Abstract
Dopamine release in cortical and subcortical structures plays a central role in reward-related neural processes. Within this context, dopaminergic inputs are commonly assumed to play an activating role, facilitating behavioral and cognitive operations necessary to obtain a prospective reward. Here, we provide evidence from human fMRI that this activating role can also be mediated by task-demand-related processes and thus extends beyond situations that only entail extrinsic motivating factors. Using a visual discrimination task in which varying levels of task demands were precued, we found enhanced hemodynamic activity in the substantia nigra (SN) for high task demands in the absence of reward or similar extrinsic motivating factors. This observation thus indicates that the SN can also be activated in an endogenous fashion. In parallel to its role in reward-related processes, reward-independent activation likely serves to recruit the processing resources needed to meet enhanced task demands. Simultaneously, activity in a wide network of cortical and subcortical control regions was enhanced in response to high task demands, whereas areas of the default-mode network were deactivated more strongly. The present observations suggest that the SN represents a core node within a broader neural network that adjusts the amount of available neural and behavioral resources to changing situational opportunities and task requirements, which is often driven by extrinsic factors but can also be controlled endogenously.
Introduction
In our everyday behavior, we are guided by environmental events that interact with our internal goals. For external events, numerous studies have shown that increased processing resources are allocated to certain stimuli because of their inherent biological relevance. Most prominently, reward-predicting stimuli encourage animals to pursue cognitive and behavioral operations necessary to receive the reward, and successful operations are reinforced. These effects have been linked to responses in mesencephalic dopaminergic areas [i.e., substantia nigra (SN) and ventral tegmental area (VTA)] that influence processing in their target structures in the basal ganglia and in cortical control areas (Wise, 2004; Pessiglione et al., 2006; Schultz, 2007; Robbins and Arnsten, 2009).
Pharmacological and lesion studies have indicated that disruptions of the dopaminergic system interfere with these activational aspects, whereas consummatory behavior is not affected (for an overview, see Salamone, 2009; see also Zink et al., 2004). Therefore, the role of dopamine in the context of reward has been conceptualized as being related to activation or incentive salience (Wise, 2004; Berridge, 2007; Robbins and Everitt, 2007). Consistent with this view, Parkinson's disease (PD) in humans has been linked to apathy, which can be counteracted by dopaminergic medication (Czernecki et al., 2002). Moreover, PD patients display deficits in a host of effortful executive functions (for an overview, see Nieoullon, 2002).
In addition to reward-related responses, dopaminergic midbrain areas have also been reported to react to environmental stimuli that are novel or otherwise perceptually salient (Redgrave et al., 1999; Horvitz, 2000; Zink et al., 2003; Bunzeck and Düzel, 2006). Crucially, all of these stimulus characteristics can be subsumed as extrinsic. Despite the large body of evidence for dopamine-system involvement in extrinsically driven resource allocation, it is as yet unknown whether the dopaminergic system can also be recruited in the absence of extrinsic factors. Importantly, although such endogenously driven recruitment of this area has been suggested (Nieoullon, 2002; Salamone et al., 2005; Braver et al., 2007; Arnsten et al., 2009), very little direct evidence for this notion has been provided thus far (Robbins and Everitt, 2007).
To test the hypothesis that dopaminergic midbrain areas also play an activating role in situations that do not entail extrinsically motivating or salient factors, we performed an fMRI experiment in which human observers performed a cued visual discrimination task with two task-demand levels. In doing so, we tried to closely parallel typical reward experiments in humans, which have observed activations in the dopaminergic midbrain areas (Knutson et al., 2005; Wittmann et al., 2005; Adcock et al., 2006). Many of these experiments used variants of the well established monetary-incentive delay task, in which a precue predicts whether reward can be obtained for fast and accurate performance in a subsequent simple task (e.g., detecting a target) (Knutson et al., 2001). In contrast, precues in the present experiment only predicted whether the upcoming task would be relatively easy or difficult, thus conceptually replacing reward prediction with the prediction of task demands.
Materials and Methods
Participants.
Fifteen subjects participated in this experiment after providing written informed consent. Two subjects were excluded from further analysis because of excessive head motion (exceeding 1° and/or 0.5 mm in abrupt movements in more than half of the runs); and an additional subject was excluded because of insufficient anatomical coverage of the midbrain regions of the functional images, leaving 12 subjects for the main analyses (mean age, 26 years; five females). Note that the inclusion of female subjects might have introduced some variability, since dopamine levels are known to vary across the menstrual cycle (Fernández-Ruiz et al., 1991), which will be addressed in future studies. The experiment was approved by the ethics committee of the Otto-von-Guericke University, Magdeburg, Germany, and subjects were paid for participation.
Paradigm.
Each trial started with a central blue or green arrow indicating the task demands of the forthcoming trial (high vs low task demands; cue-colors were counterbalanced across subjects). Cues were presented for 200 ms, followed 1000 ms later by a stimulus array (Fig. 1A). The task display consisted of two symbols synchronously presented (30 ms) in a rectangle centered 4° below and 8° right of the fixation spot. The fixation spot and the rectangle were constantly visible throughout the experiment. The stimuli were taken from an array of seven geometrical figures that were either red or white (Fig. 1B). In 60% of the trials, two randomly chosen nonidentical white figures were presented together, which represented nontargets under both conditions. In 20% of the trials, the stimulus array consisted of two randomly chosen identical white figures (targets under the high-demand condition), and the remaining 20% of the trials consisted of two randomly chosen red stimuli (targets under the low-demand condition; no shape discrimination required). Thus, the low-demand task required detecting a deviant color, whereas the high-demand task required comparing the shape of two symbols, which requires a deeper level of processing. The task (high vs low task demands) alternated in short blocks (∼25 s) to minimize the task-switching load. This, however, was only done to facilitate cue interpretation; each trial was individually cued and data analysis was performed in a fully event-related fashion. Intertrial intervals (ITI) were varied pseudorandomly between 2 and 6 s through inclusion of null events (using a geometrical distribution biased toward smaller ITIs) to allow for the separation of the different trials in an event-related fashion (Dale, 1999; Hinrichs et al., 2000; Liu, 2004). For the design matrices used in this experiment, correlations between the conditions of interest were −0.48 (averaged across runs, ±0.02), indicating that whenever signals were high in one condition they were low in the other condition; thus good separation of trial types was ensured.
Figure 1.
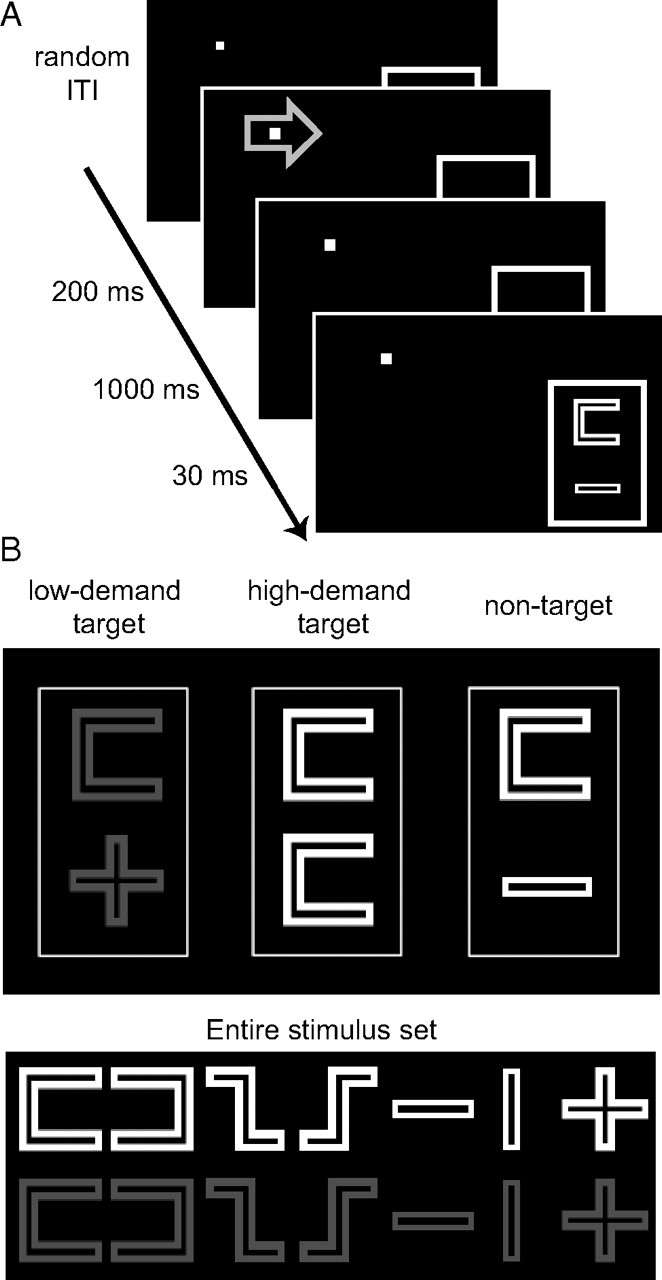
Paradigm and stimuli. A, Cues indicating which discrimination (high or low task demands, indicated by blue or green cue color, here represented in light gray) had to be performed were followed 1000 ms later by the brief presentation of two symbols within a peripheral rectangle in the right visual hemifield. B, Stimulus presentations either consisted of two red symbols (targets under the low task-demand condition; here represented in dark gray; left), two identical white symbols (targets under the high task-demand condition; middle), or two nonidentical white symbols (nontargets under both task instructions; right). Therefore, low-demand targets required detecting a color deviant, whereas high-demand targets required comparing the shape of two symbols. The full set of symbols is displayed (bottom; red stimuli again represented in dark gray). Note that fMRI analyses focused exclusively on nontarget trials that were physically identical under the high-demand versus low-demand conditions, thus stimulus differences cannot explain the observed fMRI results.
Both potential target types (relevant and irrelevant ones) appeared with equal probability after both cue colors, but subjects were only to respond to the targets of the currently cued task (right index finger button). The occurrence of both potential target types after both cue types ensured that subjects had to be aware of the preceding cue to perform the task correctly. This paradigm allowed us to compare fMRI activity in response to physically identical nontarget trials under different levels of task difficulty in the absence of any motor response. Additionally, eye movements were monitored using an MR-compatible infrared recording system (Kanowski et al., 2007). In general, subjects maintained very accurate fixation and no differential effects of eye-movement across conditions were observed.
fMRI data acquisition.
fMRI data were acquired on a Siemens 3-T Trio scanner using an eight-channel phased-array whole-head coil (Siemens); six runs with 169 volumes covering all but the uppermost part of the subjects' brains (MNI z-dimension after normalization, −30–56 mm) were recorded (repetition time, 2 s; echo time, 30 ms; flip angle, 80°; 128 × 104 × 24 at 2 × 2 × 3.6 mm). Additionally, an inversion-recovery EPI (inversion time, 1500 ms) and a proton-density-weighted image were acquired.
fMRI data analysis.
The first six volumes of each run were discarded from the analysis. The remaining functional images were analyzed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing included slice-time acquisition correction, realignment, spatial normalization, and smoothing (6 mm full width half maximum). Normalization parameters were derived from the segmented inversion-recovery EPI using standard procedures with 12 linear and 16 nonlinear transformations and medium regularization. This deformation matrix was then applied to all other volumes. Since the accuracy of standard normalization procedures for midbrain regions has been questioned (D'Ardenne et al., 2008; but see Düzel et al., 2009), we interactively checked the alignment of each subject's individual SN in the normalized PD image with the SN in the PD template provided by SPM, which were consistently in good register. After preprocessing, all experimental conditions were modeled with the canonical hemodynamic response function plus time and dispersion derivatives in an event-related model. A high-pass filter of 128 s and an autoregressive integrated moving-average model for serial correlations (equivalent to a low-pass filter) were applied to account for high- and low-frequency artifacts in the data. Task-relevant and task-irrelevant target trials and false alarms were modeled separately. The contrast of interest was calculated (high-demand > low-demand nontarget trials) for each subject and entered into a random-effects group analysis (one-sample t test). Statistical analysis was performed using cluster-based thresholding (p < 0.05 corrected cluster-size threshold with an auxiliary voxel-level threshold of t > 4.5; p < 0.0005). Results within midbrain regions were visualized by overlaying the results on the average spatially normalized proton-density weighted image of the participants using MRIcron (http://www.cabiatl.com/mricro/), on which the SN is visible as a bright stripe of increased image intensity. Additionally, whole-brain renderings of the activity maps were produced using Surfrend (http://spmsurfrend.sourceforge.net/), which were overlaid on an inflated whole-brain template using Freesurfer (http://surfer.nmr.mgh.harvard.edu/).
It has recently been argued that whole-head group-level fMRI approaches are problematic for the investigation of some midbrain areas (D'Ardenne et al., 2008; but see Düzel et al., 2009). Specifically, D'Ardenne and coworkers (2008) concluded that standard spatial resolutions and normalization strategies are insufficient to characterize hemodynamic activity in the VTA. To ameliorate such problems, we optimized our data acquisition and analysis approach to investigating midbrain activity (including small voxel sizes/high-resolution matrices and small smoothing kernels); results were overlaid on the average of the spatially normalized proton-density weighted structural scans from the same subjects, which provided excellent anatomical contrast for those midbrain areas. More importantly, however, the present study did not attempt to separate activations in the VTA from activations in the SN. In humans, these two structures build a continuous dorsal tier, which is much larger than the VTA alone (Ahsan et al., 2007), that projects to the striatum and cortical areas (Haber and Knutson, 2010). Moreover, such a distinction may not be crucial here, because in humans (in contrast to rodents), most of the dopaminergic cells are located within the SN rather than the VTA (Björklund and Dunnett, 2007).
Pupil dilation experiment.
Pupil dilation has been found to be a reliable indicator of task demands (Cabestrero et al., 2009) and can thus provide an additional window into the perceived level of task demands. Because such an analysis was not possible based on the eye-tracking data that was acquired during fMRI scanning, we ran an independent sample of subjects while recording their pupil sizes outside of the scanner (Eyelink 1000; SR-Reseach). Ten subjects were run (average age, 22.6 years; six females), using the identical experimental setup, and again counterbalancing cue colors between subjects. The resulting data were averaged across trials and baseline corrected with respect to a 200 ms precue baseline. Results for the low-demand and high-demand conditions were then compared using a sliding one-sample t test (p < 0.05, two-tailed).
Results
Behavioral results
The present experiment entailed two levels of task-demand that were precued by a colored arrow (see Materials and Methods, above) (Fig. 1). Following a short interval, two symbols were simultaneously flashed and subjects had to either compare the shapes (high task demands) or detect a deviant color (low task demands). No explicit performance feedback was provided to avoid indirect trial-based motivational factors (in addition to avoiding direct reward), and subjects were told in advance that their performance would have no influence on their compensation. Targets were rare, and the more frequent nontargets were identical under both conditions. This experimental set-up served two important functions: the target trials provided a measure of the task-demand manipulation, which was indeed very effective [high/low, 71% vs 98% correct responses; p < 0.001; response time (RT), 747 vs 560 ms; p < 0.001] and, even more importantly, it allowed us to focus our fMRI analysis entirely on nontarget trials (additionally excluding false-alarm trials, <3% of all trials) that did not entail motor responses. This approach excluded processes related to response execution that could also influence midbrain activity. Furthermore, the nontarget stimuli were identical under both instructions, so that fMRI effects also cannot be accounted for by any difference in visual stimulation. Hence, the fMRI results reported below are only related to the different levels of cognitive demand and effort during nontarget trials.
Pupil dilation results
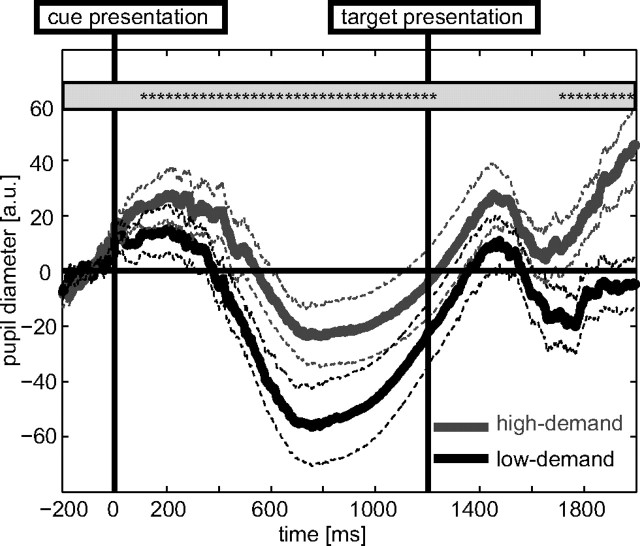
To further corroborate our notion that the behavioral differences between our main conditions indeed reflect differences in perceived task demands, we sought to provide an additional independent measure. One such measure is pupil dilation, which is found to increase with increasing task demands (Cabestrero et al., 2009). We recorded such data from an independent set of subjects (n = 10) outside of the scanner. The behavioral data of this group was similar to that of the main experiment (accuracy, 61% vs 95%; RT, 614 vs 478 ms; both p < 0.001). Figure 2 illustrates the grand-average pupil dilation results separated into the low-demand and the high-demand condition. It is obvious from this plot that pupil dilation was relatively more pronounced for the high-demand task, and that this effect was biphasic, showing clear differences both in response to the cue and to the targets. On an absolute level, pupil size appeared to shrink starting ∼400 ms after both stimuli (presumably in response to the bright visual input), but at the same time, the relative effects between high- and low-demand trials are very pronounced and significantly different over a wide time range. Note that high- and low-demand trials give rise to differential effects somewhat earlier than one would expect based on the known slow dynamics of the pupil response. It is conceivable that this is partly due to overlap from the preceding stimulus because the timing of the different events was optimized for the analysis of fMRI data, which is not optimal for the analysis of pupil responses.
Figure 2.
Grand-average pupil diameter results from an independent sample of subject that was run outside of the scanner (n = 10; ±SEM; *p < 0.05). Pupil size was larger in the high-demand relative to the low-demand condition in response to cues and to targets.
fMRI results
When comparing high minus low task-demand trials, our group-level results revealed enhanced bilateral cortical fMRI activity in the anterior cingulate cortex, the pre-supplementary motor area, the anterior insula extending into the frontal operculum, the dorsolateral prefrontal cortex, and in parietal and lateral occipitotemporal structures (corrected cluster size p value < 0.05; with an auxiliary voxel-level threshold of t > 4.5; p < 0.0005) (Fig. 3, red–yellow scale; Table 1). Additionally, large parts of the default-mode network (Raichle et al., 2001; Greicius et al., 2003), including the posterior cingulate cortex and the angular gyrus bilaterally, were deactivated more strongly for high than for low task demands (Fig. 3, blue–cyan scale; Table 2).
Figure 3.
Comparison between nontarget trials under high versus low task demands (average of all 12 subjects; corrected cluster level p < 0.05; voxel-level threshold: t >4.5, p < 0.0005). All positive differences (red–yellow scale) reflect increased fMRI signals for high task demands relative to low task demands (comparing identical nontarget trials only), whereas all negative differences (blue–cyan scale) represent areas that were deactivated more strongly during high task demands (Tables 1, 2). pre-SMA, Pre-supplementary motor area.
Table 1.
fMRI results for the comparison high > low task-demand nontarget trials
| Anatomical structure | Cluster size | Corrected cluster p value | Hemisphere | Maximum t value | MNI coordinates (x, y, z) |
|---|---|---|---|---|---|
| Subcortical areas | |||||
| Substantia nigra | 229 | <0.001 | L/R | 12.55 | 8, −20, −14 |
| Substantia nigra | 6.55 | −6, −18, −16 | |||
| Thalamus | 87 | <0.001 | L | 7.52 | −14, −22, 14 |
| Thalamus | 129 | <0.001 | R | 7.48 | 16, −6, 12 |
| Thalamus | 5.97 | 8, −10, 0 | |||
| Thalamus | 58 | 0.005 | L | 6.7 | −8, −12, −4 |
| Ventral striatum | 44 | 0.02 | L | 6.57 | −12, 10, −2 |
| Frontal cortical areas | |||||
| Anterior insula | 364 | <0.001 | L | 11.96 | −26, 30, 4 |
| Frontal operculum | 6.16 | −42, 18, 6 | |||
| Anterior insula | 390 | <0.001 | R | 10.92 | 36, 20, −2 |
| Frontal operculum | 4.88 | 50, 16, −10 | |||
| Pre-SMA | 411 | <0.001 | L/R | 9.58 | 2, 20, 48 |
| Frontal eye field | 151 | <0.001 | L | 7.33 | −26, 0, 50 |
| Frontal eye field | 5.28 | −38, −10, 52 | |||
| Inferior frontal junction | 152 | <0.001 | L | 7.28 | −38, −4, 28 |
| ACC | 55 | 0.006 | L/R | 7.24 | −4, 12, 28 |
| Inferior frontal junction | 175 | <0.001 | R | 6.97 | 48, 4, 28 |
| Frontal eye field | 91 | <0.001 | R | 6.77 | 32, −2, 56 |
| Inferior frontal gyrus | 104 | <0.001 | R | 6.56 | 50, 30, 24 |
| Middle frontal gyrus | 5.24 | 38, 44, 22 | |||
| Dorsal ACC | 59 | 0.004 | R | 6.1 | 12, 28, 34 |
| Posterior cortical areas | |||||
| Inferior parietal cortex | 927 | <0.001 | L | 10.04 | −52, −30, 44 |
| Superior parietal cortex | 7.7 | −32, −52, 56 | |||
| Inferior occipital gyrus | 405 | <0.001 | R | 8.97 | 52, −66, −14 |
| Inferior temporal gyrus | 7.74 | 52, −50, −6 | |||
| Middle occipital gyrus | 1417 | <0.001 | R | 8.88 | 46, −80, 0 |
| Inferior parietal lobule | 8.39 | 44, −40, 48 | |||
| Superior parietal cortex | 8.23 | 28, −56, 58 | |||
| Middle occipital gyrus | 1869 | <0.001 | L | 8.82 | −30, −78, 0 |
| Middle temporal gyrus | 8.55 | −46, −60, −4 |
Corrected cluster-level p < 0.05; voxel-level threshold t>4.5, p < 0.0005; distance between local maxima >16 mm for cortical and >12 mm for sub-cortical structures. Pre-SMA, Pre-supplementary motor areas; ACC, anterior cingulate cortex; L, left; R, right.
Table 2.
fMRI results for the comparison low > high task-demand nontarget trials
| Anatomical structure | Cluster size | Corrected cluster p value | Hemisphere | Maximum t value | MNI coordinates (x, y, z) |
|---|---|---|---|---|---|
| Frontal cortical areas | |||||
| Middle frontal gyrus | 153 | <0.001 | L | 8.59 | −28, 26, 50 |
| Middle frontal gyrus | 72 | 0.001 | R | 7.81 | 28, 22, 50 |
| Superior frontal gyrus | 281 | <0.001 | L | 7.21 | −10, 48, 44 |
| Superior frontal gyrus | 6 | −14, 66, 8 | |||
| Medial orbitofrontal cortex | 78 | 0.001 | L/R | 6.27 | 2, 62, −8 |
| Medial orbitofrontal cortex | 208 | <0.001 | L/R | 6.07 | −8, 26, −16 |
| Posterior cortical areas | |||||
| Angular gyrus | 842 | <0.001 | L | 11.8 | −52, −68, 40 |
| Posterior cingulate cortex | 1484 | <0.001 | L/R | 11.51 | −16, −46, 34 |
| Precuneus | 9.07 | −2, −62, 34 | |||
| Angular gyrus | 605 | <0.001 | R | 9.8 | 46, −58, 38 |
| Middle temporal gyrus | 117 | <0.001 | L | 8.14 | −60, −14, −20 |
| Parahippocampal gyrus | 46 | 0.016 | L | 6.45 | −30, −22, −20 |
| Middle temporal gyrus | 39 | 0.035 | R | 5.9 | 66, −36, −4 |
Corrected cluster level p < 0.05; voxel-level threshold t > 4.5, p < 0.0005; distance between local maxima >16 mm for cortical and >12 mm for subcortical structures. L, Left; R, right.
Most importantly, we observed enhanced fMRI signals in the high-demand task minus low-demand task bilaterally in the SN [local maxima at MNI (x/y/z): 8/−20/−14 (t = 12.55) and −6/−18/−16 (t = 6.55)] (Fig. 4), in line with our prediction. Further subcortical modulations were observed in the left ventral striatum and different parts of the thalamus. These results corroborate our hypothesis that dopaminergic midbrain structures are involved in the recruitment of processing resources even in the absence of any rewards or other salient extrinsic factors.
Figure 4.
Activity differences in the substantia nigra (average of all 12 subjects; corrected cluster level p < 0.05; voxel-level threshold: t > 4.5, p < 0.0005). The SN displayed enhanced responses to trials with high compared with low task demands (comparing identical nontarget trials only). Activations are overlaid on the averaged spatially normalized proton-density weighted image of all participants (the SN is visible as a bright stripe of enhanced image intensity).
Discussion
Here we demonstrate, for the first time, that activity in dopaminergic midbrain areas can occur in the complete absence of extrinsic factors such as reward or salient perceptual stimulus properties. Specifically, we found enhanced hemodynamic activity in the SN for nontarget trials under conditions of high compared with low task demands. The importance of this finding is underscored by current models on dopamine function that assign dopaminergic inputs a pivotal role in driving activity in frontal control regions and corticobasal ganglia processing loops related to a wide variety of executive functions (Seamans and Yang, 2004; Frank and O'Reilly, 2006; Braver et al., 2007).
In the present paradigm, the neural modulation by task demands in the SN clearly indicates that this region can be recruited volitionally in the absence of reward or highly salient stimuli, presumably via projections from prefrontal cortex (Moore et al., 1999). This finding suggests that motivational systems that are driven by extrinsic and intrinsic motivating factors overlap on the level of dopaminergic midbrain areas and functionally associated structures (most prominently the striatum, plus medial and lateral prefrontal regions). Despite this substantial overlap of neural networks, the mechanisms by which the dopaminergic midbrain areas are recruited may be distinct. Responses of dopaminergic neurons to extrinsic factors such as reward or salience are likely mediated by the superior colliculi (Dommett et al., 2005) or limbic areas, in particular the orbitofrontal cortex and the amygdala (Mohanty et al., 2008; Croxson et al., 2009; Schoenbaum et al., 2009). In contrast, volitional recruitment of the dopaminergic areas is more likely driven by higher-level prefrontal control areas (Cho and Strafella, 2009).
Although the present work focuses on dopaminergic midbrain areas, the activations observed here arise from the interplay with a broad network of cortical and subcortical regions. Some of these areas are presumably instrumental in operations that are specific to the two tasks that were compared (comparing the shape of two stimuli vs detecting a deviant color). Such specific operations, however, are unlikely to underlie the activity in high-level control areas and even less likely in the SN. Moreover, many of the identified frontal regions (including anterior–insular together with lateral and medial frontal areas) have been suggested to be part of a system that generally responds to situations of increased cognitive demand (Duncan and Owen, 2000; Dosenbach et al., 2006). Based on resting-state connectivity combined with personality-trait data, a further subdivision of this demand system into an executive-control network and a salience network has been recently suggested (Seeley et al., 2007). The latter is implicated with detecting relevant stimuli, and the SN is assumed to be a part of this network that is processing reward-related and other salient stimuli. In the context of this distinction, the present data indicate that the role of the SN goes beyond being part of a salience network.
The above findings of enhanced hemodynamic activity during high task demands were complemented by substantially stronger deactivations in large parts of the default-mode network (Raichle et al., 2001; Greicius et al., 2003), which is in accord with suggestions that dopamine is involved in task-related deactivations of this network (Argyelan et al., 2008; Engelmann et al., 2009; Tomasi et al., 2009). Together, the dopaminergic system appears to impact multiple neural targets, suggesting that its widespread innervations of many crucial control areas (Björklund and Dunnett, 2007) can be used to promote a state of enhanced availability of cognitive and behavioral resources. From a computational perspective, in particular with respect to the frontal cortex, it has been suggested that dopamine modulates the response to incoming information (Servan-Schreiber et al., 1990; Braver and Cohen, 2000; Seamans and Yang, 2004). Such a mechanism is assumed to effectively enhance the signal-to-noise ratio, but may come at the expense of higher energy demands and less influence of unattended inputs (Servan-Schreiber et al., 1990) and is therefore likely to be used in an on-demand fashion only (Robbins and Arnsten, 2009). This putative gating mechanism might be of particular relevance in situations where proactive preparation is possible (Braver et al., 2007), as in the present experiment.
On a conceptual level, our results imply that neural activity in mesolimbic reward regions observed in reward-prediction experiments, which are typically attributed directly to the reward-predicting properties of stimuli, could in fact be (at least partly) related to the cognitive effort that is exerted to achieve the reward—two factors that are inherently hard to dissociate (Maunsell, 2004). In this context, it is important to note that, because of the total absence of any direct reward or punishment, which are integral to most animal studies, the present task could only be performed by human participants. Moreover, although a direct paradigmatical combination of task difficulty and reward is appealing (Engelmann et al., 2009), it was mandatory for the purpose of the current study to avoid a reward context altogether, since neutral trials in the context of rewarded trials can be perceived as disappointing or might be avoided in a choice situation (Croxson et al., 2009; McGuire and Botvinick, 2010).
In accord with our findings, pharmacological and lesion studies in animals have indicated a central role of dopamine in the propensity to exert (physical) effort to achieve rewards (for review, see Salamone et al., 2005). Moreover, fMRI experiments have found that modulations of reward-related activity within one of the key dopaminergic target structures, the striatum, strongly depend on whether subjects have to perform a simple detection task to obtain a reward (Zink et al., 2004; Bjork and Hommer, 2007). These observations imply a more general role of this SN signal, possibly related to the amount of effort exerted (Wise, 2004; Salamone et al., 2005). Importantly, effort in animal experiments is usually operationalized in choice settings that require physical labor, thus putting it at some distance to the present study. However, more closely related studies in humans have indicated a similar role for the involved structures in tasks that invoke a form of effort that is rather cognitive in nature (Zink et al., 2004; Bjork and Hommer, 2007).
Evidently, modulations of fMRI signals in dopaminergic midbrain areas should not be equated directly with dopamine release in their target structures. Nonetheless, several observations corroborate the notion that the observed SN activity is indeed linked to enhanced dopaminergic neurotransmission: attentional deficits (especially concerning top-down, endogenous attention) have been reported for PD patients, along with deficits in other executive functions (Brown and Marsden, 1988; Yamaguchi and Kobayashi, 1998; Nieoullon, 2002; Cools, 2006). In accord with these findings, pharmacological and lesion studies in animals reported influences of the dopaminergic system on functions related to the recruitment of attentional resources (for review, see Robbins, 2005), and pharmacological investigations in humans have also yielded corroborating results (Clark et al., 1987; Servan-Schreiber et al., 1998; Bullmore et al., 2003). Moreover, demonstrations of a strong correlation between reward-related mesolimbic fMRI responses and reward-related striatal dopamine release in the same human subjects ([11C]raclopride displacement PET) suggests a strong relationship between the two measures (Schott et al., 2008).
The current findings extend our knowledge about the processes that underlie the recruitment of neural processing resources and thereby have implications on the understanding of major neurological and psychiatric disorders. Specifically, deficits in top-down resource recruitment have not only been described in PD patients, but also in attention deficit hyperactivity disorder, schizophrenia, autism, and drug abuse—conditions that have all been related to disturbed dopaminergic neurotransmission (Nieoullon, 2002; Everitt and Robbins, 2005). Additionally, cognitive deficits in normal aging have been linked to a decline of dopaminergic cells (Arnsten et al., 1995).
In summary, we conclude that dopaminergic midbrain areas play a central role in the control of neural processing resources beyond the activation by extrinsic motivating factors. Specifically, it appears that these areas can be recruited in a top-down fashion to adjust the amount of available cognitive and motor resources to be able to meet changing situational demands and opportunities without wasting energy when such resources are not required.
Footnotes
This work was supported by grants from German Research Council (BO 3345/1-1 to C.N.B., SFB-TR31/TPA8 to T.N., and SFB 779 to M.A.S, J.M.H., and H.J.H.), and the German Ministry for Education and Research (Contract 01GO0202) to the Center for Advanced Imaging, Magdeburg. We thank Dr. Michael Scholz for technical support, Johanna Starke for assistance with data collection, and Joseph A. King for valuable comments.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, Koepp MJ, Hammers A. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–270. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Ghilardi MF, Feigin A, Mattis P, Tang C, Dhawan V, Eidelberg D. Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci. 2008;28:10687–10695. doi: 10.1523/JNEUROSCI.2933-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine's influence on prefrontal cortical cognition: actions and circuits in behaving primates. In: Iversen L, Iversen S, Dunnett S, Bjorklund A, editors. Dopamine handbook. New York: Oxford UP; 2009. pp. 230–248. [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res. 2007;177:165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: the role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and performance XVIII. Cambridge, MA: MIT; 2000. pp. 713–738. [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway AR, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford Oxford Univerity; 2007. pp. 76–106. [Google Scholar]
- Brown RG, Marsden CD. Internal versus external cues and the control of attention in Parkinson's disease. Brain. 1988;111:323–345. doi: 10.1093/brain/111.2.323. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Suckling J, Zelaya F, Long C, Honey G, Reed L, Routledge C, Ng V, Fletcher P, Brown J, Williams SC. Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cereb Cortex. 2003;13:144–154. doi: 10.1093/cercor/13.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cabestrero R, Crespo A, Quirós P. Pupillary dilation as an index of task demands. Percept Mot Skills. 2009;109:664–678. doi: 10.2466/pms.109.3.664-678. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Geffen GM, Geffen LB. Catecholamines and attention. II. Pharmacological studies in normal humans. Neurosci Biobehav Rev. 1987;11:353–364. doi: 10.1016/s0149-7634(87)80007-6. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia. 2002;40:2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz JJ, Hernández ML, de Miguel R, Ramos JA. Nigrostriatal and mesolimbic dopaminergic activities were modified throughout the ovarian cycle of female rats. J Neural Transm Gen Sect. 1991;85:223–229. doi: 10.1007/BF01244947. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O'Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs H, Scholz M, Tempelmann C, Woldorff MG, Dale AM, Heinze HJ. Deconvolution of event-related fMRI responses in fast-rate experimental designs: tracking amplitude variations. J Cogn Neurosci. 2000;12(Suppl 2):76–89. doi: 10.1162/089892900564082. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Kanowski M, Rieger JW, Noesselt T, Tempelmann C, Hinrichs H. Endoscopic eye tracking system for fMRI. J Neurosci Methods. 2007;160:10–15. doi: 10.1016/j.jneumeth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT. Efficiency, power, and entropy in event-related fMRI with multiple trial types, part II: design of experiments. Neuroimage. 2004;21:401–413. doi: 10.1016/j.neuroimage.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A. 2010;107:7922–7926. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Dopamine, effort, and decision making: theoretical comment on Bardgett et al. (2009) Behav Neurosci. 2009;123:463–467. doi: 10.1037/a0015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Carter CS, Bruno RM, Cohen JD. Dopamine and the mechanisms of cognition, part II: d-amphetamine effects in human subjects performing a selective attention task. Biol Psychiatry. 1998;43:723–729. doi: 10.1016/s0006-3223(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T, Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi S. Contributions of the dopaminergic system to voluntary and automatic orienting of visuospatial attention. J Neurosci. 1998;18:1869–1878. doi: 10.1523/JNEUROSCI.18-05-01869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]