Abstract
Apraxia caused by left hemispheric stroke typically impairs skilled sequential movements. After stroke, apraxic patients need to reacquire motor skills by motor learning. The current study assessed for the first time incidental motor sequence learning in apraxic patients. Forty-eight human subjects (henceforth called “patients”) with left hemispheric stroke affecting the middle cerebral artery territory (18 with apraxia and 30 without apraxia) and 17 age-matched healthy controls were tested on a visuomanual serial reaction time task. Subjects performed four blocks consisting of repetitions of a complex six element sequence containing ambiguous pairwise transitions before a new and unfamiliar sequence was introduced in block 5. Reaction time (RT) disadvantages in this fifth block indicated incidental sequence-specific motor learning. The intentional retrieval of the learned motor knowledge was assessed subsequently with a free recall task. Voxel-based lesion-symptom mapping (VLSM) was performed to investigate for the first time the lesion correlates of deficits in learning and retrieving sequential motor knowledge. Despite generally prolonged RTs, apraxic patients showed sequence-specific motor learning as could be observed in nonapraxic patients and healthy controls. However, apraxic patients showed reduced intentional retrieval of the learned sequence. VLSM revealed that impaired intentional retrieval of motor sequence knowledge resulted from dorsal premotor cortex lesions. Apraxic patients showed a dissociation of preserved incidental motor (sequence) learning and deficient intentional retrieval of this incidentally learned motor knowledge. The data suggest that novel approaches for treating apraxia should focus on incidental motor learning, but that automatic rather than intentional retrieval strategies should be enforced.
Introduction
Apraxia, a disorder of motor cognition and frequent consequence of left hemispheric stroke, is often considered a deficit of learned movements (Rothi and Heilman, 1997; Leiguarda and Marsden, 2000). To date, few studies have examined motor learning in apraxic patients, although apraxic patients rely on motor learning processes when trying to reacquire a movement during rehabilitation. Heilman et al. (1975) reported significant learning of a simple motor skill (keeping a stylus on a rotary disc), although the improvement disappeared after a 15 min break (Heilman et al., 1975). Further studies showed that apraxic compared with nonapraxic stroke patients have deficits in intentionally learning sequences of meaningless hand postures (Motomura et al., 1989) or meaningful gestures (Rothi and Heilman, 1984; Faglioni et al., 1990). To further elucidate the role of motor learning in apraxia, the current study examined for the first time in left hemisphere stroke patients how apraxia affects incidental motor sequence learning and intentional retrieval thereof by means of the serial reaction time (SRT) paradigm and voxel-based lesion-symptom mapping (VLSM). The SRT paradigm assesses incidental motor sequence learning by performance measures; in addition a subsequent free recall task allows assessing intentional retrieval of the incidentally learned motor sequence (Nissen and Bullemer, 1987; Keele et al., 2003). Because of its spatial component and the opportunity to isolate a sequence-specific learning effect, the SRT paradigm is well suited for the investigation of apraxic patients who clinically present with spatial (Poizner et al., 1990; Clark et al., 1994) as well as sequential errors (De Renzi et al., 1983; Harrington and Haaland, 1992; Buxbaum and Schwartz, 1998; Weiss et al., 2008). The neural substrate underlying motor learning deficits in apraxic patients was revealed by quantitative VLSM, thereby extending previous studies adopting the SRT paradigm to stroke patients (Pohl and McDowd, 2006; Orrell et al., 2007), which performed no or only descriptive lesion analysis.
Materials and Methods
Subjects.
Forty-eight patients (age range: 21–77 years; 31 male, 17 female) suffering from a single (first ever) unilateral stroke affecting the left middle cerebral artery territory (two patients had a stroke additionally affecting the left anterior cerebral artery territory) and 17 healthy, age-matched controls (age range: 41–73 years; 8 male, 9 female) were enrolled after giving written informed consent. The study was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the local ethics committee of the Medical Faculty, RWTH Aachen University. Patients were recruited prospectively during the subacute phase (8–90 d poststroke) or chronic phase (>90 d poststroke) after stroke. All patients and subjects were right handed (Oldfield, 1971). Patients were divided into two groups depending on the presence or absence of apraxia as assessed by standard neuropsychological tests: tests of imitating meaningless (1) hand positions or (2) finger configurations (Goldenberg, 1996), (3) pantomiming the use of objects or actually using (4) single or (5) multiple objects (De Renzi et al., 1968; Hartmann et al., 2005) were used. Patients were classified as apraxic if they scored below the cutoff on at least one of these tests. Classification resulted in a group of 30 patients without apraxia and a group of 18 patients with apraxia. In the group of apraxic patients, the majority of patients (n = 12) suffered from both an imitation deficit (as revealed by a deficit in either imitation test) and a pantomime (or object use) deficit (as revealed by a deficit in at least one of the three following: pantomime of object use, actual object use (of single objects), or complex object use). Five patients showed an isolated imitation deficit, and one patient suffered from an isolated pantomime deficit (without imitation deficits and preserved actual object use). Note that we purposely refrain from using terms like ideomotor apraxia or ideational apraxia as the different apraxia definitions/classifications are currently under debate (Goldenberg, 2008). Rather, we describe the clinical motor deficits of the patients (impaired gesture imitation, pantomiming, and object use). The following clinical scores were assessed in both patient groups: Token Test (De Renzi and Vignolo, 1962); Medical Research Council (MRC) paresis scale for the affected hand (Medical Research Council of the United Kingdom, 1978), action research arm test (Lyle, 1981); modified Rankin scale (Rankin, 1957); and the Corsi block tapping test (Schellig, 1997). For a summary of clinical and demographic data of the three study groups (i.e., controls, and patients with and without apraxia), please see Table 1. Statistical lesion analyses adopting VLSM were used to identify lesion sites associated with hemiparesis and different apraxic symptoms (Fig. 1A). Subcortical lesions including the internal capsule were associated with degree of paresis (Verdon et al., 2010). Lesions of the inferior and superior parietal cortex were associated with deficits in imitating hand postures, while deficits in imitating finger configurations were associated with inferior parietal regions and additional smaller frontal lesions. Furthermore, lesions in temporal and parietal regions led to object use deficits. These results are in accordance with previous neuropsychological and imaging studies (Hermsdörfer et al., 2001; Mühlau et al., 2005; Goldenberg and Karnath, 2006; Randerath et al., 2010; Verdon et al., 2010). There was however, no clear association between a specific lesions site and pantomime deficits (Goldenberg, 2003). This may be due to the fact that many different areas within a widely distributed left hemispheric network are critically involved in pantomiming the use of objects (Rumiati et al., 2004; Hermsdörfer et al., 2007). Therefore, it is conceivable that the current patient sample was not large enough to reveal a clear lesion–symptom association in the VLSM analysis of the pantomime scores as it needs more statistical power to reveal the association of a lesion pattern (consisting of many different areas) with a given symptom (i.e., pantomime deficit) than, for example, to reveal a circumscribed lesion site as the inferior parietal cortex for hand gesture imitation deficits (Hermsdörfer et al., 2001; Mühlau et al., 2005; Goldenberg and Karnath, 2006).
Table 1.
Patient characteristics
| Apraxic patients (n = 18) | Nonapraxic patients (n = 30) | Healthy controls (n = 17) | |
|---|---|---|---|
| Mean age (SD) (years) | 56.8 (11.9) | 50.1 (12.3) | 53.5 (10.4) |
| Sex ratio (male/female) | 9/9 | 22/8 | 8/9 |
| Mean time poststroke (d) | 367 | 315 | |
| Range poststroke (d) | 16–1209 | 27–1506 | |
| Number of subacute/chronic patients | 8/10 | 14/16 | |
| MRC scale (Medical Research Council of the United Kingdom, 1978) (right hand) | 2.71 | 3.55 | |
| ARAT (Lyle, 1981) (right hand) | 29.65 | 42 | |
| Modified Rankin scale (Rankin, 1957)* | 2.67 | 1.77 | |
| Token Test (De Renzi and Vignolo, 1962)* | 20.92 | 6.07 | |
| Visuospatial working memorya | 4.75 | 5.28 | 5.41 |
ARAT, Action Research Arm Test.
*Significant group difference (Mann–Whitney test, p < 0.05). Groups did not differ significantly with respect to any other parameter listed (as assessed by: one-way ANOVA, χ2 test, t test, Mann–Whitney test, Fisher's exact test).
aMean of the maximum number of correctly reproduced items in the Corsi block tapping test (Schellig, 1997).
Figure 1.
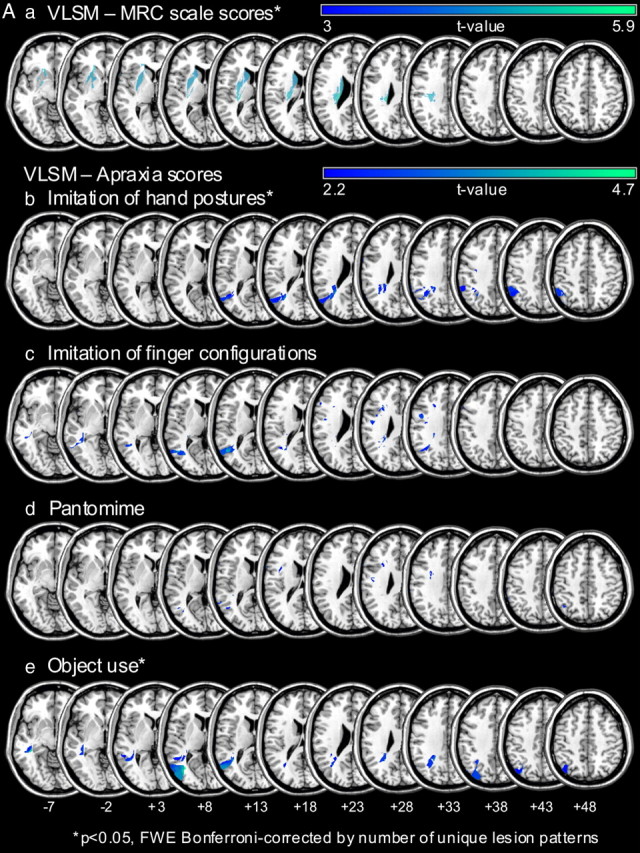
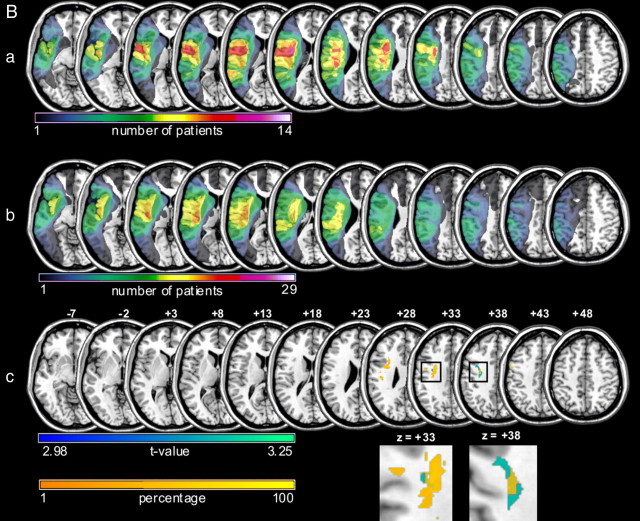
Aa–e, VLSM analyses revealing the lesion patterns for the clinical measures of paresis (a) and the different apraxia test scores (b–e) in the current patient sample. VLSM analyses are shown for apraxic and nonapraxic stroke patients combined (n = 43). a, Significant association between the degree of paresis as assessed by the MRC scale (Medical Research Council of the United Kingdom, 1978) and subcortical lesions involving the internal capsule as well as the putamen and the nucleus caudatus [t > 4.34, corrected for multiple comparisons by number of unique lesion patterns, p < 0.05, familywise error correction (FWE)]. b–e, Lesions associated with specific apraxic symptoms as assessed by the different apraxia tests are displayed. The VLSM analyses with the hand posture imitation test scores (t > 4.37) and the (single) object use test scores (t > 4.36) reached significance (corrected for multiple comparisons by number of unique lesion patterns, pFWE < 0.05). Slices with MNI z-coordinates from −7 to +48 are shown. Note that for display purposes areas with t values >3 (a) and 2.2 (b–e) are shown as indicated by the color bars. B, Lesion patterns of apraxic and nonapraxic patients and their relation to the area in the dorsal premotor cortex associated with impaired intentional retrieval of sequence items (in patients with unimpaired incidental sequence-specific learning). a, b, Lesion overlays of the patients with apraxia (n = 14, a) and without apraxia (n = 29, b). Color bars indicate the number of patients with a lesion in the region that is colored, respectively. c, A region within the dorsal premotor cortex was shown to be affected more often in apraxic compared with nonapraxic patients (areas marked with orange). The orange/yellow color bar indicates the extent (in percentage) to which a given region was more affected in patients with apraxia compared with those without apraxia. The orange areas displayed in c are those with a ≥40% difference between apraxic and nonapraxic patients. An overlapping region of the dorsal premotor cortex was associated with impaired intentional retrieval of sequence items in patients with proper incidental sequence-specific learning as revealed by VLSM (areas marked with blue, corrected for multiple comparisons by means of false discovery rate, pFDR < 0.05). For illustration purposes, the section of the slices at z = 33 and z = 38 (as indicated by the rectangles) showing the overlap within the dorsal premotor cortex was enlarged and depicted in part II. The blue color bar indicates the corresponding t values for the VLSM analysis.
Experimental setup, task, and procedure.
Task and equipment of the original SRT paradigm (Nissen and Bullemer, 1987) were adopted for the purpose of testing stroke patients. Using presentation software (Neurobehavioral Systems, version 12.1), visual stimuli (black Xs) were subsequently presented at one of three possible horizontally aligned positions on a white screen. All patients and controls were asked to respond as quickly and accurately as possible with their nondominant (i.e., left) hand by pressing the large button of a custom-made response board spatially congruent with the current stimulus. Stimuli remained visible until the subject responded (fixed response stimulus interval of 500 ms). The experiment comprised six blocks with blocks 1–4 and 6 containing the same and block 5 containing a different six item sequence with the same item frequency (Hoffmann and Koch, 1998). Importantly, subjects were not informed about the sequential order. Six item sequences were used to keep the duration of the experiment short, in view of the patients. Despite their relative shortness, the sequences were difficult to learn because of their first- and higher-order redundancies (see below). Five repetitions of the sequence per block yielded a total of 30 trials per block. Between blocks, the German word “PAUSE” (≙ break) indicated a short break. After 10 s, the color of the word PAUSE changed from black to red, signaling that the experiment continued within 2 s. Altogether, completion of the entire SRT task took no longer than 7 min for any of the subjects. After completion of the task, the amount of (incidentally) learned motor knowledge that subjects were able to retrieve intentionally was assessed by a standardized, structured interview and a free recall test. First, subjects were asked whether they had recognized something during the experiment. Second, if subjects did not mention having recognized a sequence they were asked more specifically whether they believed that stimuli had been presented randomly or followed a repetitive sequence. If subjects still denied having recognized a sequence they were informed about the sequential order and then asked to freely recall the six item sequence, indicating their answer by pressing the respective buttons. Explicit knowledge of the sequence was parameterized as the longest continuous series of button presses that matched the actual sequence.
Design.
The experiment examined incidental motor sequence learning and intentional retrieval thereof in left hemisphere stroke patients with and without apraxia as well as in healthy age-matched controls. Two different six element sequences with “ambiguous” serial transitions (Cohen et al., 1990) were used: 1-3-2-3-1-2 and 3-1-3-2-1-2, with 1 representing the left, 2 the middle, and 3 the right stimulus location. The two sequences were counterbalanced across subjects in each group, meaning that both sequences equally often served as the sequence to be learned (block: 1–4, 6) or new sequence (block: 5). Two sequences with similar first- and higher-order redundancies were used to prevent the sequence-specific learning effect from being confounded by differences in sequence structure as it occurs with (pseudo-) random sequences (Reed and Johnson, 1994; Hoffmann and Koch, 1998). Thus, in both sequences the frequency of all possible stimulus positions was the same, and both sequences contained the same number of reversals (e.g., 313) as well as the same transition probabilities. To anticipate the next response, subjects had to keep in mind at least the two preceding items.
A reaction time (RT) decrease from block 1 to block 4 indicates that learning occurs. As it is possible that this effect merely reflects habituation to the execution of the motor task rather than knowledge about the sequence, the sequence-specific learning effect was isolated in the RT difference of the block containing the new sequence (block 5) and the average RT of its preceding and succeeding block (learned sequence: blocks 4 and 6). Block 6 was included to rule out that an RT disadvantage in block 5 was simply caused by fatigue effects.
Statistical analysis.
Statistical analyses were performed with the statistical software package SPSS 18. As dependent variables were normally distributed (Kolmogorov–Smirnov test), parametric methods with Bonferroni correction were applied. After rejection of error trials, the median RT per block was calculated for each individual subject. Based on the individual median RTs, the mean RT across subjects was calculated for each block. Moreover, a learning score was computed for each subject by subtracting the average of the median RT of blocks 4 and 6 (learned sequence) from the median RT of block 5 (new sequence).
Lesion mapping.
Lesion analyses were based on 43 patients (14 apraxic and 29 nonapraxic patients) who had a clinical computed tomography or magnetic resonance imaging (MRI) scan suitable for lesion mapping [the remaining scans were not available (n = 3) or deemed insufficient (n = 2)]. All lesions were mapped using the free MRIcron software (http://www.cabiatl.com/mricro/mricron/index.html) and were drawn manually on slices of a T1-weighted template MRI scan (ch2) provided by MRIcron. Lesions were mapped onto axial slices that corresponded to the z-coordinates from −42 to +78 in steps of 5 mm. MRIcron was used to perform statistical VLSM (Bates et al., 2003; Rorden et al., 2007), which avoids grouping patients according to lesion site or behavioral cutoff scores, but rather uses continuous behavioral and lesion information (Bates et al., 2003). All analyses included only voxels that were damaged in at least 10% of the patients.
Results
SRT data
Overall error rates were not significantly different between groups (F(2,62) = 2.091, p = 0.132). The mean error rates across all blocks lay at <1% for all three groups (apraxic patients: 0.25%; nonapraxic patients: 0.63%; healthy controls: 0.13%) and 43 of 65 subjects made no errors at all. Due to this floor effect, error rates could not serve as a reasonable measure for the learning effect in the current study. Therefore, the statistical analyses of the SRT effect are confined to the reaction time data.
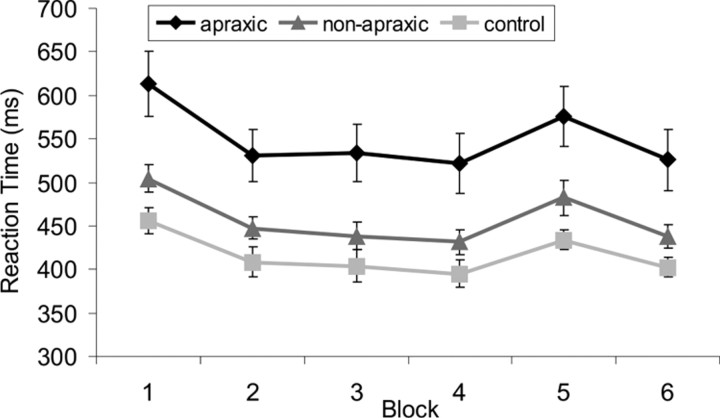
To analyze the general learning effect (within-subject factor: block 1 vs block 4) and the group effect (between-subject factor: patients with and without apraxia, healthy controls) a mixed-design ANOVA was performed. A significant main effect of group (F(2,62) = 10.04, p < 0.001) indicated general RT differences between groups. Post hoc t tests showed that apraxic patients were slower than nonapraxic patients (p < 0.01) and healthy controls (p < 0.001), while nonapraxic patients and healthy controls did not differ significantly (p = 0.460) (Fig. 2). The RT decrease from block 1 to block 4 was significant (F(1,62) = 88.431, p < 0.001), whereas the interaction term was not (F(2,62) = 1.004, p = 0.372).
Figure 2.
Average group RTs for the six blocks of the SRT paradigm. Average RT data with standard error bars for the apraxic patients (black diamonds), nonapraxic patients (dark gray triangles), and healthy control subjects (light gray squares) across the SRT paradigm. Blocks 1–4 and 6 contain the sequence to be learned, while block 5 (transfer block) contains another similar complex sequence. Note that all groups showed an unspecific practice effect (reaction times are significantly shorter in block 4 compared with block 1). More importantly, both patient groups and healthy controls showed similar sequence-specific motor learning (i.e., reaction time costs for block 5 compared with blocks 4 and 6).
To assess sequence-specific learning effects, a second mixed-design ANOVA (within-subject factor block: block 5 vs average of block 4 and 6; between-subject factor group: patients with and without apraxia, healthy controls) was performed. Again, the main effect of group was significant (F(2,62) = 8.932, p < 0.001) and post hoc t tests showed that apraxic patients responded more slowly than nonapraxic patients (p < 0.01) and healthy controls (p < 0.001), with no significant difference between the latter groups (p = 0.456). However, sequence-specific motor learning occurred in all groups (significant main effect of the within-subject factor “block”: F(1,62) = 25.042, p < 0.001; nonsignificant block by group interaction: F < 1). As apraxic patients scored worse on the Token Test (Table 1), an additional analysis including these test scores as covariates was performed for the two patient groups, which revealed similar results. Additionally, a univariate ANOVA on the proportional learning scores (raw learning score divided by individual median RT of block 5 and multiplied by 100) was performed to control for the general RT differences. This ANOVA confirmed that the three groups did not differ significantly with respect to the magnitude of the sequence-specific learning effect (F < 1). Moreover, the proportion of patients showing a positive proportional learning score was very similar: apraxic patients: 77, 8%; nonapraxic patients: 76, 7%.
An additional mixed-design ANOVA (within-subject factor block: block 5 vs average of blocks 4 and 6; between-subject factor group: no apraxia, n = 30; imitation and pantomime/object use deficit, n = 12; and pure imitation deficit, n = 5) was performed to evaluate the effect of the different apraxia subtypes on sequence-specific motor learning. This analysis revealed a significant main effect of block (F(1,44) = 9.828, p = 0.003) but no significant block-by-group interaction (F < 1), indicating that there was no significant difference in the sequence-specific motor learning among the three apraxia groups. As there was only one single patient with a pure pantomime deficit, this patient was not included in the subgroup analysis. This patient however, showed a positive SRT learning effect as well.
Free recall data
Explicit sequence knowledge was parameterized as the maximum number of sequence elements recalled in the correct order. Degree of explicit sequence knowledge differed significantly between groups (F(2,62) = 3.522, p < 0.036). Post hoc t tests showed that patients with apraxia recalled significantly fewer items of the learned sequence (mean 2.67 ± 2.06) than healthy controls (mean 4.35 ± 1.32; p < 0.05). Patients without apraxia (mean 3.37 ± 2.04) did not differ significantly from the other two groups (p > 0.05). As it seems possible that verbalization was used as a strategy to recall the learned sequence, the relationship between Token Test scores and the degree of intentionally retrievable sequence knowledge (maximal number of correctly retrieved sequence elements) was examined by assessing the correlation between these two variables. This correlation was not significant, with a correlation coefficient close to zero (r = 0.06, p = 0.707). Furthermore, it was examined whether differences in intentional retrieval were related to differences in visuospatial working memory span. Again, visuospatial working memory span was similar in all groups, as no significant group differences emerged for the Block Tapping Test (F(2,55) = 2.352, p = 0.105; means: 4.75 ± 1.24, 5.28 ± 0.89, and 5.41 ± 0.62, respectively, for apraxic patients, nonapraxic patients, and control subjects). For nonapraxic patients, a significant positive correlation between the number of correctly reproduced items of the sequence and the magnitude of the learning scores emerged (r = 0.424, p < 0.05), but not for apraxic patients (r = 0.072, p = 0.778). Similar correlation results were found for the proportional learning scores (nonapraxic patients: r = 0.496, p < 0.01; apraxic patients: r = 0.042, p = 0.868). Due to a putative ceiling effect in the free recall test (all control subjects—with one exception—reproduced three or more items of the six item sequence), such correlation analysis was not meaningful for the healthy controls.
Lesion analysis
Lesion overlay plots of apraxic and nonapraxic patients are displayed in Figure 1B. Lesion size (i.e., the number of affected voxels) did not differ significantly (t(41) < 1). However, parts of the dorsal premotor cortex (PMd) were more affected in apraxic than in nonapraxic patients (Fig. 1Bc, areas marked in orange).
VLSM analysis was performed to reveal lesion sites associated with preserved incidental sequence-specific motor learning, but impaired intentional retrieval of the learned sequence knowledge (as observed in apraxic patients). This analysis, using the maximum number of sequence elements recalled in correct order as dependent variable, was restricted to patients who showed a learning score above average (n = 18, 5 apraxic and 13 nonapraxic patients), indicating that proper sequence-specific learning, as a prerequisite for the explicit retrieval of sequence knowledge, had occurred. This analysis revealed parts of the PMd (Fig. 1Bc, areas marked in blue). Note that these subregions of PMd overlap, at least in part, with the regions more often affected in apraxic compared with nonapraxic patients (see above) (Fig. 1Bc, areas marked in orange).
Discussion
The current study investigated for the first time in apraxic patients incidental motor (sequence) learning as well as intentional retrieval thereof by means of the SRT paradigm and VSLM. Despite generally prolonged response times, apraxic patients showed preserved incidental motor learning. In contrast, apraxic patients were impaired in intentionally retrieving the previously learned motor sequence. VLSM showed that lesions of PMd were associated with impaired intentional retrieval of sequence elements in patients with preserved incidental motor learning. Similar parts of the PMd were more affected in apraxic compared with nonapraxic patients. As apraxic patients showed preserved incidental sequence-specific motor learning, but a reduced capacity to intentionally retrieve this learned motor sequence knowledge, the lesion data suggest that lesions of the PMd are the neural substrate of this intentional retrieval impairment in apraxic patients. This dissociation in apraxic patients (i.e., preserved incidental motor learning but impaired intentional retrieval of incidentally learned motor knowledge) may have important clinical implications for the development of novel therapeutic strategies for apraxia.
To our knowledge, this is the first study examining the effect of apraxia on SRT learning in a large series of stroke patients. Moreover, this is the first investigation using VLSM to quantitatively reveal the neural substrates associated with motor learning deficits in apraxic patients. The finding that (implicit) motor learning on the SRT-task is preserved in apraxic patients is in line with a previous study reporting that apraxic patients showed a significant improvement of performance on a rotary pursuit apparatus when tested five times consecutively (Heilman et al., 1975). Our results extend these findings by showing that not only simple motor skill learning, but also complex sequence-specific motor learning can be preserved in apraxic patients. This is in accordance with previous studies showing that (circumscribed) cortical lesions do not necessarily result in impaired SRT task performance (Koch et al., 2006; Pohl and McDowd, 2006; Orrell et al., 2007).
Together, incidental motor learning mechanisms appear to be intact in patients with cortical lesions resulting in apraxia. Rather, group differences emerged with respect to the intentional retrieval of the learned motor sequence. That is, apraxic patients had reduced access to the structure of the incidentally learned sequence. Note that we purposely refrain from using the terminology of implicit versus explicit motor learning, referring to the level of awareness subjects have about the sequence. As the state of awareness is difficult to be assessed by objective parameters, we concentrated on incidental motor learning, defined by the fact that subjects are not informed about the learning aspect of the (SRT) task (Dienes and Berry, 1997). Thus, motor learning is taking place incidentally and can be indirectly measured by objective behavioral parameters (i.e., RT differences). Moreover, the number of correctly retrieved items of the incidentally acquired motor sequence reflects the intentional access to the incidentally learned motor knowledge. Note that this objective parameter may well dissociate from the level of awareness documented in the structured postlearning interview; that is, awareness of the sequential pattern is not a necessary requirement for proper retrieval of the incidentally acquired motor sequence knowledge.
It remains to be tested whether the observed retrieval deficit in apraxic patients is restricted to (uncued) free recall tasks, or whether apraxic patients are also impaired in cued recall or recognition tasks. However, the apraxic dissociation of unimpaired incidental motor sequence learning, when responding to the visually presented stimuli, and impaired free recall of the respective motor sequence, is in accordance with the clinical observation that apraxic patients generally perform better when external cues are provided. Tests of pantomime, which provide fewer cues than actual object use, are more sensitive to reveal apraxia-specific deficits in stroke patients (Goldenberg et al., 2004; Weiss et al., 2008).
Another relevant finding is the positive correlation between the number of sequence items reproduced in correct serial order and the magnitude of the sequence-specific learning effect in nonapraxic patients, which is in line with previous studies in healthy subjects reporting a more pronounced sequence-specific learning effect in subjects who retrieved a high number of sequence items (Eimer et al., 1996; Rüsseler and Rösler, 2000; Zirngibl and Koch, 2002). In contrast, no such correlation was observed for apraxic patients. Thus, while apraxic patients were unimpaired in incidentally learning structured sequences, they failed when intentionally trying to access this motor knowledge. Note that the intentional retrieval of sequence elements in apraxic patients was significantly reduced only compared with healthy controls but not when being compared with nonapraxic patients with left hemisphere damage. Thus, one could argue that this pattern of results cannot exclude a nonspecific effect of lesion per se on intentional recall of motor sequence knowledge. However, the fact that there is also no significant difference between the recall scores of the patients without apraxia and those of the control subjects indicates that a left hemispheric lesion per se does not suffice to significantly impair the intentional retrieval of motor sequence knowledge. Together, the current results rather suggest that both aspects (i.e., the combination of a left hemisphere lesion and apraxia) are relevant.
VLSM revealed that lesions of the PMd were significantly associated with reduced intentional retrieval of incidentally learned motor knowledge. These PMd regions were more affected in apraxic vs nonapraxic patients. The data therefore suggest that the impaired intentional retrieval of incidentally learned motor knowledge in apraxic patients is caused by PMd lesions. Note that intentional retrieval of motor sequence knowledge involves internally selecting appropriate actions, converting a learned motor sequence into an explicit motor plan, and predicting forthcoming actions. Converging evidence from electrophysiological studies in monkeys and human functional imaging studies suggests that the PMd is involved in these motor cognitive processes. For example, lesions to the monkey PMd did not impair working memory processes per se but rather the conversion of a learned sequence into a motor plan (Ohbayashi et al., 2003). Consistent with these data, visuospatial working memory was preserved in our apraxic patients despite their PMd lesions. Human functional imaging studies demonstrated an involvement of PMd in selecting appropriate actions (Schluter et al., 2001; Johansen-Berg et al., 2002) and predicting actions (Stadler et al., 2011). Furthermore, left PMd was shown to be dominant for action selection, even for movements of the ipsilateral left hand (Schluter et al., 1998).
In view of the study by Heilman et al. (1975), in which motor improvement on the rotary pursuit apparatus in apraxic patients was reported to be extinguished after a 15 min break, future studies should also investigate whether the automatic retrieval of incidentally learned motor sequence knowledge can be preserved over longer retention intervals. Our data cannot answer this question as the break between blocks 4 and 6 was shorter than 2 min, but future studies could, for example, assess SRT task performance on consecutive days to investigate the retention of motor sequence knowledge in apraxia.
The findings of the current study have important implications for the development of novel treatment strategies of apraxia (Buxbaum et al., 2008; Dovern et al., 2011). The data suggest that such strategies should include incidental motor learning and thereby make use of the remaining motor learning resources of patients with apraxia. Triggering implicit processing of motor information (e.g., by giving visual cues rather than verbal instructions) could provide a powerful tool to overcome intentional motor learning difficulties in apraxia.
Footnotes
A.D. is a scholarship holder of the L'Oréal-UNESCO “For Women in Science” initiative in cooperation with the Christiane Nüsslein-Volhard Stiftung. G.R.F. serves as an editorial board member of Cortex, Zeitschrift für Neuropsychologie, and Fortschritte der Neurologie Psychiatrie; receives royalties from the publication of the books Funktionelle MRT in Psychiatrie und Neurologie, and Neurologische Differenzialdiagnosen; received honoraria for speaking engagements from TEVA, GlaxoSmith-Kline, Boehringer Ingelheim, and Desitin; and received research support from the Bundesministerium für Bildung und Forschung, the Deutsche Forschungsgemeinschaft, and the Volkswagen Stiftung. I.K. is associate editor for Experimental Psychology and Journal of Experimental Psychology: Learning, Memory, and Cognition, member of the editorial board of Acta Psychologica, Advances in Cognitive Psychology, BMC Neuroscience, European Journal of Cognitive Psychology, and Journal of Experimental Psychology: General, and receives research support from the Bundesministerium für Bildung und Forschung and the Deutsche Forschungsgemeinschaft. J.S., H.K., and P.H.W. report no disclosures.
References
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Schwartz MF, Montgomery MW. Ideational apraxia and naturalistic action. Cogn Neuropsychol. 1998;15:617–643. doi: 10.1080/026432998381032. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Haaland KY, Hallett M, Wheaton L, Heilman KM, Rodriguez A, Gonzalez Rothi LJ. Treatment of limb apraxia: Moving forward to improve action. Am J Phys Med Rehabil. 2008;87:149–161. doi: 10.1097/PHM.0b013e31815e6727. [DOI] [PubMed] [Google Scholar]
- Clark MA, Merians AS, Kothari A, Poizner H, Macauley B, Gonzalez Rothi LJ, Heilman KM. Spatial planning deficits in limb apraxia. Brain. 1994;117:1093–1106. doi: 10.1093/brain/117.5.1093. [DOI] [PubMed] [Google Scholar]
- Cohen A, Ivry RI, Keele SW. Attention and structure in sequence learning. J Exp Psychol Learn Mem Cognit. 1990;16:17–30. [Google Scholar]
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Pieczuro A, Vignolo LA. Ideational apraxia: a quantitative study. Neuropsychologia. 1968;6:41–52. [Google Scholar]
- De Renzi E, Faglioni P, Lodesani M, Vecchi A. Performance of left brain-damaged patients on imitation of single movements and motor sequences. Frontal and parietal injured patients compared. Cortex. 1983;19:333–343. doi: 10.1016/s0010-9452(83)80004-5. [DOI] [PubMed] [Google Scholar]
- Dienes Z, Berry D. Implicit learning: below the subjective threshold. Psychon Bull Rev. 1997;4:3–23. [Google Scholar]
- Dovern A, Fink GR, Weiss PH. How to diagnose and treat limb apraxia. Fortschr Neurol Psychiatr. 2011 doi: 10.1055/s-0029-1246097. Advance online publication. Retrieved May 7, 2011. PMID: 21480158. [DOI] [PubMed] [Google Scholar]
- Eimer M, Goschke T, Schlaghecken F, Stürmer B. Explicit and implicit learning of event sequences: evidence from event-related brain potentials. J Exp Psychol Learn Mem Cognit. 1996;22:970–987. doi: 10.1037//0278-7393.22.4.970. [DOI] [PubMed] [Google Scholar]
- Faglioni P, Basso A, Botti C, Aglioti S, Saetti C. Gesture learning and apraxia. In: Jeannerod M, editor. Motor representation and control. Erlbaum: Mahwah, NJ; 1990. pp. 837–856. [Google Scholar]
- Goldenberg G. Defective imitation of gestures in patients with damage in the left or right hemispheres. J Neurol Neurosurg Psychiatr. 1996;61:176–180. doi: 10.1136/jnnp.61.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G. Pantomime of object use: a challenge to cerebral localization of cognitive function. Neuroimage. 2003;20:S101–S106. doi: 10.1016/j.neuroimage.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia. In: Goldenberg G, Miller BL, editors. Neuropsychology and behavioral neurology. Amsterdam: Elsevier; 2008. pp. 323–338. [Google Scholar]
- Goldenberg G, Karnath HO. The neural basis of imitation is body part specific. J Neurosci. 2006;26:6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Hentze S, Hermsdörfer J. The effect of tactile feedback on pantomime of tool use in apraxia. Neurology. 2004;63:1863–1867. doi: 10.1212/01.wnl.0000144283.38174.07. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Motor sequencing with left hemisphere damage. Are some cognitive deficits specific to limb apraxia? Brain. 1992;115:857–874. doi: 10.1093/brain/115.3.857. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Goldenberg G, Daumüller M, Hermsdörfer J. It takes the whole brain to make a cup of coffee: the neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43:625–637. doi: 10.1016/j.neuropsychologia.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Schwartz HD, Geschwind N. Defective motor learning in ideomotor apraxia. Neurology. 1975;25:1018–1020. doi: 10.1212/wnl.25.11.1018. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Goldenberg G, Wachsmuth C, Conrad B, Ceballos-Baumann AO, Bartenstein P, Schwaiger M, Boecker H. Cortical correlates of gesture processing: clues to the cerebral mechanisms underlying apraxia during the imitation of meaningless gestures. Neuroimage. 2001;14:149–161. doi: 10.1006/nimg.2001.0796. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Terlinden G, Mühlau M, Goldenberg G, Wohlschläger AM. Neural representations of pantomimed and actual tool use: evidence from an event-related fMRI study. Neuroimage. 2007;36:T109–T118. doi: 10.1016/j.neuroimage.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Koch I. Implicit learning of loosely defined structures. In: Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: SAGE Publications; 1998. pp. 161–199. [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychol Rev. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Koch I, Reverberi C, Rumiati RI. Learning hierarchically structured action sequences is unaffected by prefrontal-cortex lesion. Exp Brain Res. 2006;175:667–675. doi: 10.1007/s00221-006-0584-6. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD. Limb apraxias. Higher-order disorders of sensorimotor integration. Brain. 2000;123:860–879. doi: 10.1093/brain/123.5.860. [DOI] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Medical Research Council of the United Kingdom. Aids to examination of the peripheral nervous system: memorandum No 45. Palo Alto, CA: Pedragon House; 1978. [Google Scholar]
- Motomura N, Seo T, Asaba H, Sakai T. Motor learning in ideomotor apraxia. Int J Neurosci. 1989;47:125–129. doi: 10.3109/00207458908987424. [DOI] [PubMed] [Google Scholar]
- Mühlau M, Hermsdörfer J, Goldenberg G, Wohlschläger AM, Castrop F, Stahl R, Röttinger M, Erhard P, Haslinger B, Ceballos-Baumann AO, Conrad B, Boecker H. Left inferior parietal dominance in gesture imitation: an fMRI study. Neuropsychologia. 2005;43:1086–1098. doi: 10.1016/j.neuropsychologia.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- Ohbayashi M, Ohki K, Miyashita Y. Conversion of working memory to motor sequence in the monkey premotor cortex. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orrell AJ, Eves FF, Masters RS, MacMahon KM. Implicit sequence learning processes after unilateral stroke. Neuropsychol Rehabil. 2007;17:335–354. doi: 10.1080/09602010600832788. [DOI] [PubMed] [Google Scholar]
- Pohl PS, McDowd JM, Filion D, Richards LG, Stiers W. Implicit learning of a motor skill after mild and moderate stroke. Clin Rehabil. 2006;20:246–253. doi: 10.1191/0269215506cr916oa. [DOI] [PubMed] [Google Scholar]
- Poizner H, Mack L, Verfaellie M, Rothi LJ, Heilman KM. Three-dimensional computergraphic analysis of apraxia. Neural representations of learned movement. Brain. 1990;113:85–101. doi: 10.1093/brain/113.1.85. [DOI] [PubMed] [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage. 2010;53:171–180. doi: 10.1016/j.neuroimage.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scott Med J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- Reed J, Johnson P. Assessing implicit learning with indirect tests: determining what is learned about sequence structure. J Exp Psychol Learn Mem Cognit. 1994;20:585–594. [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Heilman KM. Acquisition and retention of gestures by apraxic patients. Brain Cogn. 1984;3:426–437. doi: 10.1016/0278-2626(84)90032-0. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Heilman KM. Introduction to limb apraxia. In: Rothi LJG, Heilman KM, editors. Apraxia: the neuropsychology of action. Hove, UK: Psychology Press; 1997. pp. 1–6. [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21:1224–1231. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Rösler F. Implicit and explicit learning of event sequences:evidence for distinct coding of perceptual and motor representations. Acta Psychol (Amst) 2000;104:45–67. doi: 10.1016/s0001-6918(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Schellig D. Block-tapping-test. Frankfurt, Germany: Swets Test Services; 1997. [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Stadler W, Schubotz RI, von Cramon DY, Springer A, Graf M, Prinz W. Predicting and memorizing observed action: differential premotor cortex involvement. Hum Brain Mapp. 2011;32:677–687. doi: 10.1002/hbm.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Rahbari NN, Hesse MD, Fink GR. Deficient sequencing of pantomimes in apraxia. Neurology. 2008;70:834–840. doi: 10.1212/01.wnl.0000297513.78593.dc. [DOI] [PubMed] [Google Scholar]
- Zirngibl C, Koch I. The impact of response mode on implicit and explicit sequence learning. Exp Psychol. 2002;49:153–162. doi: 10.1027//1618-3169.49.2.153. [DOI] [PubMed] [Google Scholar]