Abstract
Behavioral studies in humans and electrophysiological recordings in nonhuman primates have suggested the existence of a specific representation of the space immediately surrounding the body. In macaques, neurons that have visual receptive fields limited to a region of space close around a body part have been found in premotor and parietal areas. These cells are hypothesized to encode the location of external objects in coordinate systems that are centered on individual body parts. In the present study, we used an fMRI adaptation paradigm on healthy participants to reveal areas in the anterior part of the intraparietal sulcus, the inferior parietal lobe (supramarginal gyrus), and the dorsal and ventral portions of the premotor cortex that exhibit selective BOLD adaptation to an object moving near the right hand. Crucially, these areas did not manifest adaptation if the stimulus was presented in far space (100 cm) or when the hand was retracted from the object. This hand-centered selectivity could not be detected when a traditional fMRI analysis approach was used. These findings are important as they provide the most conclusive neuroimaging evidence to date for a representation of near-personal space in the human brain. They also demonstrate a selective mechanism implemented by human perihand neurons in the premotor and posterior parietal areas and add to earlier findings from humans and nonhuman primates.
Introduction
The space surrounding the body is of particular importance for survival. Harmful objects near the body can represent deadly threats, and the targets of goal-directed actions are typically located within reaching distance. It is therefore of fundamental importance to know where objects are located with respect to the body. Evolution has provided the brain with an efficient mechanism to represent visual information with respect to the limbs, using the limb itself as a reference (Hyvärinen and Poranen, 1974; Rizzolatti et al., 1981a,b; Graziano et al., 1994). Electrophysiological experiments in macaques have described neuronal populations that encode the location of visual stimuli in body-part-centered reference frames in a set of anatomically connected areas in the inferior parietal lobe, intraparietal sulcus, premotor cortex, and putamen (Colby et al., 1993; Graziano and Gross, 1993). These neurons integrate multisensory information at the single-cell level, presenting both tactile and visual receptive fields (RFs), the latter limited to the region of space close to the body surface (Fogassi et al., 1996). The location of RFs is independent of eye movements but follows arm movements, suggesting that these neuronal populations encode an object's location in body-part-centered coordinates (Graziano et al., 1997; Graziano and Gross, 1998a).
Here, we used BOLD adaptation (Grill-Spector et al., 2006) to probe the existence of a peripersonal space-coding mechanism in humans. Adaptation is a robust phenomenon in electrophysiology (Li et al., 1993; Miller and Desimone, 1994; Sobotka and Ringo, 1996) that has been extended to fMRI (Sayers and Grill-Spector, 2005; Tal and Amedi, 2009). It is based on the premise that the repeated presentation of identical stimuli leads to a reduction in the measured signal from neuronal populations selective to specific stimulus features. The main advantage of fMRI adaptation compared with traditional fMRI methods is the capacity to reveal subpopulations of neurons within a single voxel that exhibit selectivity to such features (Avidan et al., 2002).
Behavioral evidence favors a peripersonal space representation in humans (di Pellegrino et al., 1997; Farnè et al., 2005), but it remains uncertain whether the human brain implements a selective mechanism similar to the one described in monkeys. Two fMRI studies have described enhanced BOLD responses in the intraparietal sulcus when objects are presented close to the hand (Makin et al., 2007) or the face (Sereno and Huang, 2006). However, a BOLD signal increase from an area might reflect the differential involvement of various processes and does not provide conclusive evidence for the existence of neurons with specific RF properties. We were encouraged to use fMRI adaptation by Rizzolatti and colleagues' observation that “the visual response tended to disappear with repetitive stimulation” when recording from premotor neurons (Rizzolatti et al., 1981b, p 151). This suggests that peripersonal space neurons adapt to visual stimulation. Here, we found significant BOLD adaptation in human premotor and parietal areas exclusively for objects near the hand. Importantly, the locations of these responses correspond well to areas where peripersonal space neurons have been found in monkeys, suggesting a common neuronal mechanism in humans and nonhuman primates for the representation of peripersonal space.
Materials and Methods
Participants.
A total of 22 right-handed participants (ages 19–37 years, mean age = 26 ± 5 years, 17 males) with no history of neurological, psychiatric, or visual disorders took part in this study. All participants were volunteers recruited within the student population in the Stockholm region. Handedness was reported by the participants in a screening questionnaire before the experiment. Subjects were at least 175 cm tall to ensure that their hand would be accessible for visual stimulation in the constrained space of the scanner environment. Written informed consent was obtained from each participant before the experiment. The study was approved by the local ethical committee at the Karolinska Institutet.
fMRI acquisition.
fMRI acquisition was performed using a Siemens TIM Trio 3T scanner equipped with a 12-channel head coil. Gradient echo T2*-weighted EPIs with BOLD contrast were used as an index of brain activity (Logothetis et al., 2001). A functional image volume was composed of 40 continuous near-axial slices of 3 mm thickness (with a 0.1 mm interslice gap), which ensured that the whole brain was within the FOV (58 × 76 matrix, 3.0 mm × 3.0 mm in-plane resolution, TE = 40 ms). One complete volume was collected every 2.54 s (TR = 2540 ms). A total of 1320 functional volumes were collected for each participant (330 in each of the four runs, as described below). An initial baseline of 15 s and a final baseline of 15 s were included in each run. To facilitate the anatomical localization of statistically significant activations, a high-resolution structural image was acquired for each participant at the beginning of the experiment (3D MPRAGE sequence, voxel size = 1 mm × 1 mm × 1 mm, FOV = 250 mm × 250 mm, 176 slices, TR = 1900 ms, TE = 2.27 ms, flip angle = 9°).
Experimental setup.
During the brain scans, participants lay comfortably in a supine position on the MRI table with their head tilted ∼30° forward to allow a direct view of an MR-compatible table (42 × 35 cm, with an adjustable slope), which was mounted on the bed above the subjects' waist (Fig. 1a). The required tilt of the head was obtained by slanting the head coil using a custom-made wooden wedge at an angle of ∼11°. The participants' heads were tilted another 20° using pillows and foam pads. The participants wore MR-compatible headphones to dampen the scanner noise. The table was adjusted to be comfortable for the participants when placing their tested (right) hand on it with a fully extended arm (Fig. 1a). A support for the hand was placed on the table 5 cm to the right of the midline, serving two purposes. First, it assured a full view of the hand for the participants. Second, it guaranteed a perfect match of the position of each participant's tested hand across experimental replicates. Participants were instructed to fixate on a green ball (2 cm diameter), which was mounted on the tip of a wooden stick attached centrally to the table in front of the participant (Fig. 1b, black dots). The distance between each participant's right hand, as measured from the tip of the middle finger, and the fixation point was 50 cm. For each participant, the visual field accessible from the scanner bore was identified, which allowed the presentation of the stimuli in the same portion of the visual field across the different distances (see below).
Figure 1.
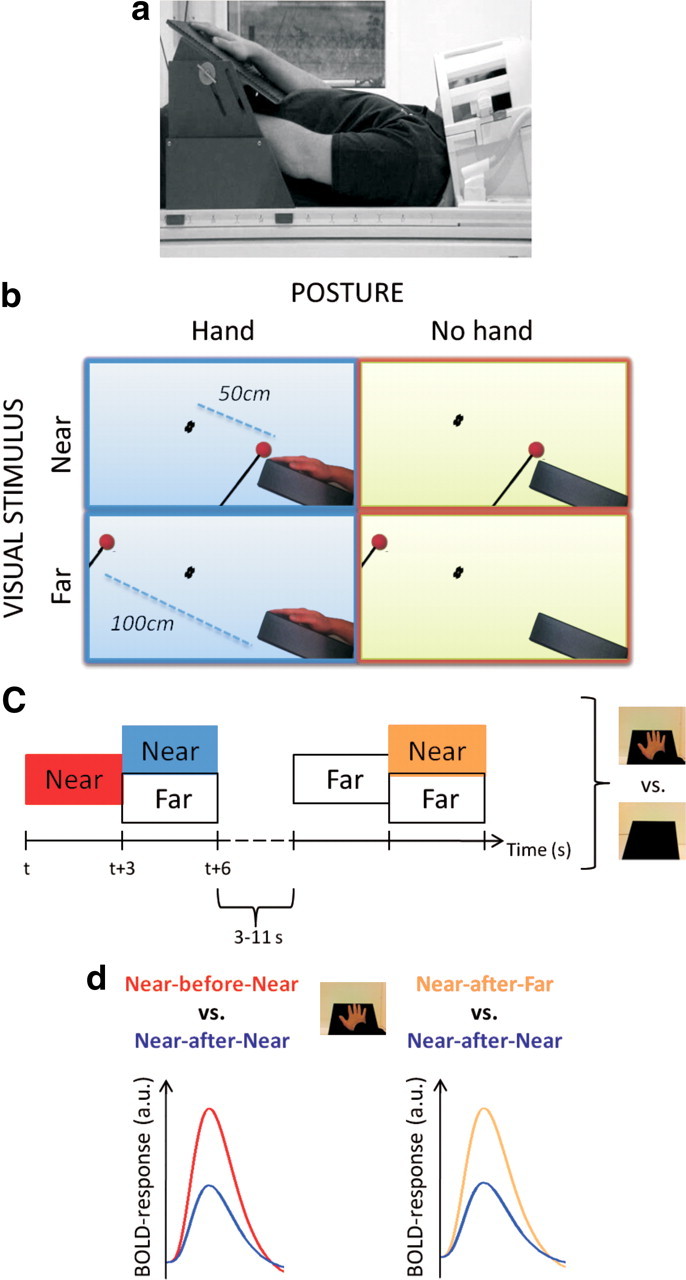
Experimental setup and design. a, Photograph of the experimental setup showing a participant lying in the scanner with the head tilted and the right hand placed on an angled table (see Materials and Methods). b, The participant was asked to maintain his or her gaze on a target point (represented as a black dot) placed 50 cm from the tip of the right middle finger throughout the whole experiment. The visual stimulus consisted of a small sphere moving close (“Near,” first row, 2 cm away from the participant's right hand) or far (“Far,” second row, 100 cm away from the participant's hand) to the participant's right hand on the table. A crucial manipulation in the study involved the posture of the arm. The arm could either be extended so that the hand was placed on the table in full view (left column) or retracted with the hand resting on the participant's chest (right column). c, The experimental design included different combinations of Near and Far stimuli: Near-Near, Near-Far, Far-Near, Far-Far. Each combination lasted 6 s and was divided into a first and second part of equal length (3 s periods). The 6 s trials were separated by an intertrial interval of 3–11 s in duration. The same experiment was performed in HAND and NO HAND runs. d, Expected results in terms of BOLD adaptation. The experimental design allowed us to study adaptation with two complementary approaches. In particular, a reduced BOLD signal is expected in the Near-after-Near half with respect to the Near-before-Near half of the stimulation (the blue and the red lines in the left plot, respectively). The plot on the right represents the expected adaptation results from the second approach. Similarly, the signal should be reduced in the Near-after-Near half with respect to the Near-after-Far half (represented with the blue and the yellow line in the right plot, respectively). Crucially, significant adaptation effects are expected in HAND runs as opposed to NO HAND runs.
The visual stimulus consisted of a red ball (3 cm diameter) mounted on the tip of a wooden stick (50 cm long). A real physical object was used rather than an artificial computer-generated stimulus because earlier single-cell recording studies reported that the former produces stronger responses in peripersonal space neurons (Graziano et al., 1997). Two trained researchers moved the ball up and down for 3 s either 2 cm away from the participant's fingertip (near position) or 100 cm away (far position). The choice of 100 cm for the far position was chosen on the basis of two previous studies. The first study, a block-design study by Makin and colleagues (2007), found significant differences in the BOLD response in intraparietal areas between stimuli presented close to the hand and stimuli appearing 70 cm from it. The second study, the seminal single-cell study of the macaque ventral premotor cortex by Rizzolatti and colleagues (Rizzolatti et al., 1981b), presented objects at a distance of 100 cm as the “far stimuli” or 2 cm as “near stimuli.” The researchers listened to audio instructions regarding the location of the forthcoming stimuli and to a metronome at 80 beats per minute, which ensured identical pace of the up and down movements of the ball at each position. In all cases, only a portion of the wooden stick and the ball attached to it were visible to the participant. To monitor eye movements, an MR-compatible camera (MRC Systems) mounted on the head coil was used. This camera recorded an image of each participant's left eye for the duration of the experiment. The image of the participant's eye was also displayed online in the scanner control room, where it could be monitored by a researcher. The video recordings were stored on a computer and analyzed offline to detect any possible eye movement away from fixation. The few trials where such an eye movement was identified were discarded. The fMRI data from these periods were modeled in a regressor of no interest to the GLM.
To monitor the alertness of the participants and the visibility of all stimuli, catch trials were presented in an unpredictable fashion during each run, interleaved with experimental conditions. Catch trials consisted of the ball suddenly stopping and appearing still for 3 s (no motion) in one of the two possible positions (near vs far). Participants were instructed to push a button with their left hand (the right hand being the one tested) as soon as they observed this occurring. The catch trials were modeled as a regressor of no interest and discarded. All instructions to the researchers were transmitted via a computer running Presentation 14.1 (Neurobehavioral Systems) and connected to an MR-compatible sound delivery system (Nordic Neuro Lab). The same software was used to record participant responses and store them on a hard drive.
Experimental design.
In this study, we aimed to identify brain areas showing BOLD adaptation selectively to visual stimuli near the hand. A moving ball was presented either near or far from the participant's hand. Four combinations of visual stimuli were presented to the participant: Near-Near, Near-Far, Far-Near, and Far-Far (Fig. 1c). Each combination lasted a total of 6 s divided into a first and second part of equal length. In each 6 s period of a stimulus combination, the ball was moved eight times: four times each in the first and second parts of the stimulation. Importantly, in the first and second parts of the stimulation, the ball was presented either in far space or in near space, resulting in the four combinations of stimuli listed above. This resulted in eight 3 s stimulation periods, which were modeled as eight separate conditions in the GLM. The crucial point of the adaptation design is whether the second part of a trial (near or far) was preceded by stimulation in the same part of space (near or far). Three conditions were of interest in the first set of analyses: Near-before-Near, Near-after-Near, and Near-after-Far (Fig. 1d). The three remaining conditions were of interest for important control analyses: Far-before-Far, Far-after-Far, and Far-after-Near (for more details, see Data analysis).
Each combination of stimuli was repeated 12 times per run. Consecutive trials were separated by a jittered intertrial baseline interval (7 ± 4 s) with no stimulation. During these baseline periods, the participants were instructed to fixate on the stationary target object, as in the stimulation trials described above. Eight catch trials per run were randomly interleaved with the experimental trials.
To assess the hand-centered nature of the selectivity for near visual stimuli, we used two different hand postures across the four experimental runs. In two runs (termed HAND), the right hand was placed on the table in front of the subject, as described above; in the remaining two runs, termed NO HAND, the participants retracted their right hand and placed it on their left shoulder. In terms of the visual stimulation produced by the moving ball on the retina, the inputs were identical in the HAND and NO HAND runs. Only the relative distance between the visual stimulation and each participant's right hand changed. The sequence of the two different types of runs with different hand postures was counterbalanced across subjects.
Data analysis.
fMRI data were analyzed with SPM8 (Wellcome Department of Cognitive Neurology). The first three volumes of each run were discarded from further analysis because of non-steady-state magnetization. Functional images were realigned to correct for head movements and coregistered with the high-resolution structural scan from each participant. The anatomical image was then segmented into white matter, gray matter, and CSF partitions and normalized to the MNI standard brain. The same transformation was then applied to all functional volumes, which were resampled to a 2.0 mm × 2.0 mm × 2.0 mm voxel size. The functional images were then spatially smoothed with an 8 mm FWHM isotropic Gaussian kernel. For each participant's dataset, a general linear regression model was fit to the data in the first-level analysis. We defined separate regressors for the first and second part of each 6 s stimulation period in the same way in the HAND and NO HAND runs. In addition, a regressor of no interest was defined, corresponding to the 6 s periods when a catch trial occurred. Each condition was modeled with a boxcar function and convoluted with the standard SPM8 hemodynamic response function. We defined linear contrasts in the GLM (see below).
The results from this analysis were given as contrast estimates for each condition for each subject (contrast images). To accommodate intersubject variability, we entered the contrast images from all subjects into a random effect group analysis (second-level analysis). To account for the problem of multiple comparisons in the statistical analysis of the whole-brain data, we reported peaks of activation surviving a significance threshold of p < 0.05, corrected using topological peak false discovery rate (FDR) as implemented in SPM8 (Chumbley et al., 2010). In addition, given the strong a priori hypotheses on the anatomical localization of regions in the human brain expected to represent perihand space (see Introduction), a significance level of p < 0.05 corrected using FWE was used with small volume corrections centered around relevant coordinates from previous studies (Ehrsson et al., 2004; Makin et al., 2007; Gentile et al., 2011). For each peak of activation, the coordinates of the peak in the MNI space, the t value, and the p value are reported. When a peak survived a threshold of p < 0.05 after correction for multiple comparisons at whole-brain level (FDR) or a small volume correction (FWE), the label “corrected” follows the p value. Alternatively, the term “uncorrected” appears after the p value in the few cases when we want to mention activations that did not survive correction for multiple comparisons but are still relevant to describe. This can occur, for example, to allow comparison with the earlier literature or to compare the results between the adaptation and a traditional approach. Importantly, all main results on which our main conclusions are drawn survived correction for multiple comparisons. In some of the figures, the effect size for the conditions of interest using histogram plots are displayed. The values on the y-axis correspond to the contrast estimates of the conditions of interest for each postural manipulation (HAND and NO HAND) separately. The error bars reflect the SE.
To identify brain regions showing significant BOLD adaptation related to visual stimulation near the hand, we used two complementary approaches. In the first analysis approach, a linear contrast was performed between the first and second parts of a Near-Near trial (Near-before-Near vs Near-after-Near) for all experimental runs when the participants' hands were placed on the support (HAND runs). To emphasize the contrast, the following nomenclature is used: (Near-before-Near vs Near-after-Near)HAND. In this contrast, the location of the moving object is constant throughout the 6 s period, which means that the visual stimulation on the retina is perfectly matched. To assess the hand-centered nature of these responses, we created an identical contrast for the runs when the participant's hand was retracted from the support and placed on the chest, named NO HAND runs, using the following nomenclature: (Near-before-Near vs Near-after-Near)NO HAND. Importantly, this allowed us to define a contrast to directly test the hypothesis of greater BOLD adaptation in the HAND runs compared with the NO HAND runs, thus identifying areas showing adaptation specifically to stimulation near the right hand: (Near-before-Near vs Near-after-Near)HAND versus (Near-before-Near vs Near-after-Near)NO HAND.
In the second analysis approach, the second parts of the stimulation periods when the object was moved close to the hand were compared, having been preceded either by 3 s of near stimulation (yielding adaption) or by 3 s of far stimulation (leading to no adaptation). To this end, the adaptation contrast was defined to compare the Near-after-Far condition with the Near-after-Near condition (Near-after-Far vs Near-after-Near)HAND. In this contrast, the second parts of the stimulation are compared, thus ruling out any possible effect of temporal order. As in the first analysis approach described above to identify hand-specific adaptation responses, the adaptation response in the runs where the hand was present on the table (HAND runs) and the response to the runs when the hand was retracted (NO HAND runs) were directly compared with the contrast (Near-after-Far vs Near-after-Near)HAND versus (Near-after-Far vs Near-after-Near)NO HAND.
To further assess whether BOLD adaptation responses in the key premotor and posterior parietal areas during stimulation in peripersonal space were significantly different from adaption in far space, we inspected the contrast between the first and the second parts of the Far-Far trials (Far-before-Far vs Far-after-Far, in both HAND and NO HAND runs) corresponding to the first analysis approach. Similarly, we also compared the second part of a Near-Far trial with the second part of a Far-Far trial (Far-after-Near vs Far-after-Far, in both HAND and NO HAND runs), corresponding to the second analysis approach. The purpose was to rule out the possibility that there were any adaptation effects in the Far conditions (e.g., that only the Near conditions led to significant adaptation in the regions hypothesized to represent peripersonal space).
Finally, to compare the fMRI adaptation approach with the classical way to analyze fMRI data, we also performed an analysis similar to that performed by Makin and colleagues (2007). The activation during the entire 6 s periods of Near stimulation (Near-Near trial) was directly contrasted with the corresponding 6 s periods of Far stimulation (Far-Far trial). Importantly, to test for activations showing evidence of hand specificity, we looked for areas showing greater activation to near stimuli compared with far stimuli in the HAND versus NO HAND runs. This latter analysis tested for areas that showed a greater difference in activity when the stimuli presented near the body rather than far from the body in the runs when the arm is extended were compared with when the arm was retracted.
Results
Behavioral results
Participants were able to complete the task of detecting catch trials throughout the experiment, as confirmed by an average accuracy of 98.7%. Moreover, recordings from the eye camera showed that participants successfully maintained fixation throughout the course of the experiment as instructed. On average, 2 of 240 total trials per participant were discarded from further analysis because of eye movements away from fixation. No difference in terms of eye movements was present between the Near and Far conditions and the HAND and NO HAND runs. Thus, the possibility that eye movements could have confounded the fMRI data can be ruled out.
Hand-centered representation of visual stimuli revealed by BOLD adaptation
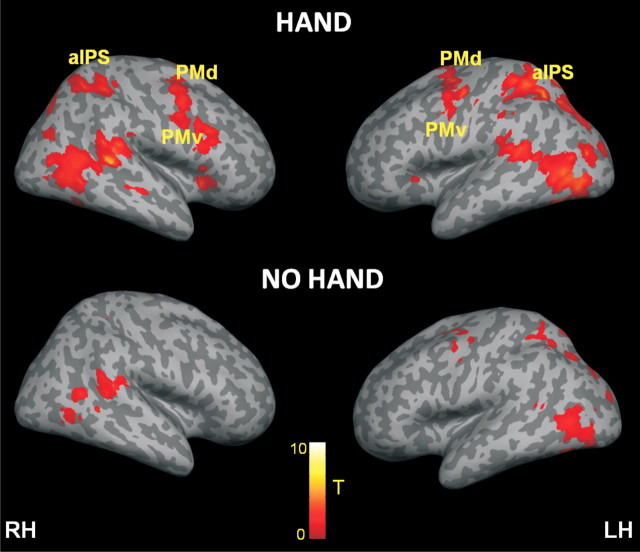
We looked for areas showing BOLD adaptation to repeated visual stimulation near the hand using the first analysis approach, comparing the first versus the second part of a near-hand stimulation trial [(Near-before-Near vs Near-after-Near)HAND]. The results from the random effects group analysis are shown in Figure 2. As expected, BOLD adaptation effects were found in visual areas reflecting low-level visual characteristics of the stimulation (left calcarine gyrus, −6, −90, −4, t = 6.10; left middle occipital gyrus, −34, −78, 14, t = 7.33; left inferior occipital gyrus, −36, −76, −8, t = 9.03; all three peaks p < 0.05 corrected, all coordinates in MNI space). Most notably, activation was also found in a set of bilateral premotor and posterior parietal areas, which showed a significant reduction of the BOLD signal when the Near stimulus was repeated near the hand. This included the bilateral anterior portions of the intraparietal sulcus (aIPS; on the left, −20, −62, 56, t = 9.20, p < 0.05 corrected; on the right, 34, −42, 50, t = 6.20, p < 0.05 corrected), the bilateral supramarginal gyrus (SMG; on the left, −56, −36, 22, t = 4.35, p < 0.05 corrected; on the right, 26, −50, 50, t = 6.88, p < 0.05 corrected) in the inferior parietal lobe, the bilateral dorsal (PMd; on the left, −36, 6, 56, t = 7.60, p < 0.05 corrected; on the right, 38, −4, 50, t = 6.27, p < 0.05 corrected), and ventral premotor cortex (PMv; on the left, −50, 0, 40, t = 5.40, p < 0.05 corrected; on the right, 44, 14, 26, t = 6.78, p < 0.05 corrected).
Figure 2.
BOLD adaptation to visual stimulation in the near position. Whole-brain rendering of the results from the adaptation contrast the near-hand visual stimulation as derived from the contrast (Near-before-Near) versus (Near-after-Near) in the HAND and NO HAND runs (top and bottom, respectively). The corresponding activation map is overlaid on an inflated canonical cortical surface of the left and right hemispheres, respectively. For display purposes, the statistical threshold was set to p < 0.001 uncorrected, with a minimum cluster size of 10 voxels. RH, Right hemisphere; LH, left hemisphere.
Crucially, we next looked for adaptation responses that were specific to the extended posture of the arm, that is, present only when the arm was extended, making the Near visual stimuli appear close to the hand [(Near-before-Near vs Near-after-Near)HAND vs (Near-before-Near vs Near-after-Near)NO HAND]. Importantly, this analysis revealed significant adaptation responses in a very similar set of premotor and parietal areas as those found in the preceding analysis: bilateral aIPS (on the left, −34, −50, 54, t = 5.87, p < 0.05 corrected; on the right, 34, −38, 54, t = 3.78, p = 0.05 corrected), bilateral PMd (on the left, −48, −6, 48, t = 4.05, p < 0.05 corrected; on the right, 40, −2, 46, t = 4.26, p < 0.05 corrected), right PMv (44, 10, 18, t = 4.48, p < 0.05 corrected), and left SMG (−58, −30, 22, t = 6.38, p < 0.001 corrected) (Figs. 2, 3; all peaks and detailed statistics are listed in Table 1). At a lower threshold, adaptation effects were also found in the right putamen (32, 6, −2, t = 3.83, p < 0.001 uncorrected), consistent with our hypothesis. As shown in Figure 3, the amplitude of the BOLD signal in the relevant brain regions was reduced when the ball was moved near the hand following a period of identical stimulation. Importantly, no such significant reduction in the BOLD signal was seen when the hand was retracted from the table. This suggests that these areas contain neurons selective for the presence of an object in the space around the hand, supporting our hypothesis.
Figure 3.
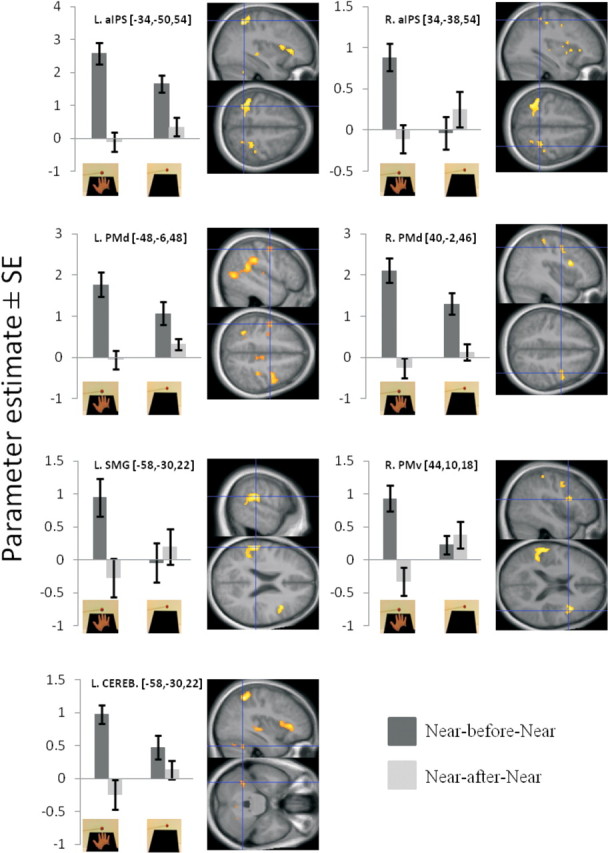
Key areas showing significant BOLD adaptation to near-hand stimuli, only when the hand is placed on the table (first analysis approach; p < 0.05 corrected). Activation maps correspond to the direct comparison of the near adaptation effects in the runs when the hand is present on the table and the runs when the hand is retracted [(Near-before-Near vs Near-after-Near)HAND vs (Near-before-Near vs Near-after-Near)NO HAND]. The plots on the left correspond to the parameter estimates for the Near-before-Near and Near-after-Near conditions, from the HAND (left) runs and the NO HAND (right) runs (as indicated by the pictures), respectively. Error bars represent SEM. The three numbers next to the titles refer to the x, y, and z coordinates in MNI space. Each plot is presented next to the corresponding activation map overlaid onto a sagittal and an axial slice of the mean high-resolution structural scan of all participants. The significant peaks of activation (p < 0.05 corrected) can be identified by the blue crosshairs. The threshold of the activation maps was set at p < 0.001 uncorrected for display purposes. CEREB, Cerebellum.
Table 1.
Hand-centered BOLD adaptation to visual stimulation (first analysis approach): (Near-before-Near vs Near-after-Near)HAND versus (Near-before-Near vs Near-after-Near)NO HAND
| Anatomical location | MNI coordinates |
Peak t | Peak pa | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L. supramarginal gyrus | −58 | −30 | 22 | 6.38 | <0.001 | 235 |
| L. anterior intraparietal sulcus | −34 | −50 | 54 | 5.87 | 0.001 | 222 |
| R. inferior part of the precentral sulcus (PMv) | 44 | 10 | 18 | 4.48 | 0.015 | 124 |
| R. precentral sulcus (PMd) | 40 | −2 | 46 | 4.26 | 0.022 | 91 |
| L. precentral sulcus (PMd) | −48 | −6 | 48 | 4.05 | 0.032 | 13 |
| L. cerebellum (Lobule VI) | −36 | −52 | −30 | 4.00 | 0.036 | 44 |
| R. anterior intraparietal sulcus | 34 | −38 | 54 | 3.78 | 0.050 | 50 |
| R. putamen | 32 | 6 | −2 | 3.83 | <0.001b | 15 |
Significant (p < 0.05 corrected) BOLD adaptation to perihand visual stimulation, modulated by the posture of the hand, as obtained from the direct comparison of the contrast Near-before-Near versus Near-after-Near across HAND and NO HAND runs.
aSmall volume correction.
bp < 0.001 uncorrected.
In the second analysis approach, we looked for adaptation by comparing the last 3 s of stimulation near the hand in the trials following stimulation far or near the hand [(Near-after-Far vs Near-after-Near)HAND]. The results of this analysis produced activation maps that were similar to those detected in the first analysis approach (Fig. 4). In particular, significant adaptations were found in the bilateral anterior portion of the IPS, contralateral left SMG, bilateral PMd, and right PMv. As in the first adaptation contrast, there were also responses in the right putamen (24, −8, 8, p < 0.001 uncorrected). Again, these adaptation responses depended on the posture of the hand and were significant only when the arm was extended [(Near-after-Far vs Near-after-Near)HAND vs (Near-after-Far vs Near-after-Near)NO HAND]. All corresponding activations are shown in Figure 4 and listed in Table 2. Thus, the results from these two complementary analyses converged onto the same set of premotor and parietal areas, underlining the robustness of the findings.
Figure 4.
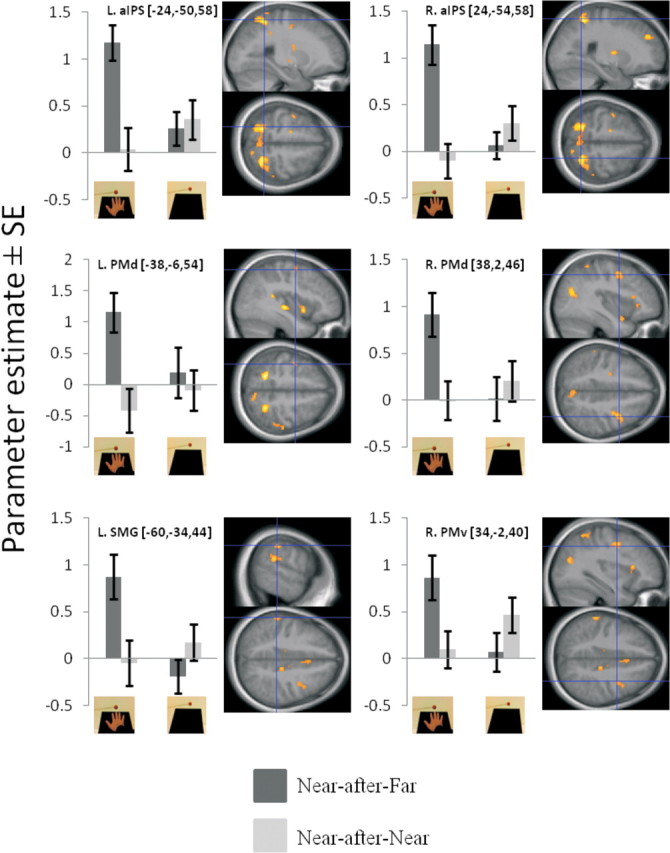
Key areas showing significant BOLD adaptation to near-hand stimuli, only when the arm is extended and placed on the table (second analysis approach; p < 0.05 corrected). The activation maps are derived from the contrast [(Near-after-Far vs Near-after-Near)HAND vs (Near-after-Far vs Near-after-Near)NO HAND]. Each plot corresponds to the parameter estimates for the Near-after-Far and the Near-after-Near conditions in the HAND (left) or NO HAND (right) runs (indicated by the pictures). Error bars represent SEM. The corresponding activation map is displayed on the sagittal and axial slices of the mean high-resolution structural scan from all participants. The significant peak of activations can be identified by the blue crosshairs; the threshold of the activation map was set at p < 0.001 uncorrected for display purposes.
Table 2.
Hand-centered BOLD adaptation to visual stimulation (second analysis approach): (Near-after-Far vs Near-after-Near)HAND versus (Near-after-Far vs Near-after-Near)NO HAND
| Anatomical location | MNI coordinates |
Peak t | Peak pa | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R. anterior intraparietal sulcus | 24 | −54 | 58 | 5.11 | 0.005 | 45 |
| L. anterior intraparietal sulcus | −24 | −50 | 58 | 4.34 | 0.020 | 36 |
| R. precentral sulcus (PMd) | 38 | 2 | 46 | 3.89 | 0.044 | 101 |
| L. supramarginal gyrus | −60 | −34 | 44 | 3.76 | 0.050 | 17 |
| L. precentral gyrus (PMd) | −38 | −6 | 54 | 3.76 | 0.050 | 17 |
| R. precentral sulcus (PMv) | 34 | −2 | 40 | 3.05 | <0.001b | 138 |
| R. putamen | 24 | −8 | 8 | 3.83 | <0.001b | 10 |
Significant (p < 0.05 corrected) BOLD adaptation to perihand visual stimulation, modulated by the posture of the hand, as obtained from the direct comparison of the contrast Near-after-Far versus Near-after-Near across HAND and NO HAND runs.
aSmall volume correction.
bp < 0.001 uncorrected.
Selectivity for perihand space: no hand-centered adaptation for visual stimuli in far space
We repeated all the analyses described above (both first and second approaches) for the visual stimulation presented in far space. Crucially, we wanted to ensure that the significant adaptation responses in the premotor–posterior parietal areas identified as being posture-dependent would not be present when objects were far from the hand. Thus, these analyses allowed us to further test the selectivity of the adaptation responses observed in the premotor and posterior parietal areas to visual stimuli presented near the hand. The Far stimuli yielded adaptation in clusters in visual areas in the occipital lobe regardless of the position of the hand, as expected from the adaption related to low-level visual features of the stimuli. These adaptation responses were very similar in location and extent to the arm-posture-independent adaptation responses that were observed for the repeated Near stimuli, suggesting that the Near and Far stimuli produced similar neural responses in the early visual areas. Similar to the first analysis approach for Near stimuli, the adaptation contrast for the repeated Far stimuli [(Far-before-Far vs Far-after-Far)HAND vs (Far-before-Far vs Far-after-Far)NO HAND] was performed. Importantly, no significant hand-centered adaptation effect was found for the Far stimuli in any portion of the IPS, the inferior parietal cortex, or in the premotor regions. Finally, the second analysis approach for the Far stimuli [(Far-after-Near vs Far-after-Far)HAND vs (Far-after-Near vs Far-after-Far)NO HAND] converged on the same results, not revealing any hand-centered adaptation effects. In conclusion, the two analysis approaches applied to the Near and Far stimuli revealed a remarkable selectivity of the premotor and posterior parietal adaptation responses to the presentation of objects near the hand.
Comparison of fMRI adaptation and a traditional analysis
To compare the fMRI adaptation approach described here with a traditional fMRI analysis looking at differences in the mean BOLD signal between conditions, we contrasted the Near and Far stimulation periods in the HAND [(Near-Near vs Far-Far)HAND] and NO HAND [(Near-Near vs Far-Far)NO HAND] runs. When the Near and Far stimulation in the HAND runs were compared, we observed activations in the left aIPS (−26, −44, 60, t = 6.87, p < 0.05 corrected), left SMG (−50, −34, 22, t = 4.11, p < 0.05 corrected), bilateral PMd (on the left, −34, −10, 54, t = 3.94, p < 0.05 corrected; on the right, 34, 8, 54, t = 3.77, p < 0.05 corrected), left thalamus (−18, −28, 0, t = 6.42, p = 0.05 corrected). We also noted activations in the right PMv (52, 14, 24, t = 3.53, p < 0.001 uncorrected) and bilateral putamen (on the left, −24, −4, −6, t = 3.69, p < 0.001 uncorrected; on the right, 30, −14, 8, t = 4.52, p < 0.001 uncorrected) that did not survive correction after multiple comparisons. This was performed under the aim of allowing the reader to better compare our results with similar approaches in the literature (e.g., Makin et al., 2007) and, more important, to allow comparison of the adaptation approach with a traditional analysis within the same dataset. The locations of these activations were thus very similar to what was observed for the adaptation responses. However, the traditional analysis could not establish whether these activations were selective for the presentation of the object near the hand. When we looked for areas showing greater BOLD signal differences between the Near and Far stimulation periods in the HAND runs compared with the NO HAND runs [(Near-Near vs Far-Far)HAND vs (Near-Near vs Far-Far)NO HAND], we found no significant activations anywhere in the brain. Even when we inspected the activation maps at p < 0.001 uncorrected, no clusters were observed in any of the relevant areas listed above (premotor and intraparietal cortices). Thus, although a traditional fMRI analysis could detect stronger premotor and posterior parietal activations when a moving ball was presented near the body compared with far from the body, only the adaptation approach could reveal neural responses that were specific to the proximity of the visual stimuli to the hand and could provide evidence for a hand-centered spatial representation.
Discussion
Selective mechanism for a hand-centered representation of space
We present converging evidence from two complementary fMRI adaptation analyses showing that human premotor and posterior parietal cortices contain neuronal populations that specifically encode visual stimuli close to the hand (perihand space). Such neuronal populations were found in the anterior part of the IPS bilaterally, the left SMG, and the PMd and PMv. These areas did not exhibit BOLD adaptation when the object was seen moving far from the hand or when the object was presented in exactly the same location close to the table but with the hand retracted. Together, these findings suggest that the human premotor and posterior parietal cortices are involved in a mechanism for the selective representation of visual stimuli near the body in hand-centered coordinates.
Adaptation is a robust phenomenon, extensively used to investigate the selectivity of neuronal populations for specific features of a visual stimulus (Grill-Spector, 2006; Grill-Spector et al., 2006; Sawamura et al., 2006). The precise mechanisms underlying BOLD adaptation are not yet fully understood (Krekelberg et al., 2006; Bartels et al., 2008); nevertheless, this approach has proven to be a useful tool for the study of neuronal selectivity (Grill-Spector and Malach, 2001; Avidan et al., 2002). fMRI adaptation offers the important advantage over traditional fMRI analysis in that it can reveal subpopulations of neurons within a single voxel that exhibit a selective preference for a particular stimulus feature. Indeed, when we used a traditional analysis comparing the Near and Far visual stimuli, we did not observe any areas exhibiting hand-centered preference for objects close to the hand. This could be due to the presence of intermingled neuronal populations with RFs centered on different body parts (e.g., eye-centered, face-centered, etc.) within the same voxels. fMRI adaptation overcomes this limitation by targeting neurons selective for a particular dimension of the stimulus; in our case, the proximity of the object to the hand. In light of this, our study provides compelling evidence for the existence of groups of neurons in the premotor, intraparietal, and inferior parietal cortices that have visual receptive fields restricted to the space near the hand. This represents a considerable advance compared with previous fMRI studies (Lloyd et al., 2003; Makin et al., 2007).
An additional strength of the present design is that it allowed many potential confounding factors to be eliminated. Importantly, an initial attentional shift toward the spatial location where the object appears at the beginning of the stimulation cannot explain our results (Kastner and Ungerleider, 2000; Greenberg et al., 2010). The adaptation effect is present only when the stimulus appears near the hand and only when the hand is placed on the table, whereas attentional shifts occur in all conditions. Thus, in the direct comparison between the adaptation contrasts in the HAND and NO HAND sessions, we effectively matched for spatial attentional load (besides, crucially, all low-level characteristics of the stimulation). Similarly, the simple effect of seeing the hand on the table or a putative modulation of visual attention driven by the mere presence of the hand cannot explain our results as we found no adaptation when the hand was visible but the object was presented far from it. Moreover, when comparing the second parts of the stimulation periods, as in the second analysis approach, we were also effectively controlling for any possible contribution of the mere presence of the hand in the visual field. Finally, the location and size of retinal stimulation of the moving ball were controlled by having the subjects fixate and by perfectly matching the effect of visual stimulus size when defining the direct adaptation contrasts between the HAND and NO HAND runs. Therefore, we conclude that our results reflect adaptation of neurons with visual receptive fields centered on the hand and restricted to peripersonal space.
A spatial representation for hand–object interactions
Even though caution should be exerted when comparing studies in nonhuman primates and humans, the premotor and parietal regions we found adapting in the present study match very well the electrophysiological data in macaque monkeys (Graziano, 2001). Indeed, peripersonal space neurons have been described in area 7b of the inferior parietal cortex (Hyvärinen and Poranen, 1974; Hyvärinen and Shelepin, 1979), at several foci in the IPS (Duhamel et al., 1998; Avillac et al., 2005; Grefkes and Fink, 2005), in the ventral premotor cortex (Rizzolatti et al., 1981a,b; Graziano et al., 1997), and subcortically in the putamen (Graziano and Gross, 1993). These neurons are multisensory because they integrate visual, tactile, proprioceptive, and even auditory information from a body part and its surrounding space (Rizzolatti et al., 1981a,b; Graziano et al., 1997, 1999; Avillac et al., 2005, 2007). One of the main properties of these neurons is that their tactile RFs are in spatial register with visual RFs covering a portion of space extending up to few centimeters (5–30 cm) from the tactile ones (Rizzolatti et al., 1981a; Fogassi et al., 1996). They typically abut on the tactile RF, forming a single responsive unit that includes the skin and the surrounding space. Similar RF properties have been described for auditory stimuli in peripersonal space (Graziano and Gandhi, 2000). Moreover, these neurons have been shown to receive proprioceptive inputs so that when the upper limb moves, the visual RF moves with it (Graziano and Gross, 1995; Graziano et al., 1997). In monkeys, this system of peripersonal neurons has been suggested to support many important functions ranging from sensory–motor transformations during the execution of goal-directed actions, such as reaching and grasping (Rizzolatti et al., 1981b; Iriki et al., 1996; Graziano and Gross, 1998b; Graziano et al., 2002), facilitating defense reactions from aversive stimuli (Graziano et al., 2002; Cooke et al., 2003; Graziano and Cooke, 2006) and the localizing limbs in space (Graziano, 1999).
In the present study, we did not investigate the multisensory properties of the neuronal populations responding to visual stimuli near the hand. However, several previous fMRI studies have shown that the same brain regions respond to multisensory stimuli in peripersonal space (Bremmer et al., 2001; Lloyd et al., 2003; Ehrsson et al., 2004; Macaluso and Driver, 2005; Makin et al., 2007; Tal and Amedi, 2009; Gentile et al., 2011). Importantly, Gentile and colleagues (2011) used an experimental setup very similar to the one used in the current study to compare bimodal visuotactile stimulation of the right hand with unimodal visual stimulation near the hand and unimodal tactile stimulation on the hand. We found that the bimodal conditions elicited the greatest activity in the premotor cortex (both PMv and PMd), intraparietal cortex, and inferior parietal cortex at sites close to the present peaks. For example, superadditive effects were identified in the anterior intraparietal sulcus (−32, −46, 62 in Gentile et al., 2011; −34, −50, 54 in the present study) and portions of the premotor cortex (−34, −16, 62 in Gentile et al., 2011; −48, −6, 48 in the present study). Furthermore, illusory binding of visual and tactile signals from the hand during the rubber hand illusion is associated with activity in similar parts of the ventral premotor cortex and left intraparietal cortex (Ehrsson et al., 2004). The present results indicate that the multisensory responses detected in these regions in previous studies may be specific to the space near the hand.
What human behaviors would depend on the perihand mechanism? In humans, activity in aIPS and premotor regions is associated with the preparation and execution of object-directed actions (Ehrsson et al., 2000, 2001; Culham et al., 2003; Fogassi and Luppino, 2005; Grefkes and Fink, 2005; Culham and Valyear, 2006). Brain stimulation studies of aIPS, for example, have shown its crucial role in the preparation phase of grasping an object; transcranial magnetic stimulation (TMS) disruption of aIPS activity has been shown to affect the correct selection of precision or power grip as a function of the visual information available (Davare et al., 2010). This suggests that in humans, as in primates, the selective perihand mechanism is used as an important interface for guiding hand actions toward objects within reaching distance (Brozzoli et al., 2011). Thus, the representation of the location of objects with respect to the hand could be used both to move the hand away from aversive objects (Makin et al., 2009) and to guide hand actions toward objects during reach-to-grasp actions (Brozzoli et al., 2009, 2010).
Conclusion
Using an fMRI adaptation design, we revealed a set of anatomically interconnected areas in the premotor and posterior parietal cortices that encode the location of objects near the body in hand-centered coordinates. This network includes the anterior portion of IPS and the SMG in the parietal lobe and the ventral and the dorsal premotor cortices in the frontal lobe. To the best of our knowledge, our study is the first to demonstrate selective adaptation to visual stimuli in space near the body, providing the most conclusive evidence to date for the existence of a neuronal representation of peripersonal space in the human brain.
Footnotes
This study was funded by the European Research Council, the Swedish Foundation for Strategic Research, Söderbergska Stiftelsen, the James S. McDonnell Foundation, the Human Frontier Science Program, and the Stockholm Brain Institute. All scans were performed at the MR-Centre at the Karolinska University Hospital in Huddinge, Sweden.
The authors declare no competing financial interests.
References
- Avidan G, Hasson U, Hendler T, Zohary E, Malach R. Analysis of the neuronal selectivity underlying low fMRI signals. Curr Biol. 2002;12:964–972. doi: 10.1016/s0960-9822(02)00872-2. [DOI] [PubMed] [Google Scholar]
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8:941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Avillac M, Ben Hamed S, Duhamel JR. Multisensory integration in the ventral intraparietal area of the macaque monkey. J Neurosci. 2007;27:1922–1932. doi: 10.1523/JNEUROSCI.2646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Logothetis NK, Moutoussis K. fMRI and its interpretations: an illustration on directional selectivity in area V5/MT. Trends Neurosci. 2008;31:444–453. doi: 10.1016/j.tins.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Pavani F, Urquizar C, Cardinali L, Farnè A. Grasping actions remap peripersonal space. Neuroreport. 2009;20:913–917. doi: 10.1097/WNR.0b013e32832c0b9b. [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Cardinali L, Pavani F, Farnè A. Action-specific remapping of peripersonal space. Neuropsychologia. 2010;48:796–802. doi: 10.1016/j.neuropsychologia.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Makin T, Cardinali L, Holmes N, Farnè A. Peripersonal space: a multisensory interface for body–objects interactions. In: Murray MM, Wallace MT, editors. Frontiers in the neural basis of multisensory processes. London: Taylor & Francis; 2011. pp. 447–464. [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CS, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Davare M, Rothwell JC, Lemon RN. Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr Biol. 2010;20:176–181. doi: 10.1016/j.cub.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Làdavas E, Farné A. Seeing where your hands are. Nature. 1997;388:730. doi: 10.1038/41921. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Farnè A, Demattè ML, Làdavas E. Neuropsychological evidence of modular organization of the near peripersonal space. Neurology. 2005;65:1754–1758. doi: 10.1212/01.wnl.0000187121.30480.09. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Gentile G, Petkova VI, Ehrsson HH. Integration of visual and tactile signals from the hand in the human brain: an fMRI study. J Neurophysiol. 2011;105:910–922. doi: 10.1152/jn.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci U S A. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. A system of multimodal areas in the primate brain. Neuron. 2001;29:4–6. doi: 10.1016/s0896-6273(01)00174-x. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:2621–2635. doi: 10.1016/j.neuropsychologia.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gandhi S. Location of the polysensory zone in the precentral gyrus of anesthetized monkeys. Exp Brain Res. 2000;135:259–266. doi: 10.1007/s002210000518. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. A bimodal map of space: somatosensory receptive fields in the macaque putamen with corresponding visual receptive fields. Exp Brain Res. 1993;97:96–109. doi: 10.1007/BF00228820. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. The representation of extrapersonal space: a possible role for bimodal, visuo-tactile neurons. In: Gazzaniga M, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. pp. 1021–1034. [Google Scholar]
- Graziano MS, Gross CG. Visual responses with and without fixation: neurons in premotor cortex encode spatial locations independently of eye position. Exp Brain Res. 1998a;118:373–380. doi: 10.1007/s002210050291. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. Spatial maps for the control of movement. Curr Opin Neurobiol. 1998b;8:195–201. doi: 10.1016/s0959-4388(98)80140-2. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Yap GS, Gross CG. Coding of visual space by premotor neurons. Science. 1994;266:1054–1057. doi: 10.1126/science.7973661. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Reiss LA, Gross CG. A neuronal representation of the location of nearby sounds. Nature. 1999;397:428–430. doi: 10.1038/17115. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T, Cooke DF. The cortical control of movement revisited. Neuron. 2002;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K. Selectivity of adaptation in single units: implications for fMRI experiments. Neuron. 2006;49:170–171. doi: 10.1016/j.neuron.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Poranen A. Function of the parietal associative area 7 as revealed from cellular discharges in alert monkeys. Brain. 1974;97:673–692. doi: 10.1093/brain/97.1.673. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Shelepin Y. Distribution of visual and somatic functions in the parietal associative area 7 of the monkey. Brain Res. 1979;169:561–564. doi: 10.1016/0006-8993(79)90404-9. [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD-signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Shore DI, Spence C, Calvert GA. Multisensory representation of limb position in human premotor cortex. Nat Neurosci. 2003;6:17–18. doi: 10.1038/nn991. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Multisensory spatial interactions: a window onto functional integration in the human brain. Trends Neurosci. 2005;5:264–271. doi: 10.1016/j.tins.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Zohary E. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J Neurosci. 2007;27:731–740. doi: 10.1523/JNEUROSCI.3653-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Brozzoli C, Rossetti Y, Farnè A. Coding of visual space during motor preparation: approaching objects rapidly modulate corticospinal excitability in hand-centered coordinates. J Neurosci. 2009;29:11841–11851. doi: 10.1523/JNEUROSCI.2955-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behav Brain Res. 1981a;2:125–146. doi: 10.1016/0166-4328(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav Brain Res. 1981b;2:147–163. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the fMRI-adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Sayers R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J Neurophysiol. 2005;95:995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Sobotka S, Ringo JL. Mnemonic responses of single units recorded from monkey inferotemporal cortex, accessed via transcommissural versus direct pathways: a dissociation between unit activity and behavior. J Neurosci. 1996;16:4222–4230. doi: 10.1523/JNEUROSCI.16-13-04222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal N, Amedi A. Multisensory visual–tactile object related network in humans: insights gained using a novel crossmodal adaptation approach. Exp Brain Res. 2009;198:165–182. doi: 10.1007/s00221-009-1949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]