Abstract
Encoding of real-life episodic memory commonly involves integration of information as the episode unfolds. Offline processing immediately following event offset is expected to play a role in encoding the episode into memory. In this study, we examined whether distinct human brain activity time-locked to the offset of short narrative audiovisual episodes could predict subsequent memory for the gist of the episodes. We found that a set of brain regions, most prominently the bilateral hippocampus and the bilateral caudate nucleus, exhibit memory-predictive activity time-locked to the stimulus offset. We propose that offline activity in these regions reflects registration to memory of integrated episodes.
Introduction
In episodic encoding, an experienced event is transformed into a memory trace that has the potential to be consciously recollected (Tulving, 1983). The brain substrates of this process can be revealed by examining the correlation between brain activity during encoding and subsequent retrieval success (Paller et al., 1987; Brewer et al., 1998; Wagner et al., 1998; Paller and Wagner, 2002; Spaniol et al., 2009). In theory, encoding of real-life events that unfold over time as cohesive episodes requires integration of information and is likely to engage offline processes as well. Previous studies have distinguished between prestimulus and intrastimulus processes in episodic encoding (Fernández et al., 1999; Otten et al., 2006; Turk-Browne et al., 2006; Park and Rugg, 2010). However, thus far no distinction has been reported between online, intrastimulus encoding processes (initiated while perceiving the event) and offline, immediate poststimulus processes (time-locked to the offset of the episode), a distinction that may be crucial to understanding the neural mechanisms of episodic encoding. Although there is not yet direct evidence linking such offline neural processes with episodic encoding in humans, findings from studies of retroactive interference (Dudai, 2004; Wixted, 2004) and working memory (WM) (Ranganath and D'Esposito, 2001; Schon et al., 2004; Nichols et al., 2006; Olsen et al., 2009) suggest such a link.
To date, the types of stimuli predominantly used in functional neuroimaging studies of encoding rendered it difficult to identify putative higher order offline processes required for encoding of real-life events. The reason for this is that most studies of episodic memory use brief, discrete, static stimuli that are quite different from the complex experiences of real life (Nyberg et al., 2002; Cabeza et al., 2004; McDermott et al., 2009) and hinder the differentiation of online and offline processes. Dynamic, real-life-like stimuli may thus be more amenable for studying offline encoding processes. Recent attempts have been made to simulate real-life episodes in laboratory settings, using movies as memoranda (Furman et al., 2007; Hasson et al., 2008). However, when using continuous stimuli, it is difficult to separate blood oxygenation level-dependent (BOLD) activity related to distinct movie episodes, as brain activity related to each event may be masked by brain activity related to the following event.
To overcome this difficulty and investigate online and offline encoding processes, we designed a functional magnetic resonance imaging (fMRI) subsequent memory paradigm using as memoranda brief scenes from narrative movies (Gelbard-Sagiv et al., 2008), intercalated with rest periods. Narrative audiovisual clips resemble real-life experiences much more than words or pictures, and at the same time they are separated into discrete units, enabling the differentiation of intrastimulus and poststimulus brain activity. This allowed us to identify brain regions exhibiting immediate postevent activation correlated with subsequent memory performance. We propose that this poststimulus activity reflects an offline encoding process whereby experiences are bound into cohesive representations and registered to memory. We discuss potential brain mechanisms that might operate in the aforementioned time window. Further analysis of this immediate offline process may contribute to our understanding of success and failure in encoding human episodic memory.
Materials and Methods
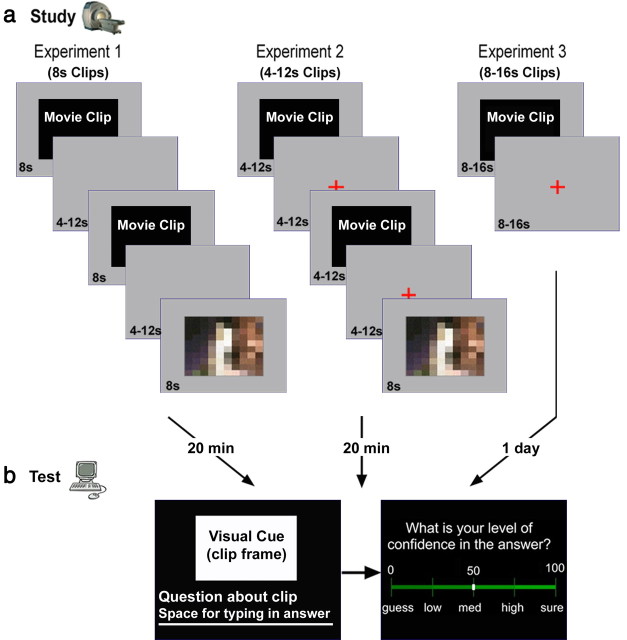
The study was divided into three separate experiments. Each experiment consisted of a Study phase, which took place in an fMRI scanner (Fig. 1a), and a subsequent Test phase outside the scanner (Fig. 1b). Experiment 1 served to identify brain regions exhibiting delayed encoding-related activation (i.e., a higher BOLD response to subsequently remembered vs forgotten clips, initiated at clip offset), using clips of a fixed length. To verify that delayed activity was indeed time-locked to clip offset rather than clip onset, we ran two additional experiments (experiments 2 and 3) with clips of varying lengths. The experimental protocols were approved by the Institutional Review Board of the Sourasky Medical Center, Tel-Aviv, and the Ministry of Health, Jerusalem.
Figure 1.
The experimental protocol. a, Study sessions of experiments 1–3. In all experiments, participants underwent brain fMRI scanning during the Study session. In experiments 1 and 2, participants viewed 160 Movie clips and 20 Scrambled clips, intermixed in random order. Movie clips were of fixed length (8 s) in experiment 1 and varying length (4–12 s) in experiment 2; all Scrambled clips were of fixed length (8 s). In experiment 3, participants viewed 112 Eventful clips of varying lengths (8 s/12 s/16 s) and 16 Uneventful clips, intermixed in random order. Before Study, participants were informed that they would subsequently be tested on the clips' gist. b, A Test session took place in a separate room, outside the scanner, 20 min (experiments 1 and 2) or 1 d (experiment 3) after Study. The Test session consisted of a computerized cued-recall test in Hebrew. One open-ended question was presented about each of the Movie clips, along with a still frame that served as a visual cue. Participants typed in their answers in the allotted space below the question. Following each question, participants estimated their degree of confidence in the previous answer, on a VAS ranging from 0 to 100.
Participants
Sixty-six right-handed Hebrew speakers, with normal or corrected-to-normal vision, participated in the experiments. Of these, 25 (10 males, mean age of 27.7 ± 3.3 years) took part in experiment 1, 20 (13 males, mean age of 27.5 ± 3.6 years) took part in experiment 2, and 21 (12 males, mean age of 26.3 ± 2.8 years) took part in experiment 3. Participants were recruited from the Weizmann Institute of Science and from the Hebrew University Faculty of Agriculture in Rehovot. Informed consent was obtained from all participants.
Stimuli
Experiment 1.
The goal of experiment 1 was to identify brain regions with a higher BOLD response to remembered versus forgotten stimuli, at stimulus offset. The stimuli used in experiment 1 consisted of 180 short (8 s) audiovisual clips. Of these clips, 160 were narrative movie clips (Movie), and 20 were visually scrambled clips (Scrambled) that were accompanied by nondistinctive background noises. The Movie clips were all edited from realistic movies in Hebrew, containing no subtitles. The clips were taken from video streaming websites and were unfamiliar to the participants. All clips (Movie and Scrambled) were of visual angle 7.5° × 5.7° and were presented on a gray background.
Experiment 2.
After identification of regions exhibiting delayed encoding-related activation, experiment 2 served to determine whether the response onset in these regions depended on stimulus length. The stimuli used in experiment 2 were based on the stimuli of experiment 1. The Movie clips of experiment 1 were divided into five groups (G4, G6, G8, G10, G12), each containing 32 clips, counterbalanced for their elicited memory performance in experiment 1 (such that the mean memorability was equal across groups). The clips in each group were then re-edited to be of new lengths (4 s/6 s/8 s/10 s/12 s) such that all clips in a given group were of identical length (e.g., all clips in G4 were of length 4 s). In the process of re-editing, the informational content of the clips was kept as similar as possible to the original Movie clips of experiment 1. This was achieved mainly by editing periods of time in the clips with little to no occurrence (shortening or lengthening pauses and removing or adding sentences that added little new information). Thus, the stimuli of experiment 2 consisted of 160 different length Movie clips, in addition to the 20 Scrambled clips used in experiment 1 (all of length 8 s).
Experiment 3.
The purpose of experiment 3 was to examine whether the activation of the regions defined in experiment 1 was time-locked to stimulus offset. In this experiment, a new set of clips was used (edited from the same pool of original movies). The stimuli consisted of 128 clips of lengths 8 s, 12 s, and 16 s. Of these clips, 96 had two versions—an 8 s version and a 12 s version, edited such that the first 8 s were identical in both versions. In addition, there were 32 single-version clips (unrelated to the clip pairs), consisting of sixteen 16 s clips and sixteen 8 s uneventful clips (Uneventful). There were no scrambled clips in this experiment, but rather Uneventful clips whose entire gist could be perceived in the first frame (these clips were not included in the subsequent test). Each participant viewed 32 of the clip pairs in the 8 s version, 64 in the 12 s version, and all 16 s and Uneventful clips.
Experimental protocol
All experiments consisted of a Study session and a Test session. During the Study session of each experiment (Fig. 1a), which took place in an fMRI scanner, participants were presented with randomly ordered audiovisual clips. Each clip was followed by a rest period during which a gray screen was presented (4–12 s in experiments 1 and 2, with a mean duration of 6.30 s in experiment 1 and 5.97 s in experiment 2; 8–16 s in experiment 3, with a mean duration of 10.75 s). In experiments 2 and 3, during the rest periods a red fixation cross was situated in the middle of the gray screen; in each experiment, the mean duration of rest periods following different clip types was the same. Before clip presentation, a 16 s audiovisual sequence was presented to allow accommodation to the fMRI environment. Each Study session was divided into two scanning runs. In experiment 3, a simple task was presented twice in the course of each run, during which participants were requested to respond with a button press to all presented squares aside from red squares. The sole purpose of this task was to ensure the alertness of the participants. All stimuli were presented (Presentation software V.13.0, Neurobehavioral Systems) on a screen situated behind the participants' head and the corresponding audio was presented through MRI-compatible headphones (MR Confon). Before the Study session, participants were instructed to view the clips attentively (yet not to engage in active rehearsal) as there would be a subsequent test on the main occurrence in the clips (i.e., their gist). They were informed beforehand about the format of the subsequent Test session. In a postexperiment questionnaire, participants were requested to report whether they engaged in conscious rehearsal during the presentation of the blank screens [experiment 1: 18 participants reported that on the whole they did not engage in active rehearsal of the clips (No-Rehearsal), five participants reported some degree of rehearsal (Rehearsal), and data were not obtained for two participants; experiment 2: 19 No-Rehearsal, one Rehearsal; experiment 3: 19 No-Rehearsal, two Rehearsal].
The Test session of each experiment took place 20 min (in experiments 1 and 2) or 1 d (in experiment 3) after the Study session, in a separate room, outside the scanner. The Test session consisted of a series of computerized questions concerning each of the Movie clips, one question per clip. All questions referred to the gist of the movies (i.e., requiring integration of information over the course of the clip), and not to small details (e.g., “What did the parents say to their son?” or “Why was the little girl upset?”). Each question was accompanied by a still frame from the clip to serve as a visual cue, and participants were instructed to type their answer in the allotted space below the question (the questions were all open-ended questions; participants were not provided with possible answers). In cases where it was not completely clear whether an answer was correct, the corresponding clip was removed from analysis. After the question was answered, a confidence level screen appeared, and the participants rated their degree of confidence in the previous answer using a visual analog scale (VAS) ranging from 0 to 100 (Fig. 1b). The questions, answers, and confidence level screens were all in Hebrew. Experiments 1 and 2 included additional memory tests (performed after the Test session; data not shown).
fMRI acquisition and data analysis
All imaging was performed at the Ascher Imaging Center, Weizmann Institute, on a 3T Trio Magnetom Siemens scanner. The functional images (T2*-weighted) were acquired using a gradient-echo EPI sequence (TR = 2000 ms, TE = 30 ms, flip angle = 75°, 36 slices with no gap, at 30° toward coronal from anterior commissure–posterior commissure (ACPC), slice thickness = 4 mm, voxel size = 3 × 3 × 4 mm, matrix = 80 × 80, FOV = 24 cm) in two separate scanning sessions. Following the functional scans, high-resolution (1 mm3) 3D anatomical images were acquired using a T1-weighted MP-RAGE pulse sequence (TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°). Both functional and anatomical scans covered the whole cerebrum. All data were preprocessed and analyzed using BrainVoyager QX 1.10 (Brain Innovation) in addition to in-house code (MATLAB 7.10 R2010a, The MathWorks). The first 15 volumes from the beginning of each scan were removed (including volumes acquired during the initial audiovisual sequence). Preprocessing of the images included removal of head-motion artifacts, correction of slice-scan timing, and high-pass frequency filtering. The functional and anatomical data were then spatially normalized to Talairach space (Talairach and Tournoux, 1988). The normalized functional data were spatially smoothed using a 6 mm FWHM Gaussian kernel. In all general linear model (GLM) analyses, regressors were added to account for head movements and baseline differences between scans.
Onset calculation method
Determining the onset of BOLD responses can often be problematic due to a large variation in signal among both individuals and brain regions (Handwerker et al., 2004). This is further complicated when attempting to determine the onset for a single stimulus due to extensive noise masking the BOLD signal. Given an estimated pattern of brain activity (represented as a boxcar function), the shape of the expected BOLD response is commonly estimated by convolving the canonical hemodynamic response function (HRF) with the boxcar model (Friston et al., 1994). Detecting the onset of activity leading to the observed BOLD response can be performed by running a reverse process (i.e., testing which boxcar function, when convolved with a canonical HRF, results in a model that best fits the observed BOLD response). We used the following method to calculate the optimal boxcar function:
We created a set of possible boxcar functions, each defined by two parameters, length and onset, designed to represent potential brain activity that could result in the observed BOLD response. Specifically, we created boxcar functions with lengths ranging from 1 TR to clip length and onsets ranging from TR = 0 (relative to clip start) to maximal clip duration plus 2 TRs, limiting the boxcar offset to maximal clip duration plus 2 TRs.
Each of the boxcar models defined above was convolved with the canonical HRF implemented in BrainVoyager (two-gamma function with time to response peak = 5 s, time to undershoot peak = 15 s, response undershoot ratio = 6, dispersion = 1) to create a set of convolved models.
For each convolved model, we created a GLM with two regressors (the model and a constant) to fit the model to the data.
The model with the minimal sum of the squared residual error (among all models with a positive regression coefficient) was chosen as the optimal model (the best fit for the data), and its onset was used as an estimate for the activation onset.
Statistical analysis
The experimental ROIs were defined by two contrasts [Remembered > Forgotten clips; R-Blank > F-Blank (blank screens following remembered and forgotten clips, respectively) in conjunction with F-Blank > baseline; see Results]. The conjunction analysis was performed by creating an intersection map of the two separate contrasts, yielding only voxels that were significant in both contrasts. This method of conjunction is conservative and constitutes a valid method for conjunction inference (Nichols et al., 2005). After defining the set of ROIs, all subsequent analyses performed on these regions were corrected for multiple comparisons (multiple ROIs) using the Holm–Bonferroni method. X ± Y represents mean ± SEM.
Sample stimulus
To demonstrate the type of stimuli used, below are a description and verbatim script of one audiovisual clip displayed in two versions (8 and 12 s long, from experiments 1 and 2, respectively), translated into English.
Experiment 1 version (8 s): A young man and woman are walking. She asks him, “So how was the flight?” He answers, “Uh … You know, the usual.” She says, “Come on, what do you mean ‘the usual’? Tell me.”
Experiment 2 version (12 s): A young man and woman are walking. After a few moments she asks him, “So how was the flight?” He answers, “Uh … You know, the usual.” She says, “Come on, what do you mean ‘the usual’? Tell me.” After a short pause, he says, “So. …”
Results
Memory performance
Participants in experiment 1 successfully recalled 42.7 ± 3.8% of the clips. In experiment 2, the overall percentage of remembered clips was 33.3 ± 3.3%, with better memory for longer clips (22.5 ± 2.9%, 28.1 ± 3.3%, 35.3 ± 4.3%, 35.3 ± 3.7%, and 45.3 ± 3.7% of the clips recalled, for clips of length 4 s, 6 s, 8 s, 10 s, and 12 s, respectively). A similar pattern was observed in experiment 3, where the mean percentage of remembered clips was 23.6 ± 3.5% (20.4 ± 3.4%, 23.4 ± 3.8%, and 30.6 ± 3.7% of 8 s, 12 s, and 16 s clips, respectively).
Experiment 1: memory performance is predicted by delayed activity in a set of brain regions
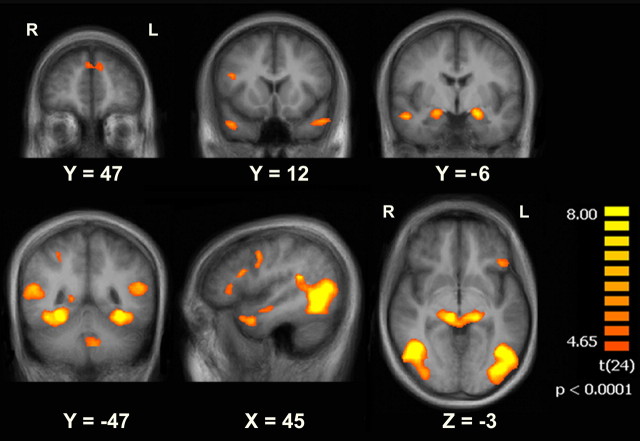
The purpose of experiment 1 was to identify ROIs exhibiting a difference-in-memory (DM) effect (i.e., higher activation for remembered vs forgotten stimuli) at stimulus offset; thus, we compared activity following the offset of remembered versus forgotten stimuli. Participants were presented with narrative Movie clips in addition to visually Scrambled clips (accompanied by nondistinctive background noises). The fMRI data acquired during the presentation of movie clips were divided into subsequently Remembered and Forgotten events. Similarly, fMRI data acquired during presentation of blank screens following the clips were divided into R-Blank, F-Blank, and S-Blank (blank screens following Remembered, Forgotten, and Scrambled clips, respectively) periods. We first examined the activity during the presentation of the clips, contrasting Remembered > Forgotten clips (p < 0.0001, uncorrected; minimal cluster size of five contiguous functional voxels) (Fig. 2) using a group-level random effects GLM. This contrast yielded multiple ROIs, including the right temporoparietal junction, the bilateral inferior frontal gyrus, and the bilateral fusiform gyrus, which have previously been associated with subsequent memory (Hasson et al., 2008; Spaniol et al., 2009; Kim, 2011) (Table 1). In concordance with the manner in which the ROIs were defined, the amplitude of the BOLD response was higher for Remembered versus Forgotten clips, with a rise in BOLD signal typically 2–4 s after clip onset, regardless of clip memorability (Fig. 3c).
Figure 2.
Brain regions showing intraclip activity correlated with subsequent memory. Regions showing significantly stronger BOLD activity for Remembered clips when compared with Forgotten clips (Early ROIs; p < 0.0001, uncorrected; minimal cluster size = five contiguous functional voxels; GLM with a random effects group analysis; n = 25). Data are shown on coronal, sagittal, and axial slices of the group-average brain.
Table 1.
Regions with increased brain activation during encoding of Remembered clips compared with Forgotten clips
| Lat | Region | BA | Peak x, y, z | Cluster sizea | t value |
|---|---|---|---|---|---|
| R | Temporoparietal junctionb | 22 | 52, −43, 16 | 3821 | 12.64 |
| R | Superior temporal sulcus | 21 | 48, −7, −14 | 743 | 7.35 |
| R | Inferior precentral sulcus | 6 | 45, −1, 40 | 672 | 6.57 |
| R | Inferior frontal sulcus | 46 | 42, 14, 22 | 518 | 6.68 |
| R | Inferior frontal gyrus | 45 | 42, 29, 7 | 405 | 6.23 |
| R | Temporal pole | 38 | 42, 5, −23 | 1246 | 6.9 |
| R | Fusiform gyrusb | 37 | 42, −55, −8 | 13,749 | 13.36 |
| R | Middle occipital gyrusb | 19 | 36, −79, 7 | 18,661 | 12.34 |
| R | Precuneus | 7 | 30, −49, −49 | 227 | 5.46 |
| R | Amygdala | NA | 18, −7, −11 | 933 | 6.91 |
| R | Anterior calcarine sulcus | 30 | 15, −49, 4 | 451 | 7.36 |
| R | Superior colliculus | NA | 6, −28, −5 | 3245 | 10.9 |
| R | Dorsomedial PFC | 9 | 3, 47, 37 | 73 | 5.49 |
| NA | Cerebellum | NA | −6, −46, −41 | 982 | 6.3 |
| L | Dorsomedial PFC | 9 | −9, 47, 34 | 191 | 5.24 |
| L | Superior colliculus | NA | −18, −28, −2 | 2808 | 9.5 |
| L | Amygdala | NA | −21, −7, −11 | 1496 | 8.28 |
| L | Middle occipital gyrusb | 19 | −33, −85, 10 | 25,549 | 15.04 |
| L | Fusiform gyrusb | 37 | −42, −61, −5 | 13,485 | 11.87 |
| L | Inferior frontal gyrus | 47 | −42, 26, −2 | 789 | 6.32 |
All regions arising from the Remembered > Forgotten contrast, at a threshold of p < 0.0001 (uncorrected, with a minimal cluster size of 5 contiguous voxels).
aCluster size in anatomical voxels (mm3).
bThe middle occipital gyrus activation was adjacent to the fusiform gyrus activation and the temporoparietal junction activation; the separation into three regions was performed manually, according to anatomical landmarks.
Figure 3.
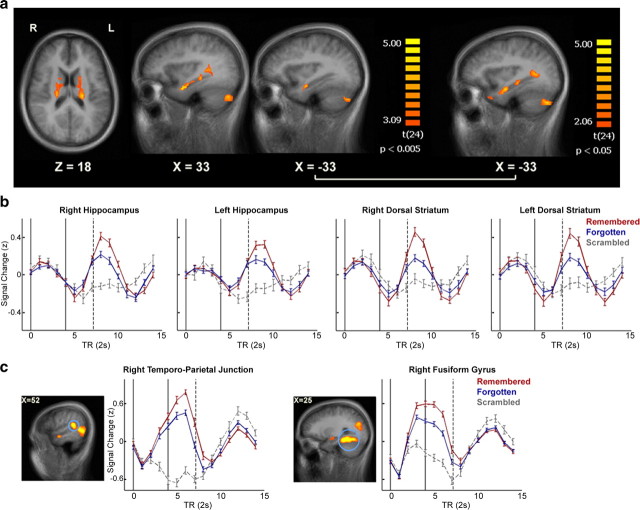
Brain regions showing postclip activity correlated with subsequent memory. a, Regions showing a significant difference in BOLD activity in experiment 1 between blank screens following Remembered clips (R-Blank) and blank screens following Forgotten clips (F-Blank) in conjunction with F-Blank > 0 (Delayed ROIs; p < 0.005 for each contrast, uncorrected; minimal cluster size = five contiguous functional voxels; GLM with a random effects group analysis; n = 25). Data are shown on axial and sagittal slices of the group-average brain. On the right, a slice including the left hippocampus is shown with the same contrast at a more relaxed threshold (p < 0.05). b, c, Mean group BOLD signal (after z scoring each time course) during and following Remembered, Forgotten, and Scrambled clips. Error bars indicate SEM. The black lines indicate the onset (left line) and offset (right line) of clip presentation, and the dashed line indicates the mean onset of the following clip. b, Results are shown for the bilateral hippocampus bodies and bilateral dorsal striatum (dorsal caudate nucleus). c, Results are shown for the right temporoparietal junction and the right fusiform gyrus, which display intraclip activity onset, for comparison.
We then searched for regions exhibiting a DM effect during the blank periods following the clips rather than during the clips themselves. To identify such regions, we created a conjunction contrast of F-Blank > baseline (p < 0.005, identifying regions active during the presentation of blank screens) and R-Blank > F-Blank (p < 0.005, identifying regions with significantly higher activity for blank screens following Remembered vs Forgotten clips). This contrast yielded several brain regions (combined p < 0.000025, uncorrected; minimal cluster size of five contiguous functional voxels) (Fig. 3a), including the right hippocampal body, bilateral hippocampus head (extending to the amygdala–hippocampal junction), right optic radiations (directly posterior to the hippocampus), bilateral dorsal caudate nucleus, and bilateral posterior cerebellum (Delayed ROIs) (Table 2).
Table 2.
Regions with increased brain activation during R-Blank compared with F-Blank in conjunction with a contrast of F-Blank > 0
| Lat | Region | BA | Peak x, y, z | Cluster sizea | t value |
|---|---|---|---|---|---|
| R | Hippocampus headb | NA | 33, −1, −23 | 507 | 5.72 |
| R | Hippocampus body | NA | 30, −31, −5 | 263 | 4.99 |
| R | Posterior cerebellum | NA | 30, −73, −35 | 1846 | 5.59 |
| R | Caudate nucleus | NA | 21, −22, −16 | 1773 | 4.55 |
| R | Optic radiationsc | NA | 33, −40, 7 | 213 | 4.14 |
| L | Caudate nucleus | NA | −21, −22, −16 | 1736 | 4.84 |
| L | Posterior cerebellum | NA | −30, −70, −38 | 705 | 4.53 |
| L | Hippocampus headb | NA | −33, −10, −17 | 317 | 4.68 |
| L | Hippocampus body | NA | −36, −28, −5 | 489 | 3.74 |
All regions arising from a contrast of R-Blank > F-Blank in conjunction with F-Blank > 0, at a threshold of p < 0.005 for each contrast (uncorrected, with a minimal cluster size of 5 contiguous functional voxels). The region in the left hippocampus was taken from the same contrast with a threshold of p < 0.05.
aCluster size in anatomical voxels (mm3).
bExtending to the amygdala–hippocampal junction.
cAdjacent to the posterior hippocampus.
The aforementioned regions all exhibited a similar pattern of activity (Fig. 3b), consisting of a delayed hemodynamic response function with an onset around the end of the clip (rise in BOLD signal 8–10 s after clip onset). Although the onset of activity was the same for both Remembered and Forgotten clips, the signal elicited by the Remembered clips was of higher amplitude. This differentiation occurred only after clip offset, with no difference in activity levels during clip presentation. Following Scrambled clips, devoid of narrative, these regions showed little to no activity compared with baseline. When relaxing the threshold to 0.05 for each of the contrasts, the left hippocampal body was also found to be significant, exhibiting activity similar to the rest of the Delayed ROIs (Fig. 3b). As the hippocampus is known to play a key role in memory encoding and consolidation (Eichenbaum, 2004; Squire et al., 2004), this region was included in the set of Delayed ROIs used for analysis. In subsequent analyses, comparison of activity patterns in ROIs arising from the Remembered > Forgotten contrast (Early ROIs) to those of the Delayed ROIs served to highlight the unique features of activity in the Delayed ROIs.
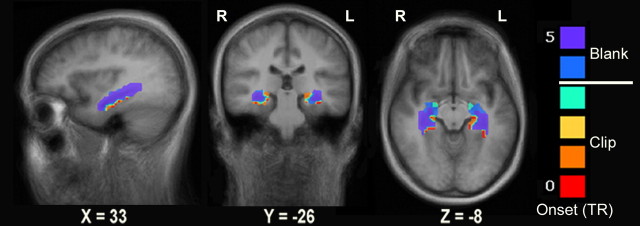
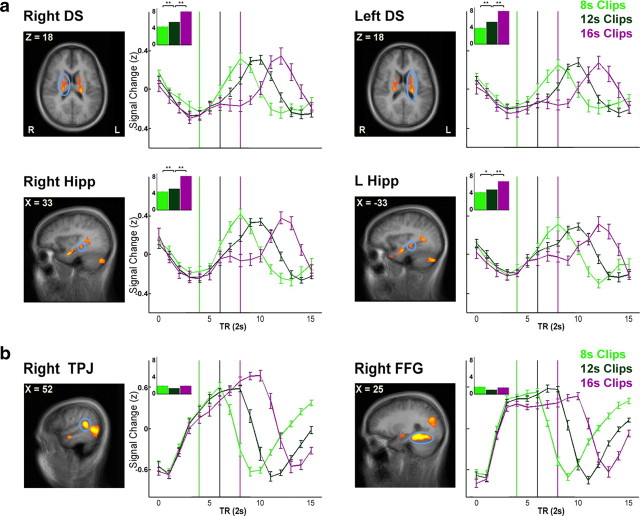
It is noteworthy that hippocampal activation was not detected in the Remembered > Forgotten contrast and only in the R-Blank > F-Blank contrast, despite the use of a more relaxed threshold in the former. As this could be due to the manner in which we probed memory (cued recall of episodic gist), we examined the onset of hippocampal activation, regardless of clip memorability. We manually defined two anatomically based ROIs, encompassing the entire hippocampus of each hemisphere, based on anatomical landmarks of the group-average anatomy (an average of the individual anatomical scans). We then estimated the onset of the brain activity most likely to yield the observed BOLD signal in each voxel (averaged over participants and clips, with no prior spatial smoothing), using the onset calculation method (see Materials and Methods). This analysis revealed that in both hemispheres almost the entire hippocampus was activated in an offline manner, with a response onset 0–2 s following the clip end (Fig. 4).
Figure 4.
Distribution of BOLD response onset in the hippocampus. The onset of the average response to clip presentation (regardless of memorability, averaged over clips and participants) for each voxel in manually defined ROIs (based on the average group anatomy) encompassing the bilateral hippocampus. Data are shown on sagittal, coronal, and axial slices of the group-average brain. The onset is calculated in TRs (TR = 2 s) from the beginning of clip presentation (with clip onset at TR = 0 and blank onset at TR = 4). An estimated onset of TR = X in a given voxel corresponds to an initial rise in BOLD signal at TR = X (see Materials and Methods for onset calculation method).
Experiment 2: onset of delayed encoding activity varies with stimulus length
Although the Delayed ROIs exhibited activation with an onset circa the end of the clips, it was not clear whether the observed response was simply delayed relative to clip onset or time-locked to the end of the clip. We therefore conducted an additional experiment (experiment 2) with clips of varying lengths to determine whether the onset of the BOLD response varied with clip length. The Movie clips in experiment 2 were created by dividing the Movie clips of experiment 1 into five groups of equal memorability and re-editing them to be of variant lengths.
First, we examined whether the results of experiment 1 were replicated in experiment 2, despite the differences in stimuli lengths. We constructed an ROI-specific GLM similar to that of experiment 1, using separate regressors for blank screens following Remembered, Forgotten, and Scrambled clips (R-Blank, F-Blank, and S-Blank, respectively), regardless of clip length. A contrast of R-Blank > F-Blank was significant in all Delayed ROIs, as was a contrast of F-Blank > baseline and a contrast of F-Blank > S-Blank (p < 0.05 for each contrast in all regions, one-tailed t test, corrected for multiple comparisons using Holm–Bonferroni) (Fig. 5a). After verifying replication of the results, all further analysis of experiment 2 data was performed on the ROIs derived from experiment 1. Our main question was whether the response onset was time-locked to clip onset or offset. If the latter were correct, we would expect a positive correlation between clip length and response onset. To test this, we compared the mean BOLD response to each clip group in experiment 2 (group G# consisted of clips of length # s), with the mean response to the corresponding group in experiment 1 (where all groups consisted of 8 s clips). The onset of the BOLD response in experiment 2, relative to the onset in experiment 1, was later for longer clips (e.g., Fig. 6a), with the best fit between the signals of the two experiments being in G8 (in which the clips of both experiments were of the same length). In most Early ROIs, the response onset was independent of clip length (Fig. 6b), although a few of these regions (including the bilateral amygdala and right temporal pole) showed a similar pattern to that of the Delayed ROIs. To test the statistical significance of this phenomenon, we calculated, for each participant, the onset of the mean signal elicited by each clip group (for elaboration of the onset calculation, see Materials and Methods). We then calculated the regression line for each participant (with response onset as the dependent variable and clip group as the independent one) and compared the slopes of participants in experiment 1 with those of experiment 2. If the response onset depended on clip length, we would expect the mean slope for participants in experiment 2 to be significantly larger than that of experiment 1 (where the mean slope is expected to be zero, as all clips were of the same length). Indeed, a comparison of the slopes calculated in the two experiments revealed a significant difference in all Delayed ROIs aside from the left cerebellum and in several of the Early ROIs (p < 0.05, corrected for multiple comparisons).
Figure 5.
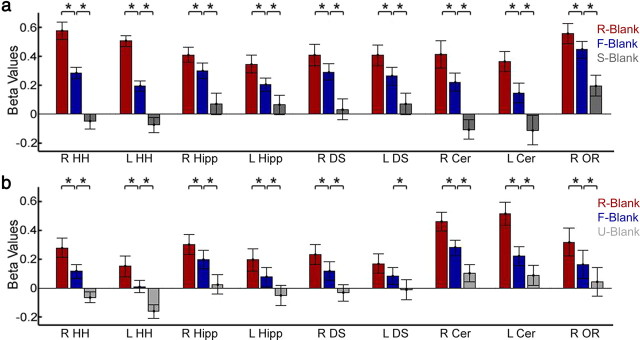
Delayed ROIs exhibit a DM effect regardless of clip length. a, Beta values of R-Blank, F-Blank, and S-Blank conditions in experiment 2 (for Delayed ROIs, derived from experiment 1). Contrasts of R-Blank > F-Blank, F-Blank > baseline, and F-Blank > S-Blank were all statistically significant in all ROIs (p < 0.05, corrected). b, Beta values of R-Blank, F-Blank, and U-Blank conditions in experiment 3 (for Delayed ROIs). A contrast of R-Blank > F-Blank was significant in all ROIs (p < 0.05, corrected, except the left caudate nucleus with p = 0.055) and a contrast of F-Blank > U-Blank was statistically significant in all ROIs (p < 0.05, corrected). Results are shown for all Delayed ROIs—bilateral hippocampal body (Hipp), bilateral hippocampus head (HH), right optic radiations (OR), bilateral dorsal striatum (DS), and bilateral cerebellum (Cer).
Figure 6.
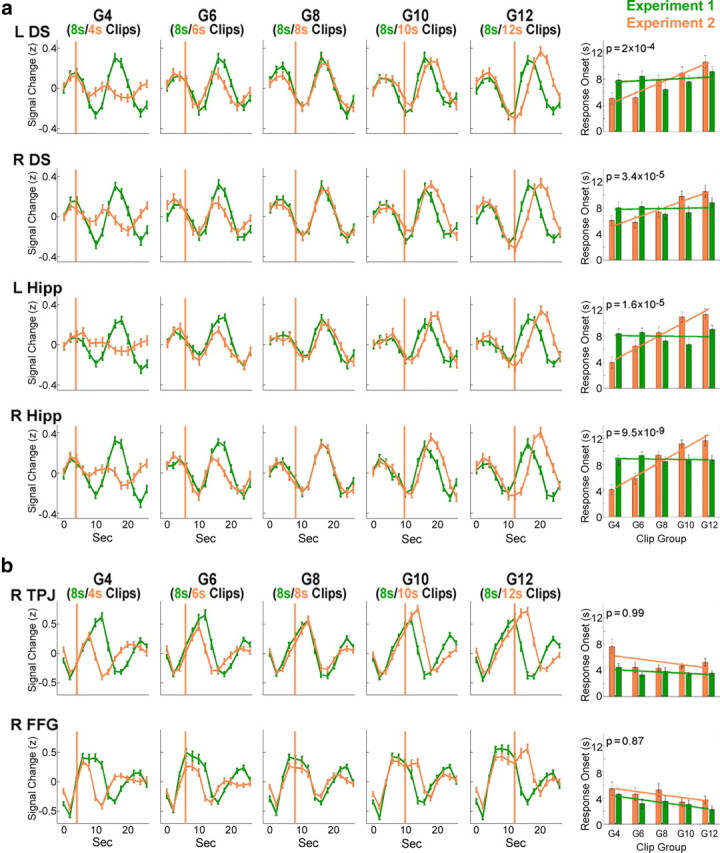
Correlation of BOLD response onset to clip length. Mean group BOLD signal (after z scoring each time course) during and following clips of each group (divided by length) in experiment 1 (all groups include 8 s clips, depicted in green) and in experiment 2 (each group includes clips of a different length, depicted in orange). Data include both Remembered and Forgotten clips, averaged from clip onset (time-point 0 represents clip onset). The orange lines represent the clip offset in experiment 2 (the clip offset in experiment 1 was always at 8 s). Error bars indicate SEM. The right column includes an automated determination of signal onset for each length group and a statistical comparison of the onset between experiments. The slope of the regression line (of response onset to clip group) in experiment 2 was compared with the slope in experiment 1 (one-tailed t test, corrected); the p values of the comparison are denoted. a, Results are shown for a subset of the Delayed ROIs—the bilateral hippocampus bodies (Hipp) and the bilateral dorsal striatum (DS). b, Results are shown for a subset of the Early ROIs—the right temporoparietal junction (TPJ) and the right fusiform gyrus (FFG).
Experiment 3: delayed encoding is an offline process, time-locked to stimulus offset
Experiment 2 served to differentiate between regions where the response onset was time-locked to clip onset and regions where the response onset was linked to clip length. However, the possibility remained that the response onset in the latter regions was not determined by clip offset, but rather by a different property of the clips, indirectly related to the clip length. To identify regions where the activation was truly time-locked to clip offset, an additional step was required—a comparison of different length clips with an identical beginning. To this end, we constructed an additional experiment (experiment 3), with pairs of clips (an 8 s version and a 12 s version) that shared the same beginning (the first 8 s were identical in both versions). This ensured that any observed difference in signal onset could not be due to early differences in the clips and could be attributed to clip offset. In addition to the clip pairs (each participant saw only one version of each pair), 16 s clips and Uneventful clips were also presented (see Materials and Methods).
As in experiment 2, we first examined whether the results of experiment 1 were replicated. A contrast of R-Blank > F-Blank proved to be significant in all Delayed ROIs (p < 0.05, corrected for multiple comparisons) (Fig. 5b), aside from the left caudate nucleus, which approached significance (p = 0.055). The same ROIs also showed a significant difference (p < 0.05, corrected for multiple comparisons) (Fig. 5b) for F-Blank > U-Blank (blanks following Uneventful clips), strengthening the hypothesis that whereas the onset of the response is determined by clip offset, the amplitude is determined by the amount of encoded information. The contrast of F-Blank > baseline did not reach significance in most regions (after correction for multiple comparisons), yet the mean F-Blank betas were positive for all Delayed ROIs, indicating that these regions were indeed activated (rather than deactivated) after clip termination (in concordance with the results of experiment 1).
We then used the onset calculation method to compare the onsets of the responses to the three eventful conditions (8 s, 12 s, 16 s clips). We first performed a one-way ANOVA with clip length as the independent variable and response onset as the dependent variable, to identify regions demonstrating a significant effect for clip length. All of the Delayed ROIs showed a significant effect (p < 0.05, corrected for multiple comparisons) (Fig. 7a shows the regions exhibiting the most marked difference), whereas only one of the 20 Early ROIs showed an effect (for representative Early ROIs, see Fig. 7b). To test the hypothesis of a positive correlation between clip length and response onset, we compared the response between 12 s and 8 s clips (one-tailed, repeated-measures t test) and between 16 s and 12 s clips (one-tailed, non-repeated-measures t test) in each of the ROIs that showed an effect. In all Delayed ROIs, both comparisons were significant (p < 0.05, corrected for multiple comparisons), whereas in the single Early ROI that showed an effect, neither comparison proved to be significant. Hence, we concluded that the delayed subsequent memory-related activity, most prominently in the bilateral hippocampus and bilateral caudate nucleus, is an offline process, time-locked to clip offset.
Figure 7.
Differences in BOLD response onset as a function of clip offset. Mean group BOLD signal (after z scoring each time course) during and following 8 s, 12 s, and 16 s clips (data from experiment 3). Data include both Remembered and Forgotten clips averaged from clip onset (time-point 0 represents clip onset). Error bars indicate SEM. The light green, dark green, and purple lines indicate the clip offset (for 8 s, 12 s, and 16 s clips, respectively). The inset graphs show a statistical comparison of response onset between the three conditions (the y-axis depicts the response onset and the x-axis depicts the clip lengths). a, Results are shown for a subset of the Delayed ROIs—the bilateral hippocampus bodies (Right Hipp: F(2,205) = 29.3, p = 6 × 10−12; Left Hipp: F(2,205) = 11.9, p = 10−5) and the bilateral dorsal striatum (Right DS: F(2,205) = 29.7, p = 5 × 10−12; Left DS: F(2,205) = 33.2, p = 3 × 10−13). b, Results are shown for a subset of the Early ROIs—the right temporoparietal junction (Right TPJ: F(2,205) = 4, n.s.) and the right fusiform gyrus (Right FFG: F(2,205) = 3.1, n.s.).
Discussion
We investigated whether brain activity immediately following the offset of a brief novel narrative episode that evolves over time is correlated with subsequent memory. We reasoned that this type of experience is likely to require binding of information as it unfolds up to a point where the episode can be registered as a cohesive unit. Therefore, immediate offline processing might be essential for the memory to be formed. We designed our paradigm in a way that allowed us to distinguish brain activations that are initiated during stimulus presentation from activations that are time-locked to the offset of the stimulus. As we were interested in higher order encoding processes, requiring integration of information over time, we probed memory for the gist of the event (gist defined as a summary of the main occurrence) rather than separate details. Extraction of gist, in this sense, requires binding of information over the course of the episode to create an internal representation of the episodic elements as a cohesive unit. We found that brain activity time-locked to the offset of the stimulus, occurring within seconds after the stimulus in a set of regions including the hippocampus and the caudate nucleus, is correlated with subsequent memory performance.
Converging evidence indicates that the hippocampus is involved in relational encoding (Henke et al., 1997; Cohen et al., 1999; Davachi and Wagner, 2002; Davachi et al., 2003), a process in which separate episodic elements are bound into an integrated memory trace (Davachi and Wagner, 2002). Such episodic binding associates features over gaps not only in space but also in time (Staresina and Davachi, 2009; Paz et al., 2010), enabling the encoding of complex experiences as integral events. Studies examining this type of binding have typically focused on relatively simple stimuli to differentiate item encoding and relational encoding. Therefore, to date, it has not been possible to determine whether this reflects online episodic binding (initiated during stimulus presentation) or offline binding whereby the integrated episode is bound into existing long-term memory (LTM). Although the same offline processes may take part in the encoding of simple associations, the gist of such stimuli is likely to be processed very quickly, possibly resulting in a small temporal separation between online and offline processes. In concordance with previous findings (Henke et al., 1997; Cohen et al., 1999; Davachi and Wagner, 2002; Davachi et al., 2003; Staresina and Davachi, 2009), we found differential hippocampal activity for successful versus unsuccessful encoding of associative information in the hippocampus, and we propose that the DM effect we observed reflects episodic binding. In support of this notion, we did not find significant postclip activation for uneventful clips, which should not require episodic binding. Furthermore, almost the entire hippocampus (bilaterally) was activated upon clip offset, indicating that offline encoding is a general property of the hippocampus, not limited to specific ROIs within the hippocampus.
The question arises as to the nature of this offline hippocampal activity. It has been reported that in rodents during periods of rest or sleep, hippocampal neurons rapidly replay sequences of firing that were observed during learning (Wilson and McNaughton, 1994; Kudrimoti et al., 1999; Nádasdy et al., 1999), resulting in sharp bursts of hippocampal activity that are less common during periods of exploration (Buzsáki, 1989). Studies have shown that these firing sequences can be divided into forward and reverse replays, whereby neurons that were sequentially active during learning fire in rapid succession during rest, in either the same order or the reverse order (Foster and Wilson, 2006; Diba and Buzsáki, 2007). It has been proposed that reverse replay may underlie immediate binding of episodic sequences, whereas forward replay may underlie mechanisms of systems consolidation (Carr et al., 2011), the process in which long-term memories become less dependent on the hippocampus and more reliant on neocortex (Dudai, 2004). Previous studies that examined poststimulus mnemonic processes in humans focused on processes occurring minutes to hours following exposure to new experiences, and their findings are construed to reflect systems consolidation (Peigneux et al., 2006; Axmacher et al., 2008; Stevens et al., 2010; Tambini et al., 2010; van Kesteren et al., 2010). Here, we focused on immediate postevent activation and suggest that our findings may reflect a similar mechanism to that reflected in the rodent hippocampus reverse replay during brief resting periods immediately following exploration episodes (Foster and Wilson, 2006). Although in real life there are commonly no intercalated artificial rest periods in ongoing events, natural event boundaries may cue the transfer of event memories from WM to LTM (Kurby and Zacks, 2008; Ezzyat and Davachi, 2011). However, when using a continuous episodic stream as memorandum, the brain activity related to each event might be masked by activity related to the following event. It is noteworthy that a leading model of human WM suggests that the transfer of episodic information from WM to LTM involves an “episodic buffer” that enables immediate binding of multimodal information into cohesive representations (Baddeley, 2000). The possibility should be considered that our protocol unveils the engagement of such a buffer. However, other possibilities exist, not precluding the above, for example, that the poststimulus activity reflects the initial stages of memory consolidation (Dudai, 2004).
Whereas the hippocampus is considered to subserve declarative memories, the caudate nucleus is known to play a role in habit formation and incremental learning (Knowlton et al., 1996; Poldrack et al., 1999; Yin and Knowlton, 2006), particularly in feedback-driven learning (Shohamy et al., 2004, 2008). Although several studies show a dissociation between the forms of memory related to these two structures (McDonald and White, 1993; Packard et al., 1994; Poldrack and Packard, 2003), there is also evidence pointing to the involvement of the caudate nucleus in declarative memory (Schott et al., 2006; Blumenfeld et al., 2011). The current study supports the latter suggestion, as we examined declarative memory of movie clips, with no explicit feedback or creation of stimulus–response associations.
How might the offline activation in the caudate nucleus contribute to the formation of declarative memory? One possibility is that closure of a narrative episode serves as a reward, promoting a form of internal-feedback-based learning that engages the striatum (Shohamy et al., 2008). Another is that the process of binding elements of a complex unfolding episode relies on networks that subserve incremental learning, including the caudate nucleus. Indeed, the caudate nucleus has been found to be more strongly activated during formation of discontinuous associations than simultaneous ones (Qin et al., 2007). Two recent studies found increased correlation between the activity of the dopaminergic system and the hippocampus during successful encoding that required integration of information. The first study (Shohamy and Wagner, 2008) showed an increased correlation between activity in the hippocampus and the dopaminergic midbrain during successful versus unsuccessful generalization of associations, and the second study (Sadeh et al., 2011) found an increased correlation between the putamen and the hippocampus during successful episodic encoding. Although these studies did not report such correlations in the caudate nucleus, as part of the dopaminergic system, it is strongly connected to both the dopaminergic midbrain and the putamen. Here, we complement this picture by providing evidence that the caudate nucleus contributes to offline encoding of episodic memory.
It is noteworthy that although we focused here on brain regions exhibiting offline encoding, we also found a large brain network whose online activity was correlated with subsequent memory performance (termed Early ROIs), in concordance with previous studies (Hasson et al., 2008; Spaniol et al., 2009; Kim, 2011). It is possible that these online processes subserve different functions than the offline processes described above. For example, the observed increased memory performance for clips of longer lengths may stem, in part, from the increased duration of online processing. Beyond this distinction between online and offline processes, our data suggest that there might be an additional, more subtle temporal division within the Early ROIs, differentiating early-online and late-online encoding processes. Specifically, we found that a small subset of the Early ROIs also exhibited rather late BOLD responses, yet with an onset preceding clip end. Some of these regions—mainly the bilateral amygdala and the right temporal pole—showed a positive correlation between clip length and response onset (in experiment 2). As the response onset preceded the clip end, it could not be time-locked to the clip end, but rather to properties of the clips indirectly related to clip length. When comparing the response to different length clips that shared the same first 8 s (experiment 3), the response onset was no longer dependent on clip length, clearly differentiating these regions from those exhibiting offline encoding. Regions exhibiting such activation patterns may be involved in encoding complex aspects of the episode, such as emotional expressions and social interactions, which can be processed in an online manner, yet not immediately upon clip onset. A caveat is, however, appropriate: although we demonstrate in this study a correlation of online and offline activity in specific brain regions with subsequent memory, the necessity of the identified regions for processes of episodic memory encoding and consolidation has yet to be determined.
All in all, we propose that the immediate poststimulus activity that we detect in the hippocampus and the caudate nucleus, time-locked to the offset of brief narrative episodes, is involved in registering integrated episodes to memory as cohesive internal representations. Further analysis of the mechanisms and function of such immediate offline processes could contribute to the understanding of human episodic memory formation.
Notes
Control analyses and supporting information for this article are available at http://www.weizmann.ac.il/neurobiology/labs/dudai/supplemental. This material has not been peer reviewed.
Footnotes
This work was supported by grants from the U.S.–Israel Binational Science Foundation and The Nella and Leon Benoziyo Center for Neurological Diseases (Y.D.). We are grateful to Ilan Dinstein, Micah Edelson, Maytal Flitter, Orit Furman, Kelly Ludmer, Avi Mendelsohn, Alex Pine, Uri Nili, Shiri Ron, and Amnon Yacoby for valuable comments. We also thank Edna Furman-Haran, Fanny Attar, and Nachum Stern from the Norman and Helen Asher Center for Brain Imaging at the Weizmann Institute.
The authors declare no competing financial interests.
References
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci. 2011;23:257–265. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. What constitutes an episode in episodic memory? Psychol Sci. 2011;22:243–252. doi: 10.1177/0956797610393742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Furman O, Dorfman N, Hasson U, Davachi L, Dudai Y. They saw a movie: long-term memory for an extended audiovisual narrative. Learn Mem. 2007;14:457–467. doi: 10.1101/lm.550407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;57:452–462. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, Zacks JM. Segmentation in the perception and memory of events. Trends Cogn Sci. 2008;12:72–79. doi: 10.1016/j.tics.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: between-systems similarities and within-system differences. Cogn Brain Res. 2002;13:281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD, Wagner AD. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 2009;29:11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. Prestimulus hippocampal activity predicts later recollection. Hippocampus. 2010;20:24–28. doi: 10.1002/hipo.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. A neural substrate in the human hippocampus for linking successive events. Proc Natl Acad Sci U S A. 2010;107:6046–6051. doi: 10.1073/pnas.0910834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Qin S, Piekema C, Petersson KM, Han B, Luo J, Fernández G. Probing the transformation of discontinuous associations into episodic memory: an event-related fMRI study. Neuroimage. 2007;38:212–222. doi: 10.1016/j.neuroimage.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A. Cooperation between the hippocampus and the striatum during episodic encoding. J Cogn Neurosci. 2011;23:1597–1608. doi: 10.1162/jocn.2010.21549. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Düzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal–midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. New York: Oxford/Clarendon; 1983. [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Fernández G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal–neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]