. 2B). When analyzed by weight status, the mean bias for REE overestimation by the Molnar equation was greater for subjects with obesity/overweight [14.3% ± 8.8%, 2.7 ± 1.6 kcal/(kg·d)] than those with normal weight [7.8% ± 7%, 2.1 ± 2 kcal/(kg·d)] and higher than the MedGem.
Table 2.
Bias and Concordance Correlations Between Reference System and Prediction Equations
| Equation | Total sample (n = 46) | Normal weight (n = 32) | Overweight/obese (n = 14) | |||
|---|---|---|---|---|---|---|
| Bias (%) | Correlation | Bias (%) | Correlation | Bias (%) | Correlation | |
| Henry-1 | 16.7 ± 7.3 | 0.864 | 15.5 ± 7.7 | 0.810 | 19.4 ± 5.6 | 0.507 |
| Henry-2 | 15.0 ± 7.6 | 0.885 | 13.7 ± 8.2 | 0.840 | 18.2 ± 4.9 | 0.558 |
| Schofield | 16.4 ± 7.5 | 0.863 | 16.6 ± 8.5 | 0.788 | 15.8 ± 5.0 | 0.626 |
| WHO | 18.4 ± 7.7 | 0.845 | 16.7 ± 8.0 | 0.790 | 22.2 ± 5.4 | 0.451 |
| Muller | 16.2 ± 8.1 | 0.853 | 17.9 ± 7.7 | 0.765 | 12.4 ± 7.9 | 0.654 |
| Molnar | 9.8 ± 8.1 | 0.931 | 7.8 ± 7.0 | 0.916 | 14.3 ± 8.8 | 0.588 |
| Herrmann | 6.9 ± 24.3 | 0.801 | 6.0 ± 21.7 | 0.773 | 8.9 ± 30.3 | 0.267 |
| Schmelzle | 26.1 ± 41.5 | 0.510 | 33.6 ± 43.9 | 0.378 | 9.0 ± 30.0 | 0.428 |
| Harris–Benedict | 13.8 ± 8.9 | 0.886 | 12.5 ± 9.8 | 0.833 | 16.9 ± 5.8 | 0.569 |
Prediction equation analysis only included subjects who were ≤18 years old. Bias as presented as a percent ± standard deviation.
Figure 2.
(a) Plot of Molnar vs. Parvo. (b) Bland–Altman plot for Molnar vs. Parvo.
Discussion
Despite the growing value of REE as a tool for treatment of obesity1–4 and other chronic illnesses,5–11 there are notable inconsistencies in the literature regarding the most accurate, feasible, and cost-effective method to measure REE in children in a clinical setting. Our aim was to determine the accuracy of a HHIC and PEs for measurement of relative REE in youth, hypothesizing that the handheld analyzer will have greater accuracy than the age-specific PEs.
We found the handheld MedGem device to provide reasonably accurate REE measurements for children and adolescents with and without obesity. In our study, the observed overestimation in REE by the MedGem compared to the reference measure was much less in individuals with overweight/obesity than those with normal weight status [6.8% ± 16.2%, 1.3 ± 2.9 kcal/(kg·d) vs. 10.3% ± 14.9%, 2.8 ± 3.7 kcal/(kg·d), respectively].
Our observed overestimation of ∼2 kcal/(kg·d) is in agreement with original pediatric and adult findings in children and adults.4,9,13,35 Specifically, the mean bias observed for the MedGem vs. the reference in our study (9.1%) was similar to Fields et al.,16 which noted a positive bias of 8%, but higher than the study by Nieman et al.,13,35 which found a negative bias of 1.2% in children. These differences may be explained by notable methodological differences between studies. Nieman et al. used Douglas bags for collection of expired gasses, which are considered the “gold standard” method for indirect calorimetry.35 In this study, as well as the studies by Woo et al.,15 Fields et al.,16 and Frankenfield and Coleman,36 standard metabolic cart systems (ParvoMedics TrueOne 2400, Vmax Encore VE29n, Deltatrac II, and Deltatrac MB101, respectively) were used as reference measures for REE.
Although these metabolic carts are generally accepted as appropriate reference systems,4 each comes with its own degree of bias,37,38 which may be additive when comparing REE results to another device, such as the MedGem. Since the MedGem device only measures VO2, without measure of CO2 production (VCO2), REE is calculated using a modified Weir equation, which assumes a fixed RQ of 0.85. The difference between this assumed RQ and the RQ measured by the reference device may be substantially different depending on the reference device used (e.g., Douglas bag vs. metabolic cart). We observed that the MedGem bias was 3.5% less in subjects with overweight/obesity compared to subjects with normal weight.
This difference may be partially explained by differences in measured vs. assumed RQ, as a higher resting RQ is expected for individuals with increased body weight and fat mass.39 In our study, the mean RQ measured by the Parvo metabolic cart was 0.896 (∼5% higher than the RQ assumed by the MedGem). However, when we applied a fixed RQ of 0.85 to the measured oxygen consumption generated by the Parvo system, the mean observed REE was 25.73 kcal/(kg·d), which is 0.04 kcal/(kg·d) lower than the mean reference REE generated by the Parvo.
Inconsistencies in trends toward over- or underestimation of REE by the MedGem among studies may also be secondary to variability in body position during REE measurement. Nieman et al.13 assessed both the reference measure and the MedGem while subjects were in a seated upright position, with subjects holding the device, whereas Woo et al.15 and Fields et al.16 assessed the reference measure while supine and MedGem while seated upright, with subjects holding the device. When Fields et al.16 tested the difference between the seated vs. supine position, they found a 56 kcal/day difference between body positions for the MedGem. Melanson et al. found that holding the MedGem while seated resulted in a 60 kcal/day increase in energy expenditure compared to the supine position.40 To minimize external bias between the two systems evaluated in our study, we specifically collected both the reference measure and the MedGem measure while subjects were supine, with the weight of the analyzer resting on their chest.
Similar to Marra et al.,12 we found substantial differences in REE among the nine REE PEs evaluated. Much of this variability may be explained by notable differences in subject populations for which these equations were originally developed and validated. Of the nine equations tested, only Henry-2, Schofield, WHO, Muller, and Herrmann were validated in a heterogeneous sample of youth, similar to the population in our study. The other equations were developed exclusively for adolescents (Molnar, Henry-1), adults (Harris–Benedict), or specifically for youth with obesity (Schmelzle).
Interestingly, although the Molnar equation was developed for children 10–17 years of age with normal or overweight, but not obese weight status, it had the strongest agreement with REE measured by the reference system in our study population of 6–18 years old with weight status across the BMI spectrum. When the MedGem and the Molnar equation were compared, we found that both overestimated REE compared to the reference system, but the Molnar overestimated 0.65% more compared to the MedGem. Importantly, the difference between the MedGem and Molnar equation were highest for children with overweight/obesity, for whom the Molnar equation predicted REE was 7.1% higher than REE measured by the MedGem, with a correlation coefficient of only rc = 0.59 compared to the Parvo reference system. The correlation coefficient for the Schmelzle equation, which was developed specifically for children with obesity, was even lower compared to the reference system.
Although REE is typically reported as kcal/day, this method may not be appropriate in youth. Absolute measures of REE increase as the child ages, while relative measures of REE [kcal/(kg·time)] decrease secondary to the expected 50%–60% reduction in basal metabolic rate relative to lean body mass associated with aging.27,30–32 Studies suggest that expressing REE in relative terms may aid clinicians in identifying individuals who are hypo- or hypermetabolic by accounting for degree of variability in body mass.41 In addition, it is common for clinical medical dietitians to prescribe specific macronutrient and micronutrient recommendations relative to kg of body weight. Although we believe relative measures are superior to absolute measures of energy expenditure in children and adolescents, future studies to investigate this relationship in youth and evidence-based recommendations are needed.
Limitations
This study has some limitations that should be addressed. Our sample size is modest (n = 53), but comparable to other recent studies commenting on the validity of the MedGem device for youth.15 Although the metabolic cart system is highly regarded as a sufficient method for measuring REE,17,18 technically, the Douglas bag method has higher accuracy and is considered the “gold standard” for measuring REE. Specialized equipment is necessary for expiratory gas analysis with a Douglas bag which is not readily available/accessible. We elected to perform MedGem testing first, to prevent participant fatigue before MedGem administration, as the Parvo measurement requires 30 minutes of participant cooperation. Thus, the order of testing between MedGem and the Parvo system was not varied or randomized, which is a limitation and may have presented some bias between outcomes due to order effects.
Conclusions
Measures of resting metabolic demand and caloric needs have become an increasingly valuable tool in clinical management of obesity and chronic illness. High quality methods for measuring REE are too expensive and time consuming for a clinical setting,13 where handheld devices and PEs provide cost-effective alternatives. We conclude that, compared to PEs, including ones developed specifically for children with overweight/obesity, the handheld MedGem device provides the most accurate estimate of REE for children across age and BMI spectrums, with a small positive bias compared to the reference measure. When using this device, clinicians should recognize that there may be some overestimation of a child/adolescent's REE, and if the data are used to recommend specific caloric goals (in cases of weight management), some adjustment or correction may be necessary. The Molnar equation may also provide acceptable accuracy, as the overall discrepancy in REE overestimation between the MedGem and Molnar equation (0.65%) may not be clinically significant for most children, but is likely to be significant for children with overweight/obesity.
Acknowledgments
The authors thank the subjects and families for participating in this study. This research was supported by the Marion Merrell Dow Clinical Scholar Award from the Children's Mercy Kansas City (V.S., PI) and support from the NIH Loan Repay Program (L40 TR000598; V.S., PI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ekelund U, Franks PW, Wareham NJ, et al. . Oxygen uptakes adjusted for body composition in normal‐weight and obese adolescents. Obesity 2004;12:513–520 [DOI] [PubMed] [Google Scholar]
- 2. Molnár D, Jeges S, Erhardt E, et al. . Measured and predicted resting metabolic rate in obese and nonobese adolescents. J Pediatr 1995;127:571–577 [DOI] [PubMed] [Google Scholar]
- 3. Müller M, Geisler C. From the past to future: From energy expenditure to energy intake to energy expenditure. Eur J Clin Nutr 2017;71:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDoniel SO. A systematic review on use of a handheld indirect calorimeter to assess energy needs in adults and children. Int J Sport Nutr Exerc Metab 2007;17:491–500 [DOI] [PubMed] [Google Scholar]
- 5. Martincevic I, Mouzaki M. Resting energy expenditure of children and adolescents with nonalcoholic fatty liver disease. JPEN J Parenter Enteral Nutr 2017;41:1195–1201 [DOI] [PubMed] [Google Scholar]
- 6. Fuentes D, Suarez L, Soto T, et al. . Resting energy expenditure in children with cystic fibrosis: Measurement with a new handheld calorimeter. Clin Nutr 2003;22:S72–S73 [Google Scholar]
- 7. Schols A, Schoffelen P, Ceulemans H, et al. . Measurement of resting energy expenditure in patients with chronic obstructive pulmonary disease in a clinical setting. JPEN J Parenter Enteral Nutr 1992;16:364–368 [DOI] [PubMed] [Google Scholar]
- 8. Fullmer S, Benson-Davies S, Earthman CP, et al. . Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet 2015;115:1417 .e2–1446.e2 [DOI] [PubMed] [Google Scholar]
- 9. Hipskind P, Glass C, Charlton D, et al. . Do handheld calorimeters have a role in assessment of nutrition needs in hospitalized patients? A systematic review of literature. Nutr Clin Pract 2011;26:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Compher C, Hise M, Sternberg A, et al. . Comparison between MedGem and Deltatrac resting metabolic rate measurements. Eur J Clin Nutr 2005;59:1136. [DOI] [PubMed] [Google Scholar]
- 11. Tan SY, Poh BK, Jamal R, et al. . Predicting energy requirements of pediatric patients with disease: Which methods are appropriate? Ped Health 2010;4:479–489 [Google Scholar]
- 12. Marra M, Montagnese C, Sammarco R, et al. . Accuracy of predictive equations for estimating resting energy expenditure in obese adolescents. J Pediatr 2015;166:1390 .e1–1396.e1 [DOI] [PubMed] [Google Scholar]
- 13. Nieman DC, Austin MD, Chilcote SM, et al. . Validation of a new handheld device for measuring resting metabolic rate and oxygen consumption in children. Int J Sport Nutr Exerc Metab 2005;15:186–194 [DOI] [PubMed] [Google Scholar]
- 14. Henry C, Dyer S, Ghusain-Choueiri A. New equations to estimate basal metabolic rate in children aged 10–15 years. Eur J Clin Nutr 1999;53:134. [DOI] [PubMed] [Google Scholar]
- 15. Woo P, Murthy G, Wong C, et al. . Assessing resting energy expenditure in overweight and obese adolescents in a clinical setting: Validity of a handheld indirect calorimeter. Pediatr Res 2016;81:51. [DOI] [PubMed] [Google Scholar]
- 16. Fields DA, Kearney JT, Copeland KC. MedGem hand‐held indirect calorimeter is valid for resting energy expenditure measurement in healthy children. Obesity 2006;14:1755–1761 [DOI] [PubMed] [Google Scholar]
- 17. Woods AL, Garvican-Lewis LA, Rice AJ, et al. . The ventilation-corrected ParvoMedics TrueOne 2400 provides a valid and reliable assessment of resting metabolic rate (RMR) in athletes compared with the Douglas Bag method. Int J Sport Nutr Exerc Metab 2016;26:454–463 [DOI] [PubMed] [Google Scholar]
- 18. Crouter SE, Antczak A, Hudak JR, et al. . Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol 2006;98:139–151 [DOI] [PubMed] [Google Scholar]
- 19. Yeh MP, Adams TD, Gardner RM, et al. . Turbine flowmeter vs. Fleisch pneumotachometer: A comparative study for exercise testing. J Appl Physiol 1987;63:1289–1295 [DOI] [PubMed] [Google Scholar]
- 20. Yeh MP, Gardner RM, Adams TD, et al. . Computerized determination of pneumotachometer characteristics using a calibrated syringe. J Appl Physiol 1982;53:280–285 [DOI] [PubMed] [Google Scholar]
- 21. Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition 1990;6:213–221 [PubMed] [Google Scholar]
- 22. Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henry C. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr 2005;8:1133–1152 [DOI] [PubMed] [Google Scholar]
- 24. Schofield W. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39:5–41 [PubMed] [Google Scholar]
- 25. Food and Agriculture Organization, World Health Organization, United Nations. Energy and protein requirements. Report of a joint FAO/WHO/UNI expert consultation. In: Health W. (ed), WHO Technical Report. World Health Organization, Geneva, Switzerland, 1985 [PubMed] [Google Scholar]
- 26. Müller MJ, Bosy-Westphal A, Klaus S, et al. . World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr 2004;80:1379–1390 [DOI] [PubMed] [Google Scholar]
- 27. Herrmann SD, McMurray RG, Kim Y, et al. . The influence of physical characteristics on the resting energy expenditure of youth: A meta‐analysis. Am J Hum Biol 2017;29:e22944. [DOI] [PubMed] [Google Scholar]
- 28. Schmelzle H, Schroder C, Armbrust S, et al. . Resting energy expenditure in obese children aged 4 to 15 years: Measured versus predicted data. Acta Paediatr 2004;93:739–746 [DOI] [PubMed] [Google Scholar]
- 29. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A 1918;4:370–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malina RM, Bouchard C, Bar-Or O. Growth, Maturation, and Physical Activity. Human Kinetics, Champaign, IL, 2004 [Google Scholar]
- 31. Gallagher D, Belmonte D, Deurenberg P, et al. . Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol Endocrinol Metab 1998; 275:E249–E258 [DOI] [PubMed] [Google Scholar]
- 32. Lee S, Arslanian SA. Fat oxidation in black and white youth: A metabolic phenotype potentially predisposing black girls to obesity. J Clin Endocrinol Metab 2008;93:4547–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255–268 [PubMed] [Google Scholar]
- 34. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data 2000;314:1–27 [PubMed] [Google Scholar]
- 35. Nieman DC, Trone GA, Austin MD. A new handheld device for measuring resting metabolic rate and oxygen consumption. J Am Diet Assoc 2003;103:588–593 [DOI] [PubMed] [Google Scholar]
- 36. Frankenfield DC, Coleman A. An evaluation of a handheld indirect calorimeter against a standard calorimeter in obese and nonobese adults. JPEN J Parenter Enteral Nutr 2013;37:652–658 [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Wang Y, Ding Z, et al. . Relative validity of an indirect calorimetry device for measuring resting energy expenditure and respiratory quotient. Asia Pac J Clin Nutr 2018;27:72. [DOI] [PubMed] [Google Scholar]
- 38. Cooper JA, Watras AC, O'Brien MJ, et al. . Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc 2009;109:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shook R, Hand G, Paluch A, et al. . High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur J Clin Nutr 2016;70:1197. [DOI] [PubMed] [Google Scholar]
- 40. Melanson EL, Coelho LB, Tran ZV, et al. . Validation of the BodyGem hand-held calorimeter. Int J Obes Relat Metab Disord 2004;28:1479–1484 [DOI] [PubMed] [Google Scholar]
- 41. Battezzati A, Viganò R. Indirect calorimetry and nutritional problems in clinical practice. Acta Diabetol 2001;38:1–5 [DOI] [PubMed] [Google Scholar]