Abstract
Retinal ganglion cells (RGCs) are the projection neurons from the eye to the brain and their loss results in visual impairment in a number of diseases. Transcription factors with a homeodomain can translocate between cells and, in at least one reported case, can stimulate neuronal survival. Otx2 is a homeoprotein transcription factor expressed in the retina that is taken up by RGCs. We thus hypothesized that Otx2 capture could regulate the survival of adult RGCs. We report that Otx2 stimulates the survival of adult mouse and rat RGCs in vitro and protects RGCs against NMDA-induced toxicity in vivo in mice. In the latter model, Otx2 also preserves visual acuity.
Introduction
Retinal ganglion cells (RGCs) provide the sole link between the retina and central visual structures. Loss of RGCs leads to profound and irreversible blindness. In humans, RGC loss leading to visual impairment occurs in diabetic retinopathy, optic neuropathies, and glaucoma (Osborne et al., 1999; Levin, 2007; Kern and Barber, 2008). Thus, a factor that promotes the survival of damaged RGCs might be of therapeutic interest.
Homeoprotein transcription factors can translocate between cells and regulate transcription and translation in the recipient cell (for review, see Prochiantz and Joliot, 2003; Joliot and Prochiantz, 2004). The homeoprotein Otx2 is expressed in the retina, where its mRNA is detected in photoreceptors and bipolar cells (Koike et al., 2007; Rath et al., 2007; Sugiyama et al., 2008; Glubrecht et al., 2009). Otx2 protein is detected in photoreceptors, bipolar cells, and cells in the ganglion cell layer (GCL) (Koike et al., 2007; Rath et al., 2007; Sugiyama et al., 2008; Glubrecht et al., 2009). The presence of Otx2 protein in cells in the GCL in which the locus is not active (Sugiyama et al., 2008) suggests that the protein can be taken up by RGCs. Recently, it has been demonstrated that exogeneous Otx2 injected into the eye is taken up by RGCs and trans-synaptically transferred to parvalbumin cells in the visual cortex, where the protein initiates the opening of the critical period for ocular dominance plasticity (Sugiyama et al., 2008). In light of evidence that the homeoproteins Engrailed 1 and 2, which are expressed in the midbrain, are survival factors for mesencephalic dopaminergic neurons (Sonnier et al., 2007), we tested the hypothesis that Otx2 promotes the survival of adult RGCs.
Materials and Methods
Recombinant protein.
Wild-type mouse Otx2 (Otx2wt) with a myc tag and a 6×His tag in C-terminal position was cloned into pTrac plasmid (Invitrogen). Mutated mouse Otx2YL (WF85/86YL) was obtained by site-directed mutagenesis from the Otx2wt expression plasmid by Dr. Ariel Di Nardo (Centre National de la Recherche Scientifique, Collège de France, Paris, France). Recombinant Otx2wt and Otx2YL proteins were produced in bacteria BL21 CodonPlus RP (Stratagene) at 37°C overnight in auto-induced medium (MagicMedia, Invitrogen). After washing the bacterial mass with PBS, two different procedures were used to purify the proteins. In the nondenaturing procedure, the bacterial mass was resuspended in 20 mm phosphate, 0.5 m NaCl, and 5 mm imidazole. The bacteria were disrupted with a French press and centrifuged at 40,000 × g for 30 min. The supernatant was passed through an affinity chromatography column charged with 100 mm NiSO4 (HiTrap Chelating HP column, GE Healthcare). The recombinant protein was then eluted with 20 mm phosphate, 0.5 m NaCl, and a gradient of 0.05–0.5 m imidazole. The fraction with the protein was immediately dialyzed against 20 mm phosphate, 0.5 m NaCl and then the protein solution was complemented to 10% glycerol and frozen. For the denaturing procedure, the protein was extracted with buffer A+ (10 mm Tris, 100 mm Na2HPO4, 20 mm imidazole, 6 m guanidine HCl, pH 8). The lysed bacteria were centrifuged for 20 min at 20,000 × g and the supernatant passed through an affinity chromatography column charged with 100 mm NiSO4 (HiTrap Chelating HP column, GE Healthcare). The recombinant protein was then eluted with 6 m guanidine HCl, 0.2 m acetic acid and immediately dialyzed against 20 mm phosphate, 0.5 m NaCl. The protein solution was complemented to 10% glycerol and frozen. Otx2 purified under denaturing conditions retained its ability to bind a known Otx2 binding sequence. Otx2 purified under nondenaturing conditions (see Figs. 2, 4A,C) and denaturing conditions (see Figs. 1, 3, 4B,D, 5–7) had RGC survival-promoting activity.
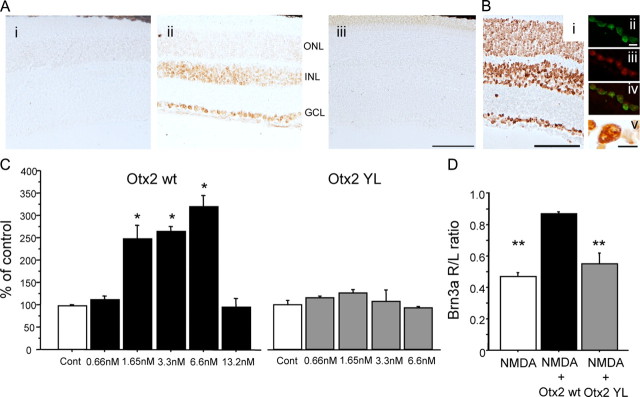
Figure 2.
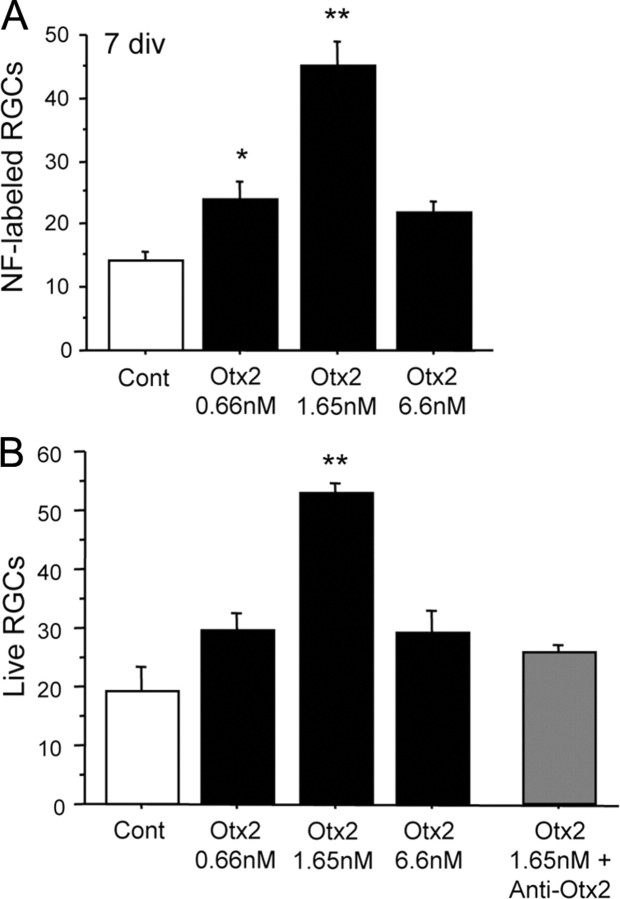
Otx2 promotes survival of adult RGCs in culture in a dose-dependent manner. A, Otx2 significantly increased the number of surviving NF+ RGCs after 7 days in culture in a dose-dependent manner (ANOVA, F = 21.712, p < 0.0001; *p < 0.05, **p < 0.005 compared to control (Cont), post hoc test, Fisher's PLSD). These results are representative of 20 independent experiments. B, Otx2 stimulates the survival of immunopurified adult rat RGCs 6 days in culture, whereas an anti-Otx2 abrogates the survival effect (ANOVA, F = 7.014, p = 0.001; **p < 0.005 compared to control, post hoc test, Fisher's PLSD). These results are representative of six independent experiments. Four to six coverslips were analyzed for each condition in A and B.
Figure 4.
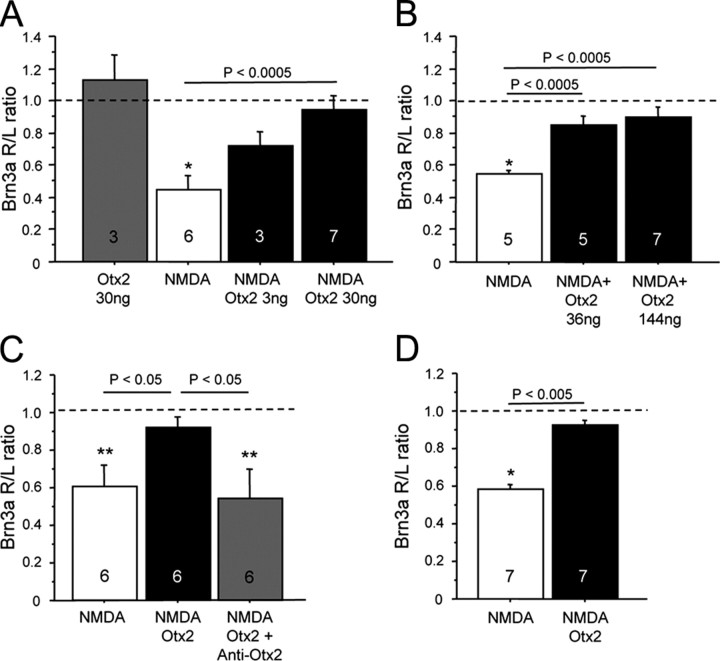
Otx2 protects adult RGCs against NMDA excitotoxicity. A, Otx2 injected into the eye does not alter Brn3a mRNA levels. NMDA significantly reduces Brn3a levels compared to the uninjected eye (*p < 0.005, one sample t test against null hypothesis = 1.0). In eyes injected with NMDA and 30 ng of Otx2, the levels of Brn3a in the injected eye are indistinguishable from those in the uninjected eye. Three nanograms provided 50% protection. B, In a separate experiment, 144 ng of Otx2 provided the same full protection as 36 ng. C, Preincubation of Otx2 with anti-Otx2 blocked the ability of Otx2 to protect against excitotoxic decrease in Brn3a levels (**p < 0.05, one sample t test against null hypothesis = 1.0). Injection of the antibody alone did not alter Brn3a mRNA levels (data not shown). D, A single injection of Otx2 at the time of NMDA (2 mm final) protected against NMDA-induced decrease in Brn3a expression for up to 21 d. R/L ratio, Right/left ratio.
Figure 1.
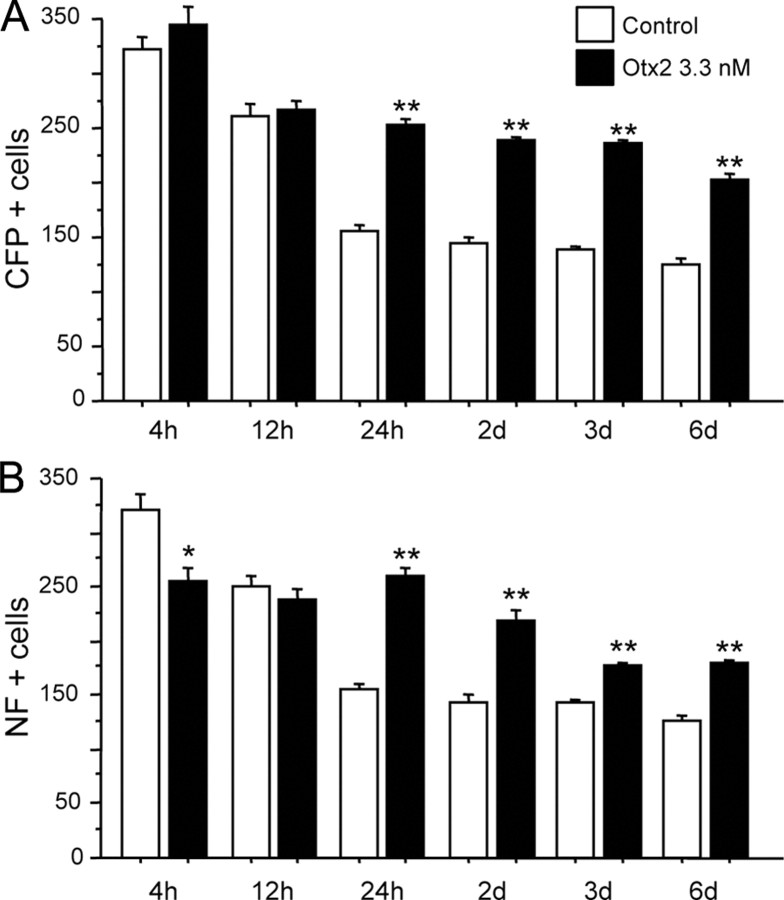
Time course of RGC survival in culture. A, B, Dissociated retinal cells from adult B6.Cg(Thy1-CFP)23Jrs/j mice were cultured in the presence or absence of 3.3 nm Otx2 for 4 h to 6 d. CFP+ (A) or NF+ (B) cells were counted. A significant survival effect of Otx2 was observed starting at 24 h. (*p < 0.005 and **p < 0.001 compared to control, 2-tailed t test). Six coverslips were analyzed for each condition.
Figure 3.
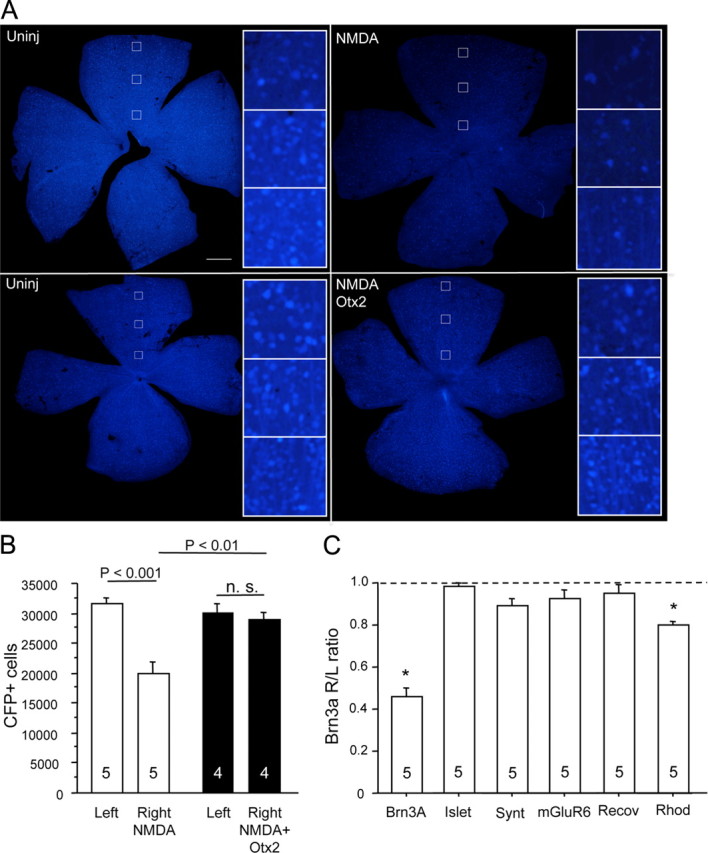
NMDA excitotoxicity of RGCs. A, Four days after intraocular injection of NMDA (2 mm final) in B6.Cg(Thy1-CFP)23Jrs/j mice, the number of CFP-positive RGC profiles appeared reduced throughout the retina compared to the uninjected (Uninj) retina (top). When Otx2 (30 ng) was injected at the time of NMDA, fewer CFP+ cells appeared lost (bottom). Scale bar, 500 μm. B, Counting CFP-positive RGCs in the experiment depicted in A showed a significant reduction of about 33% compared to the uninjected left eye (2-tailed t test). Thirty nanograms of Otx2 fully protected fully against the NMDA-induced loss of CFP+ RGCs. C, NMDA (2 mm final) significantly reduced Brn3a mRNA, while mRNAs specific for other cell types were little or not affected. (*p < 0.005, one sample t test against null hypothesis that the right eye to left eye ratio = 1.0). The numbers at the bottom of each bar represent the number of mice in each condition.
Figure 5.
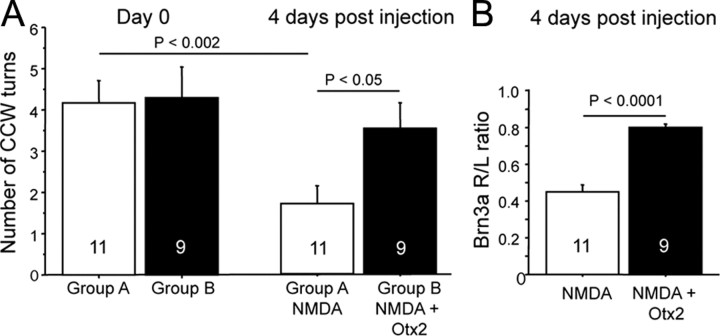
Otx2 protects RGCs against the loss of visual acuity. A, Mice were separated into two groups and were tested on the optomotor with a square wave grating of 0.375 cpd before intraocular injection. Four days after intraocular injection of NMDA, the number of head turns was significantly reduced compared to day 0 (group A, t test). In mice injected with Otx2 and NMDA (group B), the number of head turns was significantly greater than mice injected with NMDA alone and was not significantly different from the number of head turns before eye injection. B, The protection by Otx2 against visual acuity loss due to NMDA is associated with significantly greater Brn3a mRNA.
Figure 6.
Otx2YL does not promote RGC survival in vitro or in vivo. A Otx2wt or Otx2YL were produced with a myc tag and were detected using an anti-myc antibody. Anti-myc signal is detected in the GCL and INL and to a lesser extent in the outer ONL of mice injected with Otx2wt, but not in mice injected with Otx2YL. Scale bar, 100 μm. B, Cellular localization of Otx2wt-FITC injected into the eye. Bi, Six hours after injection of 156 ng of Otx2, exogenous protein detected by anti-myc was detected in all cell body layers of the retina. Bii, Confocal image of a single focal plane of the FITC signal revealed Otx2 in cells in the GCL. Biii, TO-PRO-3 fluorescence delimited the cell nuclei and nucleoli, and the merged image (Biv) shows Otx2 in GCL nuclei. Bv, DAB precipitate of exogenous Otx2 detected by the myc-tag in and around the nucleus of a cell in the GCL. Scale bars: Bi, 100 μm; Bii, Bv, 10 μm. C, Otx2wt promotes the survival of adult mouse RGCs 6 d in vitro. [ANOVA, F = 27.538, p < 0.0001; **p < 0.0005 compared to control (Cont), Fisher's PLSD]. Otx2YL did not promote the survival of adult mouse RGCs. D, Brn3a mRNA was significantly decreased 4 d after intraocular injection of NMDA compared to mice injected with Otx2wt and NMDA. Brn3a mRNA in mice injected with Otx2YL was significantly reduced compared to mice injected with Otx2wt and NMDA (ANOVA, F = 21.287, p < 0.0001; **p < 0.005, Fisher's PLSD). Three to eight coverslips were analyzed for each condition.
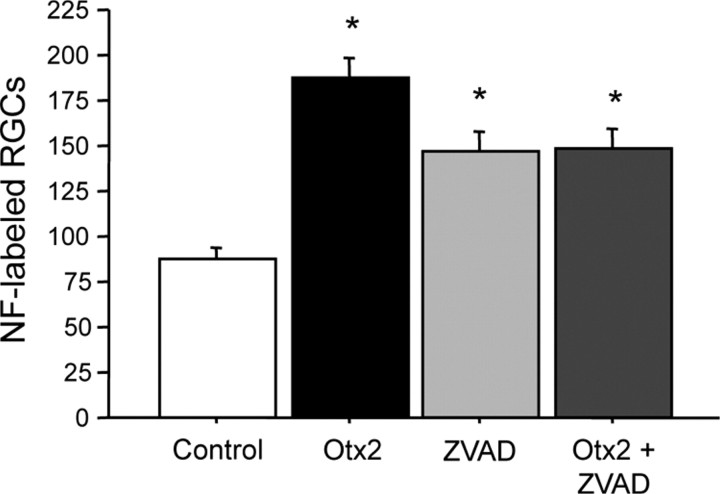
Figure 7.
RGCs in mixed culture are lost via apoptosis. Dissociated cells from adult C56BL/6 mice were cultured for 24 h with 3.3 nm Otx2, 20 μm ZVAD, or both and NF+ RGCs were counted. Otx2 promoted the survival of RGCs as did ZVAD. There was no additive effect of Otx2 + ZVAD (ANOVA, F = 18.674, p < 0.0001; *p < 0.005, Fisher's PLSD). Six coverslips were analyzed for each condition.
Otx2 proteins were solubilized in gel solubilization buffer (80 mm Tris, pH 6.8, 4 mm DTT, 1% glycerol, 1.2% SDS) and SDS-PAGE was carried out on a 12% gel. Dilutions of BSA were separated in adjacent lanes. Proteins on the gel were revealed with Bio-Safe Coomassie staining solution (Bio-Rad) or SYPRO Orange (Invitrogen). Otx2 concentration was estimated by comparison of the intensity of the Otx2 band with BSA. To confirm the identity of Otx2, after separation by SDS-PAGE the proteins were transferred to polyvinylidene difluoride (PVDF) and Otx2 was detected with anti-Otx2 raised in rat (Sugiyama et al., 2008) and chemiluminescence (ECL Plus, GE Healthcare).
Recombinant Otx2wt protein was conjugated with FITC by successive dialysis of 80 μg of protein in the following buffers: 0.5 m NaCl, then 50 mm bicarbonate, 0.5 m NaCl, pH 8.2, then 50 mm bicarbonate, 0.5 m NaCl, pH 9.2, then 50 mm bicarbonate, 0.5 m NaCl, 100 μg/ml FITC, pH 9.2, and finally 20 mm phosphate, 0.5 m NaCl. After centrifugation the supernatant was frozen at −80°C.
Mixed cultures.
Male B6.Cg(Thy1-CFP)23Jrs/j mice 8–12 weeks of age expressing cyan fluorescent protein (CFP) under the control of the Thy1 promoter (The Jackson Laboratory) or C57BL/6 mice 8 weeks of age were killed by cervical dislocation, the eyes removed, and the retinae dissected in CO2-independent medium (Invitrogen). In the B6.Cg(Thy1-CFP)23Jrs/j mice, 80–90% of RGCs strongly express CFP and only about 9% of CFP-positive cells in the GCL are labeled with the amacrine cell marker syntaxin (Raymond et al., 2008). Retinae were rinsed with PBS (50 mm phosphate, 155 mm NaCl containing 0.6% glucose and 0.5 mm EDTA) and then incubated with 13.5 U of papain (Worthington, product no. LSO 3124) per retina in the presence of DNase I for 30 min at 37°C. After inactivation of the enzyme, the retinal tissue was mechanically dissociated by trituration through a fire-polished Pasteur pipette. Dissociated cells were cultured on glass coverslips previously coated with poly-lysine and laminin (18.5 and 10 μg/ml, respectively) in Neurobasal-A (Invitrogen) complemented with B27 serum-free supplement (Invitrogen), 500 μm glutamine, 25 μm glutamate, 25 μm aspartate, and antibiotic/antimycotic. Otx2 and other treatments were added at the time of plating, and the cells were cultured at 37°C in 5% CO2 humidified atmosphere. The control condition consisted of adding the same volume of diluent without the recombinant protein. After 4 h to 18 d, the cells were fixed in buffered 4% paraformaldehyde and the RGCs were visualized by immunocytochemistry for NF200 (1:500, Sigma, catalog #N4142) and anti-rabbit Alexa Fluor 545 (Invitrogen). Images corresponding to one coverslip diameter were acquired in for both NF200 and CFP channels using a Nikon 90i microscope. Image files were opened in ImageJ, thresholded, and binarized, and objects with a circularity of 0.5–1 and an area of 8–32 μm2 were counted using the analyze particle command. Particle masks for the CFP and NF200 images were overlaid to determine the percentage of double-labeled cells. In other experiments NF200-positive cells were manually counted in one diameter of the coverslip. The data presented are the mean ± SEM of the number of CFP- or NF200-positive cells in at least four coverslips per condition. One-way ANOVA was used to determine a condition effect followed, if justified, by a Fisher's post hoc test [protected least significant difference (PLSD); StatView].
Immunopurification of rat RGCs.
Adult rat RGCs were purified from adult (8 weeks) male Long–Evans rats as described by Fuchs et al. (2005) based on work by Barres et al. (1988). Briefly, rats were killed by CO2 and cervical dislocation and the retinae were collected and washed two times with a PBS-glucose buffer (Invitrogen). The retinae were incubated with 13.5 U of papain per retina for 30 min at 37°C in the presence of DNaseI and then the action of the enzyme was stopped with PBS-glucose ovomucoïd, 0.15%. Retinas were mechanically dissociated with a fire-polished Pasteur pipette. After two stages of depletion of macrophages with an anti-macrophage (Accurate Chemicals), the RGCs were positively selected with anti-thy1 antibody (supernatant of hybridoma TIB-103, LGC Promochem). After recovery of the cells, they were cultured on glass coverslips previously coated by poly-d-lysine and laminin (18.5 and 10 μg/ml, respectively) in Neurobasal-A supplemented with B27, glutamate, aspartate, glutamine, and antibiotic/antimycotic as described above. Otx2 or vehicle was added at the time of plating and cells were cultured for 6 d in a 5% CO2 humid atmosphere at 37°C. The cells were incubated with 2 μm calcein acetoxymethyl ester (Invitrogen) for 1 h at 37°C, and the number of living cells on six coverslips per condition was counted on an epifluorescence microscope with a 488 nm filter. For antibody neutralization experiments, anti-Otx2 antibody (Neuromics) was dialyzed against PBS, and then Otx2 (25 ng in 500 μl) was incubated with anti-Otx2 (0.5 μg) in culture medium at 37°C for 30 min.
Intraocular injections.
C57BL/6 or B6.Cg(Thy1-CFP)23Jrs/j mice 8–12 weeks of age were anesthetized with a mix of xylazine/ketamine (Imalgène 500, 1.5 ml/kg/Rompun 2%, 0.5 ml/kg). The right pupil was dilated by instillation of atropine or tropacamide. A 21 gauge needle was used to make a hole in the sclera about 1 mm from the limbus. The needle of a 10 μl Hamilton syringe was inserted into the hole and the needle tip position controlled by visual guidance through the dilated pupil. A drop of mineral oil placed at the needle entry site prevented the sclera from drying and adhering to the needle. One microliter of NMDA (final concentration 2 mm) alone or with Otx2 (3–144 ng) in PBS was injected over ∼20–30 s, and the needle was maintained in the eye for a further minute. After the needle was withdrawn, the area was wiped with a cotton swab and the mice were allowed to recover under the surveillance of an experimenter and returned to its home cage.
RGC counting.
Four days after intraocular injection, CFP-Thy1 mice were killed by cervical dislocation and retinae were flat mounted, fixed with buffered 4% paraformaldehyde, and imaged with an Eclipse 90i microscope (Nikon) equipped with a CFP filter. The images were then analyzed as described (Danias et al., 2002). Briefly, Tiff files were imported into Adobe Photoshop and converted to grayscale, a high pass filter was applied, and then the same threshold was applied to all images. The files were renamed, saved, and exported to ImageJ, and objects were counted using the analyze particle command.
Quantitative PCR of mRNA.
RNA was extracted from frozen retinae using the RNaesy lipid tissue mini-kit (Qiagen), reverse transcribed to generate cDNA with the Quantitect reverse transcription kit (Qiagen), and the samples were analyzed in duplicate with a LightCycler 480 II (Roche) and 2−ΔΔCt method. The following primers (5′ to 3′) were used. Primer Brn3a pF (forward): agg cct att ttg ccg tac aa, primer Brn3a pR (reverse): cgt ctc aca ccc tcc tca gt; primer Islet 1 pF: gca tac tga tga agc agc tcc, primer Islet 1 pR: caa ggc gaa gtc act cag tac t; primer Syntaxin pF: ctc agt gag atc gag acc ag, primer Syntaxin pR: gat cat gat ctt ctt cct gcg; primer mGluR6 pF: tct gcc tct gcc tct tga g, primer mGluR6 pR: ccc ctc tct tta tcc cct tc; primer Recoverin pF: tcc cca tcc ttc aca ctt tac, primer Recoverin pR: acc tgc tta ccc agc aat c, primer HPRT pF: agc agg tgt tct agt cct gtg g, and primer HPRT pR: acg cag caa ctg aca ttt cta a. The duplicates for each sample were averaged and then normalized to HPRT (hypoxanthine-guanine phosphoribosyl transferase). The normalized value of the injected eye (i.e., right eye) was divided by the value in the uninjected eye (i.e., left eye). One sample t test was used to compare each group to the null hypothesis, i.e., a ratio equal to 1.0. Comparison between groups was carried out by t test.
Optomotor testing.
Changes in visual acuity were assessed by optomotor response as described (Abdeljalil et al., 2005) with modifications. Briefly, mice adapted to ambient light were placed on a raised grid platform centered in a well lit motorized drum with 100% contrast black and white vertical stripes. The drum rotated at 2 rpm. In preliminary experiments we determined the minimal spatial frequency [0.375 cycles per degree (cpd)] that would reliably evoke head turns in 70–75% of C57BL/6 mice and found that head turns to both clockwise and counterclockwise drum rotation could be elicited from both eyes. To test the treated eye in isolation, mice were anesthetized with xylazine/ketamine as described above and the left eye was removed. Mice were closely observed for bleeding from the wound and then returned to their cage for recovery under the surveillance of an experimenter. The retina of the left eye was removed and frozen on dry ice. One day later, the mice were tested with the 0.375 cpd optotype rotated in the counterclockwise direction, and those that made fewer than four head turns were excluded from further study. Mice were separated into two groups, anesthetized as described above, and injected with NMDA alone or with NMDA/Otx2 as described above. Four days later the mice were tested on the optomotor test, killed by cervical dislocation, and the right retinae were removed and frozen on dry ice.
Detection of injected Otx2 in the retina.
Mice were injected with 30 ng of Otx2wt or Otx2YL as described above. Six hours later the mice were killed by cervical dislocation, the eyes were removed, and the lens and cornea were removed. Eye cup preparation was fixed in buffered 4% paraformaldehyde for 1 h at room temperature and cryoprotected in 25% sucrose in PBS overnight at 4°C. Eye cups were embedded in OCT mounting medium (Tissue-Tek), frozen, and 10 or 14 μm cryostat sections were transferred to SuperFrost (Thermo Scientific) glass slides. Endogenous peroxidase activity was neutralized by 0.3% H2O2 in methanol for 10 min. Slides were rinsed three times with TBS and then nonspecific binding was blocked by incubation in TBS with 1% Triton X-100, 0.2% Tween 20, and 10% calf serum. Peroxidase-conjugated anti-myc (Roche) was diluted in the same buffer and slides were incubated overnight at 4°C. Slides were rinsed three times in PBS and peroxidase activity was revealed with diaminobenzedine (Roche). The reaction was stopped by rinsing in PBS. All images were acquired on an Eclipse 90i microscope (Nikon) using the same settings.
In a second experiment, mice were injected with 154 ng of Otx2 conjugated to FITC. Eye cups and retinal sections were prepared as described above. Sections were permeabilized and counterstained in 200 nm TO-PRO-3 (Invitrogen) or revealed with diaminobenzedine (Roche) as described above. Images were obtained by a Leica TCS SP5 confocal microscope operated on the Leica Application Suite system (Leica) or Nikon 90i.
Detection of endogeneous Otx2 in the retina.
Two retinae from adult C57Bl/6 mice were homogenized in 200 μl 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mm NaCl, and 50 mm Tris, pH 8.0, using a Pellet Pestle micro grinder (Kimble/Kontes) for 50 s. Twenty microliters of the retinal lysate or various dilutions of purified Otx2 proteins were separated on NuPAGE 4–12% Bis-Tris precast gels (Invitrogen), electrophoresed according to the manufacturer's instructions, and transferred to PVDF. Otx2 protein was detected using anti-Otx2 as described above. The chemiluminescent signal was captured on a Fujifilm LAS-4000 (Fujifilm). The ROI Otx2 signal was delimited in ImageJ, a dilution curve of the purified Otx2 protein signal was determined, and the endogenous Otx2 quantity was interpolated from the dilution curve.
Animal procedures.
All procedures were designed to minimize animal suffering and were carried out in accordance with the recommendations of the European Economic Community (86/609/EEC) and the French National Committee (87/848) for the use of laboratory animals.
Results
Mice heterozygous for the homeoprotein Engrailed, which is expressed in mesencephalic dopaminergic (mDA) neurons, suffer from a progressive loss of these neurons. This loss that starts at 6 weeks postnatal and reaches a plateau of 40% at 48 weeks (Sonnier et al., 2007). We therefore considered the possibility that RGCs, which can take up Otx2 from other cell types in the retina, most likely bipolar cells, might be sensitive to a diminished supply of Otx2 in situ. Contrary to our expectation, mice heterozygous for Otx2 showed no gross disorganization of the retina, and there was no significant cell loss in the GCL at 11 months of age (data not shown; J. Rheey, R. Torero Ibad, A. Prochiantz, and K. L. Moya, manuscript in preparation). This suggested that RGC survival normally does not depend on full Otx2 expression, but it does not eliminate the possibility of a contribution of Otx2 to RGC survival in stress conditions.
Neurofilament (NF) immunofluorescence has been used to identify RGCs in a number of studies (Kong and Cho, 1999; Luo et al., 2001; Ruiz-Ederra et al., 2004; Fuchs et al., 2005); however, NF protein has also been reported in some horizontal cell process endings but not soma (Peichl and Gonzalez-Soriano, 1993). We therefore performed a time course study using cells dissociated from B6.Cg(Thy1-CFP)23Jrs/j adult mouse retinae. Dissociated cells were cultured in the presence or absence of 3.3 nm Otx2 for 4 h to 6 days and compared the survival of CFP-labeled and NF-labeled cells. Assuming that retinal neurons survive dissociation in proportion to their numbers in the retina, we estimate that 510 of the 75,000 cell plated in each well are RGCs. Dissociation of the retina disrupts the adult tissue, and most of the RGCs do not recover from this stress when cultured. In control cultures we observed a time-dependent decrease in CFP+ and NF+ cells so that 4 h after plating about one-half of the estimated RGCs put in culture were present, about 15% at 24 h, and 10% or less at 6 days (Fig. 1). Adding Otx2 to the cultures at the time of plating about doubled the number of CFP+ and NF+ cells starting at 24 h in culture, and this survival effect was maintained through 6 days. At 24 h we found that 93.7% of CFP+ cells were also NF+ in control wells and 97.7% in Otx2-treated wells. Conversely, 88.4% of NF+ cells were also CFP+ in control wells and 90.6% in Otx2-treated wells. This suggests a close correspondence between the two populations. Therefore, we considered NF immunofluorescence to be a reliable marker for surviving adult mouse RGCs. Because the B6.Cg(Thy1-CFP)23Jrs/j mice did not reproduce well in our laboratory, subsequent mouse mixed culture experiments were performed with C57BL/6 mice and NF immunofluorescence.
In an experiment to examine the effect of different concentrations of Otx2, we observed a dose-dependent increase in the number of NF-positive RGCs with about a threefold increase for Otx2 at 1.65 nm compared to the control condition (Fig. 2A). We have replicated the survival effect of Otx2 in mixed retinal cultures in 20 independent experiments using seven different productions of Otx2 with a maximal survival of up to 4.5-fold over control with 1.65–6.6 nm Otx2, depending on the batch. To determine whether the effect of Otx2 on RGC survival could last longer, we maintained the retinal cells in defined culture conditions for 18 d without changing the medium. A single application of Otx2 at the time of plating led to significantly more NF-labeled RGCs in the cultures compared to control (86.8 ± 8.79 1.65 nm Otx2 vs 33.8 ± 2.69 control, p < 0.0005).
These results clearly show that Otx2 has survival-promoting effects on adult central neurons in mixed cell culture conditions. In these cell conditions, photoreceptors, horizontal cells, Müller glia, bipolar, and amacrine cells are plated with the RGCs. It is possible that the effects of Otx2 on RGC survival are indirect, since the exogenous Otx2 added to the culture medium might stimulate the production of (a) survival factor(s) in one of the other cell types that is then available to the RGCs. To determine whether the effects were due to a direct action of Otx2 on RGCs, we used immunopanning to purify RGCs from the adult rat retina based on findings by Barres et al. (1988) and Fuchs et al. (2005).
Purified adult rat RGCs were cultured for 6 d in defined conditions as described above for the mixed retinal cell cultures. We used the calcein live assay (Invitrogen) and counted live cells. Otx2 stimulated a significant increase in the survival of purified RGCs in a dose-dependent manner that was strikingly similar to that observed with mixed cultures (Fig. 2B). Otx2 (1.65 nm) significantly increased the number of calcein-bright RGCs about 2.5-fold compared to control 6 days after plating. Since the preparation of recombinant Otx2 contains protein bands in addition to Otx2, we verified that the survival effects are due to Otx2. Preadsorption of Otx2 with an equimolar amount of anti-Otx2 antibody completely blocked its survival promoting effect, proving that this effect is only due to Otx2 in our recombinant protein preparation (Fig. 2B). The antibody alone added to cultures had no effect on RGC survival compared to control (data not shown). Together, these results demonstrate that Otx2 acts directly on the RGCs to stimulate their survival.
Although the survival effect of Otx2 is robust in terms of percentage of control, the absolute number of RGCs is low. In our mixed cultures, assuming that all retinal cell types are initially plated in proportion to their abundance in the retina, we estimate that each well contains about 500 RGCs at the time of plating. Six to seven days after treatment with Otx2, the total number of surviving RGCs varies between 20 and 30% of the initial number (3–5 times the number that survive without Otx2 treatment). In purified RGC cultures, the number of RGCs that survive is extremely low (0.5–1%), possibly because of the multistep panning procedure that adds to the initial stress caused by adult tissue dissociation.
To avoid the mechanical damage of tissue dissociation and preserve the tissue architecture, we turned to an in vivo protocol based on NMDA excitotoxicity. To validate the NMDA model, we used transgenic mice expressing CFP under the control of the Thy1 promoter (Feng et al., 2000). Four days after intraocular injection of NMDA in thy1-CFP mice, many fewer CFP-labeled profiles were observed in the retina (Fig. 3A). The decrease appeared to be distributed throughout the retina. Semiautomated counting revealed that the number of CFP-labeled profiles was significantly reduced by a third compared to that in the noninjected eye (Fig. 3B). Furthermore, 30 ng of Otx2 injected into the eye at the time of the NMDA fully protected against this loss. (Fig. 3A,B). Using Western blotting of the whole adult retina, we estimate the endogenous Otx2 to be about 9 ng. Therefore the effective dose of 30 ng injected into the vitreous is about three times the endogenous level of Otx2.
Because this transgenic mouse line has a low reproduction rate (i.e., small and infrequent litters), we became interested in a surrogate marker for RGCs that would allow for higher throughput analysis. Brn3a, also known as Pou4f1, is a POU domain transcription factor that in the retina is expressed in RGCs and that recently has been validated as an ex vivo maker for RGC number (Quina et al., 2005; Nadal-Nicolas et al., 2009). We used reverse transcription-quantitative PCR (RT-qPCR) to evaluate levels of Brn3a mRNA in the retina after intraocular injection of NMDA. Four days after NMDA, the levels of Brn3a mRNA were significantly reduced by about 55% compared to the retina from the noninjected eye (Fig. 3C). We used primers for genes in other retinal cell types to examine whether the NMDA excitotoxicity was specific for RGCs and found no change in Islet 1 mRNA that is expressed in RGCs, amacrine cells, and bipolar cells (Elshatory et al., 2007). Based on findings by Jeon et al. (1998), RGCs are only about 4% of the Islet-producing retinal cells in the retina and therefore a 50% loss of RGCs would result in a 2% reduction of Islet 1 mRNA, a change that is likely beyond the sensitivity of the method. We also observed little or no change in syntaxin 1 that is expressed in amacrine and horizontal cells (Alexiades and Cepko, 1997) and mGluR6 and recoverin mRNA specific for bipolar cells (Milam et al., 1993; Hartveit et al., 1995) (Fig. 3C). We observed a slight but significant decrease in the mRNA for rhodopsin that is expressed in rod photoreceptors. This point was not explored further in the present study.
In addition to Brn3a, γ-synuclein has been used to identify RGCs (Soto et al., 2008; Surgucheva et al., 2008). In a separate experiment in which we used 1 mm NMDA, Brn3a and γ-synuclein mRNA levels were both reduced significantly by 40% each (40.7 ± 0.11 and 39.7 ± 0.0.9, respectively, p < 0.05, single sample 2-tailed t test). In a retina that received 30 ng of Otx2 at the same time as NMDA, the levels of Brn3a and γ-synuclein were significantly greater than in NMDA-injected retina (p < 0.05, 2-tailed t test). Together, our data suggests that Brn3a expression can be taken as an index of RGC number.
Since Otx2 is a transcription factor and could regulate Brn3a expression independently of cell death, we tested whether Otx2 injected into the eye of C56BL/6 wild-type mice altered Brn3a transcript levels. Thirty nanograms of Otx2 had no effect on retinal Brn3a mRNA 4 days after injection, demonstrating that Otx2 does not positively or negatively regulate Brn3a expression levels in the retina in vivo (Fig. 4A). The ratio of Brn3a in the injected eye compared to the noninjected eye was significantly reduced after NMDA. Thirty nanograms of Otx2 injected at the same time as NMDA completely protected against NMDA-induced reduction in Brn3a mRNA. Assuming a vitreal chamber volume of 8 μl, 30 ng corresponds to 110 nm. Three nanograms (11 nm) of Otx2 provided partial (about 50%) protection. Because our in vitro results showed a bell-shaped dose–response RGC survival curve with Otx2, we wanted to determine whether higher concentrations of Otx2 would have diminished survival promoting activity in vivo. In the experiment depicted in Figure 4B, NMDA reduced Brn3a mRNA by about 50%. Thirty-six and 144 ng injected at the same time as NMDA both provided significant protection against this decrease in Brn3a mRNA levels to the same extent. Thus, in vivo Otx2 offers half to full protection against NMDA excitotoxicity over a 50-fold range of concentrations (from 3 to 144 ng).
When Otx2 was preincubated with the anti-Otx2 antibody, protection against NMDA excitotoxicity was abolished (Fig. 4C). A single administration of Otx2 at the time of NMDA injection protects against a decrease of Brn3a mRNA for at least up to 3 weeks (Fig. 4D).
While these results show that Otx2 protects against NMDA excitotoxic RGC loss, they do not provide information on whether the surviving RGCs are functional. For this, we used the optomotor test that provides a measure of visual acuity (Prusky et al., 2004; Abdeljalil et al., 2005). In preliminary studies we noted that the finest square wave contrast grating that would reliably induce head movements in a majority of wild-type C56BL/6 mice was an optotype of 0.375 cpd (data not shown). We pretested mice with this optotype and selected those that made at least four head fixation and movements in 1 min. Four days after intraocular injection of NMDA, the number of head movements was significantly reduced (Fig. 5A). In mice that received Otx2 at the time of the NMDA injection, the number of head turns was not significantly reduced compared to the number before treatment and was significantly greater than that in the mice treated with NMDA alone. RT-qPCR for Brn3a in the retinae of these same mice confirmed that NMDA reduced Brn3a mRNA and thus the number of RGCs and that Otx2 prevented this decrease (Fig. 5B). These results show that Otx2 protects against the loss of visual acuity due to NMDA-induced excitotoxic loss of RGCs and that there is a good correspondence between RGC number as assessed by Brn3a expression levels and visual acuity.
In all homeoproteins tested, the amino acids WF in the third helix of the homeodomain are necessary for translocation across the plasma membrane (Joliot et al., 1998). We expressed and purified Otx2 mutated in the corresponding WF of the homeodomain of Otx2 (WF85/86YL). Six hours after intraocular injection, retinal sections were treated with detergent and Otx2wt or Otx2YL was visualized using an anti-myc antibody. We observed that Otx2wt accumulates in the ganglion nuclear layer (GCL), inner nuclear layer (INL), and to a lesser extent in the outer nuclear layer (ONL), while Otx2YL does not (Fig. 6A). In a separate experiment we injected 156 ng of Otx2wt into the eye and visualized the protein in retinal sections 6 h later. We observed important accumulation of Otx2wt protein in soma in all cell layers (Fig. 6Bi). High magnification revealed that exogenous Otx2 was present in nuclei and, to a lesser degree, in the cytoplasm of cells in the GCL (Fig. 6Bii,Biii,Biv,Bv).
We then tested Otx2YL on cultures of dissociated mouse retinal cells. Whereas Otx2wt significantly increased the number of NF-positive RGCs after 6 d in culture, the number of NF-positive cells after treatment with Otx2YL was no different than in control cultures (Fig. 6C). We then tested Otx2YL with NMDA in vivo and found that, as opposed to wild type Otx2, Otx2YL did not protect against NMDA excitotoxicity of RGCs in vivo (Fig. 6D). Thus, Otx2YL does not increase RGC survival in vitro or in vivo.
Finally, we asked whether Otx2 survival-promoting activity involved antiapoptotic activity. Dissociated cells from adult mouse retina were cultured for 24 h in the presence of 3.3 nm Otx2 and/or 20 μm ZVAD (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone), an anti-apoptotic caspase inhibitor. Otx2 more than doubled the number of NF+ cells (Fig. 7). ZVAD also increased RGC survival significantly but to a lesser extent, indicating that some RGC death in dissociated culture occurs by apoptosis. We observed no synergistic or additive effect when both Otx2 and ZVAD were added to the culture medium. The similar effect of Otx2 and ZVAD coupled with the absence of any additive effect suggests that survival-promoting activity of Otx2 in vitro involves anti-apoptotic activity.
Previous studies have reported that intraocular NMDA injected leads to RGC loss through apoptosis and that RGC death can be partially protected with anti-apoptotic compounds Z-YVAD and Z-DEVD (Barnett et al., 2009; Lebrun-Julien et al., 2009). However, other studies have suggested that RGC death after NMDA also involves necrosis (Goebel, 2009; Saggu et al., 2010). We tested ZVAD for RGC protection in vivo against NMDA excitotoxicity. In a meta analysis of two experiments we found that NMDA reduced Brn3a mRNA by over 70%, and with ZVAD the levels were only reduced about 45% (t = −2.679, p < 0.05). These results confirm that at least some RGC death after NMDA occurs through apoptosis and thus that Otx2 protects against apoptosis also in vivo, not precluding protection against other cell death mechanisms, since protection by Otx2 can reach 100%.
Discussion
Although first categorized as developmental genes, homeobox genes encoding homeoprotein transcription factors are often expressed in the adult where their functions are still relatively poorly known. In this study we show that Otx2 homeoprotein can act as a survival factor for adult RGCs in two distinct experimental paradigms. It protects RGCs from tissue disruption-induced death during the dissociation step preceding plating in vitro and also against NMDA-induced death in vivo. Most importantly, the in vivo protection is paralleled by the preservation of visual acuity.
This study was initiated by two observations. The first is that Otx2 is present in adult RGCs despite the absence of detectable gene expression and is thus imported into RGCs, probably from bipolar cells (Koike et al., 2007; Rath et al., 2007; Sugiyama et al., 2008; Glubrecht et al., 2009). Although this Otx2 import has an important physiological function in the regulation of the critical period in the cortex (Sugiyama et al., 2008), the import could have local physiological functions in the retina. Among them, we considered neuronal survival based on the survival activity of Engrailed for mDA neurons (Sonnier et al., 2007).
Second, in Engrailed-1 heterozygous mice mDA cells start to die after 6 weeks, and this progressive death (38% after 1 year in the substantia nigra and 20% in the VTA) can be antagonized by infusion of exogenous Engrailed and its internalization in vivo (Sonnier et al., 2007). In the retina in contrast, we did not observe any significant loss of cells in the GCL in Otx2 heterozygous mice at 11 months of age. This difference may reflect the fact that Engrailed expression is cell autonomous in mDA cells, whereas Otx2 is taken up by RGCs and this may lead to variable levels of Otx2. Also, although in normal conditions RGC maintenance may not depend on high Otx2 content, we reasoned that Otx2 might become essential for the survival of RGCs when they are placed in a life-threatening situation.
In experiments involving the dissociation of adult retinas, RGC death results from the severe insult to the cells, including a loss of local and distal cell partners. Previous studies that examined RGC survival in culture used embryonic animals or pups up to the second postnatal week, and in the best cases reported a 5- to 7-fold increase in RGC survival (de Melo Reis et al., 2008). To our knowledge the present study is unique in using RGCs from adult mice and rats, and we found that Otx2 promoted RGC survival 2- to 5-fold above untreated cultures. Although this is highly significant, the number of surviving RGCs as a percentage of the RGCs initially plated is low.
We were encouraged by these results and sought a more physiological situation. Genetic models of RGC loss, such as the DBA/2J mouse, take long to develop and have between-animal and within-animal variability in retinal pathology (Schlamp et al., 2006). As a way to cause rapid and reproducible RGC cell death in vivo, we used NMDA-induced excitotoxicity and assessed RGC survival by measuring Brn3a mRNA levels in a number of experiments. The reduction of Brn3a mRNA after NMDA was corroborated by a reduction of another RGC marker, γ-synuclein, and Otx2 countered this reduction. NMDA reduced Brn3a mRNA in a manner similar to that of a number of CFP RGCs, and Otx2 was able to fully protect against the reduction of both. Finally, Brn3a mRNA levels correlated with the loss of visual acuity after NMDA. For these reasons we consider Brn3a expression to be a reliable surrogate marker for RGC number in the NMDA model of excitotoxicity and perhaps other acute models of RGC loss. It remains to be validated if Brn3a levels can be used to assess RGC number in chronic long-term models such as DBA/2J mice.
Many other groups have used this NMDA model for RGC loss, and it was initially thought that NMDA acted directly on the RGCs. A recent report now suggests that NMDA excitotoxicity of RGCs acts on Müller glia cells that then release TNFα that triggers RGC death via apoptosis (Lebrun-Julien et al., 2009). Other studies have also attributed NMDA-induced RGC death to apoptosis (Barnett et al., 2009; Goebel, 2009), while some laboratories have reported that in this model RGC death involves other mechanisms (Saggu et al. 2010). Our results clearly show Otx2 and ZVAD, an anti-apoptotic drug, have similar and nonadditive effects in vitro and that, in the NMDA model of RGC excitotoxicity in vivo, Otx2 saves 100% of the cells and fully preserves visual acuity in the optomotor test. The two sets of data, in vitro and in vivo, indicate that Otx2 has antiapoptotic activity. They do not eliminate the possibility that it acts on other death mechanisms as well.
A difference between our in vitro and in vivo results is the bell-shaped dose–response in vitro compared to an Otx2 effective dose range of about 50-fold in vivo. One possible explanation is that the Otx2 preparations contain toxic compounds that, above a certain concentration, overwhelm the in vitro survival effects of Otx2. In vivo, the toxin could be neutralized and/or diluted via the circulation. Local inactivation in the eye and/or rapid dilution may also explain the higher Otx2 doses necessary in vivo compared to in vitro.
We note important similarities between our in vitro and in vivo results. First, Otx2 in vitro and in vivo survival-promoting activity is abrogated by a point mutation that interferes with internalization. Current studies are underway to determine whether the survival-promoting activity is dependent on transcription and/or translation (Nedelec et al., 2004; Brunet et al., 2005; Topisirovic and Borden, 2005) and to identify the molecular targets of Otx2. Second, Otx2 acts directly on RGCs. This is directly shown when pure RGC cultures were used. This may also be the case in vivo, because the geometry of the eye is such that RGCs are the first cells encountered by Otx2 following its injection, and at lower although active concentrations its diffusion into the retinal parenchyma is mainly in the innermost layers. A third important similarity between our in vitro and in vivo experiments is the long-lasting survival effect of a single treatment with Otx2 (at least 18–21 d). It is at present unknown how a single administration of Otx2 engages an RGC survival program. In light of the nonautonomous effect of Otx2 on the maturation of visual cortex parvalbumin interneurons during critical period onset (Sugiyama et al., 2008), which is paralleled by an epigenetic switch in visual cortex class cells (Putignano et al., 2007; Fagiolini et al., 2009), the protein may act similarly in RGCs. Regardless of the precise mechanism, identification of Otx2 target genes in RGCs is paramount for understanding its survival promoting effects.
In humans, RGC loss leading to visual impairment and blindness occurs in diabetic retinopathy, optic neuropathies including ischemic optic neuropathy, Leber hereditary optic neuropathy and traumatic optic neuropathy, and glaucoma (Osborne et al., 1999; Levin, 2007; Kern and Barber, 2008). Our results show that Otx2 promotes the survival of RGCs that have been mechanically damaged and challenged by excitotoxicity. Therefore, Otx2 may provide a useful therapeutic approach to slow or prevent RGC death in glaucoma and other diseases.
Footnotes
This work was supported by the Consortium National de Recherche en Génomique via le Programme National de Recherche “Vision,” Fovea-Pharmaceuticals, and Global Research Laboratory Program Grant 2009-00424 from the Korean Ministry of Education, Science, and Technology. S.P. receives support from the Agence Nationale de la Recherche (ANR GLAUCOME). We acknowledge Olivier Calvetti for participating in initial experiments, Nicole Quenech'du for assistance in cell counting, Christelle Davrinche for training in the use of the optomotor test, and the Nikon Imaging Centre at Institut Curie-CNRS. We also thank Olivier Stettler and Michel Volovitch for discussions and Prof. José Sahel for his insight. S.P., A.P. and K.L.M. designed experiments. R.T.I., J.R., S.M., V.F., and K.L.M. carried out research. R.T.I., J.R., S.M., A.P., and K.L.M. analyzed data. A.P. and K.L.M. developed the concept, directed experiments, and wrote the manuscript.
References
- Abdeljalil J, Hamid M, Abdel-Mouttalib O, Stéphane R, Raymond R, Johan A, José S, Pierre C, Serge P. The optomotor response: a robust first-line visual screening method for mice. Vision Res. 2005;45:1439–1446. doi: 10.1016/j.visres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. doi: 10.1242/dev.124.6.1119. [DOI] [PubMed] [Google Scholar]
- Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc Natl Acad Sci U S A. 2009;106:9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J, Shen F, Goldblum D, Chen B, Ramos-Esteban J, Podos SM, Mittag T. Cytoarchitecture of the retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci. 2002;43:587–594. [PubMed] [Google Scholar]
- de Melo Reis RA, Cabral-da-Silva MC, de Mello FG, Taylor JS. Müller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res. 2008;1205:1–11. doi: 10.1016/j.brainres.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007;503:182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fuchs C, Forster V, Balse E, Sahel JA, Picaud S, Tessier LH. Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2983–2991. doi: 10.1167/iovs.04-1177. [DOI] [PubMed] [Google Scholar]
- Glubrecht DD, Kim JH, Russell L, Bamforth JS, Godbout R. Differential CRX and OTX2 expression in human retina and retinoblastoma. J Neurochem. 2009;111:250–263. doi: 10.1111/j.1471-4159.2009.06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel DJ. Selective blockade of CaMKII-alpha inhibits NMDA-induced caspase-3-dependent cell death but does not arrest PARP-1 activation or loss of plasma membrane selectivity in rat retinal neurons. Brain Res. 2009;1256:190–204. doi: 10.1016/j.brainres.2008.12.051. [DOI] [PubMed] [Google Scholar]
- Hartveit E, Brandstätter JH, Enz R, Wässle H. Expression of the mRNA of seven metabotropic glutamate receptors (mGluR1 to 7) in the rat retina. An in situ hybridization study on tissue sections and isolated cells. Eur J Neurosci. 1995;7:1472–1483. doi: 10.1111/j.1460-9568.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Joliot, Maizel A, Rosenberg D, Trembleau A, Dupas S, Volovitch M, Prochiantz A. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Curr Biol. 1998;8:856–863. doi: 10.1016/s0960-9822(07)00346-6. [DOI] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Nishida A, Ueno S, Saito H, Sanuki R, Sato S, Furukawa A, Aizawa S, Matsuo I, Suzuki N, Kondo M, Furukawa T. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol Cell Biol. 2007;27:8318–8329. doi: 10.1128/MCB.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WC, Cho EY. Antibodies against neurofilament subunits label retinal ganglion cells but not displaced amacrine cells of hamsters. Life Sci. 1999;64:1773–1778. doi: 10.1016/s0024-3205(99)00115-0. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Duplan L, Pernet V, Osswald I, Sapieha P, Bourgeois P, Dickson K, Bowie D, Barker PA, Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J Neurosci. 2009;29:5536–5545. doi: 10.1523/JNEUROSCI.0831-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA. Mechanisms of retinal ganglion specific-cell death in Leber hereditary optic neuropathy. Trans Am Ophthalmol Soc. 2007;105:379–391. [PMC free article] [PubMed] [Google Scholar]
- Luo X, Heidinger V, Picaud S, Lambrou G, Dreyfus H, Sahel J, Hicks D. Selective excitotoxic degeneration of adult pig retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1096–1106. [PubMed] [Google Scholar]
- Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993;10:1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Cánovas-Martínez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- Nédélec S, Foucher I, Brunet I, Bouillot C, Prochiantz A, Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2004;101:10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, González-Soriano J. Unexpected presence of neurofilaments in axon-bearing horizontal cells of the mammalian retina. J Neurosci. 1993;13:4091–4100. doi: 10.1523/JNEUROSCI.13-09-04091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron [Erratum (2007) 54:177] 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O'Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25:11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath MF, Morin F, Shi Q, Klein DC, Møller M. Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp Eye Res. 2007;85:65–73. doi: 10.1016/j.exer.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Raymond ID, Vila A, Huynh UC, Brecha NC. Cyan fluorescent protein expression in ganglion and amacrine cells in a thy1-CFP transgenic mouse retina. Mol Vis. 2008;14:1559–1574. [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, García M, Hicks D, Vecino E. Comparative study of the three neurofilament subunits within pig and human retinal ganglion cells. Mol Vis. 2004;10:83–92. [PubMed] [Google Scholar]
- Saggu SK, Chotaliya HP, Blumbergs PC, Casson RJ. Wallerian-like axonal degeneration in the optic nerve after excitotoxic retinal insult: an ultrastructural study. BMC Neurosci. 2010;11:97. doi: 10.1186/1471-2202-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnier L, Le Pen G, Hartmann A, Bizot JC, Trovero F, Krebs MO, Prochiantz A. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J Neurosci. 2007;27:1063–1071. doi: 10.1523/JNEUROSCI.4583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–561. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Surgucheva I, Weisman AD, Goldberg JL, Shnyra A, Surguchov A. Gamma-synuclein as a marker of retinal ganglion cells. Mol Vis. 2008;14:1540–1548. [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Borden KL. Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): an unexpected relationship. Histol Histopathol. 2005;20:1275–1284. doi: 10.14670/HH-20.1275. [DOI] [PubMed] [Google Scholar]