Abstract
Motivation improves the efficiency of intentional behavior, but how this performance modulation is instantiated in the human brain remains unclear. We used a reward-cued antisaccade paradigm to investigate how motivational goals (the expectation of a reward for good performance) modulate patterns of neural activation and functional connectivity to improve preparation for antisaccade performance. Behaviorally, subjects performed better (faster and more accurate antisaccades) when they knew they would be rewarded for good performance. Reward anticipation was associated with increased activation in the ventral and dorsal striatum, and cortical oculomotor regions. Functional connectivity between the caudate nucleus and cortical oculomotor control structures predicted individual differences in the behavioral benefit of reward anticipation. We conclude that although both dorsal and ventral striatal circuitry are involved in the anticipation of reward, only the dorsal striatum and its connected cortical network is involved in the direct modulation of oculomotor behavior by motivational incentive.
Introduction
Actions are faster when rewards are expected (Ramnani and Miall, 2003; Milstein and Dorris, 2007). Neural activity in numerous brain areas is modulated by expected reward, but it remains unclear where and how the reward-modulated signal is transformed into altered motor outputs. Reward anticipation increases activation in a complex network including the striatum (nucleus accumbens, putamen and caudate), amygdala, and orbitofrontal cortex (Knutson et al., 2001; Gottfried et al., 2003; Knutson and Cooper, 2005; Haber and Knutson, 2010). By interfacing with cortical and subcortical structures involved in motor control, reward signals may bias or modulate activity in motor structures, thus boosting motor function.
The oculomotor network includes subcortical structures [caudate, substantia nigra pars reticulata (SNr), thalamus, superior colliculus (SC)], cortical eye fields [frontal (FEF) and parietal eye fields], the presupplementary motor area, and parts of the dorsolateral prefrontal cortex (Petit et al., 1996; Law et al., 1997; Pierrot-Deseilligny et al., 2003; Lynch and Tian, 2006). For simplicity, we use the term “oculomotor control structures,” although, in addition to their shared eye-movement control functions, the described regions also participate in other functions such as memory, decision making, attention, and planning of motor actions (Lynch and Tian, 2006). These control structures can bias saccades directly by interfacing with the superior colliculus, as well as indirectly through the caudate, which can render the superior colliculus more receptive for cortical input (Hikosaka et al., 2006; Grahn et al., 2008).
Thus, reward anticipation and oculomotor networks overlap in the caudate. Indeed, in nonhuman primates the caudate incorporates reward anticipation into movement-related decision making (Pasquereau et al., 2007) and into the control of oculomotor action (Lauwereyns et al., 2002). Moreover, primate caudate neurons whose firing patterns correspond to the speed and accuracy of imminent saccades also respond to reward cues (Takikawa et al., 2002a; Kawagoe et al., 2004). Similarly, reward-anticipation signals in the human caudate may lead to enhancement of signal transmission within the oculomotor network and thus boost oculomotor control.
Here we investigated whether the human caudate acts as an interface between the reward and oculomotor circuits that can improve saccade performance. We manipulated incentive motivation during goal-directed oculomotor action in the antisaccade task. For the generation of antisaccades, subjects inhibited eye movements toward a peripheral stimulus and instead saccaded in the opposite direction (Hallett, 1978; Munoz and Everling, 2004). This requires deliberate control over automatic reflexes. Reward-anticipation cues informed participants that quick and accurate saccades will be rewarded. Using fMRI, we verified the engagement of both dorsal and ventral striatal circuitry in processing reward anticipation. Moreover, we tested whether reward-anticipation cues modulated activation of the striatum and enhanced functional connectivity between the striatum and cortical eye fields. Specifically, because the caudate plays a prominent role in the oculomotor network, whereas the role of the nucleus accumbens in saccades is less pronounced, we hypothesized that individual differences in reward-anticipation performance benefits will predict (1) caudate (rather than accumbens) activation in response to reward-prospect cues, and (2) reward-cue-modulated functional connectivity between caudate (rather than accumbens) and cortical eye fields.
Materials and Methods
Participants
Fourteen healthy right-handed volunteers (10 females; mean age, 22.7 ± 2) with normal or corrected-to-normal vision participated in the experiment. None of the participants had a history of neurological or psychiatric disorders or eye problems.
Procedures
The experiment was divided into a training session and a main experimental session. During training, participants were presented with a series of trials to familiarize themselves with the stimulus-reward associations and antisaccade response requirements. Participants completed a 25 min training block and two 25 min experimental blocks inside the scanner, each subtending 128 trials. The participants were paid €50 for participation. On top of that, they could win a monetary reward of a maximum of €3.20 per block during the practice session and a maximum of €12.80 per block during the experimental session. All experimental procedures were approved by a local ethics committee, and conducted in accordance with the Helsinki Declaration, international laws, and institutional guidelines.
Task: temporal order of stimulus presentation
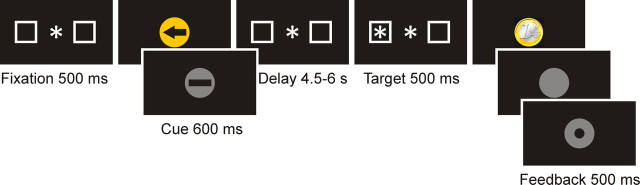
One experimental block subtended 128 trials. Each trial (Fig. 1) started with a central fixation dot surrounded by two square outlines (each subtending 3.8° visual angle) on the left and right side of the fixation dot (distance 12.4°). After this fixation display, a central visual instruction cue was presented (for 600 ms) followed by a variable delay of 4.5–6 s, terminated by a peripheral antisaccade target (a white asterisk subtending 2°). The antisaccade target was presented for 500 ms pseudorandomly in the center of the left or the right square outline. The target indicated that participants should make an immediate eye movement to the opposite side of the screen. This antisaccadic eye movement required deliberate control over automatic reflexes (Hallett, 1978; Munoz and Everling, 2004). Their response was immediately followed by presentation of a feedback image (presented for 500 ms). The length of the delay period between the visual instruction cue and the antisaccade target varied across trials between 4.5 and 6 s. A black screen with jittered duration (16, 500, 1000, and 1500 ms) was displayed between trials.
Figure 1.
Temporal order of stimuli in the antisaccade task. In reward-anticipation trials, the instruction cue was a gold circle; in no-reward-anticipation trials, the instruction cue was a silver circle. In direction-specific preparation trials, an arrow was displayed, indicating where the subsequent antisaccade target would appear; in direction- nonspecific preparation trials, a bar was presented. The length of the preparation interval between the instruction cue and the antisaccade target was varied between 4.5 and 6 s. Immediately after the antisaccade, a golden coin was displayed when a reward had been awarded; a silver circle was presented after a correct response on a nonreward trial, and after an incorrect or slow response a silver ring was displayed.
Task: instruction cues—reward anticipation and oculomotor preparation
To investigate the effect of reward anticipation and oculomotor preparation on antisaccade performance, we presented instruction cues before the appearance of the peripheral antisaccade target. In a two-by-two factorial design, the instruction cues independently manipulated the level of reward expectation (two levels: reward and no reward expected) and the level of response preparation (two levels: direction-specific oculomotor preparation or direction-nonspecific oculomotor preparation of the antisaccade response) by means of color and shape and were presented in pseudorandom order. This pseudorandom reward cuing is based on the principle that dopamine neurons in the reward system respond to the cue positively (with a phasic increase in firing) if the cue indicates an upcoming reward and they respond to the cue negatively (with a phasic decrease in firing) if the cue indicates no reward (Schultz et al., 1992; Kawagoe et al., 2004).
The level of reward expectation was manipulated by the color of the instruction cue: in reward trials the instruction cue was a gold circle; in neutral trials the instruction cue was a silver circle. The colors of the reward and the neutral cue colors were calibrated to equal luminance using Colorfacts 7 and EyeOne Monitor.
The level of oculomotor preparation was manipulated by the content of the instruction cue: in direction-specific preparation trials, an arrow was displayed in the center of the circle, indicating where the antisaccade target would appear; in the direction-nonspecific preparation trials, a bar replaced the arrow. The arrow enabled subjects to prepare for the spatial location of the antisaccade cue, whereas a bar would give no information on target location.
Task: feedback—reward, no reward, and error
The feedback indicated to participants whether the response was correct or whether the trial was rewarded. On rewarded trials, the reward was symbolically represented as an image of a golden euro coin. On neutral trials, a silver blank disk of the same size, shape, and luminance was displayed. After an incorrect or too-slow response, a silver ring with a black circle in the middle was presented. Colors of rewarded, nonrewarded, and error feedback were calibrated to equal luminance using Colorfacts 7 and the color calibration system EyeOne Monitor. Participants were informed that they would receive a monetary reward on golden reward trials in which they performed fast and correctly but not on the silver neutral trials. In line with other imaging work with monetary reward (Ramnani and Miall, 2003), the exact monetary value was not displayed in the feedback to avoid mental calculation.
Eye-tracking and stimulus delivery setup
During MRI scanning, the participant's left eye was continuously monitored with an MRI-compatible infrared oculographic limbus tracker (Resonance Technology) attached to the head coil and placed 3 cm beneath the participant's left eye. Eye movements were recorded with ViewPoint Eyetracker PC-60 (version 2.7; Arrington Research) software on a standard PC. Bidirectional communication between this PC and a second one responsible for the delivery of stimuli (using Presentation software; Neurobs) ensured that stimulus onset times were registered in the eye-movement data and that adequate feedback was provided to oculomotor responses on each trial. Eye movements were registered with a sampling rate of 60 Hz along with signals marking the stimulus onset times. Before task onset, a nine-point calibration procedure was performed. Calibration and stimuli were presented on a 66 × 88 cm screen, placed at a 4 m viewing distance at the front end of the scanner and seen through a mirror above the participants' heads. Light in the scanning environment was constrained to video presentation of stimuli against a black background. To eliminate slow drift in eye-tracking signal during the task, calibrated eye position was manually corrected to the central fixation cross. Regions of interest were defined by two peripheral outer square outlines (the endpoints of the antisaccade eye movements) surrounding the central fixation dot. The PC that tracked eye movements signaled to the stimulus presentation PC when an eye movement left the fixation region and entered one of the target regions. The Presentation PC recorded correct trials versus errors and presented feedback accordingly.
Analysis
Saccade parameters were detected with software developed in-house and implemented in Java 1.5 using minimum amplitude (>1.5°) and velocity (>30°/s) criteria, and were visually inspected and double checked for accuracy. In line with common definitions (Fischer et al., 1993), saccades with a latency of <80 ms after the display of the peripheral antisaccade target were classified as anticipatory responses. Exclusion criteria applied to trials in which participants failed to focus their eyes on the central instruction cue, trials in which gaze was not at fixation 200 ms before target appearance, trials with blinks during saccadic execution, and trials exceeding 800 ms (miss). A trial without a premature eye movement toward the peripheral antisaccade target and with a saccade landing at the location of the square outline on the opposite side of the screen executed within 800 ms was classified as a correct trial. Only correct trials (95.4 ± 1.1%) were taken into account to estimate the mean saccadic onset latency, defined as the time required to initiate a saccade toward a target after its presentation (Dorris et al., 1997; Munoz et al., 2000). Antisaccade latencies and accuracy were analyzed with a 2 × 2 × 4 × 2 × 2 within-subjects ANOVA design with Bonferroni correction. One factor was reward expectation (two levels: reward vs no reward expected for a well-performed saccade), and one factor was spatial response preparation (two levels: direction-specific cue vs direction-nonspecific cue). To control for interaction effects with reward expectation or spatial preparation, we added three factors to the analysis: (1) the delay between the cue and the target (four levels: 4.5, 5, 5.5, and 6 s), (2) cue direction (two levels: left and right), and (3) run (two levels: first and second).
fMRI acquisition, GLM, and functional connectivity
Acquisition.
Functional images were acquired on a Philips 3 T MRI system equipped with echo planar imaging (EPI) capabilities using a standard head coil for radio frequency transmission and signal reception. Functional scans of the entire brain were acquired with a single-shot, gradient-recalled EPI sequence parallel to the anterior commissure–posterior commissure plane (TE, 28 ms; TR, 2000 ms; 30 transversal slices; slice thickness, 3 mm; interslice gap, 0.7 mm; FOV, 240 mm). The first two volumes were discarded to allow for T1 equilibration effects. The duration of the antisaccade task was two times 23 min and 44 s (712 scans per scan block). High-resolution anatomical images were subsequently acquired using a three-dimensional T1-weighted scan in the steady-state sequence (TE, 4.6 ms; TR, 9.69 ms; 182 sagittal slices; slice thickness, 1.2 mm; interslice gap, 0.3 mm; FOV, 250 mm).
Preprocessing and GLM.
Preprocessing of the functional data and calculation of the contrast images for statistical analysis was done with FEAT (FMRI Expert Analysis Tool) version 5.63, a part of FSL [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library]. Functional images were realigned to compensate for small head movements, slice-time corrected, spatially smoothed with a 5 mm full-width at half-maximum Gaussian kernel, and filtered in the temporal domain using a high-pass filter with a cutoff frequency of 1/50 Hz to correct for baseline drifts in the signal and prewhitened (Woolrich et al., 2009). For each experimental run of each participant, the overall activity evoked by correct responses and error commission was modeled for reward anticipation (two levels: reward anticipation and no reward anticipation) and for the level of action preparation (two levels: direction-specific preparation and direction-nonspecific preparation), and each regressor was convolved by a prototypical synthetic hemodynamic response function. To remove any artifactual signal changes resulting from head motion, six parameters describing the head movements (three translations, three rotations) were included as confounds in the model. In the second-stage analysis, participants were treated as a fixed factor to concatenate the two experimental runs. Contrasts pertaining to the main effects of the factorial design constituted the data for the third-stage (mixed-effect) analysis, where the significance of observations was determined across the group of 14 subjects using FLAME 1 and 2 (FMRIB's Local Analysis of Mixed Effects) (Smith et al., 2004).
To investigate anticipatory reward and direction-specific preparation processes before the actual antisaccade response, we modeled evoked hemodynamic responses (EHRs) time locked to the instruction cues (reward vs nonreward and spatial response preparation vs no spatial response preparation) separately from the EHRs time locked to the antisaccade target. This was facilitated by introducing a delay period between the instruction cue and the antisaccade target varying across trials between 4.5 and 6 s. Delay lengths were evenly distributed across conditions and cue directions. A black screen with jittered duration (16, 500, 1000, or 1500 ms) and “null events” (10% of the trials with fixation only and with “real trial” duration) was displayed between trials such that the occurrence of trial events and onsets of fMRI volumes were not synchronized, allowing hemodynamic response to return to baseline during null events. Preceding the experiment, we ran simulations to establish the optimal delay lengths, intertrial lengths, and trial order. This simulation ensured that the BOLD responses to cue and target were sufficiently separated: the correlations between the different fMRI model regressors were sufficiently low to allow a reliable estimation of the hemodynamic responses on the time point of the instruction cue independent of the hemodynamic response on the actual oculomotor response (the antisaccade target). The simulation procedure was similar to that in freely available software, such as Optseq (Dale, 1999), although implemented in custom software using the design matrix engine of SPM2. Following criteria laid out by Dale (1999) and Smith et al. (2007), we created a program that generated a large number of potential delay lengths, intertrial lengths, and trial sequences, from which a convolved design matrix was crated. These design matrices were in turn evaluated with respect to the following criteria: (1) the correlations between the different regressors in the design are kept below 0.33, giving a sufficiently low shared variance between regressors and ensuring that contrasts between all regressors can be calculated reliably; (2) the lowest frequency of each of the regressors is above the high-pass filter setting, ensuring that none of the experimental effects are filtered out.
From the potential design matrices, we chose those that ensured we were able to reliably estimate the model and evaluate the contrasts of interest. This procedure is identical to the one that was used for previous studies using similar designs (Toni et al., 1999, 2002; Mars et al., 2007, 2008).
The four types of instruction cues were modeled as four separate event types (reward prospect and direction-specific oculomotor preparation; reward prospect and direction-nonspecific oculomotor preparation; no reward prospect and direction-specific oculomotor preparation; no reward prospect and direction-nonspecific oculomotor preparation). Antisaccade targets for all four trial types were treated as a single, fifth event type to model the hemodynamic responses related to the antisaccade target and the subjects' antisaccade response. Parameters describing head motion calculated during preprocessing were included as covariates to model residual effects of head motion. For each whole-brain comparison of reward-anticipation versus non-reward-anticipation trials, and for spatial preparation versus no spatial preparation trials, a cluster-corrected threshold of p < 0.001 corrected for whole-brain multiple comparisons was set using Gaussian random field theory (GRFT). In the subsequent whole-brain covariance analysis with individual oculomotor values (antisaccadic latency, antisaccadic accuracy), we report cortical regions with a threshold of p < 0.05, cluster corrected for whole-brain multiple comparisons (using GRFT).
Functional connectivity analysis.
To test for changes in connectivity between our striatal seed regions and other brain areas, we performed an analysis of functional connectivity with the psychophysiological interaction (PPI) method (Friston et al., 1997). Functional connectivity results from significantly correlated hemodynamic response patterns over time between distant brain regions as a function of the experimental task context. The PPI method does not give information about directionality. It makes inferences about regionally specific responses caused by the interaction between the experimentally manipulated psychological factor and the hemodynamic response in a specified seed region. The present PPI analysis was constructed to test for differences in the regression slope of striatal brain areas (caudate, putamen, and nucleus accumbens) on other brain areas, depending on whether participants expected reward or no reward for a oculomotor response and whether they could spatially prepare for the upcoming oculomotor response or not. Based on electrophysiological findings in nonhuman primates (Hikosaka et al., 2006), the caudate, putamen, and nucleus accumbens represent a priori regions of interest (ROIs). These seed regions were specified based on the MNI structural atlas of the FSL atlas toolbox (Mazziotta et al., 1995; Eickhoff et al., 2007). After the extraction of the ROI time courses (caudate, putamen, and nucleus accumbens) and the psychological vector of interest (weighting reward-anticipation cues with 1 and non-reward-anticipation cues with −1), their interaction term was computed (for a detailed description, see Cohen et al., 2005, 2008). The procedure was repeated for the other vector of interest (direction-specific vs direction-nonspecific oculomotor preparation cue). For each individual, this procedure yielded a functional connectivity map identifying areas where BOLD signal changes were temporally coupled with signal changes derived from striatal seeds as induced by the relative difference between the instruction cues (reward greater than no reward anticipation, and direction-specific preparation greater than direction-nonspecific oculomotor preparation). The resulting parameter estimate at each voxel thus represents the extent to which activity in each voxel correlates with the ,tk;2seed region in reward conditions more than in nonreward conditions (and in direction-specific preparation conditions more than in direction-nonspecific preparation conditions, respectively).
The two experimental runs were concatenated and third-level group analyses were conducted with a cluster-corrected statistical threshold of z > 2.3 and p < 0.05, correcting for whole-brain multiple comparisons. The individual functional activation and functional connectivity data were superimposed onto the participants' individual high-resolution anatomical images, and the resulting registration images were normalized toward a common stereotactic space (MNI) by linear scaling. Relevant anatomical landmarks were identified with the Harvard–Oxford cortical structural atlas and the Juelich histological atlas (Eickhoff et al., 2007) implemented in the FSL atlas toolbox.
Individual differences covariance analyses.
To investigate the relationship between the neural response during reward anticipation versus no reward anticipation and the behavioral benefit in the rewarded relative to the neutral condition, we computed the relative reaction time (RT) advantages as [(RTno reward − RTreward)/RTno reward] for all subjects, in line with common definitions (Schott et al., 2007). Accordingly, we computed the behavioral benefit from specific oculomotor preparation as [(RTneutral prep − RTspec prep)/RTneutral prep]. The resulting individual performance benefits in antisaccade latency were orthogonalized with the group mean separately for each instruction cue type. These demeaned condition-related benefits for each subject were incorporated as a covariate in two separate GLM regression models (fitted response to reward anticipation vs no reward anticipation and fitted response to specific oculomotor preparation vs neutral oculomotor preparation) and all voxels exceeding the cluster-corrected threshold of 0.05 in the mean z-map were determined.
The same demeaned benefit values were entered as covariates into two separate PPI analyses (fitted response to reward anticipation vs no reward anticipation and fitted response to specific oculomotor preparation vs neutral preparation), and all parameter estimates at each voxel exceeding the cluster-corrected threshold of p = 0.05 and z > 2.3 in the mean z-map were determined. Thus, apart from explaining changes in functional activation and connectivity on the basis of the instruction cue alone, it was assessed which neural changes during the display of the instruction cue covaried with the magnitude of the behavioral benefit.
We quantified the difference of the strength of the behaviorally modulated functional connectivity (caudate vs accumbens) with the cortical eye fields (frontal eye fields, intraparietal sulcus) and presupplementary motor area. The cortical eye fields and the presupplementary motor area represented a priori regions of interest as they have been linked to antisaccade control (Petit et al., 1996; Law et al., 1997; Pierrot-Deseilligny et al., 2003; Lynch and Tian, 2006). The definition of the masks for the frontal eye fields, intraparietal sulcus, and the presupplementary motor area was based on a combination of functional and anatomical landmarks. The functional landmarks were derived from the PPI-covariation analysis of the caudate. These were voxels in the frontal eye fields, intraparietal sulcus, and presupplementary motor area correlating with functional activation in the caudate during reward anticipation (vs no reward anticipation) and covarying with the magnitude of the oculomotor latency benefit (cluster corrected, p < 0.05, z > 2.3). According to PET and fMRI studies in humans (Paus, 1996; Petit et al., 1996), the human FEF is located in the vicinity of the precentral sulcus and/or in the caudalmost part of the superior frontal sulcus, just inferior and rostral to the premotor cortex. The anatomical landmarks for the precentral gyrus, superior frontal gyrus, and premotor cortex were derived from the Juelich histological atlas (premotor cortex, BA 6) (Eickhoff et al., 2007) and the Harvard–Oxford cortical structural atlas (precentral gyrus) implemented in the FSL atlas toolbox. The anatomical landmarks for the intraparietal sulcus were derived from the Juelich histological atlas (anterior intraparietal sulcus) (Eickhoff et al., 2007). The anatomical landmarks for the presupplementary motor area were derived from the Harvard–Oxford cortical structural atlas (juxtapositional lobule cortex, formerly the supplementary motor cortex) implemented in the FSL atlas toolbox (for an illustration of the masks, see supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
Statistical comparison of the strength of the dorsal striatal (caudate) behaviorally modulated functional connectivity with the strength of the ventral striatal (accumbens) behaviorally modulated functional connectivity with the cortical eye fields (frontal eye fields, intraparietal sulcus) and the presupplementary motor area was conducted by transforming the correlations at all voxels within each mask (frontal eye fields, intraparietal sulcus, and presupplementary motor area) to Z values, averaging all voxels within each mask, and then comparing the averaged Z values for dorsal striatal versus ventral striatal behaviorally mediated connectivity. Under the null hypothesis of no differences, the Z value differences are normally distributed with a mean of zero and a variance of one. Thus, the observed difference Z value can be converted to a p value and interpreted as the likelihood of significantly stronger behaviorally modulated functional connectivity between the dorsal striatum and the cortical oculomotor regions, compared to behaviorally modulated functional connectivity between the ventral striatum and the cortical oculomotor regions.
Results
Effect of reward anticipation and specific oculomotor preparation on antisaccade performance
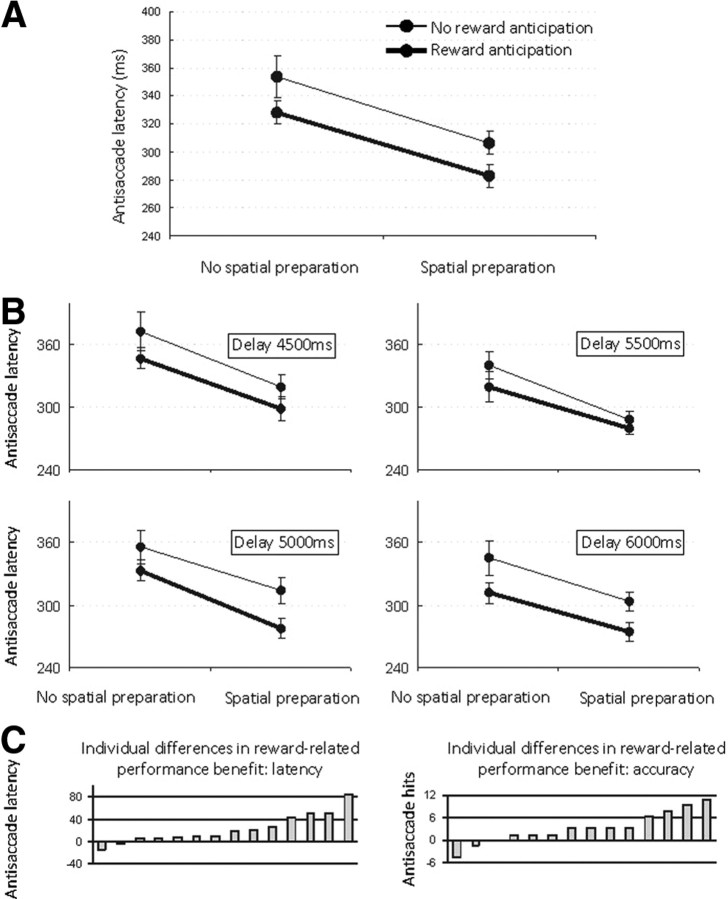
Mean onset latency and accuracy of antisaccades was 318 ± 9 ms (SE) and 95.9 ± 0.8%. Omissions rarely occurred (1.6 ± 0.6%). A 2 × 2 × 4 × 2 × 2 GLM repeated-measures analysis [reward expectation (reward prospect/no reward prospect), response preparation (direction-specific/direction-nonspecific oculomotor preparation), delay length (four levels, 4.5, 5, 5.5, and 6 s), run (1/2), and cue-direction (left/right)] revealed the following patter of outcomes. Antisaccade latency decreased in a linear fashion with the delay length between cue and target: the more time available to prepare the antisaccade, the shorter the antisaccade latency (from a mean latency of 335 ± 10 ms in the shortest delay down to 309 ± 9 ms in the longest delay; F(3,11) = 7.89; p < 0.001), suggesting that subjects used the delay between cue and target for antisaccade preparation.
There was a main effect of the instruction cues signaling reward (reward expectation/no reward expectation) and providing a priori information on the response (direction-specific/direction-nonspecific oculomotor preparation) on antisaccade performance (Fig. 2A). Subjects' mean antisaccade latency and antisaccade accuracy were significantly reduced when they expected reward for a well-performed antisaccade. The significant effect of reward expectation on antisaccade latency (F(1,13) = 12.87; p < 0.007) amounted to a 25 ms (± 6.8 ms) decrease in latency and occurred regardless of the level of specific oculomotor preparation (F(1,13) = 0.04; p < 0.854), cue-target delay length (F(3,11) = 1.30; p < 0.299) (Fig. 2B), run (F(1,13) = 0.15; p < 0.706), and cue direction (F(1,13) = 0.25; p < 0.632).
Figure 2.
Effects of instruction cues on antisaccade onset latencies. A, Average antisaccade onset latency was significantly faster on reward-anticipation trials than on trials where no reward was expected, irrespectively of the level of spatial preparation. Average antisaccade onset latency was significantly faster on trials when a priori direction-specific knowledge on the upcoming response was available than on trials with no a priori direction-specific knowledge. B, Delay length between instruction cue and target (4500, 5000, 5500, or 6000) did not interact with the effect of reward anticipation or direction-specific (spatial) response preparation on antisaccade latency. Subjects' overall antisaccade latencies decreased linearly with increasing delay lengths indicating that the delay was used for antisaccade preparation. C, Reward-related benefit on average antisaccade onset latency and on antisaccade accuracy varied across participants. Error bars indicate meant ± SEM.
The same applied to the significant effect of reward expectation on antisaccade accuracy (F(1,13) = 7.85; p < 0.023), amounting to a 3.1 ± 1.1% increase in accuracy and occurring regardless of the level of spatial response preparation (F(1,13) = 2.69; p < 0.140), cue-target delay length (F(3,11) = 0.29; p < 0.831), run (F(1,13) = 1.08; p < 0.328), and cue direction (F(1,13) = 0.33; p < 0.583). Participants differed in the extent to which reward improved their antisaccade latency and accuracy (Fig. 2C). The reward-related decrease in latency and the reward-related increase in accuracy showed a trend toward negative correlation although this correlation failed to obtain statistical significance (r = −0.474; p = 0.087).
Direction-specific oculomotor preparation cues facilitated the onset latency of antisaccades (46 ± 6.8 ms; F(1,13) = 46.01; p < 0.0001) regardless of reward expectation (F(1,13) = 0.04; p < 0.854), cue-target delay length (F(3,11) = 0.46; p < 0.71), run (F(1,13) = 4.36; p < 0.070), and cue direction (F(1,13) = 2.86; p < 0.129). Likewise, direction-specific preparation cues improved antisaccade accuracy (4.1 ±1.6%; F(1,13) = 6.99; p < 0.030) regardless of reward expectation (F(1,13) = 2.69; p < 0.140), cue-target delay length (F(3,11) = 0.37; p < 0.78), run (F(1,13) = 0.224; p < 0.648), and cue direction (F(1,13) = 2.59; p < 0.146). As cue-target delay length, run, and cue direction did not interact with incentive motivation benefits nor with the direction-specific oculomotor preparation benefits, we pooled across the levels of these factors in the remainder of the analyses.
BOLD responses
Preparation for an antisaccade when a reward (vs no reward) is at stake
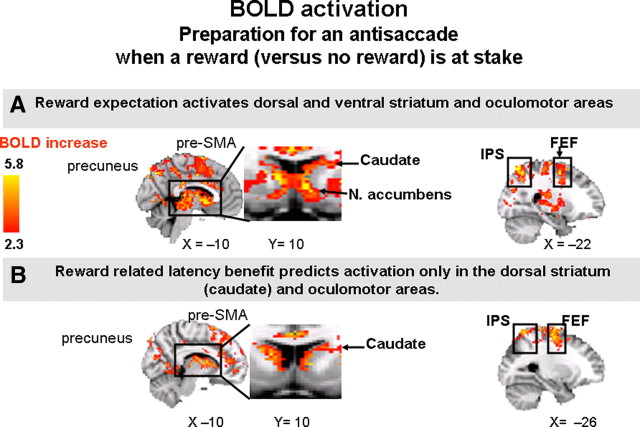
Compared to the no-reward-anticipation cues, reward-anticipation cues (main effect of reward anticipation) induced shorter antisaccade latencies and activated a widespread anticipatory subcortical–cortical network (Fig. 3A). All activation foci are listed in Table 1, including the ventral striatum (nucleus accumbens) and the dorsal striatum (caudate, putamen); additional subcortical areas (amygdala, thalamus) insula, orbitofrontal cortex, dorsal and rostral anterior cingulate, and presupplementary motor area; and cortical areas of the oculomotor control system (frontal eye fields, intraparietal sulcus, parietal occipital junction, precuneus).
Figure 3.
BOLD activation. A, Anatomical localization of regions showing significant positive increase when expecting reward for a well-performed antisaccade. Renderings (on MNI stereotactic space) are thresholded at z > 2.3. Left, Sagittal rendering (lateral view) and coronal rendering (medial view) showing both dorsal (caudate) and ventral striatum (nucleus accumbens) clusters and oculomotor clusters [presupplementary motor area (pre-SMA) and precuneus]. Right, Sagittal rendering showing activation clusters in oculomotor areas [FEFs and intraparietal sulcus (IPS)]. B, Anatomical localization of regions showing significant positive increase when antisaccade latency profits from reward expectation. Left, Sagittal rendering (lateral view) and coronal rendering (medial view) showing only dorsal striatum (caudate) clusters and oculomotor clusters (pre-SMA and precuneus). Right, Sagittal rendering showing activation clusters in oculomotor areas (FEFs and IPS).
Table 1.
Brain regions showing significant functional activation during preparation for an antisaccade when a reward (vs no reward) is at stake
| Brain region | X | Y | Z | Max z |
|---|---|---|---|---|
| R caudate nucleus | 12 | 10 | 6 | 5.83 |
| L caudate nucleus | −16 | 14 | 14 | 5.10 |
| R putamen | 20 | 8 | 4 | 6.95 |
| L putamen | −26 | 8 | 4 | 6.55 |
| R accumbens | 6 | 10 | −6 | 4.41 |
| L accumbens | −12 | 18 | −8 | 5.37 |
| L orbitofrontal cortex | −22 | 34 | −14 | 4.56 |
| R pallidum | 16 | 6 | 0 | 5.61 |
| L pallidum | −18 | 2 | 2 | 4.14 |
| L amygdala | −28 | 2 | −18 | 3.88 |
| R amygdala | 22 | 2 | −18 | 4.09 |
| R thalamus | 10 | −24 | 2 | 5.67 |
| L thalamus | −6 | −24 | 4 | 5.52 |
| L insula cortex | −44 | 10 | −4 | 6.52 |
| R insula cortex | 38 | 16 | 0 | 6.03 |
| L middle frontal gyrus | −40 | 36 | 28 | 5.64 |
| R middle frontal gyrus | 38 | 38 | 28 | 5.36 |
| Dorsal anterior cingulate | 2 | 20 | 30 | 6.62 |
| Rostral anterior cingulate | 4 | 36 | 14 | 4.24 |
| R frontal eye fields | 26 | −4 | 58 | 6.23 |
| L frontal eye fields | −28 | −8 | 60 | 6.53 |
| Presupplementary motor area | −6 | 6 | 54 | 5.53 |
| R intraparietal sulcus | 32 | −42 | 42 | 6.02 |
| L intraparietal sulcus | −32 | −48 | 42 | 5.58 |
| R inferior frontal gyrus | 38 | 34 | 2 | 5.14 |
| R superior lateral occipital cortex | 30 | −76 | 22 | 5.86 |
| L superior lateral occipital cortex | −34 | −76 | 16 | 6.56 |
| R temporal pole | 16 | 66 | 33 | 6.73 |
| L temporal pole | −56 | 6 | −8 | 5.85 |
| Precuneus | −2 | −70 | 44 | 5.45 |
Local maxima of activation of all significant clusters (at z > 2.3; p = 0.001; cluster corrected) are displayed. All coordinates are given in MNI space. R, Right; L, left.
Although the variable delay between reward cue and target was implemented as jitter to separate the BOLD response to cue and target, delay length might interact with the effects of reward versus no reward. We conducted fMRI analyses to test whether the difference in sustained activation between reward and nonreward trials (in the reward vs nonreward contrast) (Fig. 3) varied with the length of the delay (4.5, 5, 5.5, or 6 s). Similar to the lack of interaction in the behavioral results, we observed little difference in the hemodynamic response whether using a parametric sustained model or a phasic cue-locked model (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Comparing the immediate with the sustained response activations, the results showed similar striatocortical areas (cluster-corrected threshold of z > 2. 3 and p < 0.001 corrected for whole-brain multiple comparisons).
To ensure that the dorsal striatal–cortical activation patterns are specific for motivational incentives, we verified whether the activation patterns observed for behavioral benefits from reward anticipation were also seen for benefits from direction-specific oculomotor preparation. Cues with spatial a priori information on the upcoming oculomotor response (vs cues without such information) induced shorter antisaccade latencies and a higher level of preparatory cortical activation of the frontal eye fields, intraparietal sulcus, and precuneus and in temporal lobe and visual areas (supplemental Fig. 2, supplemental Table 1a, available at www.jneurosci.org as supplemental material). Importantly, however, in contrast to reward-anticipation cues, direction-specific oculomotor preparation cues failed to induce anticipatory activity in subcortical structures, a finding speaking against the possibility of generic preparatory effects.
In line with the behavioral results, neural reward anticipation affected both the direction-specific and the direction-nonspecific oculomotor preparation condition (main effect of reward anticipation). The preparatory cortical–subcortical pattern of reward anticipation was not significantly modulated by the context of direction-specific oculomotor preparation, as was evident from calculating an interaction contrast between reward versus no reward anticipation and direction-specific oculomotor versus direction-nonspecific oculomotor preparation.
Reward-anticipation BOLD signal covaries with reward-related performance benefit
To identify structures under reward anticipation that predict reward-related performance benefits, we covaried the BOLD signal during reward anticipation (compared to no reward anticipation) with individual differences in reward-related advantages in antisaccade latency and accuracy. Reward-related latency benefits covaried with activation of the caudate, cortical oculomotor control structures (frontal eye fields, intraparietal sulcus, parietal occipital junction, and precuneus) as well as posterior medial frontal, insula, and somatosensory cortex (Fig. 2B; Table 2). To ensure that these effects are attributable specifically to the beneficial effect or reward and not to a general effect of oculomotor preparation, we ran an additional covariation analysis (cluster corrected, z > 2.3; p < 0.05) to test if this subcortical–cortical coactivation during reward anticipation (compared to no reward anticipation) covaried with individual differences in latency benefits from direction-specific oculomotor preparation, and with individual differences in overall antisaccade latency. The results confirmed sucortical–cortical coactivation with individual differences in reward-related latency benefits only, suggesting that the striatal–cortical activation patterns are specific for behavioral benefits from reward anticipation. The specific dorsal striatal–cortical coactivation pattern during reward-related antisaccade latency benefits was further confirmed by covariation analysis (cluster corrected, z > 2.3; p < 0.05) with reward-related accuracy benefits. Accuracy benefits from reward anticipation predicted topographically similar patterns: activation in the caudate and in oculomotor control structures (intraparietal sulcus, frontral eye fields, presupplementary motor area, and precuneus) but not in the nucleus accumbens (supplemental Fig. 3b, available at www.jneurosci.org as supplemental material). It is however important to note that reward-related latency and accuracy benefits showed a trend toward negative correlation (r = −0.474; p = 0.087), which renders these two measures partially dependent.
Table 2.
Brain regions in which activation during reward versus no reward anticipation during antisaccade preparation, covaried with reward-related antisaccade latency benefit
| Brain region | X | Y | Z | Max z |
|---|---|---|---|---|
| R caudate | 14 | 6 | 12 | 5.81 |
| L caudate | −14 | 4 | 14 | 5.21 |
| L putamen | −26 | 4 | 4 | 4.05 |
| R insula | 38 | −8 | −4 | 3.50 |
| R frontal eye fields | 24 | −10 | 70 | 3.62 |
| L frontal eye fields | −28 | −10 | 64 | 6.17 |
| Presupplementary motor area | −6 | 6 | 54 | 6.36 |
| R intraparietal sulcus | 42 | −40 | 38 | 4.93 |
| L intraparietal sulcus | −42 | −34 | 38 | 4.19 |
| Dorsal anterior cingulate cortex | 10 | 20 | 44 | 4.99 |
| L parietal occipital junction | −28 | −62 | 62 | 5.36 |
| R parietal occipital junction | 30 | −54 | 62 | 5.11 |
| L somatosensory cortex | −30 | −32 | 58 | 3.75 |
| R somatosensory cortex | 26 | −34 | 64 | 4.25 |
| Precuneus | −14 | −44 | 46 | 4.23 |
| Cerebellum | 2 | −52 | −6 | 2.91 |
| R superior temporal gyrus | 54 | −26 | 4 | 4.11 |
| L superior temporal gyrus | −56 | −12 | 0 | 4.01 |
| R brainstem | 8 | −20 | −10 | 3.25 |
| L brainstem | −6 | −18 | −8 | 2.58 |
Local maxima of activation of all significant clusters (at z > 2.3; p = 0.05; cluster corrected) are displayed. All coordinates are given in MNI space. R, Right; L, left.
Functional connectivity between striatal and cortical regions
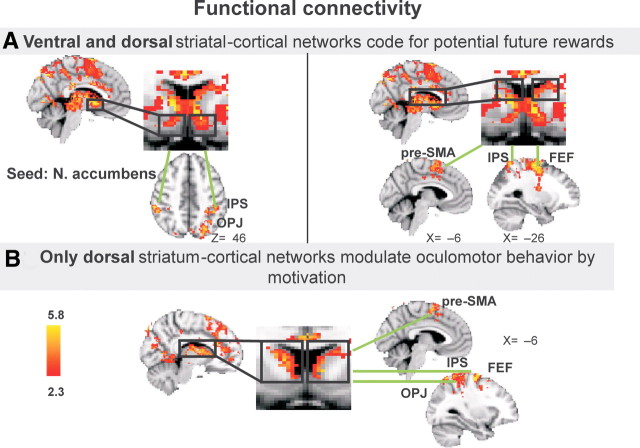
Functional connectivity (estimated through the psychophysiological interaction method) (see Materials and Methods) analyses showed increased functional connectivity during reward anticipation of the nucleus accumbens and the caudate with areas of the oculomotor system (intraparietal sulcus) and with visual and somatosensory areas (Fig. 4A; all brain areas showing functional connectivity with the caudate and the nucleus accumbens are listed in Table 3) (cluster corrected, z > 2.3; p < 0.05). The caudate displayed increased functional connectivity with the frontal eye fields, intraparietal sulcus, presupplementary motor area, parietal occipital junction, and the precuneus, whereas the nucleus accumbens showed increased functional connectivity with posterior parietal regions (intraparietal sulcus and occipital parietal junction). The putamen did not increase functional connectivity during reward anticipation (cluster corrected, z > 2.3; p < 0.05). Together, both the caudate and nucleus accumbens increase their functional connectivity with frontal and parietal oculomotor areas when a reward is at stake for an antisaccade.
Figure 4.
Functional connectivity. A, Both ventral– and dorsal striatal–cortical networks code for potential reward. Left, Anatomical localization of regions showing significant functional connectivity with the nucleus accumbens when expecting reward for a well performed antisaccade. Green lines connect regions that exhibit significant functional coupling with the seed region, placed in the nucleus accumbens (in the black boxes). Renderings are thresholded at z > 2.3. The axial rendering (top view) shows intraparietal sulcus (IPS) and occipital parietal junction (OPJ) clusters. Right, Anatomical localization of regions showing significant functional connectivity with the caudate when expecting reward for a well-performed antisaccade. Green lines connect regions that exhibit significant functional connectivity with the seed region in the caudate (in the black boxes). Renderings are thresholded at z > 2.3. The sagittal rendering (lateral view) shows FEFs, IPS, and OPJ clusters. B, Only dorsal striatal–cortical networks, and not ventral striatal–cortical networks, are involved in the modulation of antisaccade performance by motivation. Anatomical localization of regions showing significant functional connectivity with the caudate (when expecting reward for a well-performed antisaccade) and predicting antisaccade latency profits from reward expectation is shown. Green lines connect regions that exhibit significant functional connectivity with the seed region in the caudate (in the black boxes). Renderings are thresholded at z > 2.3. The sagittal rendering (lateral view) shows presupplementary motor area (pre-SMA), FEFs, IPS, and OPJ clusters. The functional connectivity of the nucleus accumbens (when expecting reward for a well-performed antisaccade) did not vary with reward-related performance benefit.
Table 3.
Brain regions showing significant functional connectivity with caudate and nucleus accumbens during preparation for an antisaccade when a reward (vs no reward) is expected
| Brain region | X | Y | Z | Max z |
|---|---|---|---|---|
| Caudate | ||||
| R frontal eye fields | 24 | −10 | 70 | 3.62 |
| L frontal eye fields | −28 | −10 | 64 | 6.17 |
| Presupplementary motor area | −6 | 6 | 54 | 6.36 |
| R intraparietal sulcus | 42 | −40 | 38 | 4.93 |
| L intraparietal sulcus | −42 | −34 | 38 | 4.19 |
| Dorsal anterior cingulate cortex | 10 | 20 | 44 | 4.99 |
| L parietal occipital junction | −28 | −62 | 62 | 5.36 |
| R parietal occipital junction | 30 | −54 | 62 | 5.11 |
| L somatosensory cortex | −30 | −32 | 58 | 3.75 |
| R somatosensory cortex | 26 | −34 | 64 | 4.25 |
| Nucleus accumbens | ||||
| R intraparietal sulcus | 58 | −38 | 48 | 3.03 |
| L intraparietal sulcus | −42 | −42 | 46 | 3.04 |
| R superior lateral occipital cortex | 40 | −76 | 14 | 2.80 |
| L superior lateral occipital cortex | −36 | −76 | 24 | 2.52 |
| L somatosensory cortex | −30 | −44 | 50 | 2.83 |
| R somatosensory cortex | 60 | −36 | 44 | 2.90 |
Local maxima of activation of all significant clusters (at z > 2.3; p = 0.05; cluster corrected) are displayed. All coordinates are given in MNI space. R, Right; L, left. The putamen is empty.
To link this functional connectivity pattern during reward anticipation to the magnitude of behavioral benefits, we covaried the striatum-seeded (caudate, putamen, and nucleus accumbens) functional connectivity results during reward anticipation (vs no reward anticipation) with individual differences in reward-related advantages in antisaccade latency and accuracy Significantly higher functional connectivity (cluster corrected, z > 2.3; p < 0.05) only between caudate and components of the oculomotor control network (frontal eye fields, intraparietal sulcus, parietal–occipital junction, and supplementary motor area) and between caudate and dorsal anterior cingulate cortex and somatosensory cortex during reward anticipation predicted higher reward-related latency benefits (Fig. 4B; all striatal functional connectivity during reward anticipation varying with reward-related latency benefits is listed in Table 4). Accumbens-seeded functional connectivity analysis confirmed the observed shift of the hemodynamic response from the ventral to the dorsal striatum when reward anticipation interfaced with antisaccade behavior. The functional connectivity pattern of the nucleus accumbens during reward anticipation with parietal oculomotor areas (intraparietal sulcus and occipital parietal junction) was unrelated to reward-related performance benefits (cluster corrected, z > 2.3; p > 0.05).
Table 4.
Brain regions in which functional connectivity of caudate during reward-versus no reward anticipation (while preparing for an antisaccade), covaried with reward-related antisaccade latency benefit
| Brain region | X | Y | Z | Max z |
|---|---|---|---|---|
| Dorsal anterior cingulate cortex | 12 | 18 | 42 | 5.09 |
| L frontal eye fields | −26 | −12 | 56 | 6.37 |
| R supplementary motor area | 8 | −6 | 64 | 5.140 |
| L supplementary motor area | −10 | −4 | 72 | 4.54 |
| L intraparietal sulcus | −36 | −52 | 40 | 4.26 |
| L parietal occipital junction | −16 | −66 | 68 | 5.75 |
| R parietal occipital junction | 18 | −62 | 66 | 4.61 |
| L somatosensory cortex | −26 | −60 | 64 | 5.32 |
| R somatosensory cortex | 32 | −46 | 68 | 4.42 |
| R premotor cortex (superior frontal gyrus) | 18 | 12 | 62 | 5.46 |
| L premotor cortex (superior frontal gyrus) | −18 | 14 | 62 | 4.73 |
Local maxima of activation of all significant clusters (at z > 2.3; p = 0.05; cluster corrected) are displayed. All coordinates are given in MNI space. R, Right; L, left. The putamen and nucleus accumbens are empty.
To ensure that the observed behaviorally (latency) modulated functional connectivity patterns during reward anticipation are attributable to the beneficial effect of reward and not to a general effect of preparation, we repeated the covariation analysis above, except instead of using reward-related benefit on latency, we used the specific oculomotor direction preparation latency benefit. The results showed that the functional connectivity of the caudate during reward anticipation was not significantly modulated by the specific oculomotor preparation benefit (cluster corrected, z > 2.3; p > 0.05).
Direct statistical comparison between the endpoints of the dorsal and ventral striatal pathways in the frontal eye fields and intraparietal sulcus, which varied with reward-related behavior, further confirmed the ventral-to-dorsal shift of striatal connectivity when it comes to reward–oculomotor action interfaces. During reward anticipation, the caudate showed significantly stronger functional connectivity with the frontal eye fields that was predictive of reward-related behavior than the nucleus accumbens (p = 0.015). The same pattern was statistically significant (uncorrected threshold, p < 0.05) in the intraparietal sulcus (p = 0.011), and was in the same direction but not statistically significant in the presupplementary motor area (p = 0.126). To further test this caudate–reward benefit functional connectivity link, we replicated this analysis using the reward cue benefit on accuracy (instead of saccade latency). Reward-related benefits in accuracy showed topographically similar functional connectivity patterns than reward-related latency benefits. Functional connectivity of putamen and nucleus accumbens during reward anticipation was again unrelated to reward-related accuracy benefits (cluster corrected, z > 2.3; p > 0.05) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
This pattern of results suggests that the nucleus accumbens may play a role in generic reward-related action monitoring, whereas the caudate plays a more specific role in using reward information to guide actions by functionally interfacing with cortical areas involved in saccade planning and execution.
Discussion
We showed that whereas both dorsal and ventral striatal circuitry are involved in the anticipation of potential future rewards, the dorsal striatum and its connections to cortical networks are involved directly in the modulation of oculomotor behavior by motivation. Subjects performed better (faster and more accurate antisaccades) when they could receive rewards for good performance. This performance increase was larger with stronger anticipatory functional connectivity between the caudate (but not the putamen or nucleus accumbens) and cortical oculomotor control structures (frontal eye fields, intraparietal sulcus, presupplementary motor area, parietal occipital junction, and precuneus). This is the first study to show how functional connectivity between the caudate and cortical oculomotor structures is key to motivated action in humans. These results bridge human and primate functional anatomy by revealing similar striatocortical oculomotor networks with a similar motivation/action interface in the caudate that transforms motivational incentive information into eye-movement preparation (Lauwereyns et al., 2002; Watanabe and Hikosaka, 2005; Lynch and Tian, 2006).
Though reward anticipation induced increased activation in both the ventral (accumbens) and dorsal striatum (caudate, putamen), only activation of the caudate was related to individual differences in the behavioral benefit from reward anticipation. Furthermore, analysis of functional connectivity confirmed that only the functional connectivity between the caudate (not accumbens or putamen) and cortical oculomotor structures was associated with reward-related benefits. As the cortical oculomotor structures are strongly interconnected, the current results do not allow for a precise characterization of the roles of the individual oculomotor structures in the motivational modulation of oculomotor performance. However, based on previous imaging work, one might interpret the observed frontal eye field activation and functional connectivity with the caudate before faster latencies as enhanced readiness and intention to perform, commonly referred to as the “preparatory set” (Connolly et al., 2002, 2005).
The PPI approach cannot be used to make inferences about directionality. Therefore, identified patterns of connectivity must be viewed in the context of known anatomical directionality analyses performed with specific anterograde and retrograde tracers in the oculomotor system of nonhuman primates (for review, see Lynch and Tian, 2006). Tracer injections in the frontal eye fields (Cui et al., 2003), parietal eye fields (Cavada and Goldman-Rakic, 1991; Baizer et al., 1993), and precuneus (Leichnetz et al., 1984) produce large and dense terminal fields in the caudate and less dense terminals in the putamen, suggesting that the cortical oculomotor structures may provide top-down signals to motor processes in the caudate. A caudate modulation of the intraparietal sulcus and the parietal occipital junction presumably reflects the motivational modulation of attentional, memory, and visual processes (Culham and Kanwisher, 2001; Valyear et al., 2006). The modulation of the presupplementary motor area, a structure involved in a priori voluntary saccadic control (Curtis and d'Esposito, 2003; Stuphorn and Schall, 2006), might reflect motivational prioritization processes in the selection of intended movement and the suppression of reflexive behavior.
This interfacing function of the caudate parallels findings from a number of primate studies that have underlined the role of the caudate in transforming motivational information into eye-movement signals (Sato and Hikosaka, 2002; Hikosaka et al., 2006; Nakamura and Hikosaka, 2006). Many caudate neurons fire not only before the onset of an expected saccade target, but also in preparatory responding to signals that predict reward. In an oculomotor task, neurons of the primate caudate were found to respond in anticipation of reward-predicting stimuli, which in turn modulated neural oculomotor signals (Kawagoe et al., 1998, 2004) and corresponded to the saccade onset latency (Takikawa et al., 2002b). Based on animal models of oculomotor control, a supporting network role of the caudate is possible for direction-specific oculomotor preparation as well. However, in line with previous imaging work (Curtis and Connolly, 2008), we observed no significant involvement of the caudate during direction-specific oculomotor preparation; increased preparatory activation was observed only in cortical oculomotor structures. Presumably, motivation-mediated oculomotor preparation capitalizes on the caudate to a greater extent, and the signal is therefore stronger and more likely to become manifest in the hemodynamic response.
When compared with network models of primate oculomotor control, the caudate–cortical functional connectivity in our human data parallels the indirect basal ganglia oculomotor pathway in nonhuman primates. This indirect pathway, involved in saccade execution, is situated as a side path, secondary to the direct pathway of the cortical oculomotor structures to the SC located on the roof of the brainstem to execute saccades (Hikosaka et al., 2006). The indirect basal ganglia pathway of the oculomotor network originates in the caudate and converges via the SNr on the SC to act as a gate for saccade execution by inducing GABAergic inhibition on the SC. When stimuli attract attention and gaze, the direct excitatory inputs from many brain areas to the SC are often incapable of, or slow in, activating SC neurons. The caudate, however, can interrupt this tonic inhibition and open the SC for the cortical inputs to increase saccade readiness (Hikosaka et al., 2000, 2006; Grahn et al., 2008). Hence, the observed increased functional connectivity between the caudate and the cortical oculomotor structures might reflect an indirect subcortical–cortical–subcortical loop in the human oculomotor system that is receptive to striatal reward signal. The current analyses of functional connectivity, however, do not allow inference about directionality of this effect. The reward expectation signal in the caudate may project “upward” to the cortical eye fields to mediate the formation of early action plans. The cortical oculomotor structures (intraparietal sulcus and frontal eye fields) in turn may project “downward” into the indirect path, back to the caudate, which can gate the SC for direct cortical input. Reward-related responses during decision making and action control have been reported previously in the primate intraparietal sulcus (Rorie et al., 2010) and posterior parietal cortex (Dorris and Glimcher, 2004). Through “looping” striatocortical interactions, the cortical and subcortical parts of the oculomotor network may communicate and coordinate their activity. This coordinated activity between the cortical oculomotor structures and the caudate under reward anticipation suggests that one of the unique functions of the indirect oculomotor pathway is subcortical cross talk with other functional basal ganglia pathways. This observed basal ganglia cross talk is consistent with new perspectives on the basal ganglia functional circuits (Haber and Knutson 2010). For a long time, the different functional circuits (reward, motor, oculomotor, and associative circuits) have been viewed to operate independently in parallel loops (Alexander and Crutcher, 1990; Alexander et al., 1990). The more recent perspective proposes a dual organization of the basal ganglia networks that permits both parallel and integrative processing (for review, see Haber and Knutson, 2010) and is consistent with recent diffusion tensor imaging (DTI) studies that support the idea that interactive networks exist also in the human brain (Draganski et al., 2008).
An exciting question for future studies may be whether this corticostriatal interface between the anticipation of motivational incentives and action control is also active during incentive learning (Dayan and Balleine, 2002). The current results suggest that actions predicted to yield reward are awarded high advantages in the caudate and its related action networks. Future incentive learning experiments may elucidate whether a change in incentive through learning (for example, in the reexposure condition of a food-aversion experiment) has an immediate impact on the neural network advantage of the associated action. The caudate may be an important player in determining changes in the interaction of rewarding incentives with the instrumental action–outcome contingency. Interfaces between motivation and cognitive control within the caudate have been shown previously in human (genetic) imaging. In an imaging study where participants received monetary rewards, after an anticipatory cue or after a button press response, Tricomi et al. (2004) showed that the caudate was activated only if the subjects perceived a contingency between a button press and a reward outcome. In line with the current oculomotor findings, these observations suggest that the interfacing role of the human caudate allowing motivation to modulate action control functions generalizes to other higher-order cognitive functions. The prominent network role of the caudate within the striatum in the integration of reward prospect with goal-directed oculomotor action converges with studies of goal-directed versus habitual control of action in humans and rodents (Dayan and Balleine, 2002; Balleine and O'Doherty, 2009; Redgrave et al., 2010). Formal tests of goal-directed and habitual control have shown that behavioral control systems can be localized, at least in part, to different regions of the striatum. Whereas the anterior caudate (the dorsomedial striatum in rats) has been associated with goal-directed action, the posterior lateral putamen (the dorsolateral striatum in rats) has been linked to the control of habitual actions (Redgrave et al., 2010). It may be particularly interesting to elucidate in future studies whether the corticostriatal networks connecting reward and action control may shift from the caudate to the putamen when actions shift from being under goal-directed to being under habitual control.
Several cognitive cortical areas have been shown previously to be sensitive to reward processes. Researchers have recently linked the left dorsolateral prefrontal cortex (Savine and Braver) and genetic polymorphisms of a dopamine transporter gene known to affect caudate dopamine (Aarts et al., 2010) to reward-related improvements in task switching. Also, motor preparatory activity in posterior parietal and premotor cortex after rewarded trials has been observed to predict subjective performance on the task (Iyer et al., 2010). As the presupplementary motor area and the dorsolateral prefrontal cortex have fiber connections with and interact with the caudate, as observed with human DTI (Lehericy et al., 2004), PET, and functional connectivity studies (Postuma and Dagher, 2006), dorsal striatum–cortical cross talk may generalize to the motivational modulation of other higher-order cognitive and motor behavior. In conclusion, we provide novel evidence that the dorsal striatum and its connected cortical control network enact motivational control over intentional behavior. The results bridge human and primate functional anatomy by showing that the caudate plays a crucial role in translating motivational incentives into voluntary eye-movement preparation and challenge traditional views on the extent to which basal ganglia functional reward and oculomotor loops operate independently.
Footnotes
This work of was supported by grants from the Netherlands Organization for Scientific Research (H.A.H, M.X.C., B.U.F., K.R.R.). Technical contributions by Marcus Spaan en Bert Molenkamp are gratefully acknowledged.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2009;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience. 1991;42:683–696. doi: 10.1016/0306-4522(91)90037-o. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn Brain Res. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during reward-guided decision-making. Neuroimage. 2008;39:1396–1407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Cui DM, Yan YJ, Lynch JC. Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys. J Neurophysiol. 2003;89:2678–2684. doi: 10.1152/jn.00501.2002. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 2004;44:365–378. doi: 10.1016/j.neuron.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H, Biscaldi M. The time of secondary saccades to primary targets. Exp Brain Res. 1993;97:356–360. doi: 10.1007/BF00228706. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Iyer A, Lindner A, Kagan I, Andersen RA. Motor preparatory activity in posterior parietal cortex is modulated by subjective absolute value. PLoS Biol. 2010;8:e1000444. doi: 10.1371/journal.pbio.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Reward-predicting activity of dopamine and caudate neurons–a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91:1013–1024. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Law I, Svarer C, Holm S, Paulson OB. The activation pattern in normal humans during suppression, imagination and performance of saccadic eye movements. Acta Physiol Scand. 1997;161:419–434. doi: 10.1046/j.1365-201X.1997.00207.x. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Smith DJ. Cortical projections to nuclei adjacent to the oculomotor complex in the medial dien-mesencephalic tegmentum in the monkey. J Comp Neurol. 1984;228:359–387. doi: 10.1002/cne.902280306. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Tian JR. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog Brain Res. 2006;151:461–501. doi: 10.1016/S0079-6123(05)51015-X. [DOI] [PubMed] [Google Scholar]
- Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cereb Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Mars RB, Coles MG, Hulstijn W, Toni I. Delay-related cerebral activity and motor preparation. Cortex. 2008;44:507–520. doi: 10.1016/j.cortex.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. J Neurosci. 2007;27:4810–4818. doi: 10.1523/JNEUROSCI.0577-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Dorris MC, Pare M, Everling S. On your mark, get set: brainstem circuitry underlying saccadic initiation. Can J Physiol Pharmacol. 2000;78:934–944. [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Nadjar A, Arkadir D, Bezard E, Goillandeau M, Bioulac B, Gross CE, Boraud T. Shaping of motor responses by incentive values through the basal ganglia. J Neurosci. 2007;27:1176–1183. doi: 10.1523/JNEUROSCI.3745-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Crivello F, Berthoz A, Mazoyer B. Functional anatomy of a prelearned sequence of horizontal saccades in humans. J Neurosci. 1996;16:3714–3726. doi: 10.1523/JNEUROSCI.16-11-03714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Rivaud-Pechoux S. Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res. 2003;142:3–17. doi: 10.1016/S0079-6123(03)42003-7. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Miall RC. Instructed delay activity in the human prefrontal cortex is modulated by monetary reward expectation. Cereb Cortex. 2003;13:318–327. doi: 10.1093/cercor/13.3.318. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorie AE, Gao J, McClelland JL, Newsome WT. Integration of sensory and reward information during perceptual decision-making in lateral intraparietal cortex (LIP) of the macaque monkey. PLoS One. 2010;5:e9308. doi: 10.1371/journal.pone.0009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci. 2002;22:2363–2373. doi: 10.1523/JNEUROSCI.22-06-02363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 30:10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duzel E. Ageing and early-stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Beckmann C, Miller K, Woolrich M. Meaningful design and contrast estimability in FMRI. Neuroimage. 2007;34:127–136. doi: 10.1016/j.neuroimage.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. J Neurophysiol. 2002a;87:508–515. doi: 10.1152/jn.00288.2001. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res. 2002b;142:284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE. Signal-, set- and movement-related activity in the human brain: an event-related fMRI study. Cereb Cortex. 1999;9:35–49. doi: 10.1093/cercor/9.1.35. [DOI] [PubMed] [Google Scholar]
- Toni I, Shah NJ, Fink GR, Thoenissen D, Passingham RE, Zilles K. Multiple movement representations in the human brain: an event-related fMRI study. J Cogn Neurosci. 2002;14:769–784. doi: 10.1162/08989290260138663. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Valyear KF, Culham JC, Sharif N, Westwood D, Goodale MA. A double dissociation between sensitivity to changes in object identity and object orientation in the ventral and dorsal visual streams: a human fMRI study. Neuropsychologia. 2006;44:218–228. doi: 10.1016/j.neuropsychologia.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hikosaka O. Immediate changes in anticipatory activity of caudate neurons associated with reversal of position-reward contingency. J Neurophysiol. 2005;94:1879–1887. doi: 10.1152/jn.00012.2005. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]