Abstract
Remyelination of the CNS involves the regeneration of mature oligodendrocytes by endogenous oligodendrocyte progenitor cells (OPCs). Previous studies have shown that bone morphogenic proteins (BMPs) inhibit the production of oligodendrocytes in the healthy CNS. However, there is currently no information on the influence of BMP signaling in vivo within demyelinated lesions of the brain or on subsequent remyelination. Here, we determine a role for BMP signaling in modulating oligodendrogliogenesis and remyelination in the brain following cuprizone-induced demyelination. We identified that BMP signaling is active in oligodendroglia and astrocytes within the demyelinated corpus callosum. Intraventricular infusion of BMP4 into the brains of mice during demyelination increased the proliferation of OPCs and, to a lesser extent, microglia and astrocytes in the corpus callosum. In contrast, infusion of Noggin, an extracellular antagonist of BMP4, increased the density of mature oligodendrocytes in the remyelinating corpus callosum. Additional evidence from myelin staining and electron microscopy indicates there is an increase in remyelinated axons in the corpus callosum of Noggin-infused mice. Thus, inhibition of endogenous BMP signaling during demyelination promotes mature oligodendrocyte regeneration and remyelination.
Introduction
In demyelinating diseases of the CNS, axons lose their myelin sheaths and consequently are vulnerable to degeneration. Apoptosis of oligodendrocytes, the cells that produce the myelin sheaths, is a key process in CNS demyelination (Barnett and Prineas, 2004). Remyelination is a natural regenerative mechanism whereby myelin sheaths are restored to the damaged axons, protecting them from further degeneration (Irvine and Blakemore, 2008). Studies using genetic lineage tracing in transgenic mice indicate that oligodendrocyte progenitor cells (OPCs) are an endogenous source of mature, myelinating oligodendrocytes in the healthy adult CNS (Rivers et al., 2008) and that in animal models of demyelination resident OPCs generate remyelinating oligodendrocytes within lesions (Zawadzka et al., 2010). In Multiple Sclerosis, OPCs are present in lesions, but often remain quiescent (Wolswijk, 1998; Kuhlmann et al., 2008). Therefore, enhancing OPC differentiation is a promising strategy to promote remyelination in lesions.
Bone morphogenic proteins (BMPs) are secreted proteins that form the largest subclass of the transforming growth factor-β superfamily of cytokines. BMP2 and BMP4, which comprise one subfamily of BMPs, bind to one of two type I signal-transducing receptors (BMPRIa and BMPRIb) and one type II ligand binding receptor (BMPRII) (Massagué, 1998). Phosphorylation of receptor-regulated SMAD1/5/8 is increased upon BMP ligand/receptor formation and is indicative of BMP pathway activation (Massagué, 1998). OPCs and oligodendrocytes derived from the CNS express BMP4 and all three BMP receptors (Kondo and Raff, 2004). Exogenous BMP4 inhibits OPC differentiation and promotes astroglial differentiation in vitro (Grinspan et al., 2000). Conversely, Noggin, an extracellular antagonist of BMP4 (Massagué, 1998), blocks the production of astrocytes by BMP4 when applied to OPC cultures (Sim et al., 2006). These studies suggest an inhibitory role for BMPs in OPC differentiation.
There is evidence that BMPs are regulated during demyelination. Within demyelinated lesions induced by ethidium bromide in the brain, BMP4 expression is increased in OPCs and is absent upon remyelination (Zhao et al., 2005). In an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis, BMPs were upregulated in the demyelinated spinal cord, with BMP4 expression being most abundant and increasing with enhanced clinical disease severity (Ara et al., 2008). The cellular sources of BMP4 included macrophages, astrocytes and oligodendrocytes within the lesions (Ara et al., 2008). While these studies suggest a possible role for BMPs within demyelinating lesions, the function of BMP signaling in demyelination and remyelination was not examined.
In this study, we report the effects of modulating BMP signaling upon adult OPCs and astrocytes during demyelination and remyelination in vivo. We show that BMP signaling is active in OPCs and astrocytes within demyelinated lesions in the corpus callosum (CC). Infusion of BMP4 increased the proliferation of glial cells, predominantly OPCs, during demyelination. We further show that infusion of Noggin increased the numbers of mature oligodendrocytes and enhanced remyelination in the CC following cuprizone-induced demyelination.
Materials and Methods
Adult female C57BL/6 mice were used for all experiments according to guidelines previously described (Cate et al., 2010). Cuprizone-mediated demyelination was induced as previously described (Cate et al., 2010). For remyelination studies, mice were returned to normal chow for 1 week following cuprizone challenge. Recombinant human BMP4 or mouse Noggin (R&D Systems) dissolved in artificial CSF (aCSF) at a dose of 400 ng per day or aCSF was delivered into the lateral ventricle by mini-osmotic pumps (ALZET, Durect Corporation) (model 1002, 14 d infusion, 0.25 μl/h flow rate; model 1007, 5 and 7 d infusions, 0.50 μl/h flow rate) using Brain Infusion Kit III as previously described (Cate et al., 2010). Mice received 1 mg/ml 5-bromodeoxyuridine (BrdU) (Sigma-Aldrich) in their drinking water for 3 d during the infusion as indicated in the experimental timelines (Fig. 1C).
Figure 1.
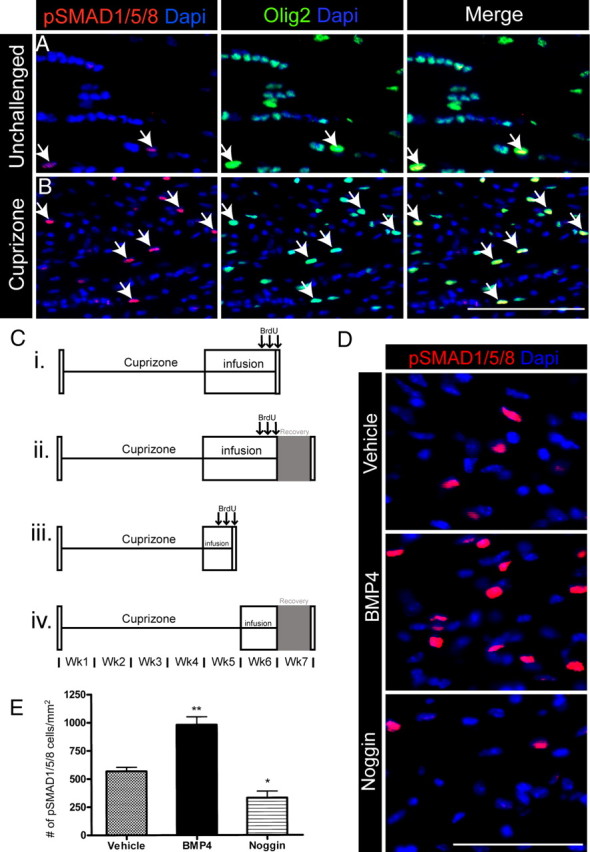
BMP signaling is active in oligodendroglial cells within demyelinating lesions. A, B, pSMAD1/5/8 and Olig2 immunoreactivity in unchallenged and cuprizone-challenged mice. Merged images reveal colabeling of pSMAD1/5/8 and Olig2 within a subset of cells in the CC of unchallenged and 6 week cuprizone-challenged mice. Arrows indicate double-positive cells. C, Timelines (i–iv) for infusion experiments detailed in Results. D, Immunostaining of pSMAD1/5/8 in the CC of 14 d infused mice after 6 weeks of cuprizone challenge. E, Quantification of the density of pSMAD1/5/8+ cells in the CC of 14 d infused mice after 6 weeks of cuprizone challenge. n = 4–6 animals per group. *p < 0.05,**p < 0.01. Scale bars: B, D, 50 μm.
Brain tissue was collected, cryosectioned, and immunoprobed as previously described (Cate et al., 2010) with the following additional antibodies: BrdU (rat; AbD Serotec; 1:40), cleaved caspase-3 (rabbit; Cell Signaling Technology; 1:200), platelet-derived growth factor α receptor (PDGFRα) (rat; eBioscience; 1:500), oligodendrocyte (clone RIP) (mouse; Millipore; 1:10,000), and ionized calcium-binding adapter molecule 1 (IBA1) (rabbit; Wako Pure Chemicals; 1:1000), or prepared for electron microscopy using standard protocols. For fluorescent myelin staining, sections were incubated with FluoroMyelin (1:200; Invitrogen) for 1 h at room temperature.
All cell counts and area analyses were performed blind to experimental treatment. Immunopositive cells and area measures were quantified as previously described (Cate et al., 2010) and data are expressed as mean value/mm2 ± SEM. Images (20×) were taken from three to six sections 50 μm apart for each animal at locations between −0.46 mm and −1.22 mm bregma with the midline CC at the epicenter and lateral borders determined by the image width. Myelinated axons quantification was performed on four 3000× images per animal. Diameter measures of G ratios (the ratio of axon diameter to the axon plus myelin sheath diameter) were calculated using ImageJ software for at least 400 fibers per animal. For FluoroMyelin intensity analysis, images were converted to grayscale and the mean gray value of the midline CC was divided by the mean gray value of the dorsal fornix.
Statistical tests were performed using GraphPad Prism. One-way ANOVA with Dunnett's multiple-comparison test was used for comparison between groups in 14 d infusion experiments, two-way ANOVA with Bonferroni's post hoc test was used for distribution of G ratios, and Student's unpaired t test was used for all other comparisons.
Results
Demyelination within the corpus callosum influences BMP signaling
We first investigated whether BMP signaling is increased in the CC during demyelination. To undertake this analysis, we used the cuprizone-induced demyelination model. We first confirmed that 6 weeks of cuprizone challenge induced both demyelination within the CC (data not shown) and a significant decrease in the number of Olig2+ oligodendroglia in the CC (unchallenged 1962 ± 111.3/mm2; cuprizone 685 ± 81.4/mm2; p < 0.0001). In addition to oligodendrocyte loss, there was recruitment of astrocytes and microglia/macrophages in the CC (data not shown).
After 6 weeks of cuprizone challenge, there was enhanced BMP signaling evidenced by an increased number of pSMAD1/5/8+ cells (unchallenged, 109 ± 9.28/mm2; cuprizone, 505 ± 83.0/mm2; p < 0.01) (Fig. 1A,B). The majority of pSMAD1/5/8+ cells colabeled with Olig2 in unchallenged (91 ± 4.2%) and cuprizone-challenged mice (54 ± 3.1%) with a significant increase in pSMAD1/5/8+Olig2+ cells in cuprizone-challenged mice (unchallenged 97 ± 9.8/mm2; cuprizone 257 ± 42.5/mm2; p < 0.05). In addition, some pSMAD1/5/8+ cells expressed GFAP in unchallenged (4.4 ± 1.6%) and cuprizone animals (21 ± 6.6%). We found no GFAP+ cells that expressed Olig2. To determine the substrate for future infusion experiments, pSMAD1/5/8+ cells were assessed at 4 weeks of cuprizone challenge in the CC and the subventricular zone (SVZ), and similar numbers of positive cells were present in the two locations (data not shown). Thus, BMP signaling is increased in cuprizone-induced demyelinated lesions and is active in the majority of oligodendroglial cells and in a subset of astrocytes.
Modulation of BMP signaling during cuprizone challenge alters glial cell numbers
To determine the consequences of BMP signaling modulation in the demyelinated CC, we implanted mini-osmotic pumps to deliver BMP4, Noggin or vehicle into the lateral ventricles for the final 14 d of a 6 week cuprizone challenge (Fig. 1Ci). Initially, we established that BMP4 infusion increased and Noggin decreased the number of pSMAD1/5/8+ cells in the CC compared with vehicle infusion (Fig. 1D,E). We also found that BMP4 infusion increased the density of pSMAD1/5/8+Olig2+ cells (p < 0.05) (vehicle 169 ± 36.0/mm2; BMP4 334 ± 42.3/mm2; Noggin 95 ± 54/mm2).
We next examined the consequences of BMP4 and Noggin infusion on the proliferation of oligodendroglial cells during cuprizone challenge. When mice were assessed after 6 weeks of cuprizone challenge (Fig. 1Ci), there was a significant increase in the density of both BrdU+ and Olig2+ cells in the CC after BMP4 infusion (BrdU+: vehicle 351 ± 77.5/mm2; BMP4 856 ± 123/mm2; p < 0.01; Olig2+: vehicle 446 ± 35.0/mm2; BMP4 858 ± 101/mm2; p < 0.01). Noggin infusion did not alter the density of BrdU+ or Olig2+ cells. In addition, BMP4-infused mice had increased BrdU+Olig2+ double-labeled cells compared with vehicle-infused mice, while there was no difference in Noggin-infused mice (Fig. 2A,E). There was no difference in the density of GFAP+ cells in the CC among the three groups of mice (Fig. 2C,F).
Figure 2.
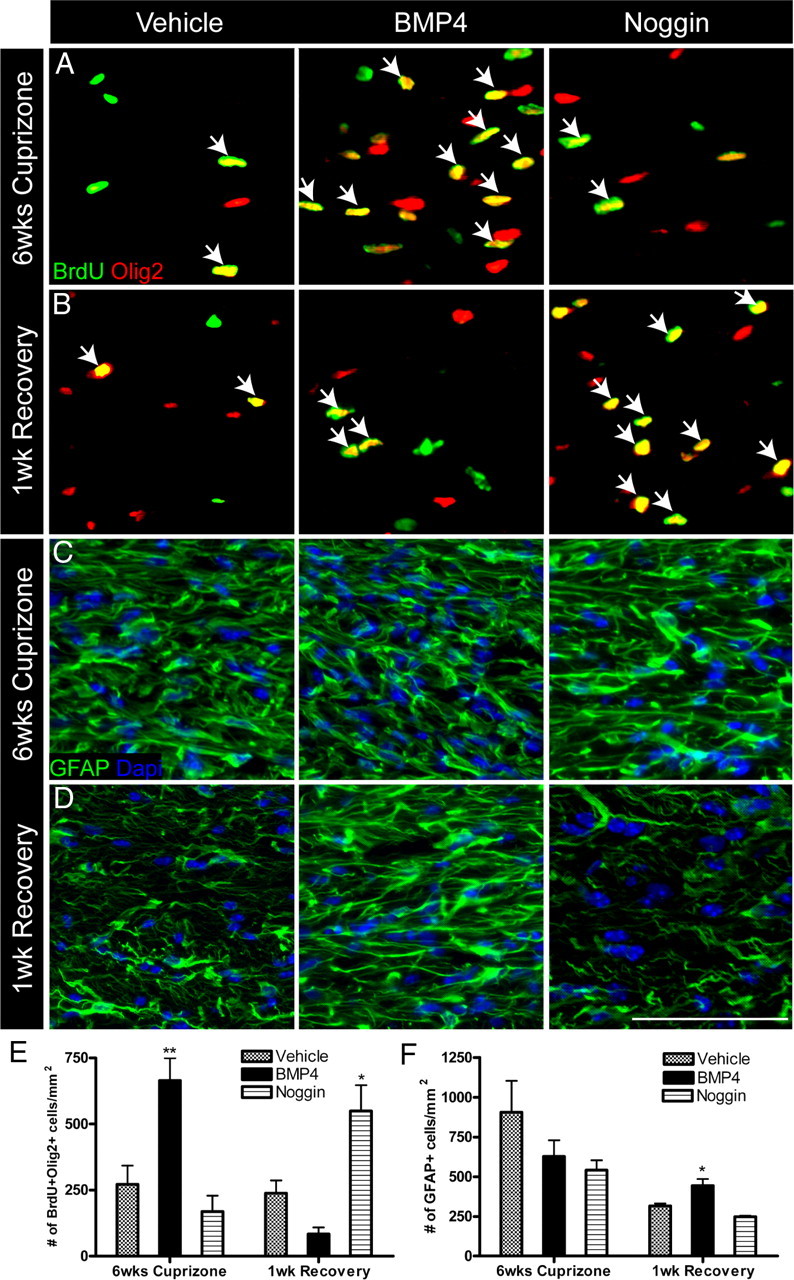
Modulation of BMP signaling during demyelination alters numbers of proliferating oligodendroglia and astrocytes. A–D, Immunostaining of BrdU-Olig2 and GFAP in the CC of 14 d infused mice after 6 weeks of cuprizone challenge and 1 week of recovery. Arrows indicate double-positive cells. E, F, Quantification of the density of BrdU+Olig2+ cells and GFAP+ cells in the CC of 14 d infused mice after 6 weeks of cuprizone and 1 week of recovery. n = 4–6 animals per group. *p < 0.05,**p < 0.01. Scale bar, 50 μm.
To assess the consequences of BMP4 and Noggin infusion on recovery, we allowed an independent cohort of mice to recover for 1 week following a 6 week cuprizone challenge (Fig. 1Cii). We identified a significant increase in the density of BrdU+ and Olig2+ cells in the Noggin-infused mice compared with vehicle (BrdU+: vehicle 430 ± 55.9/mm2; Noggin 741 ± 83.9/mm2; p < 0.05; Olig2+: vehicle 632 ± 147/mm2; Noggin 1081 ± 30.79/mm2; p < 0.05). There was no significant difference in the density of BrdU+ and Olig2+ cells between BMP4 and vehicle. However, we found an increase in the density of BrdU+Olig2+ double-labeled cells in the Noggin-infused mice (Fig. 2B,E). To determine whether the reduction of BrdU+Olig2+ double-labeled cells (between assessments at 6 weeks of cuprizone and 1 week recovery) in the BMP4-infused mice was due to migration of these cells from the midline to the lateral CC, we extended the analysis to the lateral CC at 1 week recovery. However, we found no difference in the density of BrdU+Olig2+ double-labeled cells in the lateral CC (vehicle 169 ± 42.8/mm2; BMP4 99 ± 24/mm2; Noggin 244 ± 21.7/mm2; p = 0.12). When we examined astrocytes at 1 week of recovery, we found an increase in the density of GFAP+ cells in the BMP4-infused mice within the CC, while there was no change in the Noggin-infused mice (Fig. 2D,F). Together, these data indicate that BMP4 infusion during demyelination increases OPC numbers, however, following recovery from cuprizone challenge, these BrdU-positive cells are decreased and astrocyte numbers are increased. The data suggest that Noggin infusion during demyelination results in an increase in oligodendroglia during the recovery phase.
Short-term infusion of BMP4 during demyelination increases proliferation of glial cells
Given the increased proliferation following 2 weeks of BMP4 infusion, we investigated whether short-term BMP4 infusion would have similar consequences. To expedite this, mini-osmotic pumps were implanted after 4 weeks of cuprizone challenge, and mice were examined after a 5 d infusion with continued cuprizone challenge (Fig. 1Ciii). Short-term infusion of BMP4 increased BMP signaling as determined by pSMAD1/5/8 immunostaining (vehicle 323 ± 42.9/mm2; BMP4 563 ± 70.7/mm2; p < 0.05) and increased the density of pSMAD1/5/8+Olig2+ cells (vehicle 128 ± 21.8/mm2; BMP4 326 ± 56.2/mm2; p < 0.05). Consistent with the 14 d BMP4 infusion, we found a significant increase in the density of BrdU+Olig2+ double-labeled cells compared with vehicle infusion (Fig. 3A,D). Characterization of these Olig2 cells revealed they were of the oligodendroglial lineage and all either expressed CC1, a mature oligodendrocyte marker, or PDGFRα, a marker for OPCs. There was a significant increase in Olig2+PDGFRα+ cells in BMP4-infused mice (vehicle 97 ± 8.9/mm2; BMP4 309 ± 74.3/mm2; p < 0.05).
Figure 3.
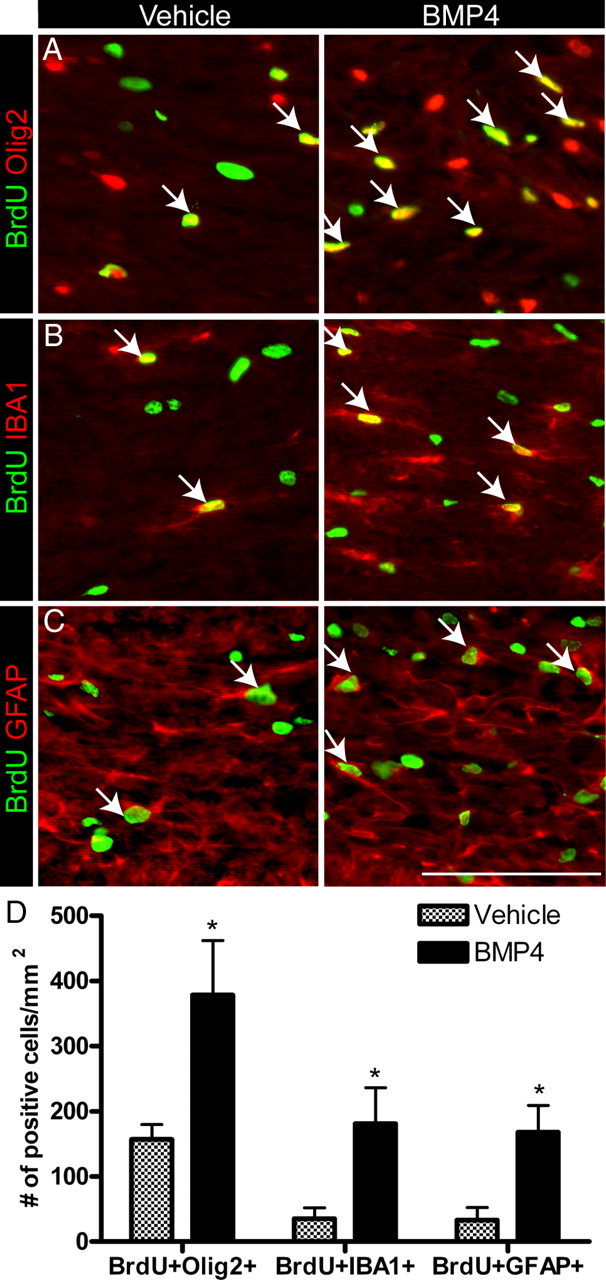
Short-term infusion of BMP4 during demyelination increases the proliferation of OPCs, microglia and astrocytes. A–D. Immunostaining and quantification of the density of BrdU+Olig2+, BrdU+IBA1+, and BrdU+GFAP+ cells in the CC after 5 d of either vehicle or BMP4 infusion. Arrows indicate double-positive cells. n = 4–5 animals per group. *p < 0.05. Scale bar, 50 μm.
Following short-term BMP4 infusion, the Olig2+ cells represented 54 ± 7.9% of the BrdU+ population, in contrast to 14 d BMP4 infusion where 80 ± 7.3% of BrdU+ cells were Olig2+. Therefore, we examined the proliferation of astroglia and microglia in the CC of short-term infused animals. We found a significant increase in the density of BrdU+IBA1+ and BrdU+GFAP+ cells (Fig. 3B–D). BMP4 infusion significantly increased the density of IBA1+ microglia/macrophages (vehicle 1278 ± 175.3/mm2; BMP4 2762 ± 204.9/mm2; p < 0.01), while the density of GFAP+ cells was unchanged (vehicle 254 ± 15.5/mm2; BMP4 313 ± 45.5/mm2; p=0.26). Thus, short-term BMP4 infusion increased the proliferation of glial cells during cuprizone-induced demyelination.
In addition, we performed caspase-3 immunostaining in the brains of mice subjected to short-term infusion. We found that there was a strong trend toward an increase in caspase-3+ cells in the CC of BMP4-infused mice (p = 0.09) (vehicle 3.6 ± 0.8/mm2; BMP4 29 ± 11/mm2; Noggin 8.7 ± 2.9/mm2). These data would suggest that exogenously administered BMP4 is inducing apoptosis in this model.
Noggin infusion during demyelination results in an increase in the number of mature oligodendrocytes in the corpus callosum
Given that exogenous delivery of Noggin for 14 d resulted in an increase in the density of Olig2+ cells at 1 week of recovery, we next examined whether there was an increase in mature oligodendrocytes in these mice. We found that Noggin infusion significantly increased the density of mature oligodendrocytes that expressed both Olig2 and CC1 at 1 week of recovery (Fig. 4A,B). We also evaluated the numbers of cells with BrdU incorporation that differentiated into oligodendrocytes. BrdU+CC1+ cells were significantly increased in Noggin-infused mice compared with vehicle (p < 0.05) (vehicle 69 ± 23/mm2; Noggin 162 ± 17.1/mm2; BMP4 9 ± 5/mm2). Thus, Noggin infusion during demyelination results in an increase in the number of mature oligodendrocytes during recovery from cuprizone challenge.
Figure 4.
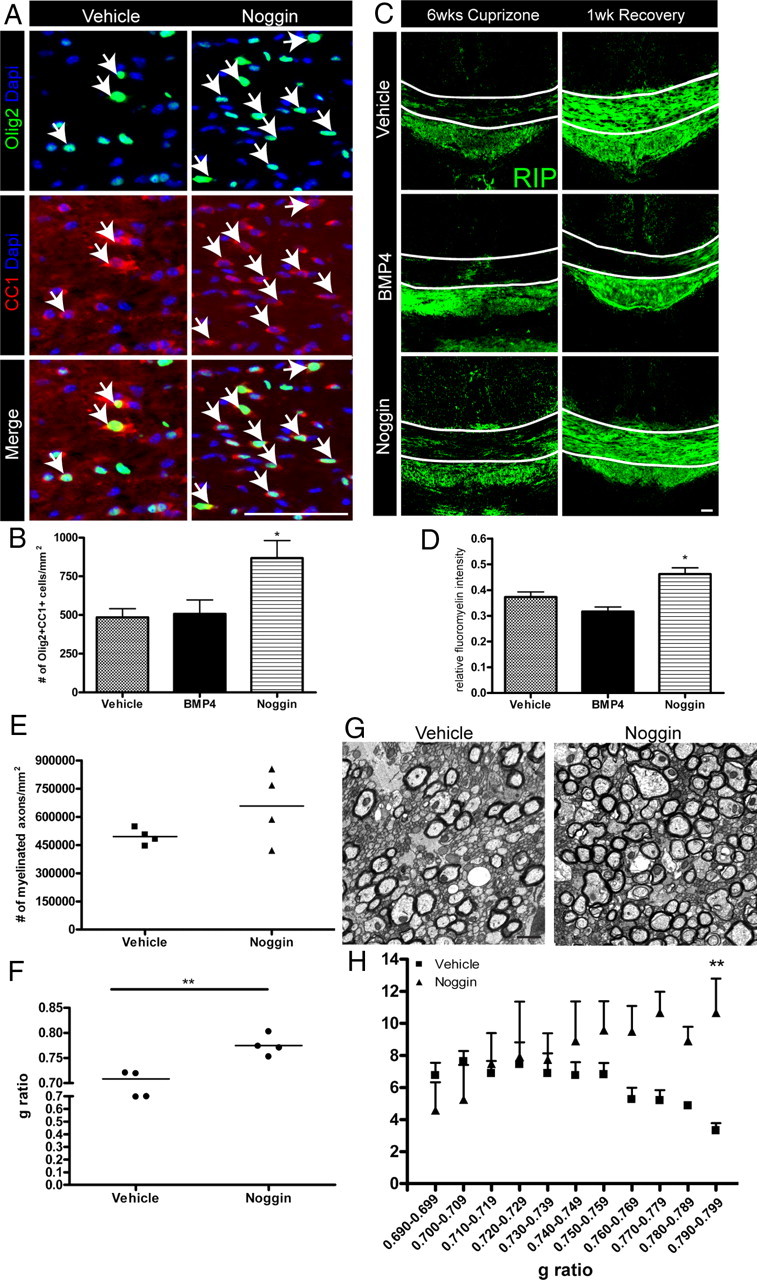
Noggin-infused mice have increased numbers of mature oligodendrocytes and remyelinated axons following recovery from cuprizone-induced demyelination. A, B, Olig2-CC1 immunostaining and quantification in the CC of 14 d infused mice after 1 week of recovery. Arrows indicate double-positive cells. C, Representative images of RIP immunostaining in the CC of 14 d infused mice. Lines indicate dorsal and ventral borders of the CC. D, Quantification of the mean relative FluoroMyelin intensity in the CC of 14 d infused mice after 1 week of recovery. E–H, Ultrastructural analysis of the CC of 7 d vehicle- and Noggin-infused mice after 1 week of recovery. Quantification of the density (E) and average G ratio (F) of myelinated axons. G, Representative electron micrographs of the caudal CC. H, Number of axons per G ratio value. n = 3–4 animals per group. *p < 0.05;**p < 0.01. Scale bar: (in A) A, C, 50 μm; G, 1 μm.
Noggin infusion during demyelination enhances remyelination in the corpus callosum
For analysis of myelination, we first performed immunostaining with the RIP antibody, which labels the myelin protein, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) (Watanabe et al., 2006). At 6 weeks of cuprizone, the three groups of 14 d infused mice displayed similar levels of RIP immunostaining (Fig. 4C). To further analyze myelination within the CC, we quantified FluoroMyelin intensity, which revealed no significant difference in the infused mice (vehicle: 0.31 ± 0.05; BMP4: 0.36 ± 0.01; Noggin: 0.34 ± 0.02; p = 0.56). In contrast, after 1 week of recovery, we found that the intensity of RIP immunostaining was greater in vehicle- and Noggin-infused animals than in BMP4-infused mice (Fig. 4C). On the other hand, at the 1 week recovery time point, FluoroMyelin intensity was significantly greater among Noggin-infused as opposed to vehicle-infused mice (Fig. 4D).
We further interrogated the effects of Noggin on remyelination by performing an additional infusion experiment with vehicle or Noggin infusion for the final 7 d of a 6 week cuprizone challenge followed by 1 week of recovery (Fig. 1Civ). There was an increased number of Olig2+CC1+ cells in Noggin-infused mice (vehicle 584 ± 99.7/mm2; Noggin 1066 ± 83.00/mm2; p < 0.05), which is consistent with the 14 d infusion experiment. We next examined the ultrastructure of the caudal CC by electron microscopy and found an increase in the density of myelinated axons in the Noggin-infused mice (Fig. 4E,G). In addition, the average G ratio of the myelinated axons was increased in the Noggin-infused mice compared with vehicle (Fig. 4F), suggesting an increase in thinly myelinated axons. We also assessed the distribution of G ratios among the myelinated axons of the vehicle-infused mice and the three Noggin-infused mice that had increased numbers of myelinated axons. There was a similar number of axons with low G ratios from vehicle- and Noggin-infused mice; however, in Noggin-infused mice, there was an increase in the number of axons with high G ratios, which are indicative of thinner myelin sheaths, a feature of remyelinated axons (Fig. 4H). In conclusion, these results suggest that Noggin infusion enhances remyelination following cuprizone-induced demyelination.
Discussion
In this study, we identified that BMP signaling is active in OPCs and astrocytes within the demyelinated CC following cuprizone challenge. We found that BMP4 infusion increased the numbers of OPCs during demyelination. However, following recovery from cuprizone challenge, oligodendroglia are decreased and astrocyte numbers are increased in BMP4-infused mice. Infusion of the BMP4 antagonist, Noggin, increased the number of mature oligodendrocytes and remyelination following recovery. Collectively, these results suggest that downregulating endogenous BMP signaling during myelin injury could have a beneficial influence on repair given that Noggin infusion increased numbers of mature oligodendrocytes and enhanced remyelination.
There is evidence for a dichotomous role of BMPs in affecting cell proliferation. Prior in vitro studies have reported that BMPs decrease the proliferation of embryonic and adult neural precursor cells (for review, see Sabo et al., 2009). In contrast, BMPs promote the proliferation of rat primary osteoblastic cells (Selvamurugan et al., 2007). In addition, recent work from our laboratory has shown that exogenous BMP4 increased the proliferation of adult SVZ neural precursor cells under differentiative conditions in vitro (Cate et al., 2010). We show here for the first time that BMP4 infusion increased the proliferation of OPCs and, to a lesser extent, microglia and astrocytes in the demyelinated CC in vivo. These data would suggest that exogenous BMP signaling could play a favorable role during demyelination since BMP4 infusion increased the pool of OPCs within demyelinated lesions.
Interestingly, however, we found a decrease in oligodendroglia numbers by 1 week of recovery in BMP4-infused mice. A key question that emerges therefore is the fate of the BrdU+Olig2+ cells in the BMP4-infused mice between 6 weeks of cuprizone challenge and 1 week of recovery. At 1 week of recovery, we observed a reduction in BrdU+ cells and we found no BrdU+ cells that expressed GFAP, suggesting that the OPCs did not differentiate into astrocytes. Furthermore, our analysis of the lateral CC, demonstrating no change in the density of OPCs, suggests that the OPCs did not migrate from the midline CC. Rather, our data showing an increase in the density of caspase-3+ cells suggests that delivery of BMP4 promoted apoptosis in vivo, which could contribute to the lack of OPCs following recovery from cuprizone challenge.
Our results support a role for Noggin in promoting remyelination after central demyelination. Noggin expanded the population of oligodendroglia during recovery from cuprizone-induced demyelination. In our experimental paradigm, BrdU was administered at the end of cuprizone challenge and, at 1 week of recovery, we observed an increase in the number of BrdU+ cells that expressed Olig2 and CC1 in the CC of Noggin-infused compared with vehicle-infused mice. Given that cuprizone challenge increased the number of pSMAD1/5/8+ cells in the CC, and that this population (present in the CC at the 4 week time point) is decreased with subsequent Noggin infusion, our study suggests that Noggin acts on cells resident in the CC. Thus, our data are consistent with there being enhanced differentiation of resident OPCs in Noggin-infused mice. Jablonska et al. (2010) found that infusion of Chordin, a BMP antagonist with slightly lower affinity for BMP4 than Noggin (Piccolo et al., 1996), induced the differentiation of DCX-expressing cells of the adult SVZ into oligodendrocytes in lysolecithin-demyelinated CC. Thus, the combined data suggest that both inhibitors of BMP signaling (i.e., Chordin and Noggin) can increase the production of oligodendrocytes in vivo in the context of demyelination and that infusion of these substances are likely to have composite effects within the SVZ and the demyelinated lesion.
Successful remyelination not only depends on OPC proliferation, but also requires OPC survival and differentiation into myelinating oligodendrocytes. At 6 weeks of cuprizone ingestion, there is endogenous OPC proliferation within the demyelinated CC, which we observed in our vehicle-infused mice. Upon withdrawal of cuprizone from the diet of mice, OPCs differentiate into myelinating oligodendrocytes and remyelination occurs (Mason et al., 2000). We found augmented remyelination following Noggin infusion that could be due to increased OPC survival or differentiation into mature oligodendrocytes. Similarly, Izrael et al. (2007) observed increased myelination in vivo when they transplanted Noggin-treated human embryonic stem cell-derived OPCs in myelin-deficient shiverer mice. While there are caveats to quantifying remyelination in the CC due to the small diameter of axons (Stidworthy et al., 2003), we provide evidence in Noggin-infused mice that there is an increase in the density of myelinated axons and a greater proportion with higher G ratios, which are indicative of thinner myelin sheaths, a feature of remyelinated axons. Therefore, our finding of an increase in thin myelinated axons in Noggin-infused mice could be attributed to an increase in the number of remyelinated axons compared with the vehicle-infused mice.
In summary, the results in our study indicate an important role for BMP signaling in modulating oligodendrogliogenesis and remyelination. We have shown that exogenous BMP signaling transiently increases the proliferation of glial cells that are rapidly cleared following recovery from a demyelinating insult. Furthermore, inhibiting endogenous BMP signaling during demyelination promoted mature oligodendrocyte regeneration and myelin repair. Given that BMPs are expressed in demyelinating lesions in humans (Deininger et al., 1995), our findings identify a potential therapeutic target to enhance repair from demyelinating disease.
Footnotes
The National Health and Medical Research Council of Australia and Holloway Family Trust supported this work. A Multiple Sclerosis Research Australia Postgraduate Scholarship supported J.K.S. The University of Melbourne, William Collie Fellowship supported H.S.C. Anna Friedhuber provided technical assistance with electron microscopy. Charityworks for MS and the Victorian Operational Infrastructure Support Program provided additional support.
The authors declare no competing financial interests.
References
- Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Cate HS, Sabo JK, Merlo D, Kemper D, Aumann TD, Robinson J, Merson TD, Emery B, Perreau VM, Kilpatrick TJ. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- Deininger M, Meyermann R, Schluesener H. Detection of two transforming growth factor-beta-related morphogens, bone morphogenetic proteins-4 and -5, in RNA of multiple sclerosis and Creutzfeldt-Jakob disease lesions. Acta Neuropathol. 1995;90:76–79. doi: 10.1007/BF00294462. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004;267:242–251. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000;61:251–262. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo JK, Kilpatrick TJ, Cate HS. Effects of bone morphogenic proteins on neural precursor cells and regulation during central nervous system injury. Neurosignals. 2009;17:255–264. doi: 10.1159/000231892. [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Vasilov A, Jefcoat SC, Partridge NC. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25:1213–1220. doi: 10.1002/jor.20409. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Waldau B, Roy NS, Schwartz TE, Pilcher WH, Chandross KJ, Natesan S, Merrill JE, Goldman SA. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 2003;13:329–339. doi: 10.1111/j.1750-3639.2003.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakurai Y, Ichinose T, Aikawa Y, Kotani M, Itoh K. Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res. 2006;84:525–533. doi: 10.1002/jnr.20950. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Fancy SP, Magy L, Urwin JE, Franklin RJ. Stem cells, progenitors and myelin repair. J Anat. 2005;207:251–258. doi: 10.1111/j.1469-7580.2005.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


