Abstract
Single-Ig-interleukin-1 related receptor (SIGIRR) is a member of the interleukin (IL)-1/Toll-like receptor (TLR) family. It negatively regulates inflammation, rendering SIGIRR−/− mice more susceptible to inflammatory challenge. This susceptibility extends to the brain, where increased responsiveness to lipopolysaccharide has been observed in SIGIRR-deficient mice. While this is likely due to enhanced TLR4-mediated signaling, the functional consequences of these changes have not yet been described. In the current study, we have investigated the impact of SIGIRR deficiency on hippocampal function, and show that novel object recognition, spatial reference memory, and long-term potentiation (LTP) were impaired in SIGIRR−/− mice. These changes were accompanied by increased expression of IL-1RI and TLR4, and upregulation of their downstream signaling events, namely IRAK1 (IL-1R-associated kinase 1), c-Jun N-terminal protein kinase (JNK), and nuclear factor κB (NF-κB). The deficit in LTP was attenuated by the endogenous IL-1 receptor antagonist (IL-1ra) and an anti-TLR4 antibody, and also by inhibition of JNK and NF-κB. We propose that IL-1RI is activated by IL-1α and TLR4 is activated by the endogenous agonist, high mobility group box 1 (HMGB1), as we identified enhanced expression of both cytokines in the hippocampus of SIGIRR−/− mice. Additionally, application of HMGB1 increased the activation of JNK and NF-κB and was found to be detrimental to LTP in a TLR4-dependent manner. These findings highlight the functional role of SIGIRR in regulating inflammatory-mediated synaptic and cognitive decline, and describe evidence of the key role of HMGB1 in this process.
Introduction
The aging process, along with neurodegenerative disorders including Alzheimer's disease (AD), is characterized by an underlying neuroinflammation (Tuppo and Arias, 2005; Glass et al., 2010), which is associated with deficits in cognitive function and hippocampal synaptic plasticity, such as long-term potentiation (LTP). Investigations into the mechanisms underlying these inflammatory processes have illustrated that proinflammatory cytokines induce an impairment in hippocampal-dependent memory (Barrientos et al., 2002; Hein et al., 2010) and LTP (Vereker et al., 2000; Curran and O'Connor, 2001). Additionally, inflammatory challenges associated with age, β-amyloid, or lipopolysaccharide (LPS) elicit similar deficits in LTP through activation of stress-activated protein kinases (O'Donnell et al., 2000; Kelly et al., 2003; Minogue et al., 2003; Costello and Herron, 2004; Barry et al., 2005).
Single-Ig-interleukin-1 related receptor (SIGIRR) is a member of the Toll-like receptor (TLR)/interleukin 1 receptor (IL-1R) family (Thomassen et al., 1999). It is abundant in peripheral tissues including lung, kidneys, and the gastrointestinal tract (Polentarutti et al., 2003; Wald et al., 2003), where it performs an anti-inflammatory role (Garlanda et al., 2009). Specifically, SIGIRR-deficient mice exhibit exaggerated symptoms of inflammatory conditions such as colitis-associated cancer (Garlanda et al., 2007), experimental autoimmune encephalitis (Gulen et al., 2010), and rheumatoid arthritis (Drexler et al., 2010). Furthermore, SIGIRR−/− mice demonstrate pronounced susceptibility to the inflammatory challenge posed by the pathogen LPS (Wald et al., 2003; Garlanda et al., 2004; Lech et al., 2007). A recent report from this laboratory provides the first evidence that these effects are extended to the brain, demonstrating exaggerated microglial activation and inflammatory regulation in hippocampus of SIGIRR−/− mice in response to LPS (Watson et al., 2010).
In addition to analysis in SIGIRR-deficient mice, overexpression of SIGIRR has proven beneficial to our understanding of how its anti-inflammatory effects may be mediated (Wald et al., 2003; Qin et al., 2005; Zhang et al., 2011). Evidence suggests that SIGIRR interacts with both IL-1RI and TLR4 to decrease the impact of proinflammatory stimuli (Qin et al., 2005; Huang et al., 2006; Gulen et al., 2010). SIGIRR overexpression reduces the production of proinflammatory cytokines and nuclear factor κB (NF-κB) activation, while kidney epithelial cells from SIGIRR−/− mice exhibit prolonged phosphorylation of the stress-activated protein kinase, c-Jun N-terminal protein kinase (JNK), leading to activation of NF-κB (Wald et al., 2003). Furthermore, Qin et al. (2005) propose that SIGIRR interaction with IL-1R and TLR4 interferes with recruitment of the adaptor molecule, myeloid differentiation factor 88 (MyD88), to downregulate the proinflammatory response (Qin et al., 2005).
In the current study, the impact of SIGIRR deficiency on hippocampal function was assessed. We demonstrate a cognitive deficit and an impairment of LTP at CA1 synapses, associated with upregulation of IL-1R- and TLR4-mediated signaling in SIGIRR−/− mice. We propose that the augmented expression of the endogenous TLR ligand, high mobility group-box 1 (HMGB1), in SIGIRR−/− mice is pivotal to the deficit in hippocampal function.
Materials and Methods
Animals.
Male and female C57BL/6 mice (2–6 months; Harlan), SIGIRR−/−/TIR8−/− mice (2–6 months; a gift from Professor A. Mantovani, Istituto Clinico Humanitas, Istituto di Ricovero e Cura a Carattere Scientifico, Milan, Italy) (Garlanda et al., 2004) and TLR4−/− mice (4–6 months; a gift from Professor P. Fallon, School of Medicine, Trinity College Dublin) were maintained in the Bioresources Unit, Trinity College Dublin. All experiments were performed under license from the Department of Health and Children (Ireland) and with local ethical approval.
Behavioral analysis.
The novel object recognition task is based on the innate tendency of rodents to differentially explore novel objects over familiar ones. The apparatus consisted of a white circular arena (diameter 200 cm, height 50 cm) placed in a dimly lit room. Mice were habituated to the arena for 20 min in the absence of objects each day for 3 d before the experiment was performed. In the training trial the mice were presented with a pair of identical objects (A and B) positioned in the center of the arena. Mice were placed into the arena at random entry points for three 5 min trials with an intertrial rest period of 5 min, and the time spent exploring each object was recorded using stopwatches. Objects were thoroughly cleaned between trials to ensure the absence of olfactory cues. In the testing trial, performed 24 h later, one of the familiar objects was exchanged for a novel object (C), placed at exactly the same position, and mice were reintroduced into the arena for a single 5 min trial; the time spent exploring each object was recorded as before. The time spent exploring each object (in seconds) is expressed as a percentage of the total exploration time. The criteria for exploration were strictly based on active exploration, in which mice had to be touching the object with their nose or head. The discrimination index for the novel object is the proportion of time mice spent exploring the novel object minus the proportion spent exploring the familiar one in the testing period.
Male and female wild-type and SIGIRR−/− mice (3–5 months old) were assessed for hippocampal-dependent spatial learning using the Y-maze task (Deacon et al., 2008; Cunningham et al., 2009). Mice were placed into one arm of a clear, perspex Y-maze containing water (2 cm deep; 20–22°C). Mice were encouraged to escape through a burrowing tube at the distal end of another arm, and returned to their home cages. Burrowing tubes were placed at the end of the remaining arms with plugged exits, to ensure all arms appeared identical. Prominent visual cues were placed throughout the room surrounding the maze. The location of the exit was fixed for each animal, and mice were placed in either one of the two possible start arms for 10 trials (15 min intertrial interval). The groups were counterbalanced with respect to the escape arm. Escape latency was measured as time taken for each mouse to enter the exit tube, and mean values were calculated across trials 1–4, 5–7, and 8–10. Male and female mice did not demonstrate behavioral differences, and results were therefore pooled. Statistical differences between wild-type and SIGIRR−/− mice across trials were determined using two-way ANOVA and post hoc Bonferroni tests.
Activity and anxiety were evaluated in wild-type and SIGIRR−/− mice by assessing their behavior in a hole-board arena (60 cm width × 60 cm length × 35 cm height), divided into 25 equal-sized squares (16 border and 8 central squares). Mice were placed on the outer corner of a square in the arena and activity was recorded using a video camera and an advanced motion-recognition software package (Mediacruise Software, Canopus Corporation) for 2 min. The distance covered and the time spent in the border and central squares were recorded.
Hippocampal slice preparation and LTP.
Female C57BL/6 and SIGIRR−/−/TIR8−/− mice, and male C57BL/6 and TLR4−/− mice were decapitated under isoflurane anesthesia (Merial); brains were rapidly removed and hippocampi dissected in ice-cold, oxygenated (95% O2/5% CO2) artificial CSF (aCSF) containing (in mm): 125 NaCl, 1.25 KCl, 1 CaCl2, 1.5 MgCl2, 1.25 KH2PO4, 25 NaHCO3, and 10 d-glucose. Hippocampal slices (400 μm) were prepared using a McIlwain tissue chopper, and incubated in a submerged chamber at room temperature for at least 1 h before experimentation. Slices were transferred to a submersion recording chamber and continually perfused (2–3 ml/min) with oxygenated aCSF containing (in mm): 125 NaCl, 1.25 KCl, 2 CaCl2, 1.5 MgCl2, 1.25 KH2PO4, 25 NaHCO3, and 10 d-glucose, at room temperature (22–23°C).
The Schaffer collateral-commissural pathway was stimulated at 0.033 Hz (0.1 ms duration) using a bipolar tungsten stimulation electrode (Advent Research Materials). Field EPSPs were recorded from the CA1 stratum radiatum using a monopolar recording electrode. Recording electrodes (∼2 MΩ) were pulled from borosilicate glass capillary tubes (Harvard Apparatus) and filled with aCSF. The stimulus intensity was adjusted to produce a response ∼50% of maximal EPSP amplitude as determined from an input–output curve for each experiment. A stable baseline of at least 10–20 min was recorded before application of theta-burst stimulation (T-BS), which consisted of 10 trains (4 pulses at 100 Hz) repeated at 5 Hz. To assess the effect of pharmacological agents on LTP, IL-1 receptor antagonist (IL-1ra) (2 μg/ml), HMGB1 (10 ng/ml) [a gift from Professor K. Mills, School of Biochemistry and Immunology, Trinity College Dublin) (see Fig. 6) or from Sigma-Aldrich (see Fig. 7)], d-JNKi1 (2 μm), wedelolactone (30 μm; Enzo Life Sciences), anti-TLR4 antibody (2.5 μg/ml; Hycult Biotechnology or Santa Cruz Biotechnology) or isotype control IgG (2.5 μg/ml; Santa Cruz Biotechnology) was applied to the perfusate 20–30 min before T-BS, and maintained for the duration of each experiment. Data were acquired using WinWCP v4.0.7 software (Dr. J. Dempster, Strathclyde, UK) and evoked EPSPs were normalized to the slope recorded in the 5 min period before LTP induction. LTP was measured as a mean value of the final 5 min of recording (55–60 min post-T-BS). Data are presented as mean percentage EPSP slope ± SEM. Sample EPSP traces represent an average of 4 consecutive EPSPs, taken immediately before T-BS, and 60 min following LTP induction.
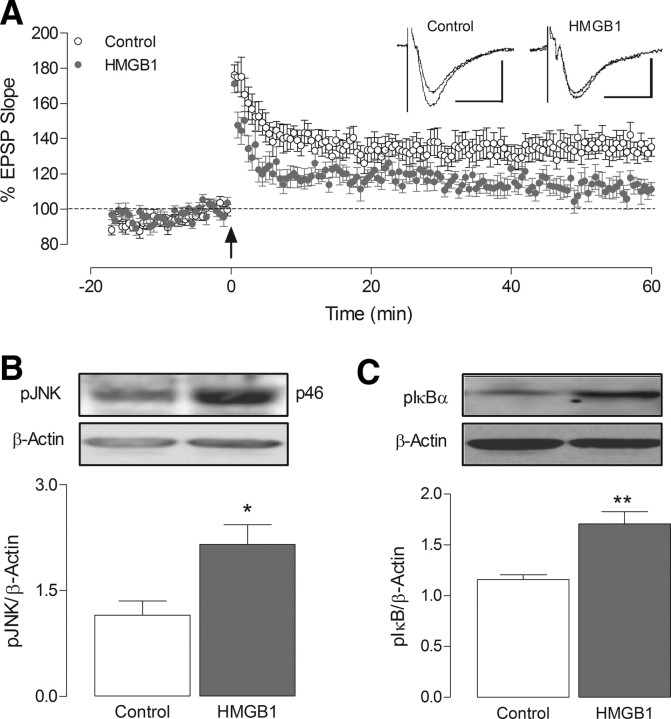
Figure 6.
Acute application of HMGB1 impairs LTP and increases hippocampal expression of pJNK and pIκBα. A, Application of HMGB1 (10 ng/ml) for 20 min substantially reduced the level of LTP obtained 60 min following induction (p < 0.01, n = 9, N = 6) relative to untreated controls (n = 8, N = 6). Arrow indicates application of theta-burst stimulation. Inset displays sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction (calibrations: 1 mV, 20 ms). B, C, Treatment of hippocampal slices with HMGB1 (10 ng/ml) for 20 min led to a significant increase in pJNK (B; *p < 0.05, n = 5–6) and pIκBα (C; **p < 0.01, n = 5–6), relative to tissue incubated in the absence of HMGB1. Protein expression was determined by Western immunoblot, and values were normalized to β-actin. Insets illustrate representative blots of pJNK (B) and pIκBα (C), along with respective β-actin blots.
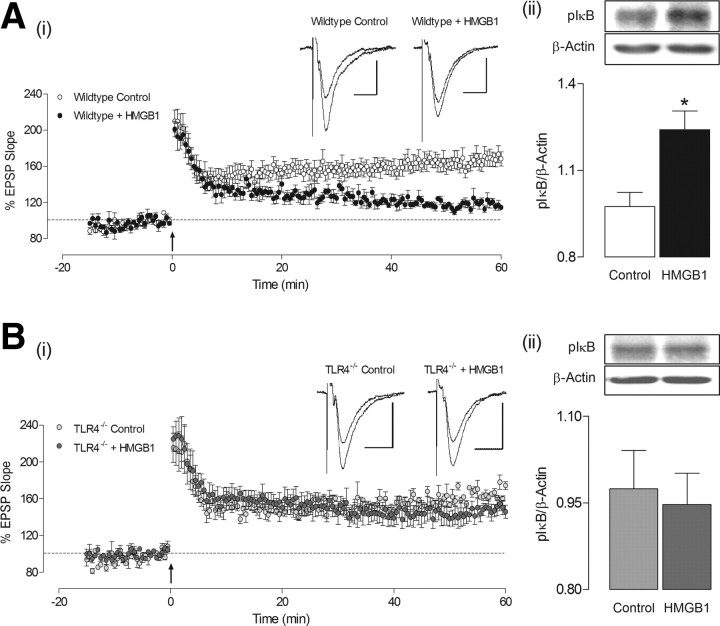
Figure 7.
HMGB1 does not alter LTP or pIκBα expression in hippocampus of TLR4−/− mice. Ai, Application of HMGB1 (10 ng/ml) for 30 min attenuated the level of LTP obtained in male wild-type mice 60 min following induction (p < 0.05, n = 4, N = 4) relative to untreated controls (n = 4, N = 4). Aii, Treatment of hippocampal slices with HMGB1 (10 ng/ml) for 30 min significantly increased pIκBα expression (*p < 0.05, n = 4), relative to tissue incubated in the absence of HMGB1. Bi, LTP recorded in hippocampal slices from male TLR4−/− mice was not significantly altered following HMGB1 application (10 ng/ml; 30 min; n = 4, N = 4) compared with control values obtained from TLR4−/− slices (n = 4, N = 4). Bii, Hippocampal slices from TLR4−/− mice treated with HMGB1 did not show altered expression of pIκBα when compared with untreated tissue from TLR4−/− mice (n = 4, N = 4). In Bi, Arrows indicate application of theta-burst stimulation. Insets display sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction (calibrations: 1 mV, 20 ms). In Bii, Protein expression was determined by Western immunoblot, and values were normalized to β-actin. Insets illustrate representative blots of pIκBα, along with respective β-actin blots.
Western immunoblotting.
Hippocampal slices not used for electrophysiological recording were placed into the recording chamber and perfused with aCSF, in the presence or absence of pharmacological agents (20–30 min) as described above. Following perfusion, tissue was harvested and stored in lysis buffer (100 μl; composition in mm: 10 Tris-HCl, 50 NaCl, 10 Na4P2O7.H2O, 50 NaF, 1% Igepal, Phosphatase Inhibitor Cocktail I and II, Protease Inhibitor Cocktail; Sigma) at −80°C. For analysis, samples were added to 2× SDS sample buffer (composition: 100 mm Tris-HCl, pH 6.8, 4% SDS, 2% bromophenol blue, 20% glycerol; Sigma) and heated to 70°C for 10 min. Samples (20 μg) were separated on 7, 10, or 12% standard SDS gels. Proteins were transferred to nitrocellulose membrane (Schleicher and Schuell) and blocked for 1 h in Tris-buffered saline-0.05% Tween 20 (TBS-T) and 5% nonfat dried milk/TBS-T at room temperature. Membranes were incubated overnight at 4°C with anti-IL-1α (1:500; R&D Systems), anti-IL-1β, and anti-IL-1RI (1:500; Santa Cruz Biotechnology), anti-IRAK1 (IL-1R-associated kinase 1) (1:1000), anti-phosphorylated (anti-p)-IκBα (ser32) (1:1000), or anti-p-JNK (1:1000; Cell Signaling Technology), anti-TLR4 (1:1000), or anti-HMGB1 (1:250; Abcam) antibody in 2% nonfat dried milk/TBS-T, washed, and incubated with a secondary anti-rabbit (1:2500) or anti-goat (1:5000; Jackson ImmunoResearch) antibody in 2% nonfat dried milk/TBS-T for 1 h. Immunoreactive bands were detected using Immobilon Western chemiluminescent substrate (Millipore), and blots were stripped (Re-blot Plus; Millipore Bioscience Research Reagents) and reprobed using anti-β-actin (1:10,000; Sigma) in 2% nonfat dried milk/TBS-T and a peroxidase-conjugated secondary anti-mouse antibody (1:5000; Jackson ImmunoResearch) in 2% nonfat dried milk/TBS-T. Images were captured using the Fujifilm LAS-3000.
One objective of this study was to assess whether HMGB1 interacted with TLR4, and to do so, hippocampal samples were equalized to provide 500 μg of protein. Samples were incubated overnight at 4°C in the presence of anti-TLR4 antibody (5 μg; Hycult Biotechnology). A/G-protein agarose beads (50 μl; Santa Cruz Biotechnology) were added, and samples were incubated for 2 h, washed in PBS containing NP-40 (0.01%), and centrifuged. Loading buffer was added to each sample and samples were heated to 70°C. Proteins were separated by gel electrophoresis as described above and HMGB1 was visualized by incubating the membrane in the presence of anti-HMGB1 antibody (as above).
Statistical analysis.
Data were assessed using two-tailed Student's t tests (paired or unpaired as appropriate) and one-way ANOVA followed by post hoc Student Newman–Keuls test to determine significant differences between multiple groups, or two-way ANOVA with post hoc Bonferroni test to assess progressive changes between groups.
Results
SIGIRR deficiency has been associated with increased inflammation in several experimental models (Drexler et al., 2010; Gulen et al., 2010), and recent data indicate that LPS induces a more profound effect on proinflammatory cytokine production in SIGIRR−/−, compared with wild-type mice in vitro and in vivo (Watson et al., 2010). These changes are paralleled by, and probably due to, increased microglial activation in SIGIRR−/− mice (Watson et al., 2010).
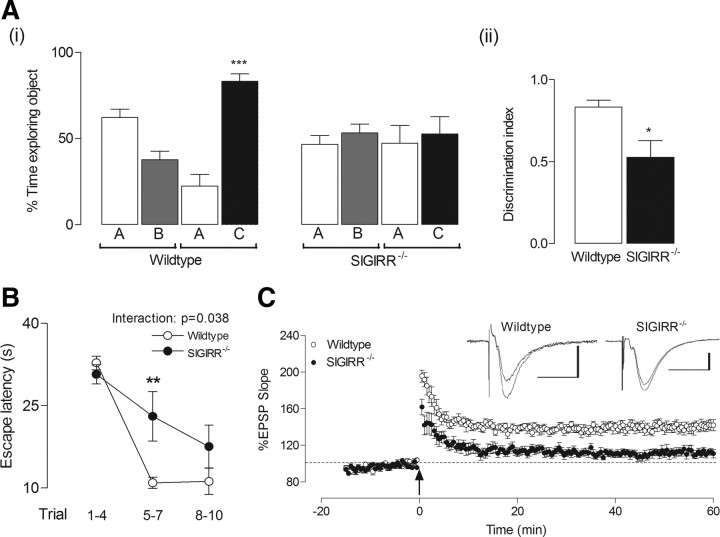
Here we demonstrate that hippocampal function is impaired in SIGIRR−/− mice. Using an object recognition task we found that, while wild-type mice spent a significantly greater amount of time exploring a novel object relative to a familiar object (***p < 0.001; ANOVA; Fig. 1A), the time spent by SIGIRR−/− mice exploring both objects was not significantly different. Accordingly, the discrimination index illustrates that the difference in exploration time between the familiar and novel objects was significantly lower in SIGIRR−/− mice than wild types (*p < 0.05; unpaired Student's t test; Fig. 1A). This suggests that the deficit identified in SIGIRR−/− mice is due to a cognitive impairment, and not simply a consequence of reduced exploratory behavior. There were no significant differences between wild-type and SIGIRR−/− mice in terms of activity; in a hole board arena (60 × 60 cm), the mean total distance traveled (±SEM) (1831 ± 195 and 2049 ± 114.4 cm) and the mean velocity (±SEM) (18.1 ± 1.5 and 20.5 ± 1.2 cm/s) were similar in wild-type and SIGIRR−/− mice. The mean percentage time spent in the outer zone (±SEM) was significantly greater in SIGIRR−/− mice compared with wild-type mice (81.5 ± 2.0 and 63.1 ± 4.6%; p < 0.01) and SIGIRR−/− mice stayed closer to the outer perimeter of the arena than wild-type mice (10.06 ± 0.25 and 15.18 ± 1.01 cm, respectively; p < 0.01). These behaviors are indicative of anxiety. Therefore we conclude that, although SIGIRR−/− mice appear more anxious than wild-type mice, their activity levels are similar.
Figure 1.
Impaired cognition and long-term potentiation in SIGIRR−/− mice. Ai, Wild-type and SIGIRR−/− mice were exposed to two identical objects (A and B) for 5 min periods, over 3 trials with 5 min intervals. When tested 24 h later, wild-type mice spent a significantly greater amount of time exploring a novel object (object C), relative to a familiar object (object A; ***p < 0.001, n = 6). However, during the testing period, SIGIRR−/− mice spent equal amounts of time exploring both the familiar and novel objects (n = 7). Aii, The same experimental data represented as the discrimination index illustrate that SIGIRR−/− mice displayed significantly less discrimination between the familiar and novel objects compared with wild types, relative to the total exploration time (*p < 0.05, n = 6–7). B, Wild-type and SIGIRR−/− mice were assessed in the Y-maze. Mean escape latency was calculated for trials 1–4, 5–7 and 8–10. SIGIRR−/− mice (n = 7) were impaired in acquiring the task with respect to wild-type mice (n = 8). Two-way ANOVA revealed significant effects across trials (p < 0.0001) and mouse strains (p < 0.05), and a significant interaction was identified between both (p < 0.05). Post hoc analysis revealed a significant difference in escape latency between wild-type and SIGIRR−/− mice during trials 5–7 (**p < 0.01). C, Theta-burst stimulation induced a consistent LTP in CA1 synapses of hippocampal slices taken from wild-type mice, which persisted for a minimum of 60 min following induction (n = 9, N = 7). However, the LTP achieved in slices from SIGIRR−/− mice, under the same recording conditions, was significantly reduced (p < 0.0001, n = 11, N = 8) compared with wild-type mice. The arrow indicates application of theta-burst stimulation. The inset displays sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction. Calibration: 1 mV, 20 ms.
To further assess cognitive function in wild-type and SIGIRR−/− mice, animals were tested for cognitive performance in the Y-maze, a task which is known to evaluate hippocampal-dependent spatial reference memory (Deacon et al., 2008; Cunningham et al., 2009). Escape latency was measured for each animal, across 10 trials, and mean values were calculated for trials 1–4, 5–7 and 8–10. Two-way ANOVA revealed that SIGIRR−/− mice were significantly impaired in acquiring the task, compared with wild types (Fig. 1B). Significant effects across trials (p < 0.0001) and mouse strains (p < 0.05) were identified, along with a significant interaction between both (p < 0.05). Post hoc analysis using Bonferroni test also revealed a significant difference in escape latency between wild-type and SIGIRR−/− mice during trials 5–7 (**p < 0.01; Fig. 1B). Similarly, the total number of arms entered by SIGIRR−/− mice before escape was also significantly greater than wild-type mice during the same period (data not shown). Intracerebroventricular injection of LPS or IL-1β exerted no significant effect on any of these measures in wild-type or SIGIRR−/− mice (data not shown). In light of these findings, we assessed LTP in CA1 synapses of hippocampal slices prepared from wild-type and SIGIRR−/− mice. T-BS reliably induced robust and reproducible LTP in wild-type mice, but the mean percentage change in EPSP slope was markedly reduced (Fig. 1C) and significantly attenuated in slices from SIGIRR−/− mice when assessed 60 min following induction (p < 0.0001; unpaired Student's t test).
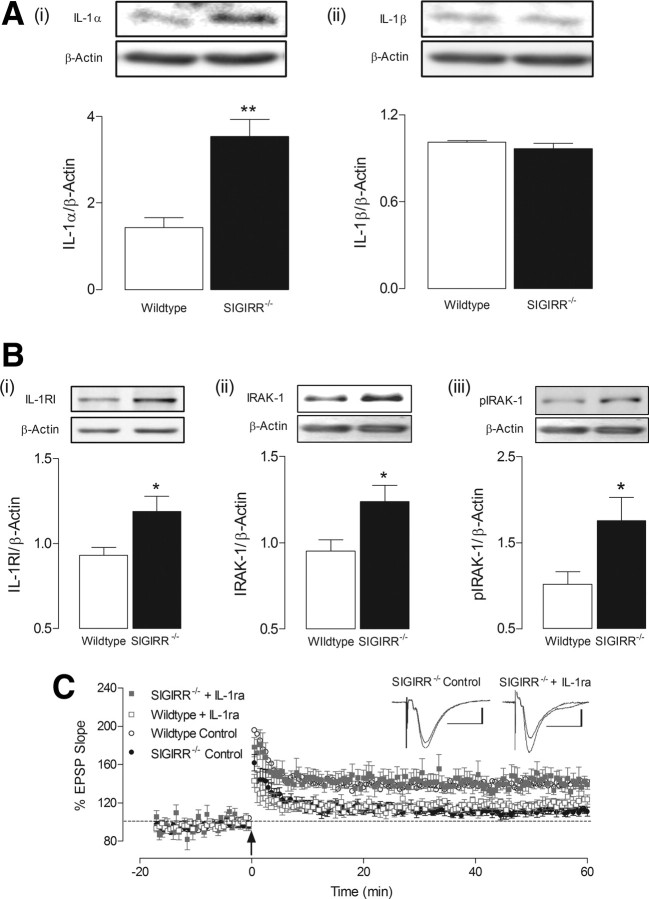
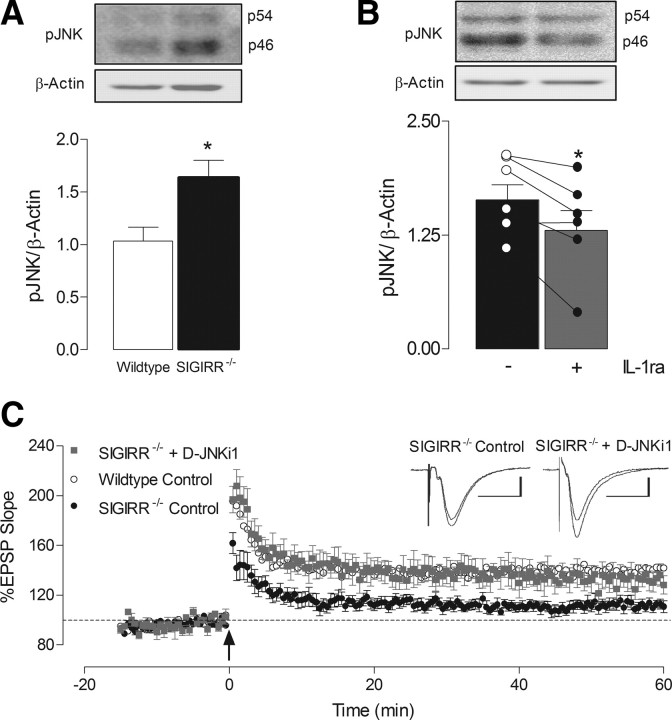
To identify the potential mechanisms responsible for these impairments in hippocampal function, we first investigated the expression of the IL-1RI-mediated signal in hippocampal tissue from wild-type and SIGIRR−/− mice. As SIGIRR is known to modulate this pathway through interaction with IL-1RI (Wald et al., 2003; Qin et al., 2005), it seemed likely that this may be affected as a result of SIGIRR deficiency. We identified a significant increase in expression of IL-1α (**p < 0.01; unpaired Student's t test; Fig. 2A) and IL-1RI in SIGIRR−/− mice relative to wild-type mice (*p < 0.05; unpaired Student's t test; Fig. 2B). Interestingly, hippocampal expression of IL-1β was not altered (Fig. 2A). Furthermore, expression of the downstream kinase, IRAK1, was also significantly enhanced in tissue from SIGIRR−/− mice (*p < 0.05; unpaired Student's t test; Fig. 2B). As these findings suggest that the IL-1RI-mediated signaling is upregulated in SIGIRR−/− mice, we used IL-1ra to investigate whether this pathway may underlie the impairment in LTP. Application of IL-1ra (2 μg/ml; 20 min) to slices prepared from SIGIRR−/− mice reversed the deficit in LTP, producing a response to T-BS which was similar to that in slices prepared from wild-type mice (p < 0.001; unpaired Student's t test; Fig. 2C). It has been demonstrated that IL-1ra can prove detrimental to hippocampal LTP (Loscher et al., 2003; Ross et al., 2003; Schmid et al., 2009), and the present data confirm that extracellular application of IL-1ra (2 μg/ml) for 20 min significantly attenuated T-BS-induced LTP in hippocampal slices of wild-type mice (p < 0.05, unpaired Student's t test; Fig. 2C).
Figure 2.
Upregulation of IL-1RI-mediated signaling impairs LTP in SIGIRR−/− mice. A, Western immunoblot analysis revealed a significant increase in the expression of IL-1α (i; **p < 0.01, n = 3–4) but not IL-1β (ii; n = 3–4) in hippocampal tissue from SIGIRR−/− mice relative to wild-type mice. B, A similar increase was identified in the expression of IL-1RI (i; *p < 0.05, n = 6) and IRAK1, both in its nonphosphorylated (ii; IRAK1) and phosphorylated (iii; pIRAK1) forms (*p < 0.05; n = 6) in hippocampus of SIGIRR−/− mice. All target proteins were normalized to β-actin. Insets illustrate representative blots for IL-1α, IL-1β, IL-1RI, IRAK, and pIRAK, along with respective β-actin for each blot. C, IL-1ra (2 μg/ml), applied for 20 min before LTP induction, significantly attenuated LTP in wild-type mice (p < 0.05, n = 5, N = 3). In the presence of IL-1ra, slices from SIGIRR−/− mice produced significantly greater LTP than that observed under control conditions (p < 0.001, n = 5, N = 4), of magnitude similar to those in LTP recorded in wild-type mice. The arrow indicates application of theta-burst stimulation. The inset displays sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction. Calibrations: 1 mV, 20 ms.
One downstream consequence of IL-1RI activation is phosphorylation of the stress-activated protein kinase, JNK. Here we demonstrate that hippocampal tissue from SIGIRR−/− mice exhibit significantly higher levels of phosphorylated JNK (p46; pJNK) compared with wild types (*p < 0.05, unpaired Student's t test; Fig. 3A). Interestingly, treatment with IL-1ra attenuated the level of pJNK in tissue prepared from SIGIRR−/− mice (*p < 0.05, paired Student's t test; Fig. 3B), but not wild types (data not shown). Importantly, incubation with d-JNKi1 (2 μm), a specific inhibitor of pJNK, significantly alleviated the impairment in LTP observed in SIGIRR−/− mice (p < 0.01; unpaired Student's t test; Fig. 3C), thus restoring it to levels observed in wild-type slices. The magnitude of LTP recorded in hippocampal slices of wild-type mice was not significantly altered by the presence of d-JNKi1 (data not shown).
Figure 3.
Inhibition of JNK rescues the deficit in LTP recorded in SIGIRR−/− mice. A, Expression of phosphorylated JNK (p46; pJNK) was significantly increased in hippocampal tissue from SIGIRR−/− mice relative to wild-type mice (*p < 0.05, n = 5–6). B, Interestingly, 20 min application of IL-1ra significantly reduced the level of pJNK in hippocampus of SIGIRR−/− mice, relative to untreated tissue (*p < 0.05, n = 6). Overlay illustrates individual values for pJNK obtained by Western immunoblot in hippocampal tissue from SIGIRR−/− mice under control conditions, and following IL-1ra treatment. Values for pJNK expression were normalized to β-actin. Insets illustrate representative blots of pJNK in wild-type and SIGIRR−/− tissue (i) and in control and IL-1ra-treated tissue from SIGIRR−/− mice (ii), with respective β-actin blots. C, Application of d-JNKi1 (2 μm) for 20 min before T-BS, significantly attenuated the deficit in LTP recorded in slices from SIGIRR−/− mice (p < 0.01, n = 4, N = 3), relative to untreated SIGIRR−/− slices. The level of LTP obtained was similar to that observed in wild-type control experiments. Arrow indicates application of theta-burst stimulation. Inset displays sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction Calibrations: 1 mV, 20 ms.
A previous report from our laboratory identified that SIGIRR deficiency was associated with increased expression of TLR4 mRNA and CD14 mRNA, coupled with NF-κB activation (Watson et al., 2010). Here we demonstrate a significant increase in TLR4 protein in hippocampal tissue of SIGIRR−/− relative to wild-type mice (**p < 0.01; unpaired Student's t test; Fig. 4A), and an associated increase in pIκBα (**p < 0.01; unpaired Student's t test; Fig. 4B). To assess the role of TLR4 activation on the LTP impairment observed in SIGIRR−/− mice, either an anti-TLR4 antibody or isotype control IgG (2.5 μg/ml) was applied to slices from wild-type and SIGIRR−/− mice. Anti-TLR4 antibody did not alter LTP recorded from wild-type slices, although a brief impairment in short-term plasticity was observed immediately following T-BS application (Fig. 4C). However, the presence of an anti-TLR4 antibody significantly increased the level of LTP recorded in SIGIRR−/− mice, relative to values obtained with control IgG (p < 0.01; unpaired Student's t test; Fig. 4D). While SIGIRR−/− LTP was enhanced by the anti-TLR4 antibody, this was still significantly less than values obtained in wild-type slices (p < 0.05; Newman–Keuls test). These findings suggest that activation of TLR4 mediates, at least partially, the deficit in synaptic plasticity in SIGIRR−/− mice. Using wedelolactone, an inhibitor of IκB phosphorylation, we also assessed whether activation of NF-κB was associated with this LTP impairment. Wedelolactone (30 μm), applied 20 min before LTP induction, markedly increased the level of LTP recorded in SIGIRR−/− mice (p < 0.0001; unpaired Student's t test), but did not significantly alter LTP in wild-type slices (Fig. 4E). Interestingly, the enhanced LTP recorded in SIGIRR−/− mice in the presence of wedelolactone was also significantly greater than LTP observed in control experiments from wild-type mice (p < 0.001; Newman–Keuls test; Fig. 4E).
Figure 4.
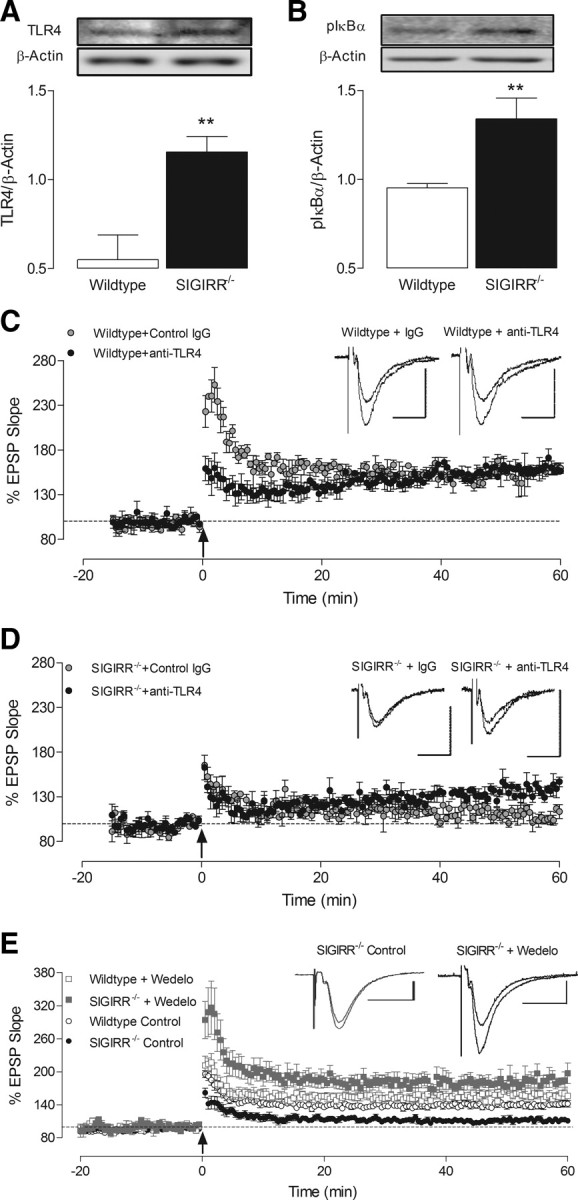
LTP impairment in SIGIRR−/− mice is associated with increased hippocampal expression of TLR4, pIκBα, and NF-κB activation. A, B, Western immunoblot revealed an increase in expression of TLR4 (A) and pIκBα (B) in hippocampal tissue prepared from SIGIRR−/− mice, relative to wild-type mice (**p < 0.01, n = 5–6). C, Anti-TLR4 antibody did not alter LTP in wild-type slices, compared with an isotype control IgG (2.5 μg/ml; n = 3, N = 2). D, LTP recorded in SIGIRR-deficient slices (n = 3, N = 3) was significantly enhanced relative to values obtained in the presence of a control IgG (p < 0.01, n = 4, N = 3). E, Application of the pIκBα inhibitor, wedelolactone (wedelo.; 30 μm), for 20 min before T-BS did not significantly alter LTP recorded in hippocampal slices from wild-type mice (n = 4, N = 3). However, the level of LTP recorded from SIGIRR−/− mice in the presence of wedelolactone was significantly increased (p < 0.0001, n = 4, N = 3), relative to untreated SIGIRR−/− slices and indeed untreated wild-type slices (p < 0.005). Arrow indicates application of theta-burst stimulation. Inset displays sample EPSP traces taken from a single experiment immediately before, and 60 min following LTP induction (calibrations: 1 mV, 20 ms). Expression levels of target proteins are normalized to β-actin. Insets illustrate representative blots of TLR4 (A) and pIκBα (B), along with respective β-actin blots.
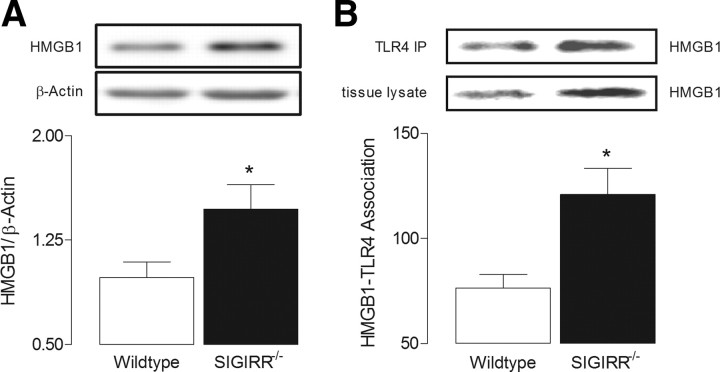
HMGB1 is a ubiquitous nuclear protein, with known affinity for TLR2 and 4 (Park et al., 2006). It is released by a number of cell types, including monocytes, macrophages and neurons, in response to inflammatory stimuli, proinflammatory cytokines (Wang et al., 1999; O'Connor et al., 2003; Qiu et al., 2008) and during necrosis (Scaffidi et al., 2002). Additionally, HMGB1 is known to augment the activity of proinflammatory stimuli such as IL-1 (Sha et al., 2008; Hreggvidsdottir et al., 2009). Here we show that expression of HMGB1 is significantly higher in hippocampal tissue from SIGIRR−/− mice compared with wild-type mice (*p < 0.05; unpaired Student's t test; Fig. 5A), suggesting that it may be responsible for the upregulated TLR4-mediated signaling and elevated IL-RI-mediated response identified in SIGIRR−/− mice. An interaction between HMGB1 and TLR4 has been reported in peripheral blood mononuclear cells (Hreggvidsdottir et al., 2009). We aimed to establish whether a similar interaction occurred in hippocampal tissue, and therefore samples were immunoprecipitated with anti-TLR4 and probed with an anti-HMGB1 antibody. The findings provide clear evidence of an interaction (*p < 0.05; unpaired Student's t test; Fig. 5B). The data suggest that a greater interaction occurs in samples from SIGIRR−/−, compared with wild-type mice, a likely consequence of increased HMBG1 and TLR4 in hippocampus of SIGIRR−/− mice. We therefore investigated the effects of acute HMGB1 application on LTP in hippocampal slices of wild-type mice, and demonstrate that recombinant HMGB1 (10 ng/ml), applied 20 min before T-BS, substantially impaired LTP of CA1 synapses (p < 0.01; unpaired Student's t test; Fig. 6A). Additionally, HMGB1 increased pJNK (*p < 0.05; paired Student's t test; Fig. 6B) and pIκBα (**p < 0.01; paired Student's t test; Fig. 6C) in hippocampal tissue.
Figure 5.
SIGIRR deficiency is associated with enhanced expression of HMGB1. A, Levels of HMGB1 were increased in hippocampus of SIGIRR−/− mice relative to hippocampal tissue from wild-type mice (*p < 0.05, n = 6). Expression levels of HMGB1 are normalized to β-actin. B, Hippocampal samples (equalized for protein; 500 μg), immunoprecipitated with anti-TLR4 antibody and probed for HMGB1, revealed an association between HMGB1 with TLR4 which was enhanced in tissue from SIGIRR−/− mice (*p < 0.05; n = 4); a sample immunoblot [upper blot (TLR4 IP)] is presented. The lower immunoblot indicates that HMGB1 expression in tissue lysate (i.e., in which no immunoprecipitation was undertaken) was greater in tissue from SIGIRR−/− mice.
To further investigate the role of TLR4 in the HMGB1-mediated impairment of LTP, we performed additional experiments on wild-type and TLR4−/− mice. Using hippocampal slices from male wild-type mice, we confirmed our previous finding in females (Fig. 6A) that HMGB1 (10 ng/ml) can significantly attenuate LTP (p < 0.05; Student's t test; Fig. 7Ai). As before, treatment of hippocampal tissue with HMGB1 (10 ng/ml; 30 min) was coupled with a significant increase in expression of pIκBα in wild-type mice (*p < 0.05; Student's t test; Fig. 7Aii), indicative of NF-κB activation. The level of LTP evoked in slices from TLR4−/− mice under control conditions was not different from that recorded in wild-type mice. However, there was no significant effect of HMGB1 application in TLR4−/− slices when compared with control values (Student's t test; Fig. 7Bi). Similarly, treatment of hippocampal tissue from TLR4−/− mice with HMGB1 (10 ng/ml; 30 min) was not associated with increased pIκBα expression (Student's t test; Fig. 7Bii).
Discussion
The current study demonstrates that SIGIRR expression in brain is essential for optimal cognitive and synaptic function. The impaired synaptic function in its absence is due to increased signaling through IL-1RI and TLR4 and the evidence indicates that increased expression of IL-1α and the TLR ligand, HMGB1, may be the primary cause of the deficit.
LTP is widely used as an indicator of healthy brain function and, accordingly, it is impaired in a number of neurodegenerative disease models which are associated with inflammatory changes. Thus deficits in LTP, accompanied by cognitive dysfunction, have been reported in models of AD where there is an overexpression of human presenilin 1 and/or human β-amyloid (Chapman et al., 1999; Puoliväli et al., 2002; Auffret et al., 2009; Townsend et al., 2010). The present data show that both LTP and hippocampal-dependent cognition were markedly decreased in SIGIRR−/− mice providing further evidence of the negative impact of inflammation on synaptic plasticity, and identifying a specific role for SIGIRR in maintenance of optimal cognitive and network function. Novel object recognition and Y-maze performances are known to be coupled with changes in LTP at hippocampal synapses (Wang et al., 2004; Clarke et al., 2010). Furthermore, impaired novel object recognition, associated with inflammatory responses, has been reported in a number of experimental models including transgenic mice expressing AD pathology (Feng et al., 2004; Heneka et al., 2006; Jardanhazi-Kurutz et al., 2010) and aged animals (Pitsikas et al., 2005; Garelick et al., 2009).
A recent report demonstrated that LPS exerted an enhanced response in glial cells and hippocampal tissue from SIGIRR−/−, compared with wild-type mice (Watson et al., 2010). In addition, intraperitoneal injection of LPS decreased exploratory behavior to a greater extent in SIGIRR−/−, compared with wild-type, mice (Watson et al., 2010). The present data indicate that, in the absence of any external inflammatory stimulus, the cognitive and synaptic deficits observed in SIGIRR−/− mice are associated with upregulation of IL-1R1- and TLR4-mediated signal transduction in hippocampus. While SIGIRR is known to regulate inflammation through interaction with both IL-1RI and TLR4 (Qin et al., 2005; Huang et al., 2006; Gulen et al., 2010), we now show that SIGIRR deficiency is associated with increased expression of these receptors as well as signaling mediated by receptor activation. Thus SIGIRR−/− mice exhibit increased expression of IL-1α, IL-1RI and IRAK relative to their wild-type counterparts. The key role of IL-1RI activation in mediating the decrease in LTP in SIGIRR−/− mice is demonstrated by the findings that LTP is restored by IL-1ra. It is important to point out that application of IL-1ra attenuates LTP under control conditions, as previously described by us (Loscher et al., 2003; Schmid et al., 2009) and others (Ross et al., 2003). The data also highlight a role for TLR4 activation in mediating the decrease in LTP in SIGIRR−/− mice since LTP was maintained in slices prepared from SIGIRR−/− mice that were perfused with an anti-TLR4 antibody. Since both IL-1ra and anti-TLR4 antibody restored LTP, it must be concluded that either TLR4 activation increases cytokine release which act in a paracrine or autocrine fashion, or that there is a convergence at key signaling events downstream of receptor activation. Consistent with the latter, the data show that activation of stress-associated signals, JNK and NF-κB, which are both upregulated downstream of IL-1RI and TLR4 activation (Ninomiya-Tsuji et al., 1999), was increased in hippocampal tissue prepared from SIGIRR−/− mice. Targeted inhibition of JNK and NF-κB restored LTP in these mice suggesting a pivotal role for these signaling events in the impaired synaptic function. At present, we must conclude that both TLR4 and IL-1RI activation combine to increase activation of JNK and NF-κB in SIGIRR-deficient mice. Enhanced activation of both have been reported in aged animals, and following LPS or Aβ administration, and is associated with impaired LTP (O'Donnell et al., 2000; Vereker et al., 2000; Kelly et al., 2003; Minogue et al., 2003; Costello and Herron, 2004; Barry et al., 2005). Similarly, inhibition of JNK or NF-κB alleviates the depression of LTP observed in response to LPS and/or Aβ (Kelly et al., 2003; Minogue et al., 2003; Costello and Herron, 2004; Barry et al., 2005). Interestingly, the evidence indicates that wedelolactone, which inhibits NF-κB activation (Kobori et al., 2004), increases LTP above control levels. One possible explanation for this is that by inhibiting NF-κB, wedelolactone also decreases production of cytokines like IL-1β, IL-6 and TNFα, which are known to exert an inhibitory effect on LTP (Tancredi et al., 1992, 2000; Vereker et al., 2000; Ross et al., 2003). While it has been known for some time that TLR4 activation plays a significant role in modulating hippocampal function (Barry et al., 2005; Cunningham et al., 2009; Czapski et al., 2010), it has recently been reported that hippocampal-dependent memory, accompanied by neurogenesis, was enhanced in TLR3-deficient mice (Okun et al., 2010).
Increased expression of TLR4 mRNA has been reported to accompany inflammation in a mouse model of AD, and brain tissue from AD patients (Walter et al., 2007), as well as in age-related conditions (Letiembre et al., 2007; Balistreri et al., 2009). Additionally, the therapeutic benefits of targeting IL-1 receptor for treatment of inflammatory diseases have been well described (Halle et al., 2008). SIGIRR deficiency is accompanied by increased expression of HMGB1 in hippocampus, a putative endogenous TLR4 ligand. HMGB1 was first described as a nuclear DNA binding protein, but more recently has been identified as a ligand for TLR2, TLR4 (Park et al., 2006) and receptor for advanced glycation end-products (Kokkola et al., 2005). In addition to its release in response to damage and inflammation (Scaffidi et al., 2002; O'Connor et al., 2003; Qiu et al., 2008; Maroso et al., 2010), HMGB1 acts as a proinflammatory cytokine within the CNS (O'Connor et al., 2003; Qiu et al., 2008). The capacity of HMGB1 to augment the activity of proinflammatory stimuli, such as IL-1 and LPS, has also been reported (Sha et al., 2008; Hreggvidsdottir et al., 2009). Accordingly, we show that HMGB1 attenuates hippocampal LTP and, consistent with its ability to activate TLR4, leads to phosphorylation of both JNK and IκB. These data suggest that the combined increases in expression of HMGB1 and TLR4 in hippocampus of SIGIRR−/− mice, and their enhanced association, may be responsible for the impairment in synaptic plasticity. A recent study by Maroso et al. (2010) proposed a role for HMGB1-TLR4 interaction in facilitating epileptiform activity in experimental models of seizure. These authors attribute this effect to targeting of the NR2B subunit of NMDA receptors (Maroso et al., 2010), an effect similar to that previously reported following IL-1RI activation (Viviani et al., 2003). Since activity-dependent regulation of the NR2A and NR2B subunits modulates the threshold for induction of synaptic plasticity at hippocampal CA1 synapses (Xu et al., 2009), it is plausible that the HMGB1-mediated reduction in LTP is a result of modulation of NMDA receptor function. However it is acknowledged that TLR4 is expressed on glial cells (Olson and Miller, 2004; Jack et al., 2005) as well as neurons (Tang et al., 2007; Maroso et al., 2010) but the cell on which HMGB1 exerts its primary effect in this study is unknown. Similarly, while HMGB1 is released from macrophages and neurons following damage or insult (Scaffidi et al., 2002; O'Connor et al., 2003; Qiu et al., 2008), the cell source of the increased HMGB1 in hippocampus of SIGIRR−/− mice remains to be determined. It is worth noting that HMGB1 is described as a late mediator of inflammation (Wang et al., 1999) and therefore it is possible that its effects are self-perpetuating.
We conclude that SIGIRR impacts upon synaptic function by negatively regulating signaling through IL-1RI and TLR4. HMGB1 is likely to be the endogenous activator of TLR4 since its expression is increased and heavily associated with the receptor in SIGIRR−/− mice. A similar increase in expression of TLR4 and HMGB1 in other tissues may explain the increased inflammation reported in EAE, colitis and arthritis in SIGIRR−/− mice, suggesting that pharmacological targeting of HMGB1 may be advantageous to the treatment of systemic inflammatory conditions (Yang et al., 2002). Of particular importance is that similar pharmacological targeting may be appropriate to attenuate the loss of cognitive function associated with neurodegenerative diseases and neuroinflammatory disorders, particularly if future studies demonstrate disruption of SIGIRR or SIGIRR-regulated signaling in these conditions.
Footnotes
This work was supported by Science Foundation Ireland, and a grant from the Sixth Research Framework Programme of the European Union (Projects NoE MUGEN LSHB-CT-2005-005203) to C.G. We thank Drs. Colm Cunningham and Éadaoin Griffin for their advice with behavioral experiments, and Prof. Padraic Fallon for the kind gift of TLR4−/− mice.
References
- Auffret A, Gautheron V, Repici M, Kraftsik R, Mount HT, Mariani J, Rovira C. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer's disease. J Neurosci. 2009;29:10144–10152. doi: 10.1523/JNEUROSCI.1856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri CR, Colonna-Romano G, Lio D, Candore G, Caruso C. TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. J Clin Immunol. 2009;29:406–415. doi: 10.1007/s10875-009-9297-5. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barry CE, Nolan Y, Clarke RM, Lynch A, Lynch MA. Activation of c-Jun-N-terminal kinase is critical in mediating lipopolysaccharide-induced changes in the rat hippocampus. J Neurochem. 2005;93:221–231. doi: 10.1111/j.1471-4159.2004.03011.x. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Herron CE. The role of c-Jun N-terminal kinase in the A beta-mediated impairment of LTP and regulation of synaptic transmission in the hippocampus. Neuropharmacology. 2004;46:655–662. doi: 10.1016/j.neuropharm.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran B, O'Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108:83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Czapski GA, Gajkowska B, Strosznajder JB. Systemic administration of lipopolysaccharide induces molecular and morphological alterations in the hippocampus. Brain Res. 2010;1356:85–94. doi: 10.1016/j.brainres.2010.07.096. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Cholerton LL, Talbot K, Nair-Roberts RG, Sanderson DJ, Romberg C, Koros E, Bornemann KD, Rawlins JN. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behav Brain Res. 2008;189:126–138. doi: 10.1016/j.bbr.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Drexler SK, Kong P, Inglis J, Williams RO, Garlanda C, Mantovani A, Yazdi AS, Brennan F, Feldmann M, Foxwell BM. SIGIRR/TIR8 is an inhibitor of Toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 2010;62:2249–2261. doi: 10.1002/art.27517. [DOI] [PubMed] [Google Scholar]
- Feng R, Wang H, Wang J, Shrom D, Zeng X, Tsien JZ. Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer's presenilin-1 and presenilin-2. Proc Natl Acad Sci U S A. 2004;101:8162–8167. doi: 10.1073/pnas.0402733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick MG, Chan GC, DiRocco DP, Storm DR. Overexpression of type I adenylyl cyclase in the forebrain impairs spatial memory in aged but not young mice. J Neurosci. 2009;29:10835–10842. doi: 10.1523/JNEUROSCI.0553-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, Sironi M, Nebuloni M, Zorini EO, Scanziani E, Mantovani A. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009;30:439–446. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, Xiao H, Delgoffe GM, Min B, Powell JD, Tuohy VK, Cua DJ, Li X. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss WD, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006;177:548–556. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jardanhazi-Kurutz D, Kummer MP, Terwel D, Vogel K, Dyrks T, Thiele A, Heneka MT. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int. 2010;57:375–382. doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- Lech M, Garlanda C, Mantovani A, Kirschning CJ, Schlöndorff D, Anders HJ. Different roles of TiR8/Sigirr on toll-like receptor signaling in intrarenal antigen-presenting cells and tubular epithelial cells. Kidney Int. 2007;72:182–192. doi: 10.1038/sj.ki.5002293. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Loscher CE, Mills KH, Lynch MA. Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. J Neuroimmunol. 2003;137:117–124. doi: 10.1016/s0165-5728(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- O'Connor KA, Hansen MK, Rachal Pugh C, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–265. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- O'Donnell E, Vereker E, Lynch MA. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. Eur J Neurosci. 2000;12:345–352. doi: 10.1046/j.1460-9568.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, Cheng A, Mughal MR, Wan R, Ashery U, Mattson MP. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE. The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat. Neurobiol Aging. 2005;26:259–264. doi: 10.1016/j.neurobiolaging.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Polentarutti N, Rol GP, Muzio M, Bosisio D, Camnasio M, Riva F, Zoja C, Benigni A, Tomasoni S, Vecchi A, Garlanda C, Mantovani A. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw. 2003;14:211–218. [PubMed] [Google Scholar]
- Puoliväli J, Wang J, Heikkinen T, Heikkilä M, Tapiola T, van Groen T, Tanila H. Hippocampal A beta 42 levels correlate with spatial memory deficit in APP and PS1 double transgenic mice. Neurobiol Dis. 2002;9:339–347. doi: 10.1006/nbdi.2002.0481. [DOI] [PubMed] [Google Scholar]
- Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–25241. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schmid AW, Lynch MA, Herron CE. The effects of IL-1 receptor antagonist on beta amyloid mediated depression of LTP in the rat CA1 in vivo. Hippocampus. 2009;19:670–676. doi: 10.1002/hipo.20542. [DOI] [PubMed] [Google Scholar]
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Antuono M, Cafè C, Giovedì S, Buè MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen E, Renshaw BR, Sims JE. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine. 1999;11:389–399. doi: 10.1006/cyto.1998.0452. [DOI] [PubMed] [Google Scholar]
- Townsend M, Qu Y, Gray A, Wu Z, Seto T, Hutton M, Shearman MS, Middleton RE. Oral treatment with a gamma-secretase inhibitor improves long-term potentiation in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2010;333:110–119. doi: 10.1124/jpet.109.163691. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Watson MB, Costello DA, Carney DG, McQuillan K, Lynch MA. SIGIRR modulates the inflammatory response in the brain. Brain Behav Immun. 2010;24:985–995. doi: 10.1016/j.bbi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen RQ, Gu QH, Yan JZ, Wang SH, Liu SY, Lu W. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci. 2009;29:8764–8773. doi: 10.1523/JNEUROSCI.1014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res. 2002;8:469–472. doi: 10.1179/096805102125001091. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu X, Zhao Y, Deng Z, Qian G. SIGIRR inhibits toll-like receptor 4, 5, 9-mediated immune responses in human airway epithelial cells. Mol Biol Rep. 2011;38:601–609. doi: 10.1007/s11033-010-0146-7. [DOI] [PubMed] [Google Scholar]