Abstract
According to the “modular” hypothesis, reading is a serial feedforward process, with part of left ventral occipitotemporal cortex the earliest component tuned to familiar orthographic stimuli. Beyond this region, the model predicts no response to arrays of false font in reading-related neural pathways. An alternative “connectionist” hypothesis proposes that reading depends on interactions between feedforward projections from visual cortex and feedback projections from phonological and semantic systems, with no visual component exclusive to orthographic stimuli. This is compatible with automatic processing of false font throughout visual and heteromodal sensory pathways that support reading, in which responses to words may be greater than, but not exclusive of, responses to false font. This functional imaging study investigated these alternative hypotheses by using narrative texts and equivalent arrays of false font and varying the hemifield of presentation using rapid serial visual presentation. The “null” baseline comprised a decision on visually presented numbers. Preferential activity for narratives relative to false font, insensitive to hemifield of presentation, was distributed along the ventral left temporal lobe and along the extent of both superior temporal sulci. Throughout this system, activity during the false font conditions was significantly greater than during the number task, with activity specific to the number task confined to the intraparietal sulci. Therefore, both words and false font are extensively processed along the same temporal neocortical pathways, separate from the more dorsal pathways that process numbers. These results are incompatible with a serial, feedforward model of reading.
Introduction
Cognitive scientists who study reading are broadly divided into “localists” and “connectionists” (Plaut et al., 1996; Coltheart, 2004). This debate has become centered on the function of a cortical region known as the visual word form area (VWFA). Destruction of this region, located at the left ventral occipitotemporal junction, or its connections to early visual cortex, results in severe alexia (Binder and Mohr, 1992; Leff et al., 2001). What remains uncertain is how this region supports reading.
Evidence from functional magnetic resonance imaging (fMRI) has led to the proposal that the VWFA stores representations of orthographically regular letter strings (Cohen et al., 2000) or whole words (Kronbichler et al., 2004), and furthermore, that there is a posterior-to-anterior hierarchy within the VWFA in response to letters, orthographically regular letter strings and pseudowords, and whole words (Dehaene et al. 2005; Vinckier et al., 2007; Glezer et al., 2009). This affords anatomical and physiological support to a serial information-processing model of reading, with encoded letter forms feeding forward into an orthographic input lexicon.
In contrast, Devlin et al. (2006) provide evidence that the VWFA stores neither prelexical nor lexical representations but acts as an interface between the abstract visual information conveyed by script and feedback from more anterior cortex that processes phonology and semantics. According to this interactive model, activity in the VWFA at the spatial and temporal resolution of fMRI is the sum of feedforward and feedback synaptic activity.
In a previous study, using positron emission tomography (PET), two reading-related pathways were demonstrated in left temporal cortex, one ventral along the fusiform gyrus and the other lateral along the left superior temporal sulcus (Spitsyna et al., 2006). At the anterior extent of both pathways, activity in response to narrative language became independent of modality, with an equal response to both written and spoken narratives. Although activity along the two pathways was stronger for written words than for equivalent arrays of false font, the design did not assess the response to false font relative to visual stimuli other than words. A response to false font distributed along both reading-related pathways and into their anterior extents would be absent if word processing occurs in a serial feedforward manner. In contrast, activity in response to false font would be compatible with an interactive, distributed model, which does not predict any script-specific visual or heteromodal activations, and does not preclude the automatic processing of arrays of false font along reading-related pathways.
The other familiar symbols conveying visual information at high spatial frequencies are numbers, but number semantics are processed in parietal cortex (Thioux et al., 2005; Cappelletti et al., 2010). The localist model would predict that activity in ventral and lateral temporal neocortex would follow the profile words > false font = numbers; in contrast, a profile of words > false font > numbers would be evidence against a localist model. Although this result would not establish the neural instantiation of a connectionist account of reading, it would be compatible with such an interpretation.
Materials and Methods
Subjects and fMRI procedures.
Nineteen right-handed subjects participated (nine females; mean age, 26 years). All subjects had normal vision and were native English speakers. None had any history of reading disorders or significant neurological or psychiatric illness. All participants gave written consent and were checked for contraindications to MRI scanning. Ethical approval was awarded by the local Research Ethics Committee.
MRI data were obtained on a Phillips Intera 3.0 tesla MRI scanner, using an eight-array head coil and sensitivity encoding with an undersampling factor of 2. Functional MRI images used a T2*-weighted gradient-echo echoplanar imaging sequence with a repetition time of 3 s. Whole-brain volumes of 48 axial slices with a slice thickness of 5 mm and in-plane resolution 2.5 × 2.5 mm were acquired in an interleaved ascending order. T1-weighted whole-brain structural images were also obtained for accurate spatial registration.
Stimuli were presented using E-Prime software (Schneider et al., 2002) on an IFIS-SA system (In Vivo Corporation). Earplugs and padded headphones were used to protect the participant's hearing. Additional foam padding was used to minimize head movements.
Functional data were acquired in a block design, using a continuous acquisition protocol. There were three repetitions of each of the five conditions within each of two runs. Conditions were presented in a pseudorandom order to prevent correlation with scanner drift and to maximize the variety of transitions between tasks. Each block lasted 39 s and consisted of a 3 s fixation period, 30 s of task stimuli, and 6 s of rest.
Visual stimuli.
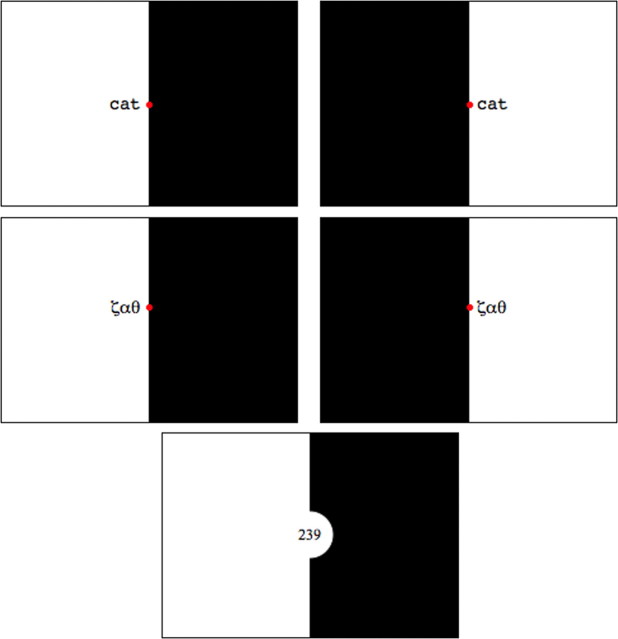
The study consisted of the following five types of stimulus: reading narratives in the left visual field (LR); reading narratives in the right visual field (RR); false font presentation in the left visual field (LF); false font presentation in the right visual field (RF); and a number decision task (ND). Stimuli for the LR and RR conditions consisted of 60-word text narratives adapted from children's books, consisting of words of higher frequency and imageability and sentences comprising simple syntax. Words were between three and six letters long, presented using rapid serial visual presentation (RSVP) to either the left or right of a central fixation point (Fig. 1). Each word was presented on screen for 500 ms with no interstimulus interval. The words were aligned to the vertical meridian; that is, words presented to the right visual field were left aligned, and those to the left visual field were right aligned. The stimuli subtended an angle of no more than 5° from fixation (foveal and parafoveal vision subtends an angle of ∼5–6° either side of fixation).
Figure 1.
Sample images from left reading (top left), right reading (top right), left false font (middle left), right false font (middle right), and number (bottom) decision conditions. Font size is not to scale.
Stimuli for the LF and RF conditions were translations of the text narratives using a Roman-to-Greek alphabet character correspondence code. Greek characters were chosen for their close visual similarity to Roman alphabetical characters and to allow easy conversion from real to false font text. Greek characters recognizable as alphabetic letters, such as ω, were excluded. No participant had an education in the Greek language, and, as an added safeguard, the conversion from Roman to Greek characters was arbitrary.
In the reading condition, participants were instructed to maintain gaze on the central fixation dot and to read the text silently. Because part of the words fell in lower acuity parafoveal vision, this made word recognition more difficult than normal and required the subjects to suppress saccades that would bring the serially presented words entirely into foveal vision. The half-field of vision opposite to that in which words were presented was rendered black. The presentation of false font “narratives” was identical. After the first one or two false font “words” had been presented, the subjects were aware that they were going to be presented with a series of meaningless stimuli, reducing the temptation to make a saccade toward each stimulus.
The ND task was designed to focus attention on processing a simple semantic property of numbers. Three-digit numbers were presented in the center of the screen (that is, within foveal vision to ensure rapid recognition) for 1500 ms, with attention focused on the last digit to decide whether the number was odd or even. The decision was signaled using a two-button pad operated with the left hand.
fMRI whole-brain analysis, using contrasts of conditions.
fMRI preprocessing and analysis were performed with the software fMRI Expert Analysis Tool FEAT by the FMRIB Software Library (FSL) (Smith et al., 2004; Woolrich et al., 2009). Preprocessing stages included skull stripping using the Brain Extraction Tool BET (Smith, 2002), motion correction using the FMRIB Linear Image Registration Tool FLIRT (Jenkinson et al., 2002), spatial smoothing using a 5 mm full-width at half-maximum Gaussian filter, high-pass temporal filtering, and registration to the high-resolution T1 anatomical image.
At the first level, design matrices were created for each run and for each subject that modeled the five experimental conditions as explanatory variables. Contrasts of parameter estimates (COPEs) were calculated for contrasts of interest using a fixed-effects design. The COPE data were then entered into the second level, in which COPEs were combined across the two runs per subject in a fixed-effects analysis. Finally, at the group level, a mixed-effects analysis was performed using the FMRIB Local Analysis of Mixed Effects FLAME tool (Beckmann et al., 2003). Statistical images were thresholded using a cluster-corrected threshold of Z >3.0, p < 0.05.
fMRI region of interest data analysis.
Region of interest (ROI) analyses were performed using the FSL Featquery tool (Smith et al., 2004; Woolrich et al., 2009) to allow between-region statistical comparisons rather than relying on the whole-brain activation maps in which apparent differences may be attributable to the use of arbitrary statistical thresholds. Spherical ROI masks with a 7 mm diameter were created symmetrically in left and right hemispheres. The regions and their peak coordinates are listed in supplemental Table 1 (available at www.jneurosci.org as supplemental material) and shown in Figure 4. The anatomical locations were confirmed using the Anatomy Toolbox contained within SPM software (Eickhoff et al., 2005).
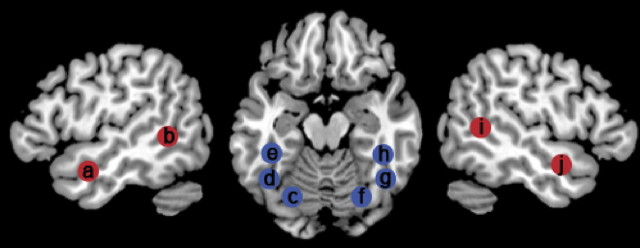
Figure 4.
A depiction of the approximate location of the regions of interest tested for main effects of stimulus type and stimulus location. The regions are left aSTS (a), left pSTS (b), left pFG (c), left fusiform gyrus in the vicinity of the VWFA (d), left mid fusiform gyrus anterior to the VWFA (e), right pFG (f), right fusiform gyrus in the vicinity of the VWFA (g), right mFG anterior to the VWFA (h), right pSTS (i), and right aSTS (j). Ventral visual stream regions are shown in blue, and lateral temporal stream regions are shown in red.
Three ROI were located along the left and right ventral visual streams: the first in the most posterior part of the fusiform gyrus (pFG); the next in a more anterior region of fusiform gyrus, with coordinates matching the VWFA; and the third in the mid fusiform gyrus anterior to the VWFA (mFG). The mFG was located at the most anterior part of the ventral temporal lobe from which the blood oxygenation level-dependent (BOLD) signal could be recovered because of the known problem of susceptibility artifact in this region (Visser et al., 2010). Two additional ROIs were located in the lateral temporal lobes, including the posterior superior temporal sulcus (pSTS) and the anterior superior temporal sulcus (aSTS).
The location of the VWFA ROI was defined according to published coordinates (Jobard et al., 2003), and the pFG and mFG ROIs were located relative to it. Functional activations were used as a guide to ensure that the VWFA, pFG, and mFG ROIs were contingent with the ventral visual stream and that the aSTS and pSTS ROIs were contingent with the lateral temporal stream. Although we acknowledge that the practice of defining ROIs according to functional activations can raise issues of statistical non-independence, this is only an issue if the purpose of the ROI analysis is to gain greater sensitivity for detecting statistical effects within the ROI. However, our intention was to use this analysis to establish interhemispheric asymmetries of activity and support impressions of apparent symmetries of activity between regions, which may be falsely inferred when viewing thresholded whole-brain statistical maps (Jernigan et al., 2003).
Featquery was used to extract COPE values for LR, RR, LF, and RF relative to ND contrasts from each individual subject. The 90th percentile voxel value within the ROI was taken rather than the maximum value to reject any artificially high outlier voxels.
Eye tracking.
An eye-tracking study was conducted on 10 participants (seven female; mean age, 35 years, 0 months), with partial overlap between the fMRI and eye-tracking participant group. The eye tracking was conducted outside the scanner to determine whether eye movements were affected by stimulus type (words or false fonts) or stimulus location (left or right visual hemifield). The LR, RR, LF, and RF stimuli and protocol (the ND task was omitted for simplicity) were replicated and presented to participants using the SR Eyelink II system (SR Research Ltd.). The stimuli were presented on a 22 inch screen with resolution of 1028 × 768 pixels, at a viewing distance of 50 cm. Stimuli were positioned with the inner extent 5 mm from the central fixation point and the outer edge up to 50 mm away, with a visual angle of 5.7° eccentricity. Eye movements were monitored using binocular tracking at a sampling rate of 500 Hz. As in the fMRI protocol, a block design was used, with a 60-word or false font narrative presented using RSVP. There were six blocks of each condition, making a total of 24 bocks split into two runs. Eye position was calibrated using a nine-point grid before each run, and drift correction was performed before each block.
The number of blinks and saccades during each block was recorded. Three “interest areas” were defined within the display screen: a rectangle covering the fixation point and two identical rectangles covering the left and right stimulus display regions. The amount of time the participant's gaze was directed toward these three areas was recorded for each trial.
Recognition memory test.
An unanticipated recognition memory test was designed to establish how well the participant was able to read and remember the texts after the eye-tracking procedure. The test consisted of 44 sentences, half of which were familiar. The familiar and unfamiliar sentences were made up from separate lists of content words, but they were broadly matched for meaning: “they drove along the quiet windy roads” (familiar) and “they walked quietly along the sandy path” (unfamiliar). Across the whole list, the familiar and unfamiliar sentences were matched for syntactic structure and length. An equal number of familiar sentences from the LR and RR blocks were used. The familiar and unfamiliar sentences were presented in random order, and the participants were required to identify them as familiar and unfamiliar as a forced two-way choice.
Results
Behavioral results
Recognition memory test
The average score on this task, performed offline, was a mean correct characterization (familiar vs unfamiliar) on 32 of 44 items, range of 25–39 (chance score, 22). The average number of “hits” (correct recognition of a familiar sentence) presented in the left visual field was 7 (range, 2–10 of 11) and in the right visual field was 7 (range, 4–11 of 11). A repeated-measures ANOVA showed no significant difference in recognition of sentences in the two visual fields (F(1,8) = 0.03, p = 0.9).
A measure of stimulus detection sensitivity was calculated for each individual (d′). This compares the likelihood of the participant correctly identifying familiar items (hits) and incorrectly identifying unfamiliar items as familiar (“false alarms”). All participants but one had a d′ score above 1, indicating that they made more hits than false alarms. The average ± SD score was 1.51 ± 0.65.
Between-subjects t tests showed no difference in hit rates (left visual field: t(7) = −0.30, p = 0.61; right visual field: t(7) = 0.32, p = 0.40) or d′ scores (t(7) = 0.02, p = 0.891) was observed between the naive participants and those who had been exposed previously to the stimuli in the fMRI study.
Eye tracking
Table 1 shows the percentage of time that gaze was directed at the fixation point or toward the stimulus for each condition (the eye movements toward the stimuli were a mixture of slow drifts or saccades, with saccades back on to the fixation point). The ability to maintain central fixation was less when the stimuli were words; gaze position was analyzed using a 2 (task) × 2 (hemifield) repeated-measures ANOVA, demonstrating a main effect of task (false font > words; F(1,8) = 9.1, p < 0.05) but not of visual hemifield (F(1,8) = 3.3, p = 0.1). The interaction between stimulus type and location was not significant (F(1,8) = 1.7, p = 0.2). Although central gaze was maintained on average for at least two-thirds of each block of 60 words, there was considerable individual variability (Table 1). Including participant group (new subjects vs participants from the fMRI study) as a between-subjects factor resulted in no significant main effects or interactions.
Table 1.
Group means ± SDs for eye-tracking measures
| Measure | LR | RR | LF | RF |
|---|---|---|---|---|
| Blinks | 23.9 ± 30.1 | 24.4 ± 33.0 | 28.7 ± 35.4 | 25.1 ± 35.6 |
| Saccades | 187 ± 87.4 | 188 ± 90.5 | 149 ± 84.2 | 162 ± 77.3 |
| Gaze at fixation | 73.1 ± 29.3% | 66.3 ± 31.7% | 84.9 ± 18.0% | 71.8 ± 28.9% |
| Gaze at stimulus | 14.8 ± 27.9% | 30.5 ± 31.1% | 7.7 ± 9.1% | 22.6 ± 25.8% |
Includes the number of blinks, number of saccades, and the percentage of time within each block in which gaze was directed toward the fixation area or toward the stimulus presentation area for each condition (n = 9).
fMRI whole-brain analysis, using contrasts of conditions
Left and right visual field presentations
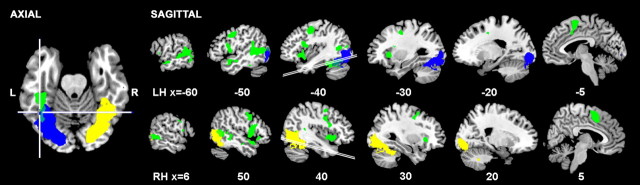
The contrast of all stimuli presented in the right visual field (RR + RF) with those presented in the left visual field (LR + LF) demonstrated peak activity in left primary visual cortex, with activity extending in a ventral direction toward the occipitotemporal junction (Fig. 2). The opposite contrast showed a similar distribution of activity in the right occipital lobe.
Figure 2.
Color-coded overlays of increased activity in three contrasts that was significant at a cluster-corrected threshold of Z > 2.3, p < 0.05, shown on a standard T1 brain template anatomically normalized into Montreal Neurological Institute stereotactic space. The views are sagittal sections in planes either side of the midline (x coordinates are in millimeters; negative is to the left, LH; positive is to the right, RH). The one axial section (left) was oriented in the plane of the ventral temporal lobes (indicated on the sagittal planes at 40 mm either side of the midline). The crosshairs locate the center of mass of the visual word form area (Jobard et al., 2003). The yellow overlay represents activity that was stronger for stimuli presented in the left visual field [(LR and LF) vs (RR and RF)], and the blue overlay shows the reverse contrast [(RR and RF) vs (LR and LF)]. The green overlay demonstrates the main effect of stimulus type, that is, regions more active during reading than during viewing false font stimuli, independent of hemifield of presentation [(RR and LR) vs (RF and LF)].
Contrasts of reading and false font
The contrast of reading (RR + LR) with false font (RF + LF) demonstrated symmetrical activity in left and right lateral temporal neocortex but a left-lateralized response in the ventral temporal lobe, in the fusiform gyrus. At the conservative statistical threshold used, the left ventral processing “stream” for words relative to false font was first evident 80 mm posterior to the anterior commissure. It extended forwards for 45 mm until the signal was lost in a region of susceptibility artifact. In lateral left temporal neocortex, activity extended from the lateral occipital cortex and thence along the entire length of the STS, with activity most evident at the posterior and anterior extents. On the right, no ventral stream was evident, even at a low statistical threshold (Z > 2.3; voxelwise correction), but the lateral temporal activity had a distribution very similar to that on the left.
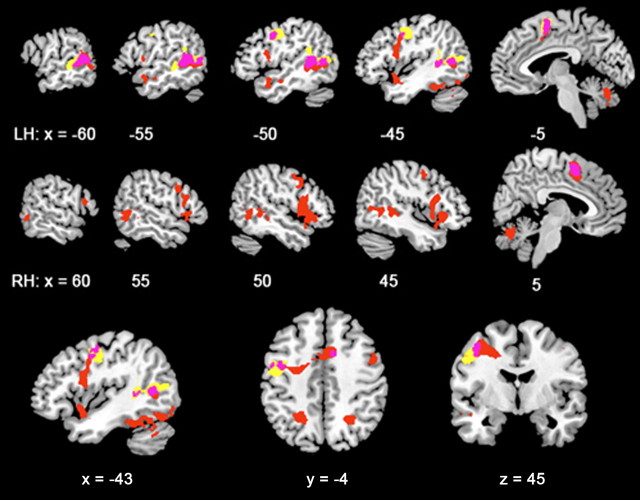
Reading relative to false font also demonstrated activity outside the occipital and temporal lobes, and this was more evident when words were presented in the left visual field (Fig. 3). In the contrast of stimuli presented in the left visual field (LR − LF), activity symmetrically distributed between the cerebral hemispheres was observed medially in the presupplementary motor area (pre-SMA) and laterally along the precentral sulcus as far ventral as the anterior insular cortex. More posterior, there were foci of symmetrical activity in both superior parietal lobes. An additional focus of activity was observed in the posterior midline cerebellum, in lobule IX.
Figure 3.
Color-coded overlays of activity for additional contrasts, depicted as in Figure 2. On the left and right sagittal views (top and middle rows, respectively), the red and yellow overlays demonstrate the effect of stimulus type in either hemifield: red demonstrates activity in the contrast of LR with LF, and yellow demonstrates activity in the contrast of RR with RF. Pink demonstrates areas of overlapping activity for the two contrasts. The bottom row shows these overlays on selected sagittal (left), axial (middle), and coronal (right) planes, with the x, y, and z planes in millimeters.
For the contrast of the stimuli presented in the right visual field (RR − RF) (Fig. 3), at the conservative statistical threshold, there was activity evident in pre-SMA and the left precentral sulcus only. However, at a lower statistical threshold, the distribution of frontoparietal activity was similar to that observed with the contrast LR − LF; in other words, the apparent differences observed for stimuli presented to the left and right hemifields was quantitative rather than qualitative.
Contrasts of number decision task
One additional contrast was investigated, that of the ND task with all word and false font conditions. The only activity evident was in the left and right intraparietal sulci.
Region of interest analysis
Five ROIs were specified in each hemisphere, three in the ventral stream (the pFG, the VWFA, and the mFG) and two in the lateral stream (the pSTS and aSTS) (Fig. 4). Using Featquery, the ROI masks were applied to each participant's parameter estimate maps for the separate contrasts of each visual presentation condition with the common baseline condition of number decision (LR vs ND, RR vs ND, LF vs ND, and RF vs ND). Within each ROI, the 90th percentile voxel value was taken as a robust maximum, avoiding sampling any artificially high outlier values. The group average values (and SDs) for each region and contrast are reported in supplemental Table 1 (available at www.jneurosci.org as supplemental material). Repeated-measures ANOVAs were performed to test specific hypotheses about the ROI. Significant results are described below; a full summary of the findings is reported in supplemental Table 2 (available at www.jneurosci.org as supplemental material).
To directly compare homologous regions in the left and right hemispheres, 2 (hemisphere) × 2 (task) × 2 (hemifield) repeated-measures ANOVAs were computed for homotopic regions in the left and right hemispheres. For this analysis, hemifield of presentation was modeled as ipsilateral or contralateral to the region of interest rather than left or right of fixation.
In the pFG, there was a main effect of hemifield only (F(1,18) = 14.6, p < 0.01). In the VWFA, there was a main effect of hemifield (F(1,18) = 25.1, p < 0.001) but also a significant hemisphere × task interaction (F(1,18) = 12.3, p < 0.01). Once the mFG was reached, there were main effects of task (F(1,18) = 10.2, p < 0.01) and hemifield (F(1,18) = 10.6, p < 0.01) and a significant hemisphere × task interaction (F(1,18) = 14.6, p < 0.01).
It was important to determine whether the persistent main effect of hemifield of stimulus presentation throughout the left and right ventral streams was related to hemifield luminance. The baseline condition had no luminance in most of the right visual hemifield, but the luminance was either to the left or the right for the reading and false font conditions. There was, however, no hemifield by hemisphere interaction in the ventral visual streams. Therefore, it was the placement of the stimuli and not the split screen luminance that predominantly affected the hemifield effect. This was further evident by investigating the response in the left pFG, early in the left ventral visual stream; if the left hemifield luminance of the baseline task relative to no luminance in the left hemifield of the RR and RF conditions had influenced activity, then negative effect sizes would have been observed in the contrasts of RR with ND and RF with ND. As is evident from supplemental Table 1 (available at www.jneurosci.org as supplemental material), the effect sizes were, in fact, weakly positive.
Importantly, the comparisons of homotopic ROIs between the left and right ventral streams directly confirmed an asymmetry of function (Jernigan et al., 2003). The left ventral stream demonstrated preferential activation for words, from the VWFA to the most rostral extent of the FG from which BOLD signal could be recovered. This was further confirmed by post hoc paired t-tests that demonstrated an advantage for reading (the average of LR and RR conditions) versus false font (the average of LF and RF conditions) in the left VWFA (t(18) = 3.3, p < 0.01) and the left mFG (t(18) = 6.1, p < 0.001) but not in the right hemisphere regions (right VWFA: t(18) = 0.08, p = 0.9; right mFG: t(18) = 0.14, p = 0.9).
The lateral streams were also assessed with 2 (hemisphere) × 2 (task) × 2 (hemifield) repeated-measures ANOVAs. In the pSTS, there were main effects of hemisphere (F(1,18) = 6.1, p < 0.05) and task (F(1,18) = 25.4, p < 0.001) and a trend for a hemisphere × task interaction (F(1,18) = 3.6, p = 0.07). In the aSTS, there was a main effect of task only (F(1,18) = 17.1, p < 0.01), with no significant interactions.
Post hoc paired t tests confirmed an advantage for reading over false font in all four of the lateral stream regions: the left pSTS (t(18) = 5.3, p < 0.001), the right pSTS (t(18) = 3.0, p < 0.01), the left aSTS (t(18) = 4.6, p < 0.001), and the right aSTS (t(18) = 2.7, p < 0.05). The main effect of hemisphere in the pSTS was attributable to a stronger response on the right.
To formally assess the transition of responses along the fusiform gyri, 3 (region) × 2 (task) × 2 (hemifield) repeated-measures ANOVAs were performed separately for the left and right ventral streams. It was predicted that both left and right ventral streams would show a lessening effect of hemifield of presentation along the caudorostral gradient and that the left ventral stream alone would become increasingly sensitive to stimulus type toward its rostral extent. This was confirmed in the left hemisphere, in which there were significant region × task (F(1,18) = 4.2, p < 0.05) and region × hemifield (F(1,18) = 4.4, p < 0.05) interactions, but in the right hemisphere, the predicted region by hemifield interaction did not reach significance (F(1,18) = 2.8, p = 0.11).
Discussion
This study has demonstrated activity for reading, relative to false font, along the ventral left temporal lobe, centered on the fusiform gyrus, and in left and right lateral temporal cortex, centered on the superior temporal sulci. This is in agreement with previous imaging studies of sentence and narrative reading (Ferstl and von Cramon, 2001; Marinkovic et al., 2003; Xu et al., 2005; Spitsyna et al., 2006). However, these reading-related pathways were also significantly active during the perception of false font stimuli relative to the number baseline; that is, the profile of activity in these regions was reading > false font > numbers. This is in contrast with the right ventral visual stream, in which activity was not relatively specific for words, and the profile of activity was words = false font > numbers.
These profiles of activity do not accord with a localist model of reading; such a hierarchical, feedforward model of reading would not predict that activation for word-like arrays of unfamiliar font would proceed beyond basic visual processing. Even if the Greek letters were partially familiar to participants (for example, from mathematical notation), they should not have activated areas in the ventral visual stream that Dehaene's model (Dehaene et al., 2005) associates with familiar bigram and quadrigram recognition. According to this model, even consonant strings, i.e., entirely familiar letters in meaningless configurations, would not be predicted to activate widespread areas of heteromodal temporal cortex to the extent that we observed using the Greek false font stimuli. The wide distribution of activity in visual and heteromodal sensory cortex during obligatory (i.e., automatic, because there was no explicit task to be performed on the false font stimuli) processing of false font is, however, compatible with a connectionist or interactive model of reading. According to this model, no cortical regions respond exclusively to written words. The interaction between feedforward and feedback processing during reading was signaled by the relative rather than absolute difference of activity in temporal lobe pathways. This contrasts with the activity in the right ventral visual stream, in which the similar activation for narratives and false font indicated that this region processed all stimuli as nonlinguistic objects.
These findings can be considered within the framework of the connectionist “triangle” model of reading (Seidenberg and McClelland, 1989; Plaut et al., 1996; Plaut, 1997; Behrmann et al., 1998; Patterson and Lambon Ralph, 1999). This model depicts three interconnected domains of orthographic, phonological, and semantic units. Words are encoded on the basis of interactions between multiple units throughout the three domains rather than as explicit lexical representations. Exposure to familiar stimuli leads to changes in the weightings between units. A familiar word will activate a stable “attractor” network, whereas a non-word may lead spreading activation that does not resolve into a stable pattern. This model has not been explicitly tested on false font, but it is compatible with the notion of widely spread activity throughout the brain before this activity declines in the absence of the stimuli mapping on to attractors. Because extrapolations from computational models to BOLD responses can only be speculative, the result of the present study can only claim to be compatible with, not evidence for, a connectionist account of reading.
Within the primate visual system, there are abundant feedback axons projecting predominantly to the superficial dendritic layer within visual association cortex (Rockland and Pandya, 1979). Recent magnetoencephalography (MEG) studies of reading have identified early left inferior frontal gyrus activity (at ∼100 ms) that precedes activation of the VWFA (Pammer et al., 2004; Cornelissen et al., 2009), which may represent prelexical phonological access (Wheat et al., 2010). This is additional support for the proposal that the VWFA could be influenced by feedback projections, direct or indirect, from as far afield as inferior frontal cortex. Reading-related activity reaches a peak at ∼150 ms (Tarkiainen et al., 1999). Although it is often implicitly assumed that this evoked response represents feedforward processing, with the current state of knowledge about the source of the MEG signal, this may represent activity in apical dendrites, transmitting feedback information, as well as feedforward information projecting to deeper cortical layers.
Although reading-related activity was left-lateralized in the ventral temporal lobe, it was bilateral along the STS. There are a number of studies indicating that verbal semantic processing is dependent on activation of both anterior temporal lobes (Xu et al., 2005; Spitsyna et al., 2006; Awad et al., 2007; Ferstl et al., 2008; Warren et al., 2009; Visser et al., 2010), with activity greatest for narratives (Xu et al., 2005). Reading-related activity in the posterior STS has been observed less frequently; Xu et al. (2005) observed such a locus of activity in response to written text presented using the RSVP technique, whereas the PET study of Spitsyna et al. (2006), in which paragraphs of text were read in the normal manner, demonstrated that activity within the caudal right temporal lobe was no greater for text than false font. This discrepancy may relate to the manner of presentation of the narratives rather than reading per se; for example, RSVP may make greater demands on sustained visual attention (Singh-Curry and Husain, 2009). However, these unexpected discrepancies between studies can only result in speculative interpretations and generate possible hypotheses for future studies.
The eye-tracking data confirmed that subjects were reasonably successful at maintaining fixation and were able to avoid the impulse to saccade toward each word. However, a significant main effect of stimulus type was observed, suggesting that the salience of the stimuli influenced the ability to inhibit saccades. Hence, in the contrast of reading versus false font, it might be expected that activity related to response inhibition would be seen. Such activity was observed in the pre-SMA and the right dorsolateral prefrontal cortex, including the inferior frontal gyrus, regions known to be activated in response inhibition tasks (Rubia et al., 2003; Aron and Poldrack, 2006; Li et al., 2006; Aron et al., 2007; Chevrier et al., 2007). In addition to frontal regions, the contrast of left hemifield reading against left hemifield false font revealed bilateral superior parietal cortex activity. A frontosuperior parietal network accords with response inhibition involving the control of eye movements. The posterior eye fields, which control visual attention and the generation of reflexive saccades, are located in superior parietal cortex, the lateral prefrontal cortex is activated during the inhibition of reflexive saccades, and the frontal eye fields trigger intentional saccades or antisaccades (Nobre, 2001; Pierrot-Deseilligny et al., 2004). The contrast of right hemifield reading against right hemifield false font revealed subthreshold bilateral frontoparietal network activation, suggesting a quantitative rather than qualitative difference between reading in the left and right hemifields. This would fit with a hypothesis that eye-movement suppression is more difficult when words are presented in the left visual field, away from the natural attentional window for reading (Leff et al., 2000).
The success of the study depended on the ND task providing a “null” baseline. We observed much higher activity in the ventral and lateral temporal streams for words and false font stimuli than for numbers, with activity specific to the ND task limited to the intraparietal sulci, suggesting that number processing is supported principally by a dorsal (rather than ventral) visual stream. The odd/even task was originally developed to visualize activity in the medial temporal lobes, because resting baselines failed to produce a null response in episodic memory studies (Stark and Squire, 2001). The present study required a baseline task that resulted in little or no activity in the reading-related pathways, which were known to include the posteroanterior extents of the left fusiform gyrus and superior temporal sulcus (Spitsyna et al., 2006). Visually presented numbers are like words and false font in that they consist of two-dimensional symbols with high contrast and spatial frequency, but processing of number-related semantics occurs in parietal cortex (Thioux et al., 2005; Cappelletti et al., 2010). Therefore, the study design was predicated on the assumption that explicit processing of visually presented numbers would result in low activity in the reading-related temporal lobe pathways, allowing the visualization of the extent of temporal lobe activity in response to both false font and words. Nevertheless, it would require additional conditions to confirm that the ND task was truly a null baseline.
In conclusion, our data demonstrated that the extensive posteroanterior temporal lobe pathways that process written narratives are also active during the passive perception of false font. The visualization of activity in response to false font was dependent on the choice of an appropriate baseline visual task, one that depended on the explicit processing of number semantics in parietal cortex. The observation that false font stimuli activate the same temporal lobe pathways as words is incompatible with the localist view of reading-related processing but accords with the hypothesis that word recognition and comprehension arise from the interaction of feedforward and feedbackward activation over a distributed system.
Footnotes
We are grateful to the three anonymous reviewers for their helpful and informative recommendations.
References
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJ. A common system for the comprehension and production of narrative speech. J Neurosci. 2007;27:11455–11464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Plaut D, Nelson J. A literature review and new data supporting an interactive account of letter-by-letter reading. Cogn Neuropsychol. 1998;15:7–51. doi: 10.1080/026432998381212. [DOI] [PubMed] [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Lee HL, Freeman ED, Price CJ. The role of right and left parietal lobes in the conceptual processing of numbers. J Cogn Neurosci. 2010;22:331–346. doi: 10.1162/jocn.2009.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Are there lexicons? Q J Exp Psychol A. 2004;57:1153–1171. doi: 10.1080/02724980443000007. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Kringelbach ML, Ellis AW, Whitney C, Holliday IE, Hansen PC. Activation of the left inferior frontal gyrus in the first 200ms of reading: evidence from magnetoencephalography (MEG) PLoS ONE. 2009;4:e5359. doi: 10.1371/journal.pone.0005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. J Cogn Neurosci. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. The role of coherence and cohesion in text comprehension: an event-related fMRI study. Brain Res Cogn Brain Res. 2001;11:325–340. doi: 10.1016/s0926-6410(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Hum Brain Mapp. 2008;29:581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Fennema-Notestine C, Ostergaard AL. More “mapping” in brain mapping: statistical comparison of effects. Hum Brain Mapp. 2003;19:90–95. doi: 10.1002/hbm.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neurolmage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Leff AP, Scott SK, Crewes H, Hodgson TL, Cowey A, Howard D, Wise RJ. Impaired reading in patients with right hemianopia. Ann Neurol. 2000;47:171–178. [PubMed] [Google Scholar]
- Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJ. The functional anatomy of single-word reading in patients with hemianopic and pure alexia. Brain. 2001;124:510–521. doi: 10.1093/brain/124.3.510. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao M, Chen X, Zhang D, Han S, He S, Hu X. Attention shift in human verbal working memory: priming contribution and dynamic brain activation. Brain Res. 2006;1078:131–142. doi: 10.1016/j.brainres.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamic of modality-specific and supramodal word processing. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC. The attentive homunculus: now you see it, now you don't. Neurosci Biobehav Rev. 2001;25:477–496. doi: 10.1016/s0149-7634(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Pammer K, Hansen PC, Kringelbach ML, Holliday I, Barnes G, Hillebrand A, Singh KD, Cornelissen PL. Visual word recognition: the first half second. Neuroimage. 2004;22:1819–1825. doi: 10.1016/j.neuroimage.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Patterson K, Ralph MA. Selective disorders of reading? Curr Opin Neurobiol. 1999;9:235–239. doi: 10.1016/s0959-4388(99)80033-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Müri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Plaut D. Structure and function in the lexical system: Insights from distributed models of word reading and lexical decision. Lang Cogn Process. 1997;12:765–806. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychol Rev. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. Pittsburgh: Psychology Software Tools; 2002. E-prime reference guide. [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJ. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Thioux M, Pesenti M, Costes N, De Volder A, Seron X. Task-independent semantic activation for numbers and animals. Brain Res Cogn Brain Res. 2005;24:284–290. doi: 10.1016/j.cogbrainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The inferior, anterior temporal lobes and semantic memory clarified: novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48:1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–3442. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat KL, Cornelissen PL, Frost SJ, Hansen PC. During visual word recognition, phonology is accessed within 100 ms and may be mediated by a speech production code: evidence from magnetoencephalography. J Neurosci. 2010;30:5229–5233. doi: 10.1523/JNEUROSCI.4448-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]