Abstract
Development of spatial memory in the rat is influenced by both maternal and nonmaternal aspects of the postnatal environment. Yet it remains poorly understood how these two aspects of the postnatal environment interact to program offspring cognitive development. By considering the joint influence of neonatal environmental novelty and maternal self-stress regulation on the development of spatial memory function in Long–Evans hooded rats, we show a persistent neonatal novelty-induced enhancement in spatial reference and working memory functions among the same individual offspring from juvenility to adulthood and a contrasting transient maternal modulatory influence on this novelty-related enhancement present during only juvenility. Specifically, at and only at juvenility, for mothers with good self-stress regulation as indexed by a low circulating basal corticosterone level, offspring showed a novelty-induced enhancement in spatial memory function, whereas for mothers with poor self-stress regulation, indexed by a high basal corticosterone level, offspring showed little enhancement or even small impairments. These findings indicate that maternal and nonmaternal postnatal environments exert separate but interacting influences on offspring cognitive development and support a maternal modulation model of cognitive development that considers maternal self-stress regulation as an important factor among the multitude of maternal influences.
Introduction
The development of spatial memory function is closely linked to the development of the hypothalamic–pituitary–adrenal (HPA) axis (Meaney et al., 1988; Catalani et al., 1993, 2000). The hippocampus, a key brain structure underlying spatial learning (Morris et al., 1982; Sutherland et al., 1982), is both a major target of the stress hormone corticosterone (CORT), an end product of the HPA axis, and one of the major driving forces that provide input to the HPA axis (McEwen and Sapolsky, 1995; de Kloet et al., 1998). Early-life stimulation and differences in maternal characteristics both result in long-lasting changes in the HPA axis and enhancement in spatial learning (Meaney et al., 1988; Catalani et al., 1993, 2000; Casolini et al., 1997; Liu et al., 1997; Tang, 2001; Tang et al., 2003, 2006; Akers et al., 2008). What remains controversial is the relative contribution of the neonatal stimulation itself and maternal influence toward the programming of the adult function (Denenberg, 1999; Pryce and Feldon, 2003; Macrì and Würbel, 2006; Parker et al., 2006; Tang et al., 2006).
An alternative to an exclusive focus on one particular source of postnatal influence is an integrative approach that aims at understanding the interaction between maternal and nonmaternal sources of environmental influences. Based on a well replicated observation that manipulation of the nursing dams' CORT via her drinking water can produce offspring with enhanced spatial memory performance (Catalani et al., 1993, 2000, 2010), the interaction model begins with the hypothesis that the maternal circulating CORT levels can influence the development of the offspring's HPA axis by creating a family-specific context, thereby allowing an identical early stimulation paradigm to produce differential effects against this varying hormonal and any other associated neural context. Early stimulation has a direct effect on the offspring's developing neural system, and maternal self-stress regulation modulates, instead of mediates, this direct effect via differential circulating corticosterone concentrations (Stanton et al., 1987; Stanton and Levine, 1988, 1990; Moriceau and Sullivan, 2006).
We tested these hypotheses in a longitudinal study with three key elements (Fig. 1A): (1) creating two contrasting postnatal experiences of environmental novelty between two halves of each newborn litter, one experiencing a 3 min daily exposure to a relatively novel nonhome environment (novel rats), and the other, only the familiar home environment (home rats); (2) assessing postnatal maternal self-stress regulation via a basal measure of dams' circulating corticosterone 5 d after weaning of her pups; and (3) assessing offspring's spatial reference and working memory function in a Morris water task in early juvenility and mid-adulthood. If maternal self-regulation plays a modulatory role in influencing this novelty effect, there should be a significant interaction effect between novelty exposure and the maternal basal CORT measure. We report a persistently detectable novelty-induced spatial memory enhancement from early juvenility to mid-adulthood. Moreover, we find a temporally limited association between this novelty effect and maternal basal CORT during juvenility only, with a lower postweaning maternal basal measure of CORT predicting a greater novelty-induced spatial memory enhancement.
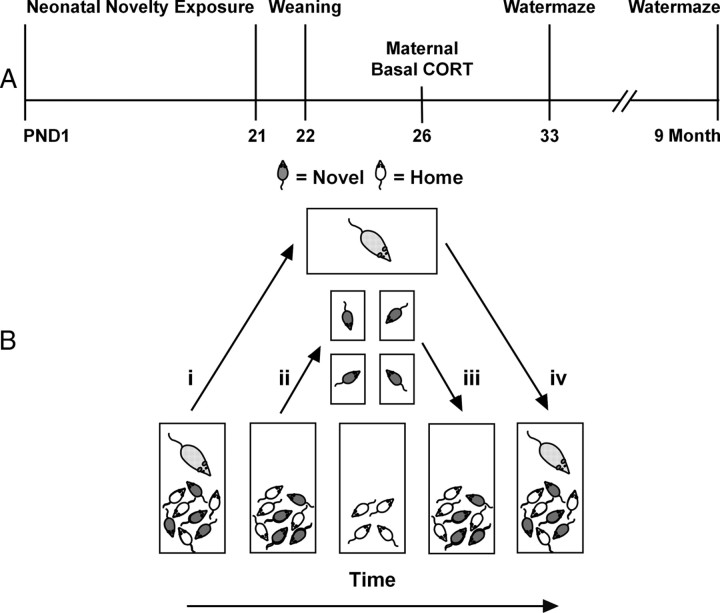
Figure 1.
A, Experimental timeline. B, Sequential steps in neonatal novelty exposure using a split-litter design. The following steps were performed for each litter. i, Removal of dam from home cage. ii, Transfer of novel pups (solid color) to individual nonhome cages (small rectangles). iii, Return of novel pups to home cage after 3 min exposure to nonhome cages. iv, Return of dam to entire litter in home cage.
Materials and Methods
Animals.
Twenty-two pregnant Long–Evans hooded dams (Charles River) arrived at the vivarium 12 d before giving birth. The dams were ∼3 months of age at the time of giving birth. Litter size at birth ranged from 9 to 16, and within 8 h after birth, litters were culled to eight pups. Pups were weaned on postnatal day (PND) 22 and were housed individually in transparent plastic cages (51 cm × 25 cm × 22 cm) with a 12 h light/dark cycle (lights on at 7:00 A.M.) and food and water ad libitum. One hundred and six males participated in the present study. All experimental procedures were in accordance with the Institutional Animal Care and Use Committee at the University of New Mexico.
Neonatal novelty exposure.
During PND 1–21, the neonatal novelty exposure procedure (Fig. 1B) was carried out in the animal housing room and involved the novel pups spending 3 min away from the familiarity of their home cage and their matched control siblings remaining in the home cage [for details of the method, see Tang (2001) and Tang et al. (2003, 2006)]. This procedure is in contrast to the commonly used neonatal handling designs (Levine, 1957, 1960; Denenberg, 1964) that use a between-litter design in which the nonhandled litters remain entirely undisturbed and the handled litters experience a combination of at least four manipulations: (1) “handling” of experimental animals by the experimenter; (2) separating the neonates from their mother; (3) increasing the mother's stress by separating her from her pups; and (4) exposing the neonates to an unfamiliar environment, i.e., novelty. Therefore, by using a split-litter or within-litter design, the novelty exposure component is isolated from the other three confounding factors, including maternal stress and associated maternal behavioral differences, inherent to the handling procedure (Tang, 2001).
Assessing maternal self-stress regulation.
Maternal self-stress regulation refers to the dams' ability to regulate her own, as opposed to her pups', stress response system. Individual differences in this ability are best characterized by trait as opposed to state measures. We indexed maternal self-stress regulation by obtaining a basal measure of CORT from the dam on PND 26, 5 d after weaning of her pups, so as to obtain a trait marker without interfering with her previous maternal role. The basal CORT measure is operationally defined as that obtained in an undisturbed state, in contrast to an evoked CORT measure after the onset of an acute stressor. The rationale is that the dams with good self-stress regulation should be able to maintain a relatively lower circulating CORT level under a basal or nondisturbed condition.
Blood collection was completed in no more than 3 min from the time of the dams' removal from the housing room between 1:00 and 5:00 P.M. using a tail-nicking procedure in a separate blood collection room. To avoid any potential effects of odor and ultrasound signaling on the remaining dams, dams were then held in a separate holding room and returned to the housing room only when blood was collected from all dams. For details of CORT assay, see Tang et al. (2003). The lower limit of detection was 10.4 ng/ml, and the intra-assay coefficient of variation was 13%. Basal CORT concentration ranged from 10.4 to 132.9 ng/ml. There was no time of day effect on this CORT measure across different dams.
Repeated measures of spatial memory performance.
To determine whether the neonatal novelty-related spatial memory enhancement (Tang, 2001, Tang et al., 2006) is a persistent phenomenon detectable repeatedly in the same individual at different stages of development and whether the influence of maternal self-stress regulation is similarly persistent, we performed spatial memory tests during both early juvenility (PND 33) and mid-adulthood (9 months of age). Specifically, we assessed spatial reference and working memory functions in a moving-platform version of the Morris water task (Morris, 1983; Panakhova et al., 1984; Whishaw, 1985; Steele and Morris, 1999) in which the rats must learn to locate and escape to a new hidden platform location on each of the 2 testing days (day 1 and day 2). For additional details on the water task, see Yang and Tang (2011).
Assessing spatial memory in a moving platform Morris water task.
We operationally defined reference memory by a quantity, referred to as TPREV_LOC_TRIAL1, computed as the amount of time the rats spent in the vicinity of the previously learned escape location (training day or day 1 location) during the first swim trial on the testing day (day 2-T1). During this first trial, rats do not yet have the knowledge that the platform will be changed and thus will use previously acquired spatial information to find the escape location. Rats with good reference memory are therefore expected to spend more time searching the previously learned escape location.
Working memory is operationally defined by a quantity, referred to as one-trial reversal, OTR = (TPREV_LOC_TRIAL1 − TPREV_LOC_TRIAL2), the reduction of time spent in the vicinity of day 1's escape location between trial 1 and trial 2 of day 2. After discovering during trial 1 on the testing day that the escape location has been changed, the rats must use this newly acquired information during trial 2 to find the new location of the hidden platform. Rats with a better working memory for the new platform location are therefore expected to give up the previous escape location more quickly, and thus show a greater reduction in the amount of time spent searching in day 1's escape location.
Other experimental control considerations.
The same cohort of rats was tested at PND 33 and 9 months of age, by experimenters blind to the novel and home group identities. Animals were tested in batches of eight, resulting in an intertrial interval ranging between 5 and 12 min. Within each batch, the order of testing was counterbalanced between the novel and home rats so that the novelty exposure treatment was not confounded by circadian variations in corticosterone.
Statistical analysis.
Litter was used as the unit of analysis. ANCOVAs were performed on spatial reference and working memory measures (TPREV_LOC_TRIAL1 and OTR) with neonatal novelty exposure as a within-litter factor and maternal basal CORT measure as a covariate. All data analysis was preceded by checking whether assumptions of normality and homogeneity of variance were violated. In the case of a violation of assumptions, data were transformed. Effect sizes are reported as Cohen's f, with small, medium, and large effect sizes being 0.10, 0.25, and 0.40, respectively (Cohen, 1988).
Results
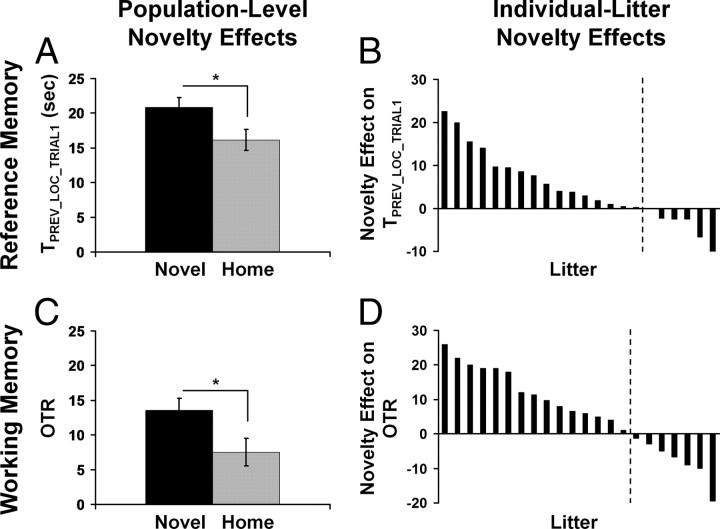
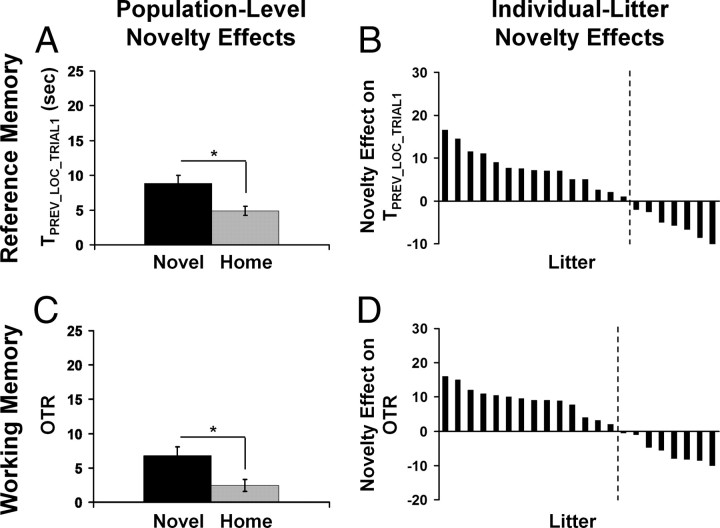
We found significant novelty exposure effects on measures of both reference memory (i.e., TPREV_LOC_TRIAL1) and working memory (i.e., OTR) with the novel rats showing better reference and working memory than the home rats during juvenility (Fig. 2A,C) (TPREV_LOC_TRIAL1: F(1,21) = 8.368, p = 0.009, f = 0.631; OTR: F(1,21) = 5.017, p = 0.036, f = 0.489) and adulthood (Fig. 3A,C) (TPREV_LOC_TRIAL1: F(1,20) = 5.668, p = 0.027, f = 0.533; OTR: F(1,20) = 5.485, p = 0.030, f = 0.523). These results indicate that repeated neonatal novelty exposure to a nonhome environment during the first postnatal weeks, although extremely brief (3 min/d), enhances both reference and working memory performance and that this enhancement can persist from the beginning of juvenility to mid-adulthood.
Figure 2.
On postnatal day 33, neonatal novelty-induced enhancement in spatial reference (A, B) and working (C, D) memory. Enhancement effects of novelty exposure at the level of rat family population (A, C) and variations in novelty effects at the level of individual rat families (B, D): a majority enhancement and a minority impairment. Error bars represent ± SEM, p < 0.05.
Figure 3.
At 9 months of age, same as for Figure 2.
In contrast to this population-level functional enhancement, we further examined how novelty exposure affected individual rat families. Litter-specific novelty-induced enhancement for each rat family was indexed by a litter-based novelty effect score (NE score), defined as Litter_AVGNOVEL − Litter_AVGHOME. For both memory measures obtained at both ages, there existed a large bidirectional range of novelty exposure effects, with the majority of the rat families showing novelty-induced enhancement (positive NE scores) and a minority showing an impairment effect of a smaller magnitude (negative NE scores) (Figs. 2B,D, 3B,D). These observations highlight the obvious, yet largely neglected, fact that a given early-life intervention may produce positive effects on average, but opposite effects may be observed on a subset of individuals. Therefore, before making a general statement based on the group averages, it is imperative to consider potential contextual variables that may modulate both the magnitude and direction of the intervention effect.
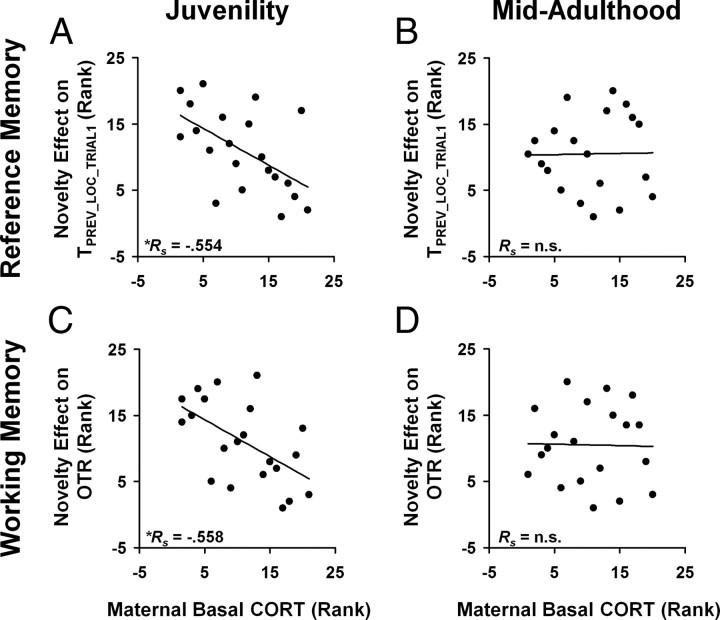
To place this bidirectional variation into the context of maternal individual differences in self-stress regulation, we computed a correlation between the NE scores and the maternal basal CORT measure. As the postnatal maternal basal CORT level can directly influence her offspring through the milk she provides them (for review, see Catalani et al., 2011), thus providing a differential physiological context for the pups to respond to and recover from the novelty exposure, we expected and found that the direction and magnitude of the novelty exposure effect were indeed correlated with the maternal basal CORT measure on PND 33 (Fig. 4A,C) (TPREV_LOC_TRIAL1: rs = −0.554, p = 0.009; OTR: rs = −0.558, p = 0.009). Specifically, reference and working memory enhancements are both observed in the context of low maternal basal CORT while a lack of such enhancement, or even small impairments, was observed in the context of high maternal basal CORT. These observations support the hypothesis that the mother's ability to regulate her own stress hormone is a critical modulatory factor in determining both the direction and magnitude of neonatal novelty exposure effects.
Figure 4.
Association between the basal CORT measure of maternal self-stress regulation and the family-to-family variations in novelty effect on working (A, B) and spatial reference (C, D) memory. A, C, At juvenility, offspring of mothers with lower postnatal basal CORT measure showed greater spatial memory enhancement induced by novelty exposure, while those with the higher CORT measure showed impairment. B, D, During mid-adulthood, this relationship was no longer detectable. *p < 0.05.
In contrast to the persistence of neonatal novelty exposure effects on reference and working memory from the early juvenile period to mid-adulthood, the maternal stress hormone-related modulatory influence was only detectable during PND 33, not at 9 months of age (compare Fig. 4A,C with B,D) (p > 0.20). Therefore, novelty-induced enhancement can persist during adulthood in the absence of a detectable concurrent maternal stress hormone-related modulatory effect. Consequently, such a novelty-induced enhancement cannot be mediated solely by individual differences in maternal self-stress regulation.
Discussion
The results support the hypothesis that environmental novelty, a nonmaternal neonatal environmental factor, is a separate and independent source of influence on offspring cognitive development and that naturally occurring variations in postnatal maternal self-stress regulation, a maternal environmental factor, are an additional source of modulatory influence that sets the context to critically determine both the direction and magnitude of the nonmaternal environmental influence. That this maternal modulatory influence was detectable only at juvenility but not at mid-adulthood may reflect a diluted maternal influence due to the offspring gaining further experience through unmonitored events during maturation and growth that may further interact with the earlier novelty exposure effect and alter adaptive capacity of the individual.
For several decades, it has been suggested that maternal care behavior mediates the effects of early stimulation (Barnett and Burn, 1967; Lee and Williams, 1974; Levine, 1975; Smotherman and Bell, 1980; Liu et al., 1997). However, there have been recent findings suggesting that maternal care alone is neither sufficient nor necessary for the production of stimulation-induced effects (Denenberg, 1999; Pryce and Feldon, 2003; Macrì and Würbel, 2006; Parker et al., 2006; Tang et al., 2006) and that other environmental variables that affect the offspring or the mother's HPA function can also powerfully influence offspring development (Moriceau and Sullivan, 2006; Parker et al., 2006; Tang et al., 2006; Benetti et al., 2007; Akers et al., 2008; Raineki et al., 2010; Catalani et al., 2011). Most importantly, in studies using the postnatal/neonatal handling procedure, maternal care is intrinsically confounded with the maternal stress hormone and with offspring's stress experience (see Materials and Methods) (Russell, 1971; Daly, 1973; Tang, 2001), and these confounding factors cannot be ruled out by using a cross-fostering experimental design as by Liu et al. (1997) and Francis et al. (1999).
The present study used the neonatal novelty exposure with a split-litter design that both isolates the novelty exposure manipulation of the offspring from maternally related and other confounding factors and allows separate observations of potential novelty-exposure-induced preferential maternal care (Tang et al., 2006; Benetti et al., 2007), and it demonstrated the following: first, separate contributions to cognitive development made by maternal self-stress regulation and postnatal environmental novelty; and second, revealing a nonmaternal early environmental factor and a modulatory, instead of mediatory, relationship between maternal individual differences in self-stress regulation and novelty effects on offspring cognitive development. Together, our findings, along with previous studies, support a broader view on the maternal contribution to offspring development—to include maternal self-stress regulation in addition to maternal care.
Early stimulation studies typically use what may be referred to as a “one-shot” functional assessment in which early stimulation during infancy is followed by a delayed assessment in either juvenility or adulthood. By using this “one-shot” approach, it cannot be ascertained whether a stimulation effect will likely have a persistent impact throughout life or an effect that may disappear or change at a later time. The present findings obtained from a repeated-measure longitudinal experimental design clarify this issue by showing that the enhancing effect of neonatal novelty exposure is persistent, with comparable effect sizes, and thus likely to afford its benefit throughout the period between juvenility and mid-adulthood. The present study also offers a novel demonstration of a more efficient and sensitive analysis method for indexing both spatial reference and working memory function in a 2 d test across different stages of development.
In consideration of possible underlying neuroendocrine mechanisms, it is important to recognize that glucocorticoid receptors (GRs) in the hippocampus play a facilitative role in spatial memory formation (Oitzl et al., 2001), at least in part via its regulation of synaptic plasticity (de Kloet et al., 1999; Joëls et al., 2006). Here, it is possible that the novelty-induced enhancement in hippocampal synaptic plasticity (Tang and Zou, 2002) and subsequent GR enhancement (Zou et al., 2001) both contribute to the observed enhancement in spatial memory among the neonatal novelty-exposed rats. Whether hippocampal glucocorticoid receptor expression is increased as a result of neonatal novelty exposure remains to be determined. Since dams are known to show an increase in corticosterone blood levels upon her reunion with her pups after a brief separation (Smotherman et al., 1977), maternal individual differences in glucocorticoid levels after novelty exposure may play a role in providing a differential hormonal context surrounding the time of novelty exposure.
While higher quantity of maternal care has been shown to be associated with increased GR expression (Liu et al., 1997; Francis et al., 1999), here it is unlikely that the memory enhancement induced by novelty exposure is due solely to a greater amount of maternal care, because dams, whether high or low caregivers, did not show preferential care toward the novelty-exposed pups (Tang et al., 2006; Benetti et al., 2007). It is possible, and remains to be determined, whether the previously reported association between quantity of maternal care and offspring GR expression may be due to differences in dams' circulating corticosterone level, as manipulation of dams' circulating corticosterone is known to result in changes in the offspring's HPA function and spatial memory, likely through the mother's milk (Catalani et al., 1993, 2000, 2010).
In conclusion, the present findings support a broader view of maternal contribution to postnatal cognitive development by considering maternal individual differences in self-stress regulation as a possible source of influence that sets differential contexts for neonatal environmental novelty, an otherwise identical nonmaternal environmental manipulation, to produce a diverse range of effect on spatial memory development. This view of maternal modulation of nonmaternal environmental influences represents an integrative model of cognitive development that takes into consideration the complex interactions between maternal and nonmaternal experiential factors and the multifaceted nature of maternal influence. Such a view contrasts with the exclusive role of maternal care as promoted by the maternal mediation hypothesis.
Footnotes
We thank Katherine Akers and Masato Nakazawa for their assistance in the collection of blood samples.
The authors declare no competing financial interests.
References
- Akers KG, Yang Z, DelVecchio DP, Reeb BC, Romeo RD, McEwen BS, Tang AC. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One. 2008;3:e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA, Burn J. Early stimulation and maternal behavior. Nature. 1967;213:150–152. doi: 10.1038/213150a0. [DOI] [PubMed] [Google Scholar]
- Benetti F, Andrade de Araujo P, Sanvitto GL, Lucion AB. Effects of neonatal novelty exposure on sexual behavior, fear, and stress-response in adult rats. Dev Psychobiol. 2007;49:258–264. doi: 10.1002/dev.20181. [DOI] [PubMed] [Google Scholar]
- Casolini P, Cigliana G, Alemà GS, Ruggieri V, Angelucci L, Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors. Neuroscience. 1997;79:1005–1012. doi: 10.1016/s0306-4522(96)00668-9. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LA, Porcu A, Korányi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Alema GS, Cinque C, Zuena AR, Casolini P. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in rodents. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ed 2. Hillsdale, NJ: Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Daly M. Early stimulation of rodents: a critical review of present interpretations. Br J Psychol. 1973;64:435–460. doi: 10.1111/j.2044-8295.1973.tb01370.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Critical periods, stimulus input, and emotional reactivity: a theory of infantile stimulation. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Commentary: is maternal stimulation the mediator of the handling effect in infancy? Dev Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lee MHS, Williams DI. Changes in licking behavior of rat mother following handling of young. Anim Behav. 1974;22:679–681. [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Stimulation in infancy. Sci Am. 1960;202:81–86. [PubMed] [Google Scholar]
- Levine S. The potential influence of infantile stimulation on emotional disorders. In: Levi L, editor. Society, stress and disease. London: Oxford UP; 1975. pp. 411–415. [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. An attempt to dissociate ‘spatial mapping’ and ‘working-memory’ theories of hippocampal function. In: Siefert W, editor. Neurobiology of the hippocampus. London: Academic; 1983. [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci U S A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakhova E, Buresová O, Bures J. Persistence of spatial memory in the Morris water tank task. Int J Psychophysiol. 1984;2:5–10. doi: 10.1016/0167-8760(84)90066-7. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioral impact of the postnatal environment in rats: manipulations, effects, and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell PA. “Infantile stimulation” in rodents: a consideration of possible mechanisms. Psychol Bull. 1971;75:192–202. [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal influences and early behavior. New York: Spectrum; 1980. pp. 201–210. [Google Scholar]
- Smotherman WP, Wiener SG, Mendoza SP, Levine S. Maternal pituitary-adrenal responsiveness as a function of differential treatment of rat pups. Dev Psychobiol. 1977;10:113–122. doi: 10.1002/dev.420100204. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Maternal modulation of infant glucocorticoid stress response: role of age and maternal deprivation. Psychobiology. 1988;16:223–228. [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Tang AC. Neonatal exposure to novel environment enhances hippocampal-dependent memory function during infancy and adulthood. Learn Mem. 2001;8:257–264. doi: 10.1101/lm.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Zou B. Neonatal exposure to novelty enhances long-term potentiation in CA1 of the rat hippocampus. Hippocampus. 2002;12:398–404. doi: 10.1002/hipo.10017. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb BC, Romeo RD, McEwen BS. Modification of social memory, hypothalamic-pituitary-adrenal axis, and brain asymmetry by neonatal novelty exposure. J Neurosci. 2003;23:8254–8260. doi: 10.1523/JNEUROSCI.23-23-08254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci U S A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. Physiol Behav. 1985;35:139–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Yang Z, Tang AC. Novelty-induced enhancement in spatial memory: is infancy a critical period? Behav Brain Res. 2011;219:47–54. doi: 10.1016/j.bbr.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Zou B, Golarai G, Connor JA, Tang AC. Neonatal exposure to a novel environment enhances the effects of corticosterone on neuronal excitability and plasticity in adult hippocampus. Brain Res Dev Brain Res. 2001;130:1–7. doi: 10.1016/s0165-3806(01)00173-0. [DOI] [PubMed] [Google Scholar]