Abstract
Processing speed (PS) training improves performance on untrained PS tasks in the elderly. However, PS training's effects on the PS of young adults and on neural mechanisms are still unknown. In humans, we investigated this issue using psychological measures, voxel-based morphometry, the n-back task [a typical task for functional magnetic resonance imaging (fMRI) studies with conditions of 0-back (simple cognitive processes) and 2-back tasks (working memory; WM)], resting-state fMRI for the analysis of functional connectivity between brain regions during rest (resting-FC), and intensive adaptive training of PS. PS training was associated with (1) significant or substantial improvement in the performance of PS measures, (2) changes in the gray matter structures of the left superior temporal gyrus and the bilateral regions around the occipitotemporal junction, (3) changes in functional activity that are related to simple cognitive processes (but not those of WM) in the left perisylvian region, and (4) increased resting-FC between the left perisylvian area and the area that extends to the lingual gyrus and calcarine cortex. These results confirm the PS-training-induced plasticity in PS and the training-induced plasticity of functions and structures that are associated with speeded cognitive processes. The observed neural changes caused by PS training may give us new insights into how PS training, and possibly other cognitive training, can improve PS.
Introduction
Processing speed (PS) is individual cognitive ability measured by how fast individuals execute cognitive tasks, particularly elementary cognitive tasks (e.g., Salthouse, 1996). Working memory (WM) is a limited capacity storage system involved in the maintenance and manipulation of information over short periods of time (Baddeley, 2003). On the other hand, PS has been proposed to be one of key cognitive components, along with WM, in a wide range of cognitive domains and in psychometric intelligence (Kail and Salthouse, 1994; Fry and Hale, 2000). PS, capacity of WM (WMC), and psychometric intelligence all correlate with one another (Fry and Hale, 2000).
Previous findings have indicated that PS or speeded cognitive processes relate to brain connectivity and to each brain region involved in a certain type of information processing. While WM and WMC are associated with functional activity and brain structures of the lateral prefrontal cortex and the parietal cortex (Klingberg, 2006), PS or speeded cognitive processes are associated with different neural systems. Higher PS in a visual PS task is related to white matter structural integrity in occipital and parietal white matter (Tuch et al., 2005). Furthermore, speeded cognitive processes of particular information are related to each region relevant to the particular information processing. For example, the left ventral prefrontal cortex is associated with linguistic output as well as fluent output (Flaherty, 2005), and the superior temporal gyrus (STG) is associated with the processing of comprehensible speech as well as rapid speech (Poldrack et al., 2001).
Previous studies of cognitive training have shown WM training's effect on psychological measures and neural systems, and PS training's effect on psychological measures in the elderly. It has been shown that performance of training on cognitive tasks, including WM tasks, can improve performance of a wide range of untrained cognitive tasks, such as nontrained WM tasks, nonverbal reasoning tasks, and response inhibition tasks [for review, see Uchida and Kawashima (2008) and Takeuchi et al. (2010d)]. Furthermore, several studies have shown that PS training can improve performance of nontrained PS tasks in the elderly (e.g., Edwards et al., 2002). Also, WM training affects brain activity, the density of cortical dopamine D1 receptors, and white matter structural integrity in frontal and parietal regions (Olesen et al., 2004; Dahlin et al., 2008; McNab et al., 2009; Takeuchi et al., 2010a).
However, to our knowledge, no previous studies have investigated the effect PS-training tasks have on the PS of young adults nor on neural mechanisms, which is the purpose of the current study. Considering the contribution of PS to human psychometric intelligence, it is important to investigate the extent of PS's plasticity. In this study, among the neural mechanisms affected by PS training, we focused on changes of functional activity, functional connectivity during rest (resting-FC) and regional gray matter volume (rGMV). We hypothesized that PS training leads to PS improvements and functional and structural changes in each region involved in rapid cognition in the young. Furthermore, the effects of PS training on the performance of other untrained cognitive tasks besides untrained PS tasks in the young are unknown. So we investigated the effects of PS training on diverse cognitive functions in an exploratory way.
Materials and Methods
Subjects.
Sixty-three healthy, right-handed individuals (32 men and 31 women) participated in this study. The mean age of subjects was 21.6 years (SD, 1.68). All subjects were university students or postgraduate students. All subjects had normal vision, and none had a history of neurological or psychiatric illness. Whether or not they had histories of neurological illnesses or psychiatric illnesses was assessed with our laboratory's routine questionnaire in which each subject answered questions about whether they had or have certain illnesses. Handedness was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971). In accordance with the Declaration of Helsinki (1991), written informed consent was obtained from each subject. This study was approved by the Ethics Committee of Tohoku University.
There were several intervention periods in which subjects participated. In each period, a group consisting of several subjects participated in pretraining (Pre) and posttraining (Post) MRI experiments and psychological experiments at the same time. This study was performed together with another intervention study investigating the effects of arithmetic training. This study and the study using arithmetic training share the subjects of the no-intervention control group, the psychological and neuroimaging outcome measures, the training period, the training time, and the training frequency. Participants were randomly assigned to either the intervention group (the PS-training or the arithmetic-training group) or the no-intervention group. However, participants in the same intervention group period were all assigned to the same training protocol group (the PS-training group or the arithmetic-training group). None of the participants were notified that there were three groups (rather than just the intervention group and the no-intervention group) until after the Post MRI and psychological experiments. The PS-training group consisted of 23 participants (11 men and 12 women) and the mean age of the subjects of the PS-training group was 22.0 years (SD, 1.6). The no-intervention group consisted of 21 participants (12 men and 9 women) and the mean age of the subjects of the no-intervention group was 21.2 years (SD, 1.7). The subjects assigned to the PS-training group and subjects assigned to the no-intervention group did not differ significantly (p > 0.1, two-tailed t tests) in basic background characteristic such as age, sex, and score on Raven's Advanced Progressive Matrix (Raven, 1998), which measures cognitive ability that is central to general intelligence (Snow, 1989), nor the scores on simple PS measures (Word–Color task and Color–Word task in the Stroop test). One participant in the PS-training group and one participant in the no-intervention group were not able to participate in the Post MRI and psychological experiments for reasons that appeared after the Pre MRI and psychological experiments. Furthermore, the computer that had been assigned to one participant in the PS-training group during the training period was later found to have not been working well, and presentation of the stimuli was slow during training. Another participant in the PS-training group did not follow the instructed button-pressing method during the training. These four subjects were excluded from further analysis of the effects of the training. Furthermore, subjects who could not understand rules of the cognitive tasks used as outcome measures (characterized by little answers) were excluded from the analyses involving those tasks (two exclusions).
Procedure.
The PS-training program consisted of computerized, in-house developed Borland C++ programs that consisted of eight tasks using computer buttons and two tasks using paper and pencil. Participants in the PS-training group undertook 5 d of training within a 6 d period. Instructions and brief practice of all training tasks was given at the beginning of the first training day before the start of that day's training. Training each day lasted ∼4 h. All participants were MRI scanned and took the psychological tests immediately before and after this 6 d period. The no-intervention group did not receive any training or perform any specific activity during the period separating the two MRI sessions. Since subjects in the intervention groups were required to participate in 5 d of training sessions during the 6 d intervention period, subjects had to go through Post experiments 1 or 2 d after completion of the intervention. This condition was the same in the two intervention groups of this experiment.
Training tasks.
The PS-training program consisted of the adaptive training of PS tasks. Training tasks involved eight tasks using computer buttons and two tasks using paper and pencil.
The first four of the eight training tasks using computer buttons resembled 0-back (Callicott et al., 1999) tasks. In all of these four tasks, a certain type of stimulus was presented successively. In each trial, subjects had to push buttons that corresponded with the presented stimuli before the next trial (before the next stimuli were presented). Stimuli were randomly presented as visual numbers (one, two, three, four) in Task 1, visual locations (a mark was presented in either one of four corners of the interface) in Task 2, auditory numbers (one, two, three, four) in Task 3, and auditory locations (a pure tone was presented in either the left ear or the right ear) in Task 4. In the first task and the third task, subjects had to push button F on the keyboard if they received the first stimulus, G for the second, H for the third, and J for the fourth. In the second task, subjects had to push button F if the stimulus was presented in the upper left, V in the lower left, J in the upper right, and N in the lower right. In the fourth task, subjects had to push button H if the stimulus was received in the left ear and J if the stimulus was received in the right ear. Task 5 was a computerized task similar to the Number Comparison task (Earles and Kersten, 1999). In each trial, a pair of number strings containing three single-digit numbers (zero to nine) was presented visually and subjects had to decide whether the strings were the same when reordered. If the strings were the same, subjects pushed H. If they were not the same, subjects pushed J. In half of the trials the strings were the same, but in the other half of the trials they were not. When the strings were not the same, one of the three numbers in the number strings was different between the pair of strings. Task 6 was a computerized task similar to the Visual Number Matching task (Woodcock, 1997). In each trial, a row of six double-digit numbers with two identical numbers in the row (for example, 37 65 43 37 28 88) was presented. Subjects had to push one of two buttons corresponding with the places of the two identical numbers in the row (D, F, G, H, J, and K corresponded to the six places in the row). In Tasks 1–6, performance of each block (a period during which stimuli were presented sequentially) was defined by the number of correct responses and each block ended after 24 trials. Task 7 was a computerized task for the rapid recognition of numbers presented visually. In this task, single-digit numbers (from 1 to 4) were visually presented rapidly. Subjects had to push the H button within 1 s after the same stimulus was presented three times in a row. One block of the task ended when the sets of three same stimuli had been presented a total of 10 times. Task 8 was a computerized task for the rapid recognition of visuospatial location. In this task, visual locations (a mark was presented in one of the four corners of the interface) were presented rapidly. Subjects had to push the H button within 1 s of the same stimulus being presented three times in a row. One block of the task ended when the sets of three same stimuli had been presented a total of 10 times. In Tasks 7 and 8, subject performance in each block was defined by the number of correct responses minus the number of inappropriate responses. In the computerized tasks, subjects were allowed to use the fingers they preferred to do the task as fast as possible. However, we instructed subjects to stick to the use of the same fingers throughout the intervention.
In all of the eight training tasks using computer buttons, difficulties (stimulus presentation rates) were modulated based on subject performance. When subject performance was below a certain level in a block (a period when stimuli were presented sequentially), the difficulty of the task in the next block decreased based on how badly subjects performed [the difficulty level decreased by one (presentation rate was multiplied by 0.99) when subject made one mistake more than the set criteria (level b), the difficulty level decreased by two when subjects made two mistakes more than the set criteria, and so on]. However, when subject performance during the block fell below a certain level (equal to the level of chance in most of the tasks; level a), the extent of decreased difficulty remained the same regardless of further decreases in performance. When subject performance rose above a certain level (which was more strict than the criteria for lowering the level; level c) during a block, the difficulty of the task in the next block increased based on how well the subjects performed. The difficulty of the task increased in a manner similar to the way it decreased. When subject performance was between the criteria to increase the level of the block and the criteria to decrease the level, the difficulty of the task did not change in the next block. In other words, if we call performance during a block in the task x, the presentation rate of stimuli in the block s, and the presentation rate of stimuli in the next block s(100/99)y, then when x ≤ a, y = −4, when a < x ≤ b, y = −(b − x + 1), when b < x ≤ c, y = 0, and when c < x, y = x − c. [a, b, c] for Tasks 1–3 is [6, 9, 12]. [a, b, c] for Tasks 4 and 5 is [12, 15, 18]. [a, b, c] for Task 6 is [8, 11, 14]. [a, b, c] for Tasks 7 and 8 is [−3, 0, 2]. These values of [a, b, c] in each task were determined by the chance accuracy of each task.
In the ninth and tenth tasks, subjects used paper and pencil. Task 9 was a paper and pencil task similar to the number comparison task (Earles and Kersten, 1999). In this task, rows of pairs of number strings containing three single-digit numbers (zero to nine) were printed on paper. Subjects had to decide whether the strings were the same when reordered. If the strings were the same, subjects drew a circle. If they were different, subjects made a check mark. In half of the rows the strings were the same, but in the other half of the rows they were not the same and one of the three numbers in the number strings was different between the two strings. Subjects were instructed to answer as many of these questions as possible in 1 min. They had to perform this task 10 times before continuing to the next task. Task 10 was a paper and pencil task similar to the Visual Number Matching task (Woodcock, 1997). In this task, rows of six double-digit numbers were printed on paper with two identical numbers in each row (for example, 37 65 43 37 28 88). Subjects were instructed to circle either of the identical digits in each row and to do this task as quickly and as accurately as possible in 1 min. Subjects had to perform this task 10 times before continuing to the next task. The stimuli of Tasks 9 and 10 were randomly generated to match the conditions of the tasks, and as a result, different stimuli were presented each time in each task. Paper and pencil training tasks (Tasks 9 and 10) were added to the training tasks, since it is known that increasing the variability of tasks, stimuli, and situations of a training leads to more successful transfer (Sweller et al., 1998; Yamnill and McLean, 2001; Green and Bavelier, 2008).
In the cases of Tasks 1–6, subjects had to complete 20 blocks of each training task before continuing to the next task, and in Tasks 7 and 8, they had to complete 10 blocks (in Tasks 9 and 10, subjects had to perform the task 10 times before continuing to the next task, as described earlier). Training for the day finished after subjects completed two cycles of each task (meaning subjects completed 40 blocks in the case of Task 1, for example). In most cases, it took 4 h at most to finish a day of training. In the computerized tasks, subjects continued the next day's training at the level they finished at on the previous day in each task.
Psychological outcome measures.
For evaluation of the pretraining and posttraining, a battery of neuropsychological tests and questionnaires was administered. This battery included the following contents: (1) Raven's Advanced Progressive Matrices (Raven, 1998), a nonverbal reasoning task. (2) Cattell's Culture Fair Test (Cattell and Cattell, 1973), a nonverbal reasoning test. (3) A (computerized) digit span task, a verbal WM task (for the detail of this task, see Takeuchi et al., 2011a). (4) A (computerized) visuospatial WM task. In the visuospatial WM task, circles were presented one by one at a rate of 1/s in a four-by-four grid-like interface. Participants had to remember the location and order of the stimuli. After the presentation of stimuli, participants indicated the location and order of the presented stimuli by clicking the grid-like interface on a computer screen with a mouse in the stimuli's presented order (forward visuospatial WM task) or in the reverse order (backward visuospatial WM task). The number of items to be remembered started with two items and progressively increased. Three sequences were given at each level, until the participants responded incorrectly to all three sequences, at which point the task was ended. The score of each test was equal to the sum of the number of items correctly repeated in both the forward visuospatial WM task and the backward visuospatial WM task. (5) Tanaka B-type intelligence test (Tanaka et al., 2003). Type 3B, which is for examinees in their third year of junior high school and older, was used in this study. This test is a nonverbal mass intelligence test that does not include story problems but uses figures, single numbers, and letters as stimuli. In all subtests, subjects had to complete as many problems as possible within a certain time (a few minutes). This test consists of a maze test (subjects had to trace a maze with a pencil from start to finish), counting cubes (subjects had to count the number of cubes piled up in three-dimensional ways), a displacement task [figures and numbers; subjects had to substitute a figure (nine figures) with a number (1–9) according to a model chart], identification versus same–different judgments (Japanese kana characters; subjects had to judge whether a pair of meaningless Japanese strings were the same), filling in a sequence of numbers (subjects had to fill in the blanks of a number sequence with suitable numbers according to the rules of the number arrangement), marking figures [subjects had to select forms that were identical to three samples from a series (sequence) of eight different forms], and filling in figures (subjects had to complete uncompleted figures so that the uncompleted figures were the same as the sample figures when rotated). (6) The Stroop task (Hakoda's version) (Hakoda and Sasaki, 1990), which measures response inhibition and impulsivity. Hakoda's version is a matching-type Stroop task requiring subjects to check whether their chosen answers are correct, unlike the traditional oral naming Stroop task. The test consists of two control tasks (Word–Color task and Color–Word task), a Stroop task, and a reverse Stroop task. In this study, we used the Word–Color and Color–Word tasks as measures of simple PS, and we used the Stroop and reverse Stroop tasks as measures for inhibition. In the Word–Color task, a word naming a color (e.g., “red”) was presented in the leftmost of six columns. The other five columns were each filled with one of five colors and subjects had to check the column matching the written word in the leftmost column. In the Color–Word task, the leftmost of six columns was filled with a color and the other five columns contained written words naming different colors. Subjects had to check the column with the word matching the color of the leftmost column. In the reverse Stroop task, in the leftmost of six columns, a word naming a color was printed in another color (e.g., “red” was printed in blue letters) and the other five columns were each filled with five different colors from which subjects had to check the column whose color matched the written word in the leftmost column. In the Stroop task, in the leftmost of six columns, a word naming a color was printed in another color (e.g., “red” was printed in blue letters) and the other five columns contained words naming colors. Subjects had to check the column containing the word naming the color of the word in the leftmost column. In each task, subjects were instructed to complete as many of these exercises as possible in 1 min. (7) Arithmetic tasks, similar to the ones constructed by Grabner et al. (2007). These tests measured multiplication performance on two forms of one-digit times one-digit multiplication problems (a simple arithmetic task with numbers between 2 and 9) and two forms of two-digit times two-digit multiplication problems (a complex arithmetic task with numbers between 11 and 19). The two forms of each task were the same, but the numbers used in the problems were ordered differently. Each form of the simple arithmetic task was presented with a time limit of 30 s and each form of the complex arithmetic task was presented for a limited time of 60 s. (8) The S-A creativity test (Society For Creative Minds, 1969), which measures creativity. A detailed discussion of the psychometric properties of this instrument and how it was developed is found in the technical manual of this test (Society For Creative Minds, 1969). The test was used to evaluate creativity through divergent thinking (Society For Creative Minds, 1969) and it involves three types of tasks. The first task requires subjects to generate unique ways of using typical objects. The second task requires subjects to imagine desirable functions in ordinary objects. The third task requires subjects to imagine the consequences of “unimaginable things” happening. The S-A test scores the four dimensions of the creative process (fluency, originality, elaboration, and flexibility). In this study, the sum of the graded scores of the four dimensions was used in the analysis. For more details, including the psychometric properties of this test, sample answers to the questionnaire, and the manner in which they were scored, see our previous works (Takeuchi et al., 2010b,c).
We collected several questionnaires designed to assess the traits or states of the subjects, but they are not described in this study. These questionnaires were, in most of the cases, self-report questionnaires including participants' behaviors in their daily lives. They were, in most of the cases, designed to assess the traits of subjects, not the effect of the 5 d intervention. In addition to self-report questionnaires, all neuropsychological assessments were performed by postgraduate and undergraduate students who were kept blind to the group membership of the participants.
fMRI tasks.
To map training-induced changes in brain activity related to simple cognitive processes and WM, functional magnetic resonance imaging (fMRI) was used. The n-back task, a typical task for fMRI studies with the conditions of 0-back (simple cognitive processes) and 2-back (WM), which uses digits as stimuli, was used to detect training-related changes in brain activity related to these cognitive processes. Subjects were instructed to press buttons as fast as possible in these tasks, which made the 0-back task a task of simple PS. During fMRI scanning, all subjects went through a simple block design n-back task that consisted of a 2-back task (a working memory task in which subjects had to push buttons after judging whether a currently presented stimulus and the stimulus presented two stimuli ago were the same) and a 0-back task (a nonmemory simple cognitive task in which subjects had to push buttons based on the currently presented stimulus). Between the n-back task periods, there were rest periods, which were used as the control condition in this study. Behavioral performance was recorded as accuracy. For details of the procedure, see our previous work (Takeuchi et al., 2011a).
Image acquisition.
All MRI data acquisition was conducted with a 3 T Philips Intera Achieva scanner. Forty-two transaxial gradient-echo images (echo time = 30 ms, flip angle = 90°, slice thickness = 3 mm, FOV = 192 mm, matrix = 64 × 64) covering the entire brain were acquired at a repetition time of 2.5 s, using an echo planar sequence. For the n-back session, 174 functional volumes were obtained. Using a MPRAGE sequence, high-resolution T1-weighted structural images (240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, FOV = 24 cm, 162 slices, 1.0 mm slice thickness) were collected. For the resting state fMRI, 34 transaxial gradient-echo images (64 × 64 matrix, TR = 2000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 cm, 3.75 mm slice thickness) covering the entire brain were acquired using an echo planar sequence. For this scan, 160 functional volumes were obtained while subjects were resting. During the resting state scans, the subjects were instructed to keep still with their eyes closed, as motionless as possible, and not to sleep or to think about anything in particular, as was done in previous studies (Greicius et al., 2003). Furthermore, data with no diffusion weighting (b = 0 image) were acquired and used for the preprocessing of the fMRI images (for details, see Takeuchi et al., 2011a). All the subjects who participated in this study also participated in other studies or projects, and MRI scannings not described in this study were performed together with the ones described in this study.
Voxel-based morphometry analysis.
To investigate the effect of PS on brain structures, voxel-based morphometry (VBM) was used. VBM is a method for the in vivo study of the human brain structures and it can detect changes in regional gray matter (GM) caused by training (Draganski et al., 2004). Across all measures, we tested the group difference in changes from the Pre to Post measures. Preprocessing of the morphological data was performed with VBM2 software (Gaser, 2007), an extension of SPM2. Default parameter settings were used (Gaser, 2007). To reduce the scanner-specific bias, we used a customized GM anatomical template and prior probability maps of gray and white matter images created from the T1-weighted structural images taken in our scanner in a previous study (Takeuchi et al., 2010c). Next, the T1-weighted structural images of each subject were segmented into gray and white matter partitions using the new gray and white matter prior probability maps. The resulting images included the extracted gray and white matter partitions in the native space. The gray matter partition was then normalized to the new gray matter probability map. The normalization parameters determined from this initial step were then applied to the native T1-weighted structural image. These normalized T1-weighted structural data were then segmented into gray and white matter partitions. In addition, we performed a correction for volume changes (modulation) by modulating each voxel with the Jacobian determinants derived from the spatial normalization, allowing us to also test for regional differences in the absolute amount of GM (Ashburner and Friston, 2000). All images were subsequently smoothed by convolving them with an isotropic Gaussian kernel of 10 mm full-width at half-maximum. Finally, the signal change in rGMV between preintervention and postintervention images was computed at each voxel for each participant. In this computation, we included only voxels that showed gray matter volume values >0.10 in both Pre and Post scans to avoid possible partial volume effects around the borders between GM and white matter, as well as between GM and CSF. The resulting maps representing the rGMV change between the Pre and Post MRI experiments (rGMV post − rGMV pre) were then forwarded to the group-level analysis described below.
Preprocessing and data analysis for functional activation data.
Preprocessing and data analysis were performed using statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (MathWorks). Before the analysis, the BOLD images from the pretraining scan and the BOLD images from the posttraining scan were realigned and resliced to the mean image of the BOLD images from the pretraining scan. They were then corrected for slice timing, coregistered to a b = 0 image of diffusion tensor imaging, and spatially normalized into our original b = 0 image template [created in Takeuchi et al. (2010b) using images of subjects who did not participate in this study] using the b = 0 image to give images with 3 × 3 × 3 mm3 voxels.
As described in our previous study (Takeuchi et al., 2011a), we did not use T1-weighted structural images for the process of coregistering because visual inspections suggest that the process of coregistering the BOLD images taken in our studies to the T1-weighted structural images used in our laboratory often fails. This failure could be attributable to differences in the two images caused by distortions of the BOLD images. The normalization procedures used b = 0 images for the following reasons. First, the process of coregistering the BOLD images taken in our studies to the b = 0 images is reliable according to a visual inspection of all images. This might be because both images are taken using an EPI sequence and have similar characteristics, including distortion of the images (Reber et al., 1998). Second, b = 0 images have clearer anatomical characteristics (compared with BOLD images), which allow for precise normalization procedures. Third, the structure of the orbitofrontal cortex (OFC) is typically lost in BOLD images taken in the 3 T scanner (Stenger, 2006). In a few cases, the direct normalization of the BOLD images to SPM5's EPI template distorted the structures around the OFC to compensate for the loss of the OFC in the BOLD images taken in our study. On the other hand, b = 0 images apparently have relatively more of these structures around the OFC according to visual inspection. Therefore, b = 0 images prevent the kind of distortion seen in BOLD images and allow for better normalization procedures. However, since b = 0 images and BOLD images in the same subjects have similar characteristics, b = 0 images and BOLD images probably also match well in the coregistration process, despite the difference in the structures around the OFC.
A design matrix was fitted to each participant with one regressor for each task condition (0-, 2-back in the n-back task) using the standard hemodynamic response function (HRF). The design matrix weighted each raw image according to its overall variability to reduce the impact of movement artifacts (Diedrichsen and Shadmehr, 2005). The design matrix was fit to the data for each participant individually. After estimation, β images were smoothed (8 mm full-width half-maximum) and taken to the second level or subjected to a random effect analysis. We removed low-frequency fluctuations with a high-pass filter using a cutoff value of 128 s. The individual-level statistical analyses were performed using a general linear model.
In the individual analysis, we focused on the activation change and compared activation related to the conditions (0-/2-back vs rest) before and after a 6 d intervention period. The resulting maps for each participant representing changes between the Pre and Post measures in brain activity during the corresponding conditions, as well as the brain activity during the corresponding conditions in the Pre measures, were then forwarded to a group analysis.
We used this design (0-/2-back conditions were compared with rest), as the simple cognitive processes we referred to must include cognitive processes such as the processing of visual stimuli. The processing of visual stimuli is usually something that should be controlled by setting appropriate control conditions in the fMRI studies. We think this view is supported by the fact that a simple reaction time task is one of the simple, typical PS tasks. Also consistent with this view is the fact that the neural correlates of the performance of the visual choice reaction time task include the white matter integrity of such areas as the optic radiation, which connects the thalamus and the visual area (Tuch et al., 2005).
Preprocessing and data analysis for functional connectivity data.
The preprocessing and the data analysis for the functional connectivity data were performed using SPM5 implemented in Matlab. Before the analysis, the BOLD images from the pretraining scan and the BOLD images from the posttraining scan were corrected for slice timing. They were then realigned and resliced to the mean image of the BOLD images from the pretraining scan. Normalizing procedures for these images were performed in the same way described in the preprocessing and data analysis for the functional activation data section above. The images were then smoothed (8 mm full-width at half-maximum).
Individual-level statistical analyses were performed using a general linear model (GLM). We removed low-frequency fluctuations with a high-pass filter cutoff value of 128 s (or 1/128 Hz). Slow signal drifts with a period longer than this, which are probably not based on brain activities, are removed by this value. A low-pass filter was not used and serial correlations in the BOLD signal were accounted for by a first-degree autoregressive correction. Several sources of spurious variances and their temporal derivatives were then regressed out by putting these variances into the following regressors: (1) six parameters obtained by a rigid body correction of head motion and (2) the whole-brain signal averaged over a whole-brain mask. Such a regression procedure removes fluctuations that are not likely to be involved in specific regional correlations. Correlation maps were produced by extracting the BOLD time course from a seed region, then computing the correlation coefficient between that time course and the time course from all other brain voxels. For the current study, we examined correlations associated with the left perisylvian area, which is the region that showed significant training-related changes in the analyses of gray matter and functional activation. The seed region in this study was a 6-mm-radius sphere centered on a focus. The peak voxel of the sphere (x, y, z = −57, −6, 9) was defined by the peak voxel of the significant result in the functional activation analysis of this study.
At the individual level analysis, contrast images representing changes in resting-FC with the seed regions following the 6 d intervention period and contrast images representing resting-FC with the seed region preceding the intervention were estimated for each subject after preprocessing. These images were then forwarded to the group analysis described below.
Statistical thresholds in the group-level analysis of imaging and behavioral data.
The behavioral data were analyzed using the statistic software SPSS 16.0. With regard to the analysis and the effects of the training on each measure, the PS-training group was compared with the no-intervention group using one-way analyses of covariance (ANCOVAs) with the difference between pretest and posttest measures as dependent variables and pretest scores (see below for the covariates of imaging data analyses) as covariates to exclude the possibility that any pre-existing difference in the measures between the groups affected the results of each measure. Since the superiority (or beneficial effects) of the intervention training was our primary interest, in our behavioral analysis, test–retest changes in the group of interest were compared to test–retest changes in the control group using one-tailed tests (p < 0.05), as was performed in the previous studies (Klingberg et al., 2002, 2005). However, in behavioral measures in which the meaning of “superiority” was not clear, two-tailed tests were used, namely, when the creativity test score was associated with an impaired selective attention system, psychosis, and cognitive disinhibition (Necka, 1999; for details, see Takeuchi et al., 2011a).
In the group-level imaging analysis of rGMV, we tested for groupwise differences in the change in rGMV across the whole brain. We used the factorial design option in SPM5. In the imaging analysis, the effects of the interventions, estimated by comparing changes in Pre to Post measures as described above, were compared between the groups at each voxel with total gray matter volume in the Pre measurement, pretest scores of measures of general intelligence (RAPM), and two measures of PS (Color–Word task and Word–Color task), as covariates. The differences between the PS-training group and the control group were investigated. This analysis corresponds to the ANCOVA. RAPM pretest score was included in covariates to correct the effect of preexisting differences of general intelligence.
Furthermore, to show more firmly that preexisting group differences in rGMV did not affect findings in the group-level imaging analysis, we extracted mean changes in Pre to Post measures in the significant clusters in the group-level whole-brain imaging analysis (ANCOVA) described above, as well as mean values of the Pre measures in these significant clusters. Then, we performed the ANOVA to compare group differences of mean changes in Pre to Post measures in the significant clusters of the group-level whole-brain imaging analysis. We also performed ANCOVA to compare group differences in mean changes in the Pre to Post measures of the significant clusters and in this analysis, we used mean values of the Pre measures in these significant clusters as covariates.
In the analysis of rGMV, applying VBM5 (Gaser, 2007), the level of statistical significance was set at p < 0.05, corrected at the nonisotropic adjusted cluster level (Hayasaka et al., 2004) with an underlying voxel level of p < 0.0025. Nonisotropic adjusted cluster-size tests can and should be applied when cluster size tests are applied to data known to be nonstationary (i.e., not uniformly smooth), such as VBM data (Hayasaka et al., 2004). We did not perform any region of interest analyses in this study.
In the group-level imaging analysis of functional activation and resting-FC with the seed region, we tested for groupwise differences in the changes in functional activation during each condition and the changes in resting-FC with the seed region across the whole brain. We performed voxelwise ANCOVAs with the difference in each measure between Pre scan and Post scan at each voxel as dependent variables and the value of each measure in the Pre scan at each voxel as independent variables. This analysis was performed using Biological Parametrical Mapping (BPM) (Casanova et al., 2007) implemented in SPM5. It used images representing changes in the functional activation of each condition/resting-FC between the Post scan and the Pre scan, as well as images representing the functional activation of each condition/resting-FC in the Pre scan, which was made as described above. This analysis using BPM was not applied to the rGMV analysis, as to our knowledge, BPM does not use the nonisotropic adjusted cluster-size test, which was used in the rGMV analysis described above.
In the group-level imaging analysis of functional activation and resting-FC with the seed region, regions with significance were inferred using cluster-level statistics (Friston et al., 1996). Only clusters with a p < 0.05, after a correction for multiple comparisons at the cluster size, with a voxel-level cluster-determining threshold of p < 0.0025 uncorrected, were considered statistically significant in this analysis.
Results
Training data
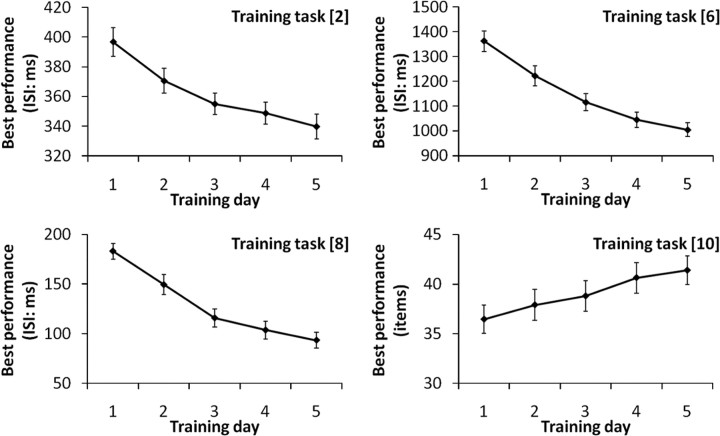
Practice resulted in a significant increase in performance across all of the 10 training tasks [in computerized tasks this was measured by the shortest interstimulus interval (ISI) of the tasks in which subjects achieved a certain level of performance in that trial and shortened the ISI in the next block; in the paper and pencil tasks, this was measured by the largest number of items answered in one trial; see Materials and Methods] from the first day of training to the last day of training (one-tailed paired t, p < 0.001; for all of the 10 training tasks) (Fig. 1). Furthermore, the best performance on each training task on the second, third, fourth, and fifth training days significantly increased from the best performance of the day before (one-tailed paired t test, p < 0.05) except Task 4 on the third training day (p = 0.052), Task 9 on the fourth training day (p = 0.054), and Task 10 on the third (p = 0.084) and fifth (p = 0.104) training days.
Figure 1.
Practice-related performance increase in PS-training tasks 2, 6, 8, 10. Practice resulted in a significant increase in performance across all of the training tasks (in computerized tasks, performance was measured in terms of the shortest ISI of the tasks in which subjects achieved a certain level of performance in that trial and shortened the ISI in the next block; in the paper and pencil tasks, performance was measured in terms of the largest number of items answered in one trial) from the first day of training to the last day of training (one-tailed paired t, p < 0.001). Error bars represent SEs.
The effect of PS training on behavioral measures
Behavioral results comparing the control group and the PS-training group showed significantly larger Pre to Post test increases for the performance of one PS measure, the Word–Color task (p = 0.042). The results also showed substantially larger Pre to Post test increases in the performance of another PS measure, the Color–Word task (p = 0.059).
Furthermore, an exploratory behavioral analysis revealed that the PS-training group showed significantly larger Pre to Post test increases for a complex arithmetic task (p = 0.017) when compared with the control group, as well as substantially larger Pre to Post test increases in the performance of the Color–Word task (p = 0.059) and of Tanaka's B type intelligence test (p = 0.055), which consists of speeded tasks (for all the results of the psychological measures, see Table 1). These results may suggest that PS training leads to a wide range of speeded tasks. However, corrections for multiple comparisons were not performed and the statistical values were only marginally significant or substantial. This statistical procedure follows the standard procedures that are taken in studies of this kind. This statistical procedure is all the more appropriate considering the exploratory nature of this analysis. However, the behavioral results of this exploratory analysis should be interpreted with caution until replicated.
Table 1.
Pre and Post test scores in psychological measures (mean ± SEM)
| PS-training |
Control |
Planned contrast | p valuea | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Simple PS | ||||||
| Word–Color task (items) | 68.5 ± 1.7 | 78.9 ± 2.2 | 73.8 ± 1.3 | 80.2 ± 1.2 | PS-training > control | 0.042** |
| Color–Word task (items) | 53.4 ± 1.7 | 58.0 ± 2.0 | 52.9 ± 1.4 | 55.3 ± 1.7 | PS-training > control | 0.059* |
| Nonverbal reasoning | ||||||
| RAPMb (score) | 26.2 ± 0.9 | 28.5 ± 0.8 | 28.1 ± 0.8 | 30.1 ± 0.9 | PS-training > control | 0.618 |
| CCFTc (score) | 30.3 ± 1.1 | 34.3 ± 0.5 | 29.8 ± 1.0 | 35.0 ± 1.3 | PS-training > control | 0.882 |
| WM | ||||||
| Digit span (score) | 33.3 ± 1.2 | 33.9 ± 1.3 | 36.5 ± 1.9 | 37.6 ± 1.7 | PS-training > control | 0.914 |
| Visuospatial WM (score) | 26.8 ± 0.8 | 26.3 ± 0.6 | 29.8 ± 1.2 | 30.6 ± 1.2 | PS-training > control | 0.984 |
| Intelligence test with speeded tasks | ||||||
| Tanaka B type intelligence test | 109.7 ± 2.6 | 123.2 ± 2.8 | 118.7 ± 2.8 | 127.6 ± 2.8 | PS-training > control | 0.055* |
| Inhibition | ||||||
| Reverse Stroop task (items) | 60.2 ± 1.3 | 65.1 ± 2.2 | 61.3 ± 1.6 | 66.0 ± 1.5 | PS-training > control | 0.418 |
| Stroop task (items) | 47.4 ± 1.5 | 51.4 ± 2.1 | 47.8 ± 1.6 | 51.0 ± 1.7 | PS-training > control | 0.349 |
| Arithmetic | ||||||
| Simple arithmetic (items) | 32.0 ± 1.2 | 33.6 ± 1.5 | 33.0 ± 1.1 | 35.0 ± 1.3 | PS-training > control | 0.636 |
| Complex arithmetic (items) | 6.20 ± 0.49 | 7.67 ± 0.54 | 7.03 ± 0.44 | 7.65 ± 0.52 | PS-training > control | 0.017** |
| Creativity | ||||||
| S-A creativity test (total grade) | 26.5 ± 1.2 | 27.5 ± 1.2 | 26.1 ± 1.4 | 27.0 ± 1.3 | two-tailed | 0.438 |
aOne-way ANCOVAs with test–retest differences in psychological measures as dependent variables and Pre test scores of the psychological measures as covariate.
bRaven's Advanced Progressive Matrices.
cCattell's Culture Fair Test.
*p > 0.1,
**p > 0.05.
The effect of PS training on gray matter structures
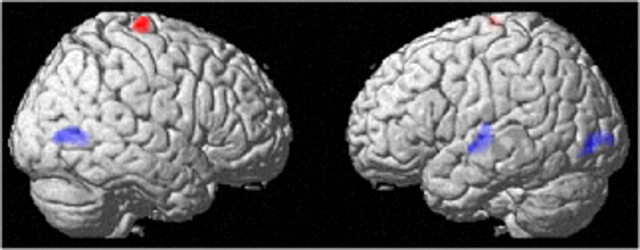
A VBM analysis revealed that, compared with the control group, the PS-training group showed a statistically significantly larger training-related decrease in the rGMV of (a) the left superior temporal gyrus (x, y, z = −59, −14, 0; t = 3.91; p = 0.001, corrected for multiple comparisons at the nonisotropic adjusted cluster level with a cluster-determining threshold of p < 0.0025, uncorrected), (b) the left middle-inferior occipital gyrus (x, y, z = −31, −84, 1; t = 4.55; p < 0.001, corrected), and (c) the right middle temporal-occipital gyrus (x, y, z = 46, −69, 4; t = 4.33; p < 0.001, corrected) (Fig. 2) [(rGMV Pre − rGMV Post)PS group − (rGMV Pre − rGMV Post)control group]. On the other hand, compared with a test–retest increase in the control group, the PS-training group showed a statistically significantly larger increase in the rGMV of (d) the right precentral gyrus (x, y, z = 16, −22, 74; t = 5.13; p < 0.001, corrected) (Fig. 2) [(rGMV Post − rGMV Pre)PS group − (rGMV Post − rGMV Pre)control group]. The p values of ANOVA (two-tailed) that compared group differences in the mean values of Pre to Post changes in the rGMV of significant clusters a–d were 0.009, 0.003, 0.0003, and 0.010. The p values of ANCOVA (two-tailed) that compared the group differences in the mean values of Pre to Post changes of rGMV with the mean values of the Pre measured rGMV in these clusters as covariates in significant clusters a–d were 0.006, 0.003, 0.0007, and 0.013.
Figure 2.
The effect of PS training on rGMV. The results are shown with p < 0.05, corrected for multiple comparisons at the nonisotropic adjusted cluster-level with an underlying voxel-level of p < 0.0025, uncorrected. Red area, Increase in gray matter volume in the group with PS training when compared with the control group. Compared with the control intervention (no intervention), PS training resulted in an increase in the rGMV of the right precentral gyrus. Blue areas, Decrease in gray matter volume in the group with PS training when compared with the control group. Compared with the control intervention (no intervention), PS training resulted in a decrease in the rGMV of the left superior temporal gyrus and the bilateral regions around the occipitotemporal junction.
The effect of PS training on functional activity
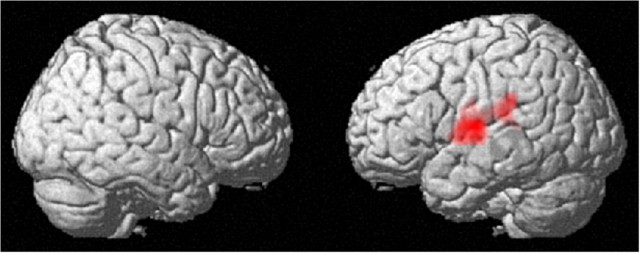
FMRI measures revealed that, compared with the control group, the PS group showed statistically significantly larger increases in functional activity associated with the 0-back condition (simple cognitive processes) from Pre to Post measures in the cluster that extends over the left Rolandic operculum and the left superior temporal gyrus (x, y, z = −57, −6, 9; t = 6.44; p < 0.001, corrected for multiple comparisons at the cluster level with a cluster-determining threshold of p < 0.0025, uncorrected) (Fig. 3). No regions showed a significantly larger PS-training-related test–retest decrease in functional activity associated with the 0-back condition. Nor did they show a significantly larger test–retest change in functional activity associated with the 2-back (WM) condition in the PS-training group compared with the change in the control group.
Figure 3.
The effect of PS training on functional activity. Increase in functional activity that is associated with simple cognitive processes (0-back) in the group with PS training when compared with the control group (p < 0.05, corrected for multiple comparisons at the cluster level with an underlying voxel level of p < 0.0025, uncorrected). Compared with the control intervention (no intervention), PS training resulted in an increase in functional activity in the cluster that extends over the left Rolandic operculum and the left superior temporal gyrus.
The effect of PS training on resting-FC with the left perisylvian area
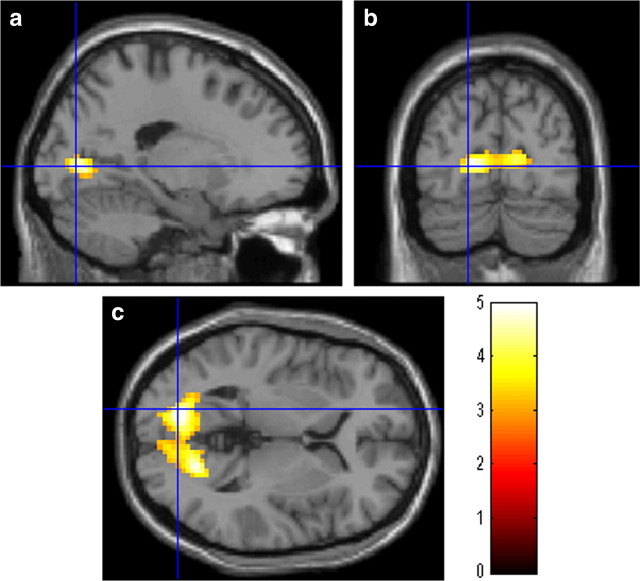
We then investigated the training-related changes in resting-FC with the left perisylvian area, which had shown PS-training-related structural and functional activity change. The analysis of resting-FC revealed that, compared with the control group, the PS group showed a statistically significantly larger increase from Pre to Post measures in resting-FC between the left perisylvian area and the cluster that extends into the bilateral calcarine cortices and the bilateral lingual gyrus (x, y, z = −18, −78, 3; t = 4.97; p < 0.001, corrected for multiple comparisons at the cluster level with a cluster-determining threshold of p < 0.0025, uncorrected) (Fig. 4a). No regions showed a significantly larger PS-training-related test–retest decrease in resting-FC with the left perisylvian area.
Figure 4.
The effect of PS training on resting-FC with the left perisylvian area. Increase in resting-FC with the left perisylvian area in the group with PS training when compared with the control group (p < 0.05, corrected for multiple comparisons at the cluster-level with an underlying voxel level of p < 0.0025, uncorrected). Compared with the control intervention, PS training resulted in an increase in resting-FC between the left perisylvian area and the cluster that extends into the bilateral calcarine cortices and the bilateral lingual gyrus.
Discussion
The present study revealed the effect of PS training on cognitive functions, functional activation, and rGMV in young adults by convergent approaches. The results showed that PS training (1) led to a significant or substantial improvement in the performance of PS measures but did not lead to the improved performance of WM, reasoning, inhibition or creativity, (2) reduced the rGMV of the left superior temporal gyrus and the bilateral regions around the occipitotemporal junction, (3) increased functional activity that was related to simple cognitive processes (but not the functional activity of WM) in the left perisylvian region (the left superior temporal gyrus and the left Rolandic operculum), and (4) increased resting-FC between the left perisylvian area and the area that extends in the lingual gyrus and calcarine cortex.
The present findings further support the notion that cognitive trainings of different cognitive functions have distinct effects (Ball et al., 2002). Previous studies of WM training and the present findings of PS training showed that, while the former affects WM, inhibition, creativity, or reasoning but not PS (for review, see Takeuchi et al., 2010d), the latter does the opposite. As for the effects of training on the brain, WM training as a whole leads to changes in the brain structures (including a reduction of rGMV) and changes in functions of the frontoparietal regions, which play a key role in WM (Olesen et al., 2004; Takeuchi et al., 2010d). On the other hand, the present imaging results show that PS training led to an increase in functional activity, a reduction in the rGMV of the left superior temporal gyrus, and a reduction in the rGMV of the bilateral regions around the occipitotemporal junction. These imaging results show that PS training has rather distinct effects on the brain (but note also that the WM training using mental calculation led to reduction of rGMV of the left superior temporal gyrus) (Takeuchi et al., 2010d).
Improvement in PS by the PS training, but lack of improvement in WM, reasoning, or inhibition, is consistent with (1) the results of previous PS-training studies of the elderly and (2) the present results showing functional activity changes in the 0-back task (simple cognitive task), but not in the 2-back task (WM task). Present psychological results showed that PS training led to significant or substantial improvement in the performance of PS measures (Word–Color task and Color–Word task in the Stroop test), but not in the performance of WM, reasoning, and creativity. This finding is congruent with the results of previous studies of PS training in the elderly that showed the effect of PS training on PS but not on other cognitive domains (e.g., Edwards et al., 2002, 2005). Also consistent with the findings of the present psychological results, the present results of functional activity revealed that PS training led to an increase in brain activity during the 0-back task (simple cognitive task), but not during the 2-back task (WM task). The results of the brain activities suggest that the change in the brain activities underlying cognitive performance is specific to simple cognitive processes without WM.
PS-training-related increases of functional activity during the 0-back task (simple cognitive task) and reduction of rGMV in the left superior temporal gyrus may underlie PS-training-related cognitive improvement in tasks using linguistic stimuli. The perisylvian regions, including the left superior temporal gyrus, may play a key role in language or linguistic processes as perisylvian regions are always activated when these cognitive processes are involved (Cabeza and Nyberg, 2000). Perhaps, among a number of training tasks, tasks using linguistic stimuli may cause an increase in functional activity and a reduction in rGMV in the left superior temporal gyrus and lead to improvements of cognitive performance in the verbal speeded tasks.
Increased resting-FC between the left perisylvian region and the regions around the lingual gyrus may reflect increased verbal information transfer between these two regions. While the left perisylvian's function is described above, the lingual gyrus is believed to play an important role in recognizing words (Kuriki et al., 1998). The lingual gyrus and the regions in the left perisylvian area strongly strengthen their functional coupling during visual spelling (Booth et al., 2008). The lingual gyrus is structurally connected with the lateral temporal regions by the inferior longitudinal fasciculus (Catani et al., 2003). Together, the observed changes may reflect increased verbal information transfer between these two regions and may underlie the improvement in cognitive speed that is related to this process.
PS-training-related rGMV reduction in the bilateral regions around the occipitotemporal junction may lead to rapid bottom-up recognition of visual information, which may in turn lead to the cognitive improvement of tasks requiring rapid recognition of visual information. These bilateral regions are consistently activated during the recognition of what is represented by some visual information (Cabeza and Nyberg, 2000) and are, more specifically, suggested to be involved in the bottom-up construction of shape descriptions from visual features. Perhaps, among a number of training tasks, ones requiring rapid recognition of what is represented by some visual information might cause a rGMV reduction in this region and lead to improvements in cognitive performance on the training tasks requiring such rapid recognition.
Decreases in rGMV after just 1 week of intense cognitive training are consistent with our previous study that involved a kind of working memory training (Takeuchi et al., 2011b). In this previous study, we suggested that cognitive training may lead to rGMV's nonlinear changes (an initial increase followed by a decrease), which are affected by training length and intensity (more intensity leads to a rapid time course for this nonlinear change). The present results also hold this pattern to be true. The speculation regarding nonlinear rGMV's changes was based on studies showing that (A) mild training examined midterm exhibited mainly an increase in rGMV (Boyke et al., 2008; Driemeyer et al., 2008; Ilg et al., 2008), (B) but a decrease in rGMV when examined long after the training has been stopped (Boyke et al., 2008; Driemeyer et al., 2008). (C) Intense training led to a decrease in rGMV in a short period of time (Takeuchi et al., 2011b). Our other two sets of unpublished data using young adults also held the pattern of A to be true (Takeuchi et al., 2011b), and one other study's unpublished data using young adults, which was described in the Materials and Methods, also showed the same pattern as C (T. Nagase, H. Takeuchi, Y. Taki, Y. Sassa, H. Hashizume, and R. Kawahsima, unpublished observation). However, it should be noted that even after mild interventions for a short period of time, there are a few or substantial decreases of rGMV (May et al., 2007; Quallo et al., 2009). As was discussed in our previous study (Takeuchi et al., 2011b), we regard the usage-dependent selective elimination of synapses (Huttenlocher and Dabholkar, 1997) as one speculated mechanism underlying the decreases of rGMV. Selective elimination of synapses helps to sculpt neural circuitry, including the circuitry supporting cognitive abilities (Hensch, 2004).
Contrary to the results of our previous study, there was an increase in rGMV (in the right precentral gyrus) related to the training. The change in the right precentral gyrus may be due to use of the left hand during the computerized task training (note that fingers from both hands were essential in the training Task 2). Although the mixture of an increase and decrease in rGMV following a short-term intervention was seen in another previous study (Quallo et al., 2009), the exact cause of this phenomenon is unknown. One possibility is that, unlike the involvement of the regions showing a decrease in rGMV, the involvement of the right precentral gyrus was not so strong and it may have been in a phase in which there was a temporal increase in rGMV before the decrease in rGMV (Boyke et al., 2008; Driemeyer et al., 2008).
This study has a limitation that it shares with previous studies of cognitive training, including the most prestigious studies described below and our previous studies. The multiple (and sometimes heterogeneous) training programs used in this study, as well as in previous studies (e.g., Klingberg et al., 2002; Hogarty et al., 2004), are supposed to strengthen transfer effects (Goldstone, 1998; Sweller et al., 1998). However, they may also make it difficult to see the effects of each training program (Takeuchi et al., 2010d).
In summary, the present study investigated the effects of PS training on the PS of young adults and on neural mechanisms. Rather consistent with our hypothesis, results confirm that both PS-training-induced plasticity in PS and the training-induced plasticity of functions and structures are associated with speeded cognitive processes. Other than PS training, a wide range of cognitive interventions involving speeded training tasks led to an improvement in cognitive functioning. Thus, the observed neural changes caused by PS training may give us new insights into how PS training, and possibly other cognitive trainings, can improve PS.
Footnotes
This study was supported by a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan Science and Technology Agency (JST)/Research Institute of Science and Technology for Society, and JST/Core Research for Evolutional Science and Technology. We thank Yuki Yamada for operating the MRI scanner, Sarah Michael for checking the English in this manuscript, the participants, the testers for the psychological tests, and all our other colleagues in Institute of Development, Aging and Cancer, Tohoku University for their support.
References
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Mehdiratta N, Burman DD, Bitan T. Developmental increases in effective connectivity to brain regions involved in phonological processing during tasks with orthographic demands. Brain Res. 2008;1189:78–89. doi: 10.1016/j.brainres.2007.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Measuring intelligence with the culture fair tests. Champaign, IL: Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. Neuroimage. 2005;27:624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning—revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles JL, Kersten AW. Processing speed and adult age differences in activity memory. Exp Aging Res. 1999;25:243–253. doi: 10.1080/036107399244011. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Myers RS, Roenker DL, Cissell GM, Ball KK. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging Ment Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Flaherty AW. Frontotemporal and dopaminergic control of idea generation and creative drive. J Comp Neurol. 2005;493:147–153. doi: 10.1002/cne.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54:1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Gaser C. VBM Toolbox for SPM2, VBM Toolbox for SPM5. 2007 [Google Scholar]
- Goldstone RL. Perceptual learning. Annu Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38:346–356. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol Aging. 2008;23:692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoda Y, Sasaki M. Group version of the Stroop and reverse-Stroop test: the effects of reaction mode, order and practice. Kyoikushinrigakukenkyu (Educ Psychol Res) 1990;38:389–394. [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Kechavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Gillberg CG, Forssberg H, Westerberg HLP. Computerized training of working memory in children with ADHD-a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Takeuchi F, Hirata Y. Neural processing of words in the human extrastriate visual cortex. Cogn Brain Res. 1998;6:193–203. doi: 10.1016/s0926-6410(97)00030-x. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Gänssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Necka E. Creativity and attention. Polish Psychol Bull. 1999;30:85–98. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JDE. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. J Cogn Neurosci. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, Lemon RN, Iriki A. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad Sci U S A. 2009;106:18379–18384. doi: 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. Manual for Raven's progressive matrices and vocabulary scales. Oxford: Oxford Psychologists; 1998. [Google Scholar]
- Reber PJ, Wong EC, Buxton RB, Frank LR. Correction of off resonance-related distortion in echo-planar imaging using EPI-based field maps. Magn Reson Med. 1998;39:328–330. doi: 10.1002/mrm.1910390223. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Snow RE. Toward assessment of cognitive and conative structures in learning. Educ Res. 1989;18:8–14. [Google Scholar]
- Society For Creative Minds. Manual of S-A creativity test. Tokyo: Tokyo shinri; 1969. [Google Scholar]
- Stenger VA. Technical considerations for BOLD fMRI of the orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. London: Oxford UP; 2006. pp. 423–446. [Google Scholar]
- Sweller J, Van Merrienboer JJG, Paas F. Cognitive architecture and instructional design. Educ Psychol Rev. 1998;10:251–296. [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010a;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage. 2010b;51:11–18. doi: 10.1016/j.neuroimage.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel-based morphometry. Neuroimage. 2010c;51:578–585. doi: 10.1016/j.neuroimage.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R. Effects of working memory training on cognitive functions and neural systems. Rev Neurosci. 2010d;21:427–449. doi: 10.1515/revneuro.2010.21.6.427. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. Failing to deactivate: the association between brain activity during a working memory task and creativity. Neuroimage. 2011a;55:681–687. doi: 10.1016/j.neuroimage.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One. 2011b doi: 10.1371/journal.pone.0023175. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Okamoto K, Tanaka H. Manual of new Tanaka B type intelligence test. Tokyo: Kaneko Syobo; 2003. [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Kawashima R. Reading and solving arithmetic problems improves cognitive functions of normal aged people: a randomized controlled study. Age. 2008;30:21–29. doi: 10.1007/s11357-007-9044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. The Woodcock-Johnson tests of cognitive ability—revised. In: Flanagan DP, Genshaft JL, editors. Contemporary intellectual assessment: theories, tests, and issues. New York: Guilford; 1997. pp. 230–246. [Google Scholar]
- Yamnill S, McLean GN. Theories supporting transfer of training. Hum Resour Dev Q. 2001;12:195–208. [Google Scholar]