Abstract
When and how newborn neurons are organized to form a functional network in the developing brain remains poorly understood. An attractive model is the gonadotropin-releasing hormone (GnRH) neuron system, master regulator of the reproductive axis. Here we show that blockage of IGF signaling, a central growth-promoting signaling pathway, by the induced expression of a dominant-negative form of IGF1 receptor (IGF1R) or specific IGF1R inhibitors delayed the emergence of GnRH2 neurons in the midbrain and GnRH3 neurons in the olfactory bulb region. Blockage of IGF signaling also resulted in an abnormal appearance of GnRH3 neurons outside of the olfactory bulb region, although it did not change the locations of other olfactory neurons, GnRH2 neurons, or brain patterning. This IGF action is developmental stage-dependent because the blockade of IGF signaling in advanced embryos had no such effect. An application of phosphatidylinositol 3-kinase (PI3K) inhibitors phenocopied the IGF signaling deficient embryos, whereas the MAPK inhibitors had no effect, suggesting that this IGF action is mediated through the PI3K pathway. Real-time in vivo imaging studies revealed that the ectopic GnRH3 neurons emerged at the same time as the normal GnRH3 neurons in IGF-deficient embryos. Further experiments suggest that IGF signaling affects the spatial distribution of newborn GnRH3 neurons by influencing neural crest cell migration and/or differentiation. These results suggest that the IGF-IGF1R-PI3K pathway regulates the precise temporal and spatial organization of GnRH neurons in zebrafish and provides new insights into the regulation of GnRH neuron development.
Introduction
When and how newborn neurons are organized to form a functional network in the CNS remains poorly understood (Ayala et al., 2007). An attractive model involves the gonadotropin-releasing hormone (GnRH) neurons, master regulators of the vertebrate reproductive axis. During development, GnRH neurons conduct a long-distance migration, starting from the olfactory region and reaching the preoptic area (POA) and hypothalamus (Schwanzel-Fukuda and Pfaff, 1989; Wray et al., 1989). Many vertebrate species have multiple forms of GnRHs. For instance, there are two forms of GnRHs in zebrafish, namely GnRH2 and GnRH3. The zebrafish GnRH3 neurons are the counterparts of the mammalian hypophysiotropic GnRH1 neurons and are located at the preoptic area–hypothalamus and olfactory bulb–terminal nerve in the adult brain (Steven et al., 2003; Palevitch et al., 2007). Hypothalamic GnRH3 neurons are found to be originated from the olfactory area (Abraham et al., 2008, 2010) and GnRH2 neurons are located in the midbrain in zebrafish (Abraham et al., 2010). Previous studies have suggested that both zebrafish GnRH3 neurons and GnRH2 neurons may originate from the cranial neural crest (Whitlock et al., 2003, 2005), although their precise embryonic origins and relationship are still under debate.
While the routes and timing of GnRH neuron migration have been elucidated, the underlying regulatory mechanisms are less clear (Tobet and Schwarting, 2006). Insulin-like growth factors (IGFs) are major embryonic growth-promoting factors (Duan and Xu, 2005). IGFs regulate many aspects of brain development in mice and zebrafish, such as neuron differentiation, survival, and migration (Bondy and Cheng, 2004; Schlueter et al., 2007; Joseph D'Ercole and Ye, 2008; Annenkov, 2009). Mammalian GnRH neurons coexpress IGF1 and the IGF1 receptor (IGF1R) in postnatal and adult stages in vivo (Daftary and Gore, 2005). IGF1 stimulates cell proliferation and GnRH gene expression in mammalian GnRH neuronal cell lines (Zhen et al., 1997; Longo et al., 1998) and modulates GnRH activities in cultured pituitary cells isolated from salmonid fish (Weil et al., 1999; Baker et al., 2000; Ando et al., 2006; Furukuma et al., 2008; Luckenbach et al., 2010). IGF1 has also been shown to regulate the migration of neural crest-derived melanoblasts in chick embryos (Schöfer et al., 2001). In young rats, pharmacological inhibition of brain IGF signaling impairs estrous cyclicity (Todd et al., 2007) and reduced brain IGF signaling in middle-aged female rats leads to luteinizing hormone surge dysfunction by affecting estrogen-dependent processes that influence GnRH neuron activation and/or GnRH release (Todd et al., 2010). Despite these studies, it is unclear whether IGF signaling plays any role in GnRH neuron development during embryogenesis.
In this study, we used the zebrafish model to test the hypothesis that IGF signaling regulates the proper spatial and temporal organization of GnRH neurons in early development.
The free-living and transparent zebrafish embryos are well suited for investigation of the regulation of GnRH neuron development. The availability of the Tg(GnRH3:EGFP) line, in which GnRH3 neurons express EGFP (Abraham et al., 2008), provides a powerful system to visualize GnRH neurons in living embryos. Our results suggest that IGF signaling is critical for the timing of both GnRH2 and GnRH3 neuron emergence as well as for the spatial distribution of GnRH3 neurons.
Materials and Methods
Materials.
All chemicals and reagents were purchased from Fisher Scientific unless noted otherwise. Superscript III reverse transcriptase and oligonucleotide primers were purchased from Invitrogen Life Technologies. The anti-total and Phospho-Akt antibodies were purchased from Cell Signaling Technology. The polyclonal anti-IGF1R antibody (C-20) was purchased from Santa Cruz Biotechnology and the fibroblast growth factor receptor 1a (FGFR1) antibody was from Ana Spec. The anti-phosphotyrosine antibody (4G10) was purchased from Millipore. A selective inhibitor of IGF1R kinase, NVP-AEW541 (García-Echeverria et al., 2004), was provided by Novartis Institutes. BMS-754807, another structurally distinct IGF1R inhibitor (Wittman et al., 2009), was purchased from Active BioChem. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY-294002 and the MEK inhibitor U0126 were purchased from Biomol. The PI3K inhibitor wortmannin was purchased from Sigma. The olfactory marker protein (OMP) and transient receptor potential channel 2 (TRPC2) cDNAs were kindly provided by Dr. N. Miyasaka and Y. Yoshihara (RIKEN Brain Science Institute, Saitama, Japan) and Prof. R. Celik (Bogazici University, Istanbul, Turkey). The sox10 and crestin cDNAs were kindly provided by Prof. P. Henion (Ohio State University, Columbus, Ohio).
Animals.
Zebrafish (Danio rerio) were maintained at 28°C on a 14:10 h (light:dark) cycle and fed twice daily. Males and females were maintained in separate tanks until the day of natural crossing to generate embryos. The fertilized eggs were raised at 28.5°C and staged according to Kimmel et al. (1995). Phenylthiourea (0.2 mm) was added to prevent pigmentation. Tg(hsp70:dnIGF1R-GFP) and Tg(hsp70:GFP) transgenic lines, which express a dominant-negative form of IGF1R-GFP (dnIGF1R-GFP) or GFP under the control of the zebrafish hsp70 promoter, were generated and maintained as reported (Kamei et al., 2011). The generation of the Tg(GnRH3:EGFP) transgenic line that expresses EGFP in GnRH3 neurons has been reported previously (Abraham et al., 2008). To study the role of IGF signaling on GnRH3 neurons, the Tg(hsp70:dnIGF1R-GFP) line was crossed with the Tg(GnRH3:EGFP) line. As a control, the Tg(hsp70: GFP) line was crossed with the Tg(GnRH3:EGFP) line. The Tg(a1-tubulin1a:EGFP) fish, in which developing neurons are labeled with expression of EGFP (Goldman et al., 2001), were provided by Dr. D. Goldman's group (University of Michigan, Ann Arbor, Michigan). All experiments were conducted in accordance with guidelines approved by the University Committee on the Use and Care of Animals, University of Michigan.
Genetic and pharmacological blockade of the IGF signaling.
To verify the effectiveness of the heat shock induction of dnIGF1R:GFP and its effect in blocking IGF signaling, Tg(hsp70:dnIGF1R-GFP) embryos were subjected to heat shock (HS) treatment. Briefly, the embryos were placed in a 0.5 ml tube containing 0.4 ml of embryo-rearing solution and then placed in a PCR machine at 37°C for 1 or 0.5 h. Wild-type and Tg(hsp70:GFP) embryos were treated at the same time and used as controls. For pharmacological inhibition experiments, drugs were dissolved in DMSO and added in the embryo-rearing solution directly.
In situ hybridization.
To generate the GnRH2 and GnRH3 probes, their partial cDNAs were cloned using the following primers: GnRH2 forward primer: 5′-GCATGGAGTGGAAAGGAAGG-3′ and reverse primer: 5′-GGAGTCCAAAAA CATGGTCT-3′; GnRH3 forward primer: 5′-ATGGTGCTGGTCTGCAGGCT-3′ and reverse primer: 5′-GAACTGCTGCA AATGGGTAC-3′. cDNA prepared from adult zebrafish brains was used as template. The PCR products were cloned into pGEM-T Easy Vector System I (Promega). The synthesis of digoxigenin-labeled RNA riboprobe and hybridization were performed as previously reported (Maures et al., 2002). Photographs were taken under a Leica MZ16F microscope (Leica Microsystems).
Reverse transcription PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen). After DNase treatment (Invitrogen), RNA samples were reverse transcribed with random hexamer primers and SuperScript III reverse transcriptase (Invitrogen). PCR was performed using TaqDNA polymerase (Biolabs) under the following conditions: 94°C for 2 min, 94°C for 30 s, 58°C for 30 s, 70°C for 30 s, 25 cycles for β-actin, and 36 cycles for GnRH3. The following primers were used: GnRH3 forward primer: 5′-CAGCACTGGTCATATGGTTGGC-3′ and reverse primer: 5′-CACTCTTCCCCGTCTGTCGG-3′; β-actin forward primer: 5′-AGGCCAACCGCGAGAAGATGACC-3′ and reverse primer: 5′-GAAGTCCAGGGCGACGTAGCAC-3′.
Immunoprecipitation and Western immunoblotting analysis.
Western immunoblotting was performed as described previously (Schlueter et al., 2007). Approximately 60 embryos for each group were dechorionated, deyolked, homogenized, centrifuged, and subjected to immunobloting analysis. For phospho-IGF1R and FGFR1, equal amounts of proteins were subjected to immunoprecipitation using the G416 phosphotyrosine antibody and followed by Western immunoblotting as described previously (Kamei et al., 2011).
Developmental rate.
The somite number was used as an indicator of developmental rate up to 26 h postfertilization (hpf). For this, embryos were anesthetized and photographed. Their somite number was determined manually.
Statistics.
Data are shown as means ± SEM. Statistical significance among experimental groups was determined by one-way ANOVA, followed by the Tukey's multiple-comparison test. Fisher's exact test, followed by theχ2 test, was used to compare the percentage of embryos with GnRH2 or GnRH3 neurons between test groups with the control group. Significance was accepted at p < 0.05 or better.
Results
Genetic and pharmacological blockade of IGF1R signaling in zebrafish embryos
Two transgenic zebrafish lines, Tg(hsp70:dnIGF1R-GFP) and Tg(hsp70:GFP), that express a dominant-negative form of the zebrafish IGF1Ra or GFP alone under the control of the heat shock-inducible hsp70 promoter (Kamei et al., 2011) were used to investigate the role of IGF signaling in GnRH neuron development. Fluorescence microscopy indicated robust expression of dnIGF1R-GFP or GFP expression after the HS treatment (1 h at 37°C) (Fig. 1A). No GFP signal in somatic cells was observed in wild-type embryos after the HS treatment. Induction of dnIGF1R-GFP expression caused growth retardation and developmental delay, as indicated by a smaller body size (Fig. 1A) and reduced somite number (Fig. 1D). Somite number is used as a quantitative parameter for determining the developmental stage during 10–24 hpf (Kimmel et al., 1995). As shown in Figure 1C, HS treatment resulted in a major reduction in the levels of phospho-IGF1R and phospho-Akt. HS treatment of the control Tg(hsp70:GFP) embryos did not alter IGF1R or Akt phosphorylation or growth (data not shown). As previously reported (Kamei et al., 2011), induction of dnIGF1R-GFP expression did not decrease the levels of phospho-FGFR1 (Fig. 1C). Although the phospho-FGFR1 levels in the HS treatment group appeared to be higher, this was likely loading/transfer variation, as indicated by similar high levels of total Akt (Fig. 1C).
Figure 1.
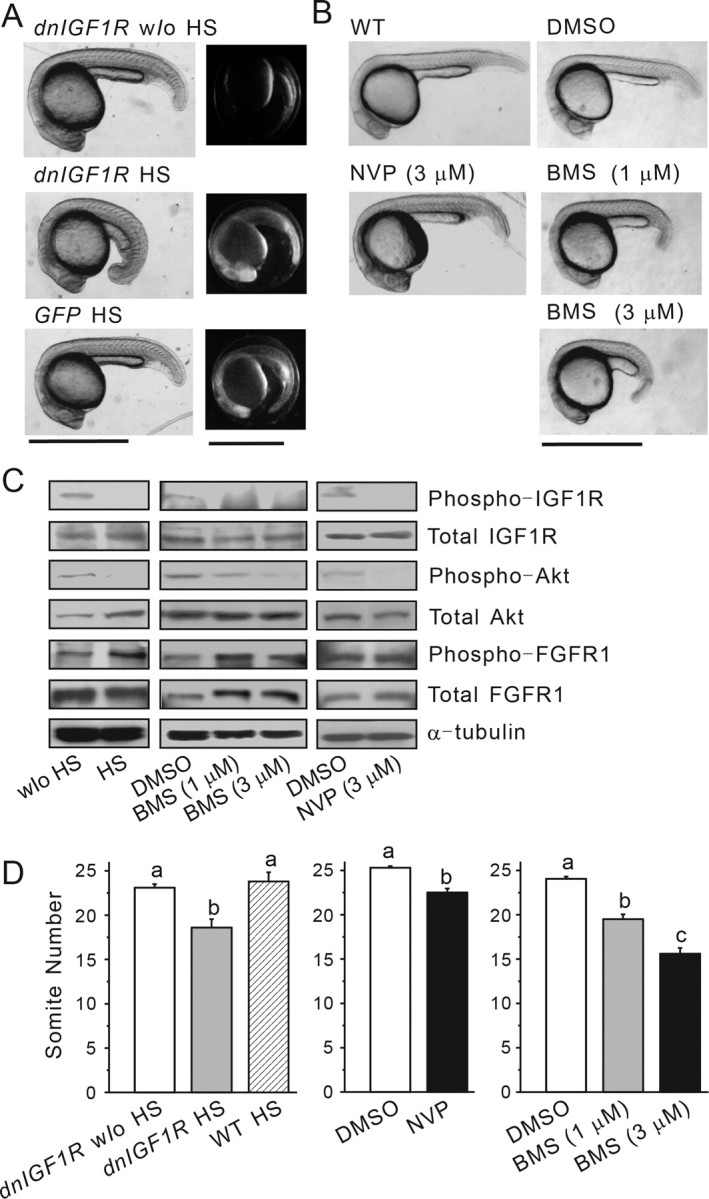
Temporally controlled genetic and pharmacological blockade of IGF signaling in zebrafish embryos. A, Inducible expression of a dominant-negative form of IGF1R. Tg(hsp70:dnigf1r-GFP) (dnIGF1R) or Tg(hsp70:GFP) (GFP) embryos were subjected to 37°C HS treatment at 4 hpf for 1 h and raised to 26 hpf and photographed. Phase-contrast views are shown in the left column and fluorescence views in the right column. Note GFP expression in somatic cells induced by HS. Yolk sac is autofluorescent. Scale bar, 1 mm. B, Pharmacological inhibition of IGF signaling. Wild-type embryos were treated with NVP-AEW541 (NVP), BMS-754807 (BMS), or the vehicle (DMSO) at the indicated concentrations from 4 to 26 hpf. A representative embryo from each group is shown. Scale bar, 1 mm. C, Efficient and specific blockade of IGF signaling. Embryos described in A and B were analyzed by Western blotting using the appropriate antibodies. For phosphorylated IGF1R and FGFR1, embryos were immunoprecipitated using an antiphosphotyrosine antibody followed by Western blotting using the appropriate antibodies. D, Effect of IGF signaling blockade on embryonic growth. Embryos were treated as described in A and B and raised to 26 hpf. Somite number was determined and shown. Data are mean ± SE, n = 7∼20. Groups labeled with different letters are significantly different from each other (p < 0.05).
As an independent and complimentary approach to block IGF signaling, we used NVP-AEW541 and BMS-754807, two specific and structurally distinct IGF1R kinase inhibitors (García-Echeverria et al., 2004; Wittman et al., 2009). Treatments of zebrafish embryos with either of these inhibitors led to marked reduction in IGF1R and Akt phosphorylation and developmental delay (Fig. 1B–D). NVP-AEW541 or BMS-754807 treatment did not decrease the levels of phospho-FGFR1 (Fig. 1C). In fact, there was an increase in the levels of phospho-FGFR in the BMS-754807 1 μm group, but the increase was smaller in the BMS-754807 3 μm group (Fig. 1C). The levels of total FGFR were also higher in these groups.
Blockade of IGF signaling delays the timing of GnRH3 neuron emergence
We next examined the possible effect of the blockade of IGF signaling on the emergence/differentiation of GnRH3 neurons. For this, the Tg(hsp70:dnIGF1R-GFP) embryos were treated with HS from 4 to 5 hpf, and embryos were sampled at 26 and 32 hpf (Fig. 2A). Reverse transcription (RT)-PCR analysis showed a strong reduction in the levels of GnRH3 mRNA in the HS-treated Tg(hsp70:dnIGF1R-GFP) embryos (Fig. 2B). As shown in Figure 2C, two clusters of GnRH3 neurons were detected in the forebrain region in 24% of wild-type embryos and 20% of untreated Tg(hsp70:dnIGF1R-GFP) embryos at 26 hpf, but none were observed in HS-treated Tg(hsp70:dnIGF1R-GFP) embryos at this stage. At 32 hpf, GnRH3 neurons were found in 90% of the HS-treated wild-type embryos and 100% of the Tg(hsp70:dnIGF1R-GFP) embryos without HS treatment (Fig. 2C). In contrast, only 22% of the HS-treated Tg(hsp70:dnIGF1R-GFP) embryos were GnRH3-positive (Fig. 2C).
Figure 2.
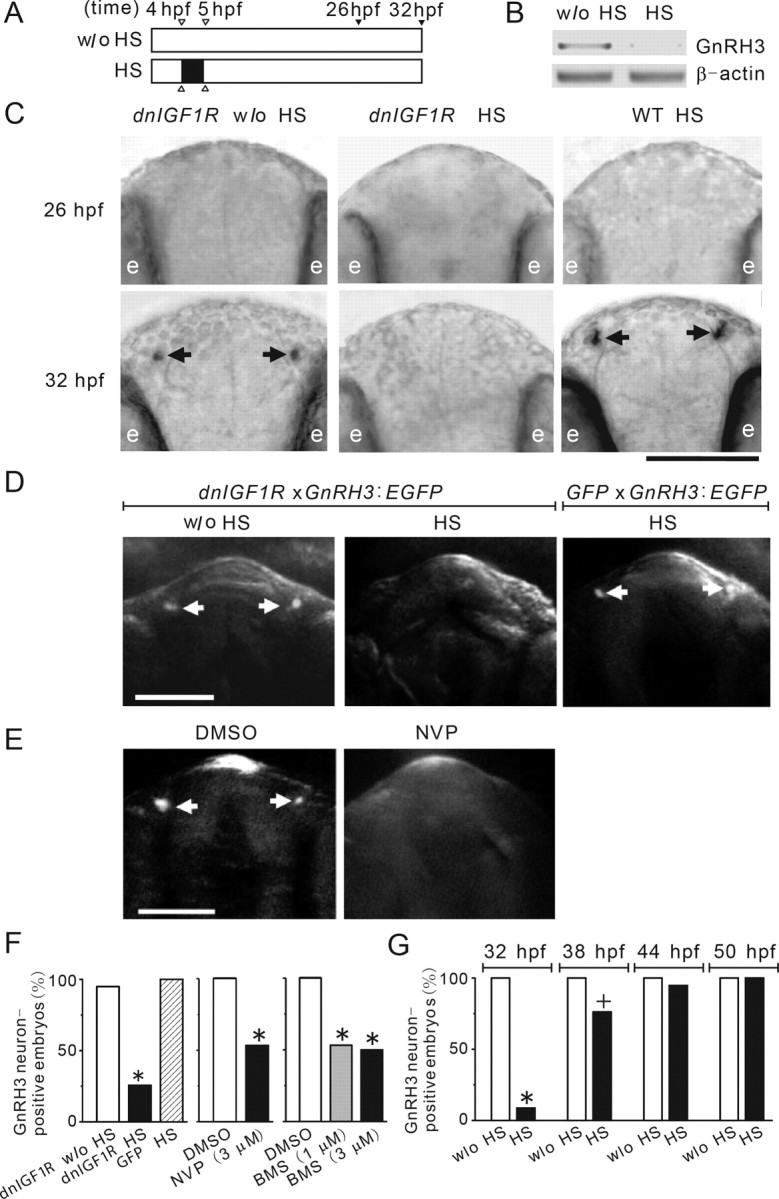
Blockade of IGF signaling delays the timing of GnRH3 neuron emergence. A, A schematic diagram illustrating the experimental design. Tg(hsp70:dnigf1r-GFP) embryos were subjected to 1 h 37°C HS treatment from 4 to 5 hpf or no HS treatment (w/o HS) and raised to 26 and/or 32 hpf and analyzed. B, RT-PCR result. Tg(hsp70:dnigf1r-GFP) described in A were raised to 26 hpf and analyzed by RT-PCR for GnRH3 and β-actin mRNA. C, Embryos described in A were analyzed by whole-mount in situ hybridization and representative views are shown in the bottom. Arrows indicate GnRH3 neurons. The locations of eyes are indicated by e. In this and all following figures, dorsal views are shown with anterior up unless stated otherwise. Scale bar, 0.1 mm. D, Tg(hsp70:dnigf1r-GFP/GnRH3:EGFP) and Tg(hsp70:GFP/GnRH3:EGFP) double-transgenic embryos were treated as described in A. GFP-positive GnRH3 neurons (arrows) were visualized in living embryos at 32 hpf. E, Tg(GnRH3:EGFP) embryos were treated with DMSO, 3 μm NVP-AEW541 (NVP) from 4 to 32 hpf and GFP-positive GnRH3 neurons (arrows) were visualized in living embryos at 32 hpf. F, Quantitative results of D and E. n = 31∼78, *p < 0.001. G, Tg(hsp70:dnigf1r-GFP) embryos were subjected to one 1 h HS treatment at 4 hpf and two 0.5 h HS treatments at 26 and 38 hpf and raised up to the indicated time points. GnRH3 neurons were detected by whole-mount in situ hybridization. n = 15∼20; +p < 0.05, *p < 0.001 compared with the without HS control group at the same time point. BMS, BMS-754807.
To further study the role of IGF signaling in GnRH3 neuron development, we generated Tg(hsp70:dnIGF1R-GFP/GnRH3:EGFP) double-transgenic embryos. Tg(hsp70:GFP/GnRH3:EGFP) embryos were also generated and used as a control. As shown in Figure 2, D and F, HS treatment significantly reduced the percentage of GnRH3/GFP neuron-positive embryos in the Tg(hsp70:GFP/GnRH3:EGFP) group at 32 hpf, but had no such effect in the control embryos. Likewise, addition of either NVP-AEW541 or BMS-754807 significantly reduced the number of GnRH3/GFP neuron-positive embryos at 32 hpf (Fig. 2E,F). These effects were gradually weakened as embryogenesis progressed. At 50 hpf, the HS-treated Tg(hsp70:dnIGF1R-GFP) embryos completely caught up (Fig. 2G). These results suggest that genetic and pharmacological blockade of IGF signaling delays the timing of GnRH3 neuron emergence in zebrafish.
IGF signaling is required for the proper spatial organization of GnRH3 neurons
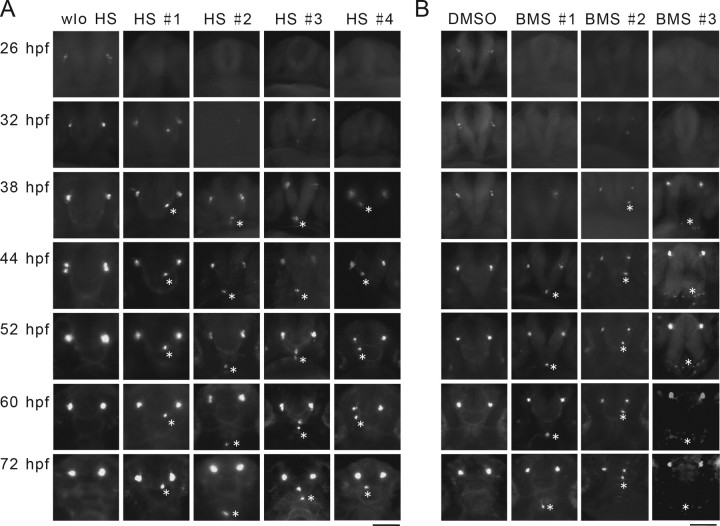
We next examined the role of IGF signaling on GnRH3 neuron spatial distribution. GnRH3 neurons formed two bilateral clusters in the olfactory bulbs at 3 d postfertilization (dpf) and began to migrate caudally toward the telencephalon and hypothalamus until 7 dpf (Abraham et al. 2008). When IGF signaling was inhibited at 4 hpf by HS treatment and the distribution of GnRH3 neurons was examined at 3 and 7 dpf, many of them were detected in ectopic positions outside of the olfactory region (Fig. 3A). Two major abnormal phenotypes were observed. In phenotype 1, some GnRH3 neurons were observed outside the olfactory bulb region. In phenotype 2, the entire GnRH3 neuron clusters were dispersed outside the olfactory bulb region. Quantitative results indicate that the blockade of IGF signaling at the blastula stage (4 hpf) resulted in the appearance of ectopic GnRH3 in >60% of the embryos at 3 dpf (Fig. 3A,C). In contrast, inhibition of IGF signaling at 30 hpf had no effect on GnRH3 neuron distribution (Fig. 3B,C). Blockade of IGF signaling at 30 hpf resulted in growth retardation (data not shown). To ascertain that these ectopic GFP-positive cells are indeed GnRH3 mRNA-expressing cells, in situ hybridization analysis was performed. As shown in Figure 3D, GnRH3 mRNA was detected in normal and ectopic GFP-positive cells in all eight embryos examined, although the intensity of GnRH3 mRNA signals in these ectopic cells appeared to be weaker compared with that of the normal GnRH3 neurons (Fig. 3D).
Figure 3.
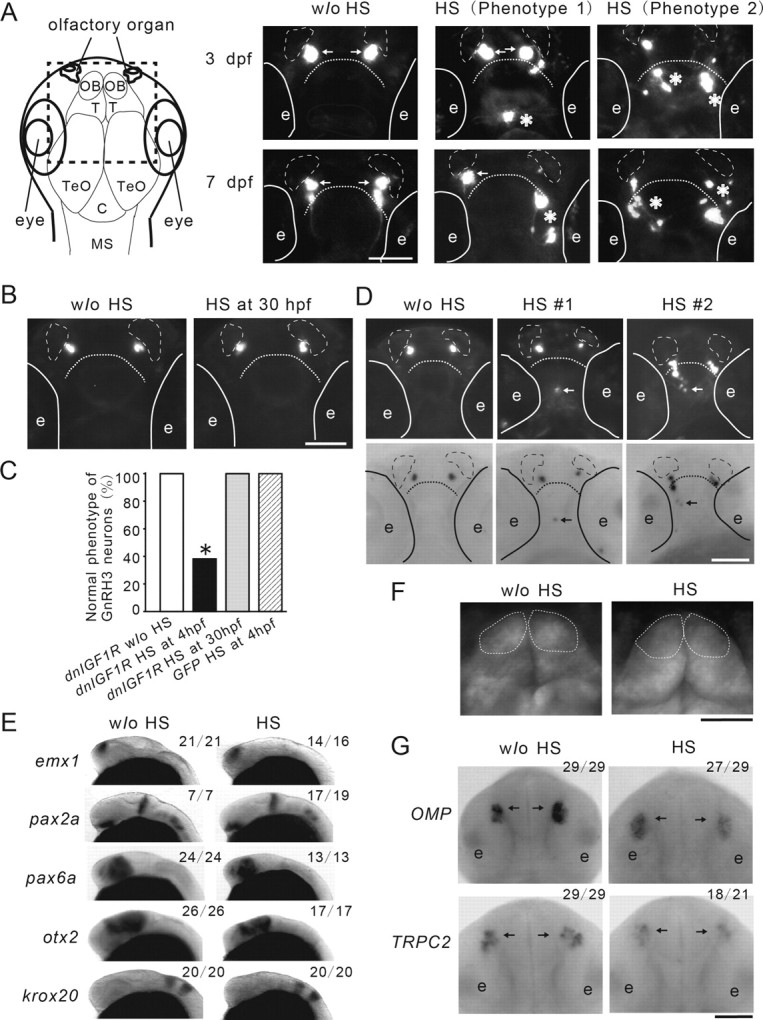
IGF signaling regulates the spatial organization of GnRH3 neurons; this action is developmental stage-dependent. A, Blockade of IGF signaling in early embryos results in abnormal distribution of GnRH3 neurons in the larval brain. Left, Diagram illustrating the orientation and anatomy of zebrafish larval brain. OB, olfactory bulb; T, telencephalon; TeO, tectum opticum; C, cerebellum; MS, medulla spinalis. Tg(hsp70:dnigf1r-GFP/GnRH3:EGFP) double-transgenic embryos were treated as described in Figure 2A and raised to 3 and 7 dpf. The location of GFP-positive GnRH3 neurons was visualized and representative images are shown (right). GnRH3 neurons at the two normal locations are indicated by arrows. Aberrant GnRH3 neurons are indicated by asterisks. Solid lines indicate the locations of eyes (e). Broken lines represent olfactory organs. Dashed line denotes the boundary between the olfactory bulb and telencephalon. B, Inhibition of IGF1R in advanced embryos has no effect. Tg(hsp70:dnigf1r-GFP/GnRH3:EGFP) double-transgenic embryos were subjected to 1 h HS treatment at 30 hpf. The embryos were raised to 3 dpf and the distribution of GnRH3 neurons was examined and shown. C, Quantitative results of A and B. n = 19∼41, *p < 0.001 compared with the no (w/o) HS group. D, The ectopic GFP-positive cells are indeed GnRH3 neurons. Larvae (3 dpf old) described in A were subjected to in situ hybridization analysis for GnRH3mRNA. Top, GFP view; bottom, in situ hybridization results. Aberrant GnRH3 neurons are indicated by arrows. Similar results were obtained in all eight embryos examined. E, Blockage of IGF signaling has no effect on global brain patterning. Tg(hsp70:dnigf1r-GFP) embryos were treated as described in A, raised to 32 hpf, and subjected to in situ hybridization analysis for the indicated marker genes. The frequency of embryos with the indicated expression patterns is shown in the upper right of each panel. Scale bar, 0.2 mm. F, Blockage of IGF signaling does not alter the morphology of olfactory bulbs. Tg (α1-tubulin1a: EGFP/hsp70:dnIGF1R-GFP) embryos are treated as described in A, raised to 3 dpf, and analyzed. Dashed line denotes the olfactory bulbs. G, Blockage of IGF signaling does not alter the location of specific sensory neurons. Tg(hsp70:dnigf1r-GFP) embryos were treated as described in A, raised to 32 hpf, and subjected to in situ hybridization analysis for the indicated marker genes. Arrows indicate locations of olfactory sensory neurons expressing OMP and TRPC2.
We next investigated whether this action of IGF signaling is specific to GnRH3 neurons or represents a more general effect in the brain and/or the olfactory bulb region. As shown in Figure 3E, blockage of IGF signaling did not change in the following general brain marker genes: emx1 expression in the forebrain; pax2a expression in the optic stalk, midbrain-hindbrain boundary, and hindbrain; pax6a expression in the eye, telencephalon, and diencephalon; otx2 expression in the midbrain; and krox20 expression in the third and fifth rhombomeres of the hindbrain. Likewise, no notable morphological change was found in the olfactory bulbs when IGF signaling was blocked in Tg(α1-tubulin1a:EGFP) embryos, in which EGFP is expressed in developing neurons under the control of the α1-tubulin1a promoter (Fig. 3F). Genes encoding the OMP and TRPC2 are known to be expressed in two distinct populations of neurons in the developing olfactory bulbs (Sato et al., 2005). As shown in Figure 3G, blockage of IGF signaling did not change the locations of omp- and trpc2 mRNA-expressing neurons. These results suggest that IGF signaling plays a specific role in regulating the proper spatial organization of GnRH3 neurons and that there is a critical time window in early development for this action of IGF signaling.
IGF signaling regulates the timing of GnRH2 neuron emergence but not their locations
We next examined whether IGF signaling also regulates the temporal and spatial organization of GnRH2 neurons. In zebrafish, GnRH2 neurons emerge in the midbrain at ∼26 hpf (Whitlock et al., 2003) (Fig. 4A). As shown in Figure 4, A and B, GnRH2 neurons were detected in the midbrain at 26 and 32 hpf in 100% of embryos in the HS-treated wild-type embryo group and the Tg(hsp70:dnigf1r-GFP)-without-HS treatment group. In comparison, only 25% of the HS-treated Tg(hsp70:dnigf1r-GFP) embryos had detectable GnRH2 neurons (Fig. 4A). RT-PCR analysis showed that the blockade of IGF signaling resulted in a major reduction in GnRH2 mRNA levels at 26 hpf (data not shown). At 32 hpf, however, GnRH2 neurons emerged in 100% of embryos in the HS-treated Tg(hsp70:dnigf1r-GFP) group. No ectopic GnRH2 neurons were detected in these IGF-deficient embryos (Fig. 4A). These data suggest that blockage of IGF signaling in early embryonic stage delays the timing of GnRH2 neuron emergence but has little effect on their location.
Figure 4.
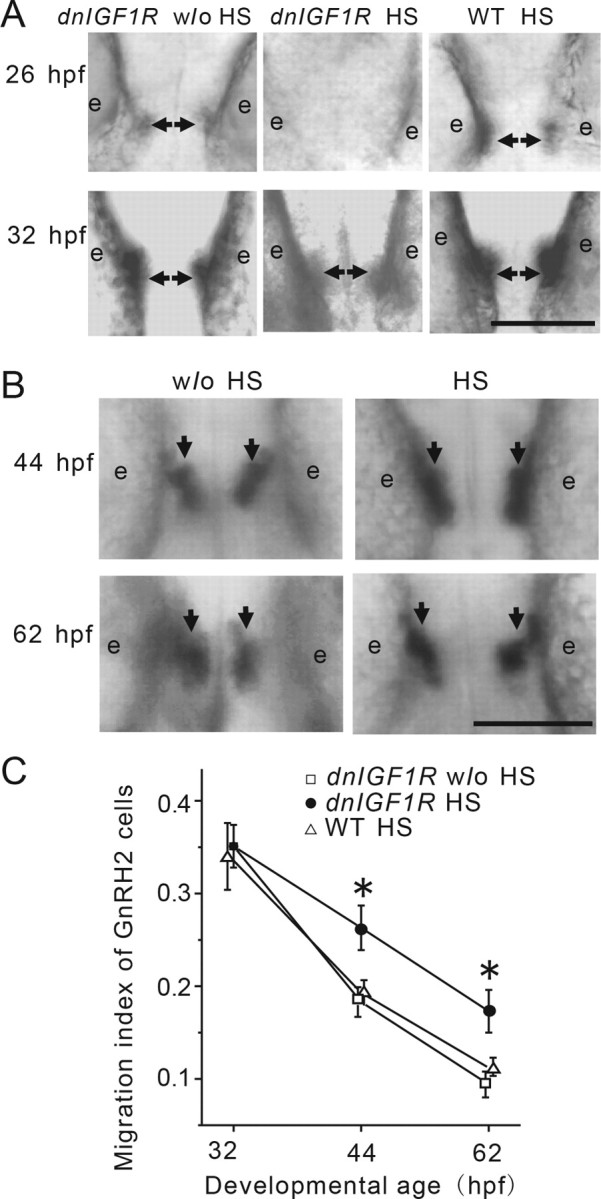
IGF signaling regulates the timing of GnRH2 neuron emergence but not their spatial locations. A, Blockage of IGF signaling delays the timing of emergence of GnRH2 neurons. Tg(hsp70:dnigf1r-GFP) embryos were treated as described in Figure 2A. GnRH2 neurons were detected by whole-mount in situ hybridization at the indicated time points. Ventral views are shown with anterior up. Arrows indicate GnRH2 neurons. The location of eyes are indicated by e. B, C, Blockage of IGF signaling in advanced embryos decreases GnRH2 neuron migration rate. Tg(hsp70:dnigf1r-GFP) embryos were treated with or without two 0.5 h HS treatments at 32 and 44 hpf. GnRH2 neurons were detected by whole-mount in situ hybridization the indicated time points. Ventral views are shown with anterior up. Arrows point to the positions of GnRH2 neurons (B). The migration index, defined as the length between right and left GnRH2 neuronal clusters divided by the length between the centers of the retinas, was quantified and shown in C. Values are shown as mean ± SE. n = 15–19, *p < 0.01 compared with the no (w/o) HS group of the same developmental age.
In zebrafish, the GnRH2 neurons migrate laterally and localize to the midline of the midbrain tegmentum by 72 hpf (Gopinath et al., 2004; Palevitch et al., 2007). We next determined the effect of the blockade of IGF signaling on GnRH2 neuron migration. For this, Tg(hsp70:dnigf1r-GFP) embryos were treated with two 0.5 h HS treatments at 32 and 44 hpf. The embryos were raised and GnRH2 neurons were detected by whole-mount in situ hybridization at 44 and 62 hpf (Fig. 4B). The migration index was determined and is shown in Figure 4C. While there was no difference between the groups at 32 hpf, the HS-treated Tg(hsp70:dnigf1r-GFP) group had a significantly greater value compared with the untreated transgenic embryo group or the HS-treated wild-type embryo group at 44 and 62 hpf. These data suggest that blockage of IGF signaling in advanced embryos slows down GnRH2 neuron migration.
IGF signaling regulates the spatial organization of newborn GnRH3 neurons by altering the migration and/or differentiation of neural crest cells
Next, we took advantage of the greater resolution of Tg(GnRH3-GFP) embryos to map the precise timing and locations of GnRH3 neurons in control and IGF-deficient embryos. For this, Tg(hsp70:dnIGF1R-GFP/GnRH3:EGFP) double-transgenic embryos were generated and used. In the control group, i.e., Tg(hsp70:dnIGF1R-GFP/GnRH3:EGFP) embryos without HS treatment, GnRH3 neurons began to emerge in two bilateral clusters in the olfactory bulb region at 26 hpf (Fig. 5A). At 32 hpf, GnRH3 neurons were clearly seen in these two normal locations in all 14 individuals examined and remained so thereafter (Fig. 5A). When Tg(hsp70:dnIGF1R-GFP/GnRH3:EGFP) embryos were treated with 1 h HS at 4 hpf, the two clusters of bilateral GnRH neurons did not appear in any of these embryos at 26 hpf (Fig. 5A). At 32 hpf, they were detected in only one of the four embryos examined. At 38 hpf, GnRH neurons were finally detected in all four embryos. As shown in Figure 5A, in all cases, the ectopic GnRH3 neurons emerged at the same time as those normal GnRH3 neurons. Next, IGF signaling in Tg(GnRH3:EGFP) embryos was inhibited by adding BMS-754807. Treatment of Tg(GnRH3:EGFP) embryos with BMS-754807 resulted in essentially the same result (Fig. 5B): a delay in the timing of GnRH3 neuron emergence and a concurrent appearance of ectopic GnRH3 neurons in all embryos examined. This result suggests that IGF signaling regulates the spatial organization of newborn GnRH3 neurons.
Figure 5.
IGF signaling regulates the proper spatial distribution of newborn GnRH3 neurons. A, Time-lapse imaging of GnRH3 neurons in Tg(hsp70:dnigf1r-GFP/GnRH3:EGFP) double-transgenic fish. Embryos were treated as described in Figure 2A. The timing of emergence and spatial location of GnRH3 neurons were monitored in the same individuals at the indicated time points. Four HS-treated fish (#1–4) are shown. B, Time-lapse imaging of GnRH3 neurons in Tg(GnRH3:EGFP) fish. Embryos were treated with DMSO or BMS-754807 (BMS; 3 μm) from 4 to 32 hpf. The timing of emergence and spatial location of GnRH3 neurons were monitored in the same individuals at the indicated time points. Three treated fish (#1–3) are shown. Aberrant GnRH3 neurons are indicated by asterisks.
GnRH3 neurons in the olfactory region are known to originate from the cranial neural crest in zebrafish (Whitlock et al., 2003, 2005). The concurrent appearance of the normal and ectopic GnRH3 neurons in IGF-deficient embryos led us to postulate that IGF signaling may regulate GnRH3 distribution by acting on their precursor cells in the cranial neural crest. To test this hypothesis, we examined the effect of IGF signaling blockade on neural crest cell migration/differentiation by analyzing sox10 and crestin expression. In zebrafish, sox10 is expressed in premigratory neural crest cells and extended caudally with the progress of embryogenesis (Dutton et al., 2001). Between 16 to 26 hpf, sox10 mRNA is maintained in migrating neural crest cells of the medial migration pathway but absent in the lateral pathway (Dutton et al., 2001). As shown in Figure 6A, the expression pattern of sox10 in 16 hpf IGF-deficient embryos resembled that of the 12 hpf control embryos (Fig. 6A). Likewise, 20 and 32 hpf IGF-deficient embryos showed a similar crestin expression pattern to those of 16 and 26 hpf control embryos, respectively (Fig. 6A), indicating a 4–6 h delay in neural crest cell development. Importantly, the blockade of IGF signaling also resulted in the disappearance of sox10 mRNA-expressing cells at 20, 26, and 32 hpf, most notably in the dorsal region (Fig. 6A). This is distinct from the crestin expression, which is a pan-neural crest marker and is expressed in all neural crest cells until 26 hpf, and is downregulated at 32 hpf (Luo et al., 2001). As shown in Figure 6A (middle), the expression pattern of crestin mRNA in 16 and 32 hpf IGF-deficient embryos resembled that of the 12 and 26 hpf control embryos, respectively. Differently from the case of sox 10, however, many crestin mRNA-expressing cells were observed in the migrating neural crest cells (likely those in the lateral pathways) at 16 and 20 hpf. We also examined the expression patterns of pax2a, a marker gene of optic stalk, mid-hindbrain boundary, and hindbrain. There was no major change between control and IGF-deficient embryos in all stages examined (Fig. 6A, right).
Figure 6.
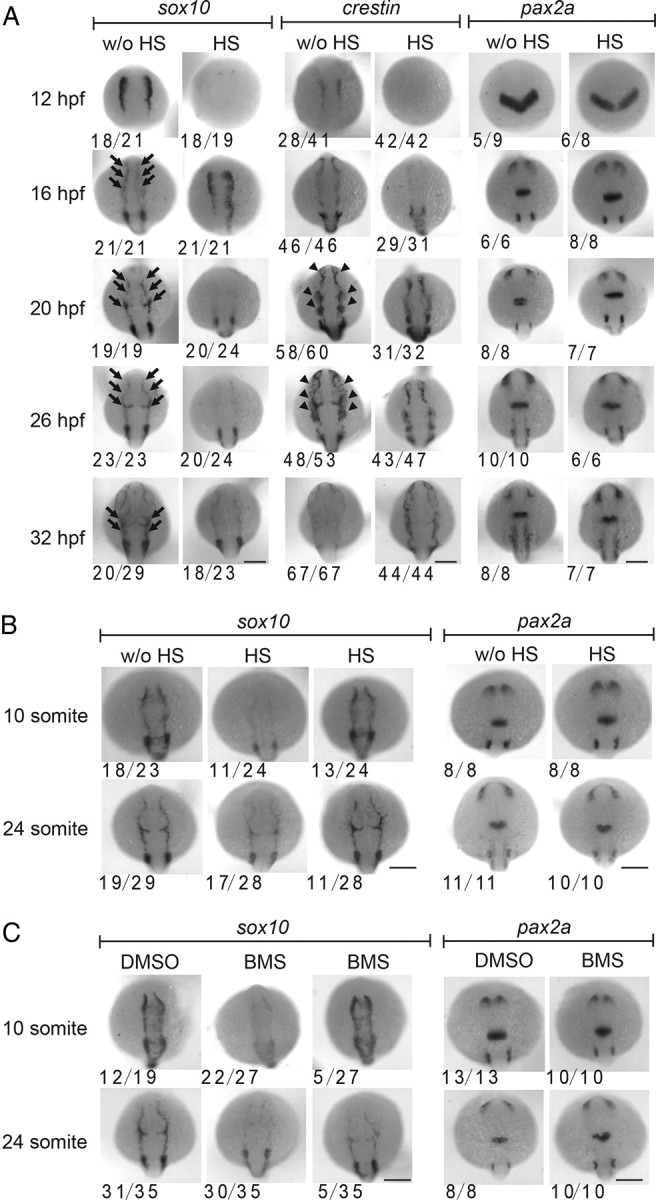
IGF signaling is important for neural crest cell differentiation and/or migration. A, Blockade of IGF signaling affects neural crest cell development and/or migration. Tg(hsp70:dnigf1r-GFP) embryos were treated as described in Figure 2A, raised to the indicated time points, and subjected to in situ hybridization analysis for the indicated marker genes. The frequency of embryos with the indicated expression patterns is shown at the bottom of each panel. Note that while sox10 mRNA-expressing cells in the medial migration pathway (arrows) disappeared in IGF-deficient embryos, crestin mRNA-expressing neural crest cells (arrowheads) were observed. B, Blockade of IGF signaling decreases neural crest cell differentiation and/or migration. Tg(hsp70:dnigf1r-GFP) embryos were treated as described in A. Control and experimental embryos of the same developmental stages were collected and analyzed as described in A. C, Wild-type embryos were treated with 3 μm BMS-754807 (BMS) or DMSO from 4 hpf to the indicated stages and analyzed by in situ hybridization analysis for the indicated marker genes. Scale bar, 0.2 mm.
To further rule out the possibility that the observed changes in sox10 expression is an indirect consequence of the global developmental delay, we analyzed sox10 expression using developmental stage-matched control and IGF-deficient embryos at the 10 and 24 somite stages. As shown in Figure 6B, 46% and 60% of HS-treated embryos at 10 and 24 somite stage had markedly reduced sox10 expression in the dorsal region. We next tested the effect of BMS-754807. Eighty-one and 87% of BMS-treated embryos at 10 and 24 somite stage had markedly reduced sox10 expression in the migrating neural crest cells. BMS-754807 treatment did not change the expression pattern of pax2a (Fig. 6C). These results suggest that the blockade of IGF signaling delays neural crest cell development and alters the migration and/or differentiation of the sox10 mRNA-expressing neural crest cells in the medial migration pathway.
IGF signaling regulates GnRH3 neuron development via the PI3K but not the MAPK pathway
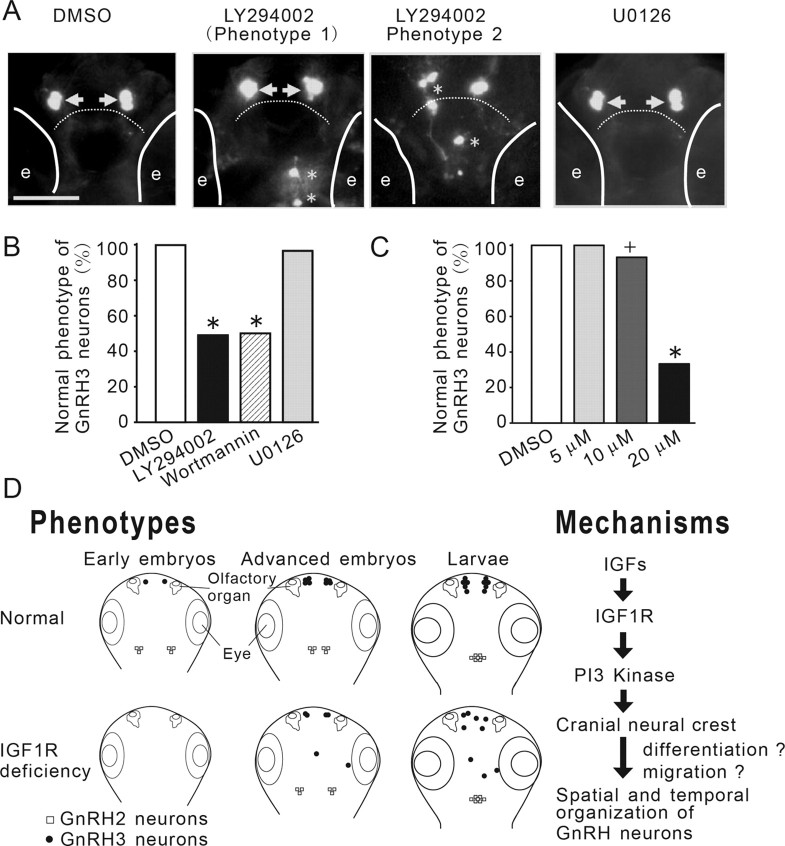
The IGF1R signaling is primarily mediated by the PI3K-Akt pathway and Mek-Erk MAPK pathway (Duan, 2002). Treatments of Tg(GnRH3:EGFP) embryos with LY294002, a PI3K inhibitor, phenocopied the IGF signaling-deficient embryos (Fig. 7A). The effect of LY294002 was concentration-dependent (Fig. 7A,C). Likewise, wortmannin, a structurally distinct PI3K inhibitor, also caused abnormal GnRH3 distribution (Fig. 7B). In contrast, U0126, a Mek inhibitor, had no effect (Fig. 7A,B). Together, our results suggest IGF signaling regulates spatial distribution of GnRH3 neuron via the PI3K-Akt, but not the MAPK, signaling pathway.
Figure 7.
The IGF action on GnRH3 neurons is mediated through the PI3K, but not the MAPK, signaling pathway. A, Inhibition of PI3K, but not MAPK, phenocopies IGF-deficient embryos. Tg(GnRH3:EGFP) embryos were treated with DMSO or the indicated inhibitors. GnRH3 neurons were visualized at 3 dpf. GnRH3 neurons at the two normal locations are indicated by arrows. Aberrant GnRH3 neurons are indicated by asterisks. Solid lines indicate the locations of eyes (e). Dashed line denotes the boundary between the olfactory bulb and the telencephalon. B, Quantitative results of A. n = 29∼37, +p < 0.05 and *p < 0.001 compared with the DMSO group. C, Dose-dependent effect of LY294002. D, A proposed model on the roles and underlying mechanisms of IGF signaling in regulating the temporal and spatial organization of newborn GnRH neurons in zebrafish embryos. Inhibition of IGF signaling delays the timing of GnRH2 and GnRH3 neuron development in early embryos and slows down the migration of GnRH2 neurons from lateral to the midline in advanced embryos. Inhibition of IGF signaling in early embryonic stage also results in abnormal distribution of GnRH3 neurons in the larval brain. Right, IGFs activate the IGF1R-PI3K pathway to regulate the migration/differentiation of cranial neural crest cells in early embryogenesis. This in turn results in altered temporal and spatial organization of newborn GnRH3 neurons.
Discussion
In this study, we have unraveled an important role of IGF signaling in regulating the timing and location of newborn GnRH neurons using the zebrafish model. By a combination of genetic, biochemical, and pharmacological tools, and in vivo real-time imaging, we show that IGF signaling is critical for the timing of GnRH2 and GnRH3 neuron emergence and the spatial distribution of GnRH3 neurons. The action of IGF signaling on GnRH3 neurons is developmental stage-dependent and mediated through the PI3K-Akt, but not the MAPK, signaling pathway (Fig. 7D).
The GnRH neuronal system has long been used for studying tangential neuronal migration. During mammalian development, hypothalamic GnRH neurons conduct a long-distance migration, starting from the olfactory region and reaching the POA and hypothalamus (Schwanzel-Fukuda and Pfaff, 1989; Wray et al., 1989). Wierman et al. (2011) recently reviewed various regulators of GnRH neuron development and migration (both cell autonomous and nonautonomous factors). Among them, two growth factor systems, FGF8/FGFR1 and hepatocyte growth factor, are important regulators of GnRH neuron proliferation and migration (Tsai et al., 2005; Giacobini et al., 2007; Falardeau et al., 2008; Chung and Tsai, 2010). The possible involvement of other embryonic growth factors, however, has not been well documented. In this study, we have provided several lines of evidence to support the notion that IGF signaling regulates the proper spatial and temporal organization of newborn GnRH neurons. First, specific inhibition of IGF1R by dnIGF1R-GFP delayed the timing of GnRH2 and GnRH3 neuron emergence and resulted in the mislocation of GnRH3 neurons. Another independent line of evidence came from the pharmacological inhibition experiments. Treatment of embryos with NVP-AEW541 and BMS-754807, two structurally distinct IGF1R inhibitors, resulted in similar changes: delaying GnRH2 emergence from 26 to 32 hpf and GnRH3 neurons emergence from 32 to ∼38 hpf, and the appearance of ectopic GnRH3 neurons. Our finding on the role of IGF signaling in regulating the timing of GnRH neuron emergence is perhaps not surprising. Previous studies have shown that inhibition of IGF signaling results in global growth retardation and developmental delays and that IGF signaling plays an important role in accelerated developing rate in zebrafish embryos in response to oxygen availability (Schlueter et al., 2007; Kamei et al., 2011). IGF signaling has also been shown to regulate many aspects of the CNS development (Bondy and Cheng, 2004; Joseph D'Ercole and Ye, 2008; Annenkov, 2009). In zebrafish embryos, IGF signaling is implicated in the development of motoneurons and the notochord (Schlueter et al., 2007; Zou et al., 2009).
A new and potentially important finding is that IGF signaling regulates the spatial organization of GnRH3 neurons. Zebrafish GnRH3 neurons are the counterpart of the mammalian hypophysiotropic GnRH1 neurons, and ablation of embryonic GnRH3 neurons can lead to impaired reproduction in adult zebrafish (Abraham et al., 2010). GnRH3 neurons localize in the olfactory regions first and then start migration toward the hypothalamus (Abraham et al., 2008). In this study, we show that genetic and pharmacological blockades of IGF signaling in early embryogenesis result in abnormal spatial organization of the GnRH3 neurons in the larval brain. This action is specific to GnRH3 neurons, as no ectopic neurons were found with GnRH2 neurons. Likewise, blockade of IGF signaling had no effect in OMP neurons and TRPC2 neurons, two other neurons in the olfactory bulb region. In addition, inhibition of IGF signaling did not alter brain patterning or the size and structure of the olfactory bulbs. The emergence of ectopic GnRH3 neurons is not due to transient or leaky gene expression because these ectopic cells continued expressing GnRH3 mRNA until at least 7 dpf, albeit at reduced levels. To our knowledge, this is the first study demonstrating that the perturbation of IGF signaling in early embryonic stage has a long-lasting effect on the proper spatial distribution of GnRH neurons later in life.
The action of IGF signaling is developmental stage-dependent. While genetic or pharmacological inhibition at an early stage (4 hpf) resulted in the appearance of ectopic GnRH3 neurons, inhibition of IGF signaling at a later stage (30 hpf) had no such effect. It should be emphasized that inhibition of IGF signaling at 30 hpf was effective because it reduced embryonic growth and slowed GnRH2 neuron migration. This suggests that there is a critical time window in early embryogenesis during which IGF signaling regulates the emergence of GnRH3 neurons at the correct locations. We considered two possible reasons for the appearance of ectopic GnRH3 neurons in IGF-deficient embryos. First, the differentiated GnRH3 neurons may mismigrate into abnormal locations. Second, GnRH3 neurons may be born in the wrong locations. Our in vivo time-lapse imaging analysis revealed that the ectopic GnRH3 neurons appeared at the same time as those in the normal place, thus supporting, although not necessarily proving, the second scenario. This is also in agreement with our finding that inhibition of IGF signaling at a later stage (30 hpf) did not result in the appearance of any ectopic GnRH3 neurons. Our findings in zebrafish may also explain previous findings made in the mouse model. While Igf-1−/− mice exhibited misplaced glutamergic neurons of the mitral cell layers in the olfactory bulb (Hurtado-Chong et al., 2009), mice with deleted IGF1R or the p85α regulatory subunit of PI3K in GnRH neurons only (under the control of the GnRH1 promoter) showed a normal number and spatial distribution of GnRH1 neurons, although they exhibited differences in GnRH morphology (Acosta-Martínez et al., 2009; Divall et al., 2010).
The precise origins of GnRH3 and GnRH2 neurons in zebrafish and their relationship are still under debate. Whitlock et al. (2005) proposed that the cranial neural crest-derived precursor cells migrate following two distinct routes: (1) anterior migration stream toward the olfactory region (presumably becoming GnRH3 neurons) and (2) lateral route passing ventrally to midbrain (presumably becoming GnRH2 neurons). In this study, we have shown that while IGF signaling regulates the timing of both GnRH2 and GnRH3 emergence, it also plays a highly specific role in determining the precise location of GnRH3 neurons. We discovered that blockade of IGF signaling in early embryos not only resulted in a delay in the neural crest cell development (as indicated by the delayed expression pattern of the pan-neural crest marker gene crestin), but also led to a major reduction in sox10 mRNA-expressing cells in the medial migration pathway. These results imply that GnRH3 neurons may arise from neural crest cells, most likely those in the medial migratory pathway. This idea is in good agreement with recent cell fate-mapping studies in chicks and mice, indicating that neural crest cells give rise to a subpopulation of GnRH1 neurons and olfactory ensheathing glial cells (Barraud et al., 2010; Forni et al., 2011). Since sox10 expression is maintained in the medial migration pathway and not on the lateral pathway, it is tempting to speculate that GnRH3 neurons may be derived from sox10 mRNA-expressing neural crest cells in the medial migration pathway, while GnRH2 neurons are derived from other populations of neural crest cells. Whether IGF signaling controls the migration and/or differentiation of neural crest cells of the medial migration pathway and how the global blockade of IGF signaling leads to a reduction in sox10-expressing cells are not clear at present. How the global blockade of IGF signaling leads to a reduction in sox10-expressing cells is still unclear. Future studies are needed to answer these questions.
The IGF1R belongs to the receptor tyrosine kinase family and it uses two major intracellular signaling pathways, namely the PI3K-Akt cascade and Ras-MEK1/2-Erk1/2 cascade (Duan and Xu, 2005). How these downstream signaling pathways are used in vivo in different tissues and/or under different conditions is not well understood. Our results suggest that PI3K-Akt signaling, but not the MEK1/2-Erk1/2 signaling, mediated the actions of IGF-IGF1R in regulating GnRH neuronal system formation in vivo. While specific inhibition of the MEK1/2-Erk1/2 pathway using U0126 had no effect on the temporal and spatial organization of GnRH neurons, LY294002 and wortmannin, two distinct PI3K inhibitors, phenocopied the IGF signaling-deficient embryos. These results suggest that the IGF-IGF1R-PI3 kinase signaling pathway regulates the precise timing and the spatial distribution of newborn GnRH neurons in zebrafish embryos.
In humans, disrupted GnRH neuronal ontogeny can lead to idiopathic hypogonadotrophic hypogonadism (IHH). For instance, mutations in KAL-1 cause X-linked Kallmann's syndrome, characterized by IHH, anosmia, synkinesis, and unilateral renal agenesis (González-Martínez et al., 2004). At present, only a small percentage (<30%) of IHH cases can be accounted for by known gene mutations, suggesting the existence of unexplored regulatory mechanisms (Falardeau et al., 2008). Therefore, our findings not only expand our knowledge of regulation of the temporal and spatial organization of GnRH neurons, but also may have implications in the understanding of the etiology of human reproductive disorders.
Footnotes
This work was supported by NSF Grants IOB-0543018 and IOS-1051034 to CD. TAO was supported in part by the Japan Society for the Promotion of Science Fellowship Program (#200609320). We thank Dr. H. Kamei for helpful discussion and assistance and Mr. John Allard, Ms. Lisa Hebda and Ms. Sarah Gaubatz for proofreading of this manuscript.
The authors declare no competing financial interests.
References
- Abraham E, Palevitch O, Ijiri S, Du SJ, Gothilf Y, Zohar Y. Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurons and the role of GnRH as an autocrine migration factor. J Neuroendocrinol. 2008;20:394–405. doi: 10.1111/j.1365-2826.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- Abraham E, Palevitch O, Gothilf Y, Zohar Y. Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: effects on neurogenesis, neuronal migration, and reproduction. Endocrinology. 2010;151:332–340. doi: 10.1210/en.2009-0548. [DOI] [PubMed] [Google Scholar]
- Acosta-Martínez M, Luo J, Elias C, Wolfe A, Levine JE. Male-biased effects of gonadotropin-releasing hormone neuron-specific deletion of the phosphoinositide 3-kinase regulatory subunit p85α on the reproductive axis. Endocrinology. 2009;150:4203–4212. doi: 10.1210/en.2008-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Luo Q, Koide N, Okada H, Urano A. Effects of insulin-like growth factor I on GnRH-induced gonadotropin subunit gene expressions in masu salmon pituitary cells at different stages of sexual maturation. Gen Comp Endocrinol. 2006;149:21–29. doi: 10.1016/j.ygcen.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Annenkov A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signaling network regulating neurogenesis. Mol Neurobiol. 2009;40:195–215. doi: 10.1007/s12035-009-8081-0. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Baker DM, Davies B, Dickhoff WW, Swanson P. Insulin-like growth factor I increases follicle-stimulating hormone (FSH) content and gonadotropin-releasing hormone-stimulated FSH release from coho salmon pituitary cells in vitro. Biol Reprod. 2000;63:865–871. doi: 10.1095/biolreprod63.3.865. [DOI] [PubMed] [Google Scholar]
- Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, Liu KJ, Baker CVH. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci U S A. 2010;107:21040–21045. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Chung WC, Tsai PS. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Front Horm Res. 2010;39:37–50. doi: 10.1159/000312692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood) 2005;230:292–306. doi: 10.1177/153537020523000503. [DOI] [PubMed] [Google Scholar]
- Divall SA, Williams TR, Carver SE, Koch L, Brüning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J Endocrinol. 2002;175:41–54. doi: 10.1677/joe.0.1750041. [DOI] [PubMed] [Google Scholar]
- Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31:6915–6927. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukuma S, Onuma T, Swanson P, Luo Q, Koide N, Okada H, Urano A, Ando H. Stimulatory effects of insulin-like growth factor 1 on expression of gonadotropin subunit genes and release of follicle-stimulating hormone and luteinizing hormone in masu salmon pituitary cells early in gametogenesis. Zoolog Sci. 2008;25:88–98. doi: 10.2108/zsj.25.88. [DOI] [PubMed] [Google Scholar]
- García-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Messina A, Wray S, Giampietro C, Crepaldi T, Carmeliet P, Fasolo A. Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration. J Neurosci. 2007;27:431–445. doi: 10.1523/JNEUROSCI.4979-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Hankin M, Li Z, Dai X, Ding J. Transgenic zebrafish for studying nervous system development and regeneration. Transgenic Res. 2001;10:21–33. doi: 10.1023/a:1008998832552. [DOI] [PubMed] [Google Scholar]
- González-Martínez D, Hu Y, Bouloux PM. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann's syndrome. Front Neuroendocrinol. 2004;25:108–130. doi: 10.1016/j.yfrne.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Gopinath A, Tseng LA, Whitlock KE. Temporal and spatial expression of gonadotropin releasing hormone (GnRH) in the brain of developing zebrafish (Danio rerio) Gene Expr Patterns. 2004;4:65–70. doi: 10.1016/s1567-133x(03)00149-2. [DOI] [PubMed] [Google Scholar]
- Hurtado-Chong A, Yusta-Boyo MJ, Vergaño-Vera E, Bulfone A, de Pablo F, Vicario-Abejón C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci. 2009;30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- Joseph D'Ercole A, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei H, Ding Y, Kajimura S, Wells M, Chiang P, Duan C. Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability. Development. 2011;138:777–786. doi: 10.1242/dev.056853. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Longo KM, Sun Y, Gore AC. Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1–7 cells. Endocrinology. 1998;139:1125–1132. doi: 10.1210/endo.139.3.5852. [DOI] [PubMed] [Google Scholar]
- Luckenbach JA, Dickey JT, Swanson P. Regulation of pituitary GnRH receptor and gonadotropin subunits by IGF1 and GnRH in prepubertal male coho salmon. Gen Comp Endocrinol. 2010;167:387–396. doi: 10.1016/j.ygcen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish crestin throughout embryonic development. Dev Dyn. 2001;220:169–174. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1097>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Maures T, Chan SJ, Xu B, Sun H, Ding J, Duan C. Structural, biochemical, and expression analysis of two distinct insulin-like growth factor (IGF)-I receptors and their ligands in zebrafish. Endocrinology. 2002;143:1858–1871. doi: 10.1210/endo.143.5.8768. [DOI] [PubMed] [Google Scholar]
- Palevitch O, Kight K, Abraham E, Wray S, Zohar Y, Gothilf Y. Ontogeny of the GnRH systems in zebrafish brain: in situ hybridization and promoter-reporter expression analyses in intact animals. Cell Tissue Res. 2007;327:313–322. doi: 10.1007/s00441-006-0279-0. [DOI] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter PJ, Peng G, Westerfield M, Duan C. Insulin-like growth factor signaling regulates zebrafish embryonic growth and development by promoting cell survival and cell cycle progression. Cell Death Differ. 2007;14:1095–1105. doi: 10.1038/sj.cdd.4402109. [DOI] [PubMed] [Google Scholar]
- Schöfer C, Frei K, Weipoltshammer K, Wachtler F. The apical ectodermal ridge, fibroblast growth factors (FGF-2 and FGF-4) and insulin-like growth factor I (IGF-I) control the migration of epidermal melanoblasts in chicken wing buds. Anat Embryol. 2001;203:137–146. doi: 10.1007/s004290000148. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Steven C, Lehnen N, Kight K, Ijiri S, Klenke U, Harris WA, Zohar Y. Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen Comp Endocrinol. 2003;133:27–37. doi: 10.1016/s0016-6480(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM. Central insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats. Biol Reprod. 2007;77:492–503. doi: 10.1095/biolreprod.107.060434. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology. 2010;151:1356–1366. doi: 10.1210/en.2009-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol. 2005;19:225–236. doi: 10.1210/me.2004-0330. [DOI] [PubMed] [Google Scholar]
- Weil C, Carré F, Blaise O, Breton B, Le Bail PY. Differential effect of insulin-like growth factor I on in vitro gonadotropin (I and II) and growth hormone secretions in rainbow trout (Oncorhynchus mykiss) at different stages of the reproductive cycle. Endocrinology. 1999;140:2054–2062. doi: 10.1210/endo.140.5.6747. [DOI] [PubMed] [Google Scholar]
- Whitlock KE, Wolf CD, Boyce ML. Gonadotropinreleasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Dev Biol. 2003;257:140–152. doi: 10.1016/s0012-1606(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Whitlock KE, Smith KM, Kim H, Harden MV. A role for foxd3 and sox10 in the differentiation of gonadotropinreleasing hormone (GnRH) cells in the zebrafish Danio rerio. Development. 2005;132:5491–5502. doi: 10.1242/dev.02158. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: Initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol. 2011;32:43–52. doi: 10.1016/j.yfrne.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittman MD, Carboni JM, Yang Z, Lee FY, Antman M, Attar R, Balimane P, Chang C, Chen C, Discenza L, Frennesson D, Gottardis MM, Greer A, Hurlburt W, Johnson W, Langley DR, Li A, Li J, Liu P, Mastalerz H, et al. Discovery of a 2,4-disubstituted pyrrolo-[1,2-f][1,2,4]triazine inhibitor (BMS-754807) of insulin-like growth factor receptor (IGF-1R) J Med Chem. 2009;52:7360–7363. doi: 10.1021/jm900786r. [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen S, Zakaria M, Wolfe A, Radovick S. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by insulin-like growth factor I in a cultured GnRH-expressing neuronal cell line. Mol Endocrinol. 1997;11:1145–1155. doi: 10.1210/mend.11.8.9956. [DOI] [PubMed] [Google Scholar]
- Zou S, Kamei H, Modi Z, Duan C. Zebrafish IGF genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One. 2009;4:e7026. doi: 10.1371/journal.pone.0007026. [DOI] [PMC free article] [PubMed] [Google Scholar]