Abstract
Prepulse inhibition (PPI) of startle is the suppression of the startle reflex when a weaker sensory stimulus (the prepulse) shortly precedes the startling stimulus. PPI can be attentionally enhanced in both humans and laboratory animals. This study investigated whether the following three forebrain structures, which are critical for initial cortical processing of auditory signals, auditory fear conditioning/memories, and spatial attention, respectively, play a role in the top-down modulation of PPI in rats: the primary auditory cortex (A1), lateral nucleus of the amygdala (LA), and posterior parietal cortex (PPC). The results show that, under the noise-masking condition, PPI was enhanced by fear conditioning of the prepulse in a prepulse-specific manner, and the conditioning-induced PPI enhancement was further increased by perceptual separation between the conditioned prepulse and the noise masker. Reversibly blocking glutamate receptors in the A1 with 2 mm kynurenic acid eliminated both the conditioning-induced and perceptual separation-induced PPI enhancements. Blocking the LA eliminated the conditioning-induced but not the perceptual separation-induced PPI enhancement, and blocking the PPC specifically eliminated the perceptual separation-induced PPI enhancement. The two types of PPI enhancements were also eliminated by the extinction manipulation. Thus, the top-down modulation of PPI is differentially organized and depends on operations of various forebrain structures. Due to the fine-tuned modulation by higher-order cognitive processes, functions of PPI can be more flexible to complex environments. The top-down enhancements of PPI in rats are also useful for modeling some mental disorders, such as schizophrenia, attention deficit/hyperactivity disorder, and posttraumatic stress disorder.
Introduction
The startle reflex, the whole-body reflexive response to sudden and intense sensory stimuli (Landis and Hunt, 1939; Koch, 1999; Yeomans et al., 2002), can disrupt cognitive/behavioral performances (Hoffman and Overman, 1971; Foss et al., 1989). Prepulse inhibition (PPI) of startle is the suppression of the startle reflex when a weaker sensory stimulus (the prepulse) shortly precedes the startling stimulus (Hoffman and Searle, 1965; Hoffman and Ison, 1980). According to the “protection of processing” theory (Graham, 1975), receiving a sensory stimulus triggers both the information processing for the stimulus signal and the gating mechanism dampening effects of disruptive inputs, and PPI may provide a protection of the early processing of the prepulse signal from interference. Thus, PPI is generally recognized as an operational measure of sensorimotor gating (Braff and Geyer, 1990; Swerdlow et al., 1991; Cadenhead et al., 1993).
Although the PPI-mediating circuitry resides in the brainstem (Davis and Gendelman, 1977; Fox, 1979; Li and Frost, 2000) (for review, see Fendt et al., 2001; Li and Yue, 2002), indicating that PPI principally reflects an automatic process at the preattentive stage, PPI can be top-down modulated by either feature-based attention or spatial attention to the prepulse in both humans and rats (for review, see Li et al., 2009). In rats, for instance, when the prepulse is fear conditioned, it draws more attention and the conditioned prepulse-induced PPI is enhanced (Huang et al., 2007; Zou et al., 2007; Li et al., 2008; Du et al., 2009b, 2010; Ishii et al., 2010). Also, a precedence effect-induced perceived spatial separation between the conditioned prepulse and the noise masker further enhances PPI by facilitating spatial attention to the prepulse (Du et al., 2009b, 2010). Clearly, the top-down attentional modulation of PPI contains various components that may involve different forebrain structures. This study investigated whether the following three forebrain structures are involved in the attentional modulation of PPI in rats: the primary auditory cortex (A1), lateral nucleus of the amygdala (LA), and posterior parietal cortex (PPC).
The A1 occupies the initial stage of cortical processing of auditory signals and provides auditory inputs to other cortical or forebrain subcortical regions including the PPC and amygdala (Romanski and LeDoux, 1993; Reep et al., 1994). It also projects directly to the auditory midbrain, such as the inferior colliculus (IC) (Herbert et al., 1991; Druga et al., 1997), which is also a structure in the PPI circuitry (Li et al., 1998a,b). The LA, which is involved in the formation of emotional learning (Romanski and LeDoux, 1992; Pitkänen et al., 1997), storage of fear memories (Blair et al., 2005; Schafe et al., 2005), and attentional bias toward the threat (Maren, 2007; Meck and MacDonald, 2007; Cisler and Koster, 2010), also plays a role in affecting PPI (Swerdlow et al., 2001), while the PPC is important in mediating spatial attention shift/orienting in humans (Kim et al., 1999; Yantis et al., 2002; Greenberg et al., 2010) and directed spatial attention in rats (Reep and Corwin, 2009).
Materials and Methods
Animal preparation.
According to the target forebrain structure (A1, LA, or PPC) and the injected agent [the broad-spectrum antagonist of glutamate receptors, kynurenic acid (KYNA), or the vehicle, Locke's solution], 86 young-adult male Sprague Dawley rats (age, 10 weeks; weight, 280–300 g) were randomly assigned to six structure/injection agent groups: (1) A1/KYNA (n = 14), (2) A1/vehicle (n = 14), (3) LA/KYNA (n = 16), (4) LA/vehicle (n = 14), (5) PPC/KYNA (n = 14), and (6) PPC/vehicle (n = 14).
To examine the anatomical specificity of KYNA injection, another 10 rats with KYNA injection within the barrel field of primary somatosensory cortex (S1BF) were used as the anatomical control group. The S1BF is located both on top of the LA area and next to the A1 area.
The surgical procedures were the same as used in our previous studies (Du et al., 2009a,c). Briefly, injection guide cannulae (C317G guide cannula; Plastics One) were bilaterally implanted into one of the four forebrain structures in each of the 10% chloral hydrate-anesthetized (400 mg/kg, i.p.) rats. Referenced to bregma, the stereotaxic coordinates of the structures were the following: (1) A1: anteroposterior, −4.6 mm; mediolateral, ±6.5 mm; depth, −4.2 mm; (2) LA: anteroposterior, −3.1 mm; mediolateral, ±5.2 mm; depth, −7.8 mm; (3) PPC: anteroposterior, −4.4 mm; mediolateral, ±3.1 mm; depth, −1.6 mm (Fox et al., 2003); (4) S1BF: anteroposterior, −3.1 mm; mediolateral, ±5 mm; depth, −2.5 mm.
Rats were given 1 week for recovery from surgery in a room with the temperature of 24 ± 2°C and a 12 h light/dark cycle, with food and water available ad libitum. These rats were treated in accordance with the Guidelines of the Beijing Laboratory Animal Center, and the Policies on the Use of Animals and Humans in Neuroscience Research approved by the Society for Neuroscience (2006).
Stimuli and apparatus.
The apparatus for PPI testing have been described in detail in our previous reports (Du et al., 2009b, 2010). Briefly, the rat's whole-body startle reflex, which was induced by an intense 10 ms broadband noise burst (0–10 kHz, 100 dB SPL) delivered by a loudspeaker above the rat's head, was measured by a custom-made electrical scale (National Key Laboratory on Machine Perception, Peking University) in a soundproof chamber. Beginning with the onset of the startling stimulus, electrical voltage signals were collected and sampled (at a frequency of 16 kHz) for a sufficiently long time (500 ms). Since a distinct waveform complex of the startle response could be reliably induced by the startling stimulus [Zou et al. (2007), their Fig. 2], in a trial, the peak-to-peak amplitude between the primary peak component (with the latency mainly between 15 and 20 ms) and the subsequent peak component (with the latency of mainly between 20 and 25 ms) were digitized and measured. The prepulse stimulus was delivered by two spatially separated (i.e., left and right) loudspeakers in the frontal field with a 100° separation angle and 52 cm away from the rat's head position.
Figure 2.
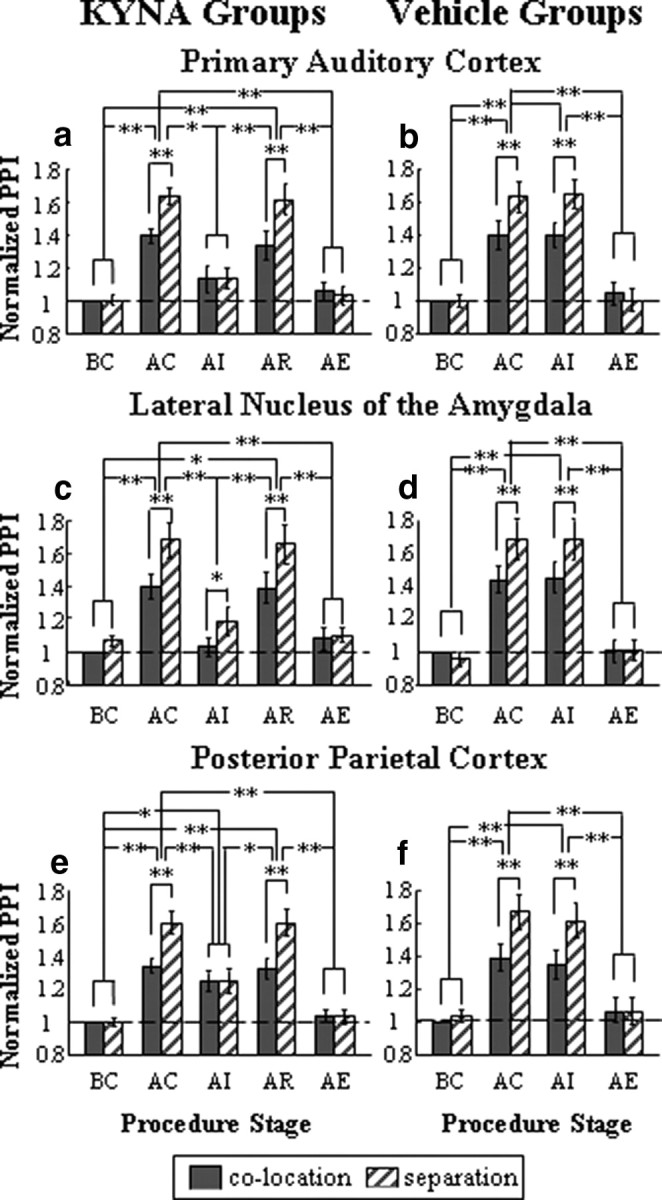
Normalized PPI induced by the conditioned prepulse at different procedure stages in A1/KYNA group (n = 12) (a), A1/vehicle group (n = 12) (b), LA/KYNA group (n = 12) (c), LA/vehicle group (n = 12) (d), PPC/KYNA group (n = 12) (e), and PPC/vehicle group (n = 12) (f). The filled bars represent the conditions when the prepulse was perceptually colocated with the noise masker, while the diagonal bars represent the conditions when the prepulse was perceptually separated with the noise masker. BC, Before conditioning; AC, after conditioning; AI, after injection; AR, after recovery; AE, after extinction. In this and the next figures, all the PPI values were normalized relative to the value at the procedure stage BC and under the prepulse/masker colocation condition. Error bars represent the SEM. **p < 0.01 and *p < 0.05 (by repeated-measures ANOVA, Bonferroni's pairwise comparisons, and paired t tests).
The prepulse, which started 100 ms before the startling pulse, was a 50 ms lower-frequency-harmonic (1.3, 2.6, and 3.9 kHz) or higher-frequency-harmonic (2.3, 4.6, and 6.9 kHz) tone complex. Each of the two prepulse signals was digitally generated by MATLAB software and converted by a custom-developed sound delivery system (National Key Laboratory on Machine Perception, Peking University) with the 16 kHz sampling rate and 16 bit resolution. Sound levels were calibrated by a sound level meter (Brüel & Kjær; type 2230) whose microphone was placed at the central location of the rat's head when the rat was absent, using a “Fast”/”Peak” meter response. The single-source sound level of the prepulse for each of the two horizontal loudspeakers was fixed at 60 dB SPL.
Procedures.
After 1 week of recovery from surgery, each rat went through the 6 d testing procedure. For the first 3 successive days, the rat was placed into the restraining cage (Zou et al., 2007), whose dimensions matched the size of the rat, and the rat could not reorient their body position. For 30 min on each of the 3 d, the rat was exposed to a broadband noise (60 dB SPL), which was continuously presented by each of the two horizontal loudspeakers. Neither the prepulse nor the startling noise was presented. This procedure was to adapt the rat to the restraining cage and testing chamber.
On the fourth day, startle responses before conditioning (procedure stage BC) was measured. The rat was placed in the restraining cage for 5 min, receiving 10 presentations of startling stimulus without prepulse presentation on the broadband-noise background whose intensity was 60 dB SPL. The interval between startling stimuli varied between 25 and 35 s (mean, 30 s). Then the two-session PPI testing was conducted with the two prepulse stimuli being randomly and evenly presented in each session (i.e., the lower- and higher-frequency prepulse stimuli were used in each session). The prepulse was presented from each of the two horizontal loudspeakers with the inter-loudspeaker onset delay being either +1 ms (left leading) or −1 ms (right leading) in each of the two testing sessions. Due to the precedence effect (Wallach et al., 1949; Litovsky et al., 1999; Li and Yue, 2002), a type of perceptual fusion of correlated leading and lagging sounds based on the attribute-capturing process (Li et al., 2005; Huang et al., 2011), a single fused prepulse image would be perceived at the left loudspeaker in some trials (when the left loudspeaker led) and at the right loudspeaker in other trials (when the right loudspeaker led). In addition to the prepulse, a broadband noise (0–10 kHz, 60 dB SPL) was continuously delivered from each of the two horizontal loudspeakers as the masker. The inter-loudspeaker onset delay for the masker was +1 ms in one session and −1 ms in the other session, leading to a fused continuous noise masker image at the left loudspeaker in one session and at the right loudspeaker in the other session. Thus, two types of perceived spatial relationships between the prepulse and the masker were created in each session: perceptual separation (when prepulse and masker had different leading loudspeakers) and perceptual colocation (when prepulse and masker shared the same leading loudspeaker). Note that a change between the precedence effect-based perceived spatial separation and colocation does not affect the impact of bottom-up sensory inputs but facilitates selective spatial attention to the attended signal (Li et al., 2004).
In a testing trial, the startling noise burst started 50 ms after the offset of the prepulse, making the interstimulus onset interval 100 ms (50 + 50 ms). Then a new trial started ∼30 s (varying from 25 to 35 s) after the offset of the prepulse. In each testing session, 10 trials were assigned to the condition of perceptual spatial separation (5 trials for each of the two prepulse stimuli), 10 trials were assigned to the condition of perceptual colocation (5 trials for each of the two prepulse stimuli), and 5 trials were assigned to the no-prepulse (startling stimulus only) condition.
Then, on the same day, after the PPI testing, rats underwent both the manipulation of fear conditioning and the manipulation of conditioning control (so simply called the conditioning/conditioning-control manipulation). The conditioned stimulus (CS) was the prepulse stimulus delivered by each of the two horizontal loudspeakers with balanced left-right leading, and the unconditioned stimulus (US) was 6 mA rectangular-pulse (duration, 3 ms) footshock using Grass S-88 stimulator (Grass) (Du et al., 2009b, 2010). For each rat, during the fear-conditioning manipulation, 10 temporally synchronized (paired) combinations of the footshock (US) and one of the prepulse stimuli (CS) were presented every 30 s (US started 3 ms before CS ending, and coterminated with CS). During the conditioning-control manipulation, 10 temporally random (unpaired) combinations of the footshock and the other prepulse were presented every 30 s. In each group, one-half of the rats received fear conditioning of the lower-frequency prepulse and conditioning control of the higher-frequency prepulse, and the other one-half of the rats received the contrary manipulations.
On the fifth day (24 h after the conditioning/conditioning-control manipulation), PPI after conditioning (procedure stage AC) was measured with the procedure described above. Note that both the conditioned prepulse and the conditioning-control prepulse were always presented in each of the two testing sessions. Then, either the KYNA (2 mm in Locke's solution; Sigma-Aldrich) or Locke's solution was injected slowly into bilateral A1 (2.0 μl on each side), LA (1.0 μl on each side), PPC (2.0 μl on each side), or S1BF (2.0 μl on each side) over a period of ∼1 min. Drug administration was made through the guide cannula, which was connected to a 5.0 μl microsyringe via polyethylene tubing (inner diameter, 0.38 mm; outer diameter, 1.09 mm; Clay Adams, division of BD Biosciences). PPI after injection (procedure stage AI) was tested 15 min after the injection. Since the blocking effect of KYNA is reversible (Li and Kelly, 1992; Malmierca et al., 2003), PPI testing was conducted again 2 h after the injection of KYNA when the injected structure recovered from blocking (procedure stage AR).
On the sixth day, all rats underwent the manipulation of fear extinction. Without pairing the US, the conditioned prepulse was presented 60 times and the conditioning-control prepulse was presented 20 times with the interstimulus interval of 30 s. For each rat, the total 80 prepulse presentations (60 for CS and 20 for CS control) were evenly divided into four extinction sessions with the intersession interval of 10 min. After the extinction manipulation, PPI was measured again (procedure stage AE).
Data analyses.
The amount of PPI was calculated with the following generally used formula: PPI = (amplitude to startling sound alone − amplitude to startling sound preceded by prepulse)/(amplitude to startling sound alone).
Since in each group, one-half of the rats were fear conditioned with the lower-frequency prepulse (when the higher-frequency prepulse was the conditioning-control stimulus) and the other one-half of the rats were fear conditioned with the higher-frequency prepulse (when the lower-frequency prepulse was conditioning-control stimulus), PPI values were averaged over the two subgroups after normalization. PPI values for each individual rat were normalized relative to the PPI value before the conditioning/conditioning-control manipulation (procedure stage BC) under prepulse/masker colocation condition. Mixed and within-subject repeated-measures ANOVAs followed by Bonferroni's pairwise comparisons (for comparisons between procedure stages) and paired t tests (for comparisons between perceived colocation and spatial separation) were performed using SPSS 13.0 software. The null-hypothesis rejection level was set at 0.05.
Histology.
When all recordings were finished, rats were killed with an overdose of chloral hydrate. Lesion marks were made via the cannula by an anodal DC current (500 μA for 10 s). Brains were stored in 10% formalin with 30% sucrose, and then sectioned at 50 μm in the frontal plane in a cryostat (−20°C). Sections were examined to determine locations of injection cannulae.
Results
Histology
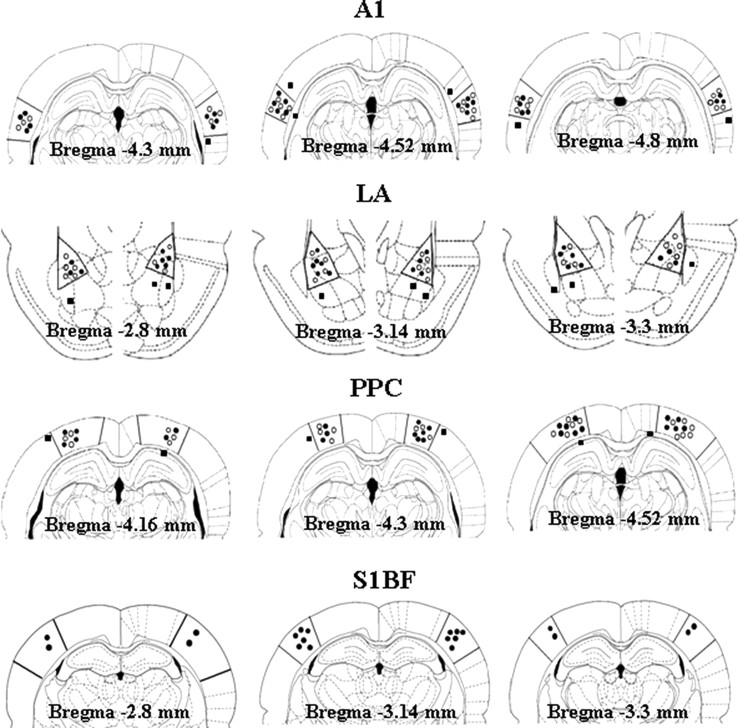
According to histological examination (Fig. 1), injection cannulae were precisely located within left A1 area in 13 rats and right A1 area in 12 rats in the A1/KYNA group (filled circle); within left A1 area in 12 rats and right A1 area in 13 rats in the A1/vehicle group (open circle); within left LA area in 14 rats and right LA area in 12 rats in the LA/KYNA group (filled circle); within left LA area in 12 rats and right LA area in 13 rats in the LA/vehicle group (open circle); within left PPC area in 13 rats and right PPC area in 13 rats in the PPC/KYNA group (filled circle); within left PPC area in 12 rats and right PPC area in 12 rats in the PPC/vehicle group (open circle); within left S1BF area in 10 rats and right S1BF area in 10 rats in the S1BF/KYNA group.
Figure 1.
Histological locations of injection cannulae in all 96 rats. Correct locations of cannulae in KYNA groups are labeled by filled circles and in vehicle control groups by open circles. Misplaced cannulae are labeled by filled squares.
Rats with unilateral or bilateral misplaced injection cannulae (filled square) were removed from data analyses. Thus, descriptions and statistical analyses here were based on the data from 12 rats in each of six groups (A1/KYNA, A1/vehicle, LA/KYNA, LA/vehicle, PPC/KYNA, and PPC/vehicle) and 10 rats in S1BF/KYNA group.
Responses to the startling stimulus alone
Table 1 shows the group mean amplitude of startle response to the startling stimulus alone (when the prepulse was not presented) in each of the rat groups. The baseline startle amplitude significantly increased after the conditioning/conditioning-control manipulation (all p < 0.05, by within-subject repeated-measures ANOVA and pairwise comparisons) and reduced to the level at the procedure stage BC after fear extinction. Injection of either KYNA or Locke's solution into one of the four brain structures did not significantly influence the startle amplitude to the startling stimulus alone (all p > 0.05).
Table 1.
Startle amplitudes to the startling stimulus alone
| Groups | Amplitude in the device scale unit |
||||
|---|---|---|---|---|---|
| Before conditioning | After conditioning | After injection | After recovery | After extinction | |
| A1/KYNA (n = 12) | 1425 ± 281 | 1640 ± 299 | 1662 ± 258 | 1644 ± 296 | 1400 ± 354 |
| A1/vehicle (n = 12) | 1486 ± 246 | 1662 ± 258 | 1720 ± 251 | N/A | 1516 ± 187 |
| LA/KYNA (n = 12) | 1104 ± 466 | 1336 ± 537 | 1354 ± 571 | 1267 ± 535 | 1055 ± 561 |
| LA/vehicle (n = 12) | 1207 ± 424 | 1400 ± 438 | 1432 ± 423 | N/A | 1267 ± 456 |
| PPC/KYNA (n = 12) | 1346 ± 355 | 1541 ± 379 | 1598 ± 406 | 1564 ± 405 | 1355 ± 460 |
| PPC/vehicle (n = 12) | 1290 ± 415 | 1449 ± 413 | 1479 ± 426 | N/A | 1268 ± 506 |
| S1BF/KYNA (n = 10) | 1109 ± 316 | 1252 ± 433 | 1286 ± 220 | 1268 ± 390 | 997 ± 212 |
Values represent mean ± SD.
Baseline PPI
In this study, two types of tone complexes (lower-frequency and higher-frequency ones) were used as the prepulse stimuli. Table 2 shows the unnormalized PPI values obtained at the procedure stage BC (before the conditioning/conditioning-control manipulation) under prepulse/masker colocation condition for all the rat groups. In each group, the values of PPI induced by the lower-frequency prepulse and those by the higher-frequency prepulse did not significantly differ (all p > 0.05 by paired t tests).
Table 2.
Group mean baseline PPI values (under perceived prepulse/masker colocation and before the conditioning/conditioning-control manipulation)
| Groups | Lower-frequency prepulse (%) | Higher-frequency prepulse (%) |
|---|---|---|
| A1/KYNA (n = 12) | 31.7 ± 7.1 | 31.5 ± 8.9 |
| A1/vehicle (n = 12) | 32.7 ± 9.4 | 32.8 ± 11.1 |
| LA/KYNA (n = 12) | 34.6 ± 12.2 | 34.6 ± 11.9 |
| LA/vehicle (n = 12) | 36.6 ± 17.4 | 36.4 ± 15.7 |
| PPC/KYNA (n = 12) | 31.2 ± 7.5 | 30.5 ± 7.9 |
| PPC/vehicle (n = 12) | 34.4 ± 7.0 | 32.0 ± 7.8 |
| S1BF/KYNA (n = 10) | 36.0 ± 7.4 | 36.9 ± 7.8 |
Values represent mean ± SD.
Effects of KYNA injection on PPI induced by conditioned prepulse
Figure 2 shows the results of PPI for rat groups with injection of KYNA (left panels) or Locke's solution (right panels) into the A1 (top panels), LA (middle panels), and PPC (bottom panels), respectively, during different procedure stages. To emphasize the most important results of the present study, we first summarize the effects of injecting KYNA into one of the three brain structures when the prepulse was the conditioned tone complex (Fig. 2a,c,e).
For each of the three rat groups with KYNA injection (A1/KYNA, LA/KYNA, PPC/KYNA), there is no evidence in Figure 2 to suggest that at procedure stage BC the perceived spatial separation between the prepulse and masker enhanced PPI. However, there is evidence to suggest that, when the prepulse became conditioned (procedure stage AC), PPI was remarkably enhanced, and the enhancement was further increased by the perceived spatial separation. Then, injection of KYNA markedly reduced the two PPI enhancements (procedure stage AI) and the degree of the reductions was brain structure dependent. Also, the injection effects disappeared 2 h after the injection (procedure stage AR). Finally, following the extinction manipulation (procedure stage AE), the PPI level returned to that at procedure stage BC.
Statistical tests were applied to examine the observations. For each of the three groups, a 5 (procedure stage: BC, AC, AI, AR, AE) × 2 (perceived spatial relationship, simply called separation type: colocation, separation) within-subject repeated-measures ANOVA shows that the interaction between the two factors was significant (all F(4,44) > 23; p < 0.001). Pairwise comparisons (for comparisons between procedure stages) and paired t tests (for comparisons between separation types) show that (1) at procedure stage BC, the effect of separation type on PPI was not significant (all t(11) < 1.7; p > 0.05); (2) the PPI level at procedure stage AC was significantly larger than that at procedure stage BC (p < 0.01); (3) at procedure stage AC, the effect of separation type on PPI was significant (all t(11) > 7.4; p < 0.001).
Following injection of KYNA into one of the three brain structures, both the conditioning-induced and separation-induced PPI enhancements were changed and the changes depended on the injected brain structure (see below).
Effects of blocking the A1 on PPI induced by conditioned prepulse
Following injection of KYNA into the A1 (Fig. 2a, procedure stage AI), perceived spatial separation-induced PPI enhancements disappeared (colocation vs separation, t(11) = 0.335, p > 0.05). Also, the PPI level at procedure stage AI became significantly smaller than that at procedure stage AC (p < 0.05), but not significantly different from that at procedure stage BC (p > 0.05). Two hours after the injection (procedure stage AR), the PPI level recovered to that at procedure stage AC (p > 0.05) and became significantly larger than that at procedure stage AI (p < 0.01). Moreover, the significant effect of separation type reappeared (t(11) = 8.152; p < 0.001). After the extinction manipulation, the PPI level returned to that at procedure stage BC (p > 0.05), and the effect of separation type became not significant (t(11) = 1.616; p > 0.05). Thus, blocking the A1 completely abolished both the conditioning-induced PPI enhancement and the perceptual separation-induced PPI enhancement.
Effects of blocking the LA on PPI induced by conditioned prepulse
Following injection of KYNA into the LA (Fig. 2c, procedure stage AI), the PPI level became significantly reduced compared with that at procedure stage AC (p < 0.01). More specifically, the group mean reduction was 26.3% under the colocation condition and 30.0% under the separation condition, leading to that the PPI level returned to that at procedure stage BC (p > 0.05). However, the effect of separation type on PPI was still significant (t(11) = 2.282; p < 0.05). Two hours after the injection (procedure stage AR), the PPI level was significantly larger than that at procedure stage AI (p < 0.01) and became not significantly different from that at procedure stage AC (p > 0.05). Also, the effect of separation type remained significant (t(11) = 7.233; p < 0.001). At procedure stage AE, the PPI level returned to that at procedure stage BC (p > 0.05), and the effect of separation type became not significant (t(11) = 0.788; p > 0.05). Thus, blocking the LA abolished the conditioning-induced PPI enhancement but not the perceptual separation-induced PPI enhancement.
Effects of blocking the PPC on PPI induced by conditioned prepulse
Following injection of KYNA into the PPC (Fig. 2e, procedure stage AI), although the PPI level became significantly smaller than that at procedure stage AC (p < 0.01), it was still significantly larger than that at procedure stage BC (p < 0.05). Also, the effect of separation type on PPI became not significant (t(11) = 0.029; p > 0.05). Two hours after the injection (procedure stage AR), the PPI level was significantly larger than that at procedure stage AI (p < 0.05) and became not significantly different from that at procedure stage AC (p > 0.05). Also, the effect of separation became significant again (t(11) = 9.973; p < 0.001). At procedure stage AE, the PPI level returned to that at procedure stage BC (p > 0.05) and the effect of separation type became not significant (t(11) = 0.150; p > 0.05). Thus, blocking the PPC abolished the perceptual separation-induced PPI enhancement but not the conditioning-induced PPI enhancement.
Effects of vehicle injection on PPI induced by conditioned prepulse
Figure 2, b, d, and f, show the PPI levels for rat groups with injection of Locke's solution into the A1, LA, or PPC at different procedure stages when the prepulse was the conditioned tone complex. Briefly, the only difference in PPI between the vehicle injection groups and the KYNA injection groups was that, in each of the three vehicle groups, the injection did not significantly change either the conditioning-induced PPI enhancement (procedure stage AI vs procedure stage AC, p > 0.05) or the separation effect (at procedure stage AI, PPI under separation condition was still larger than that under colocation condition; all t(11) > 4.5, p < 0.01).
PPI induced by conditioning-control prepulse
Figure 3 shows the PPI levels for rat groups with injection of either KYNA (left panels) or Locke's solution (right panels) into the A1, LA, or PPC at different procedure stages when the prepulse was the conditioning-control tone complex.
Figure 3.
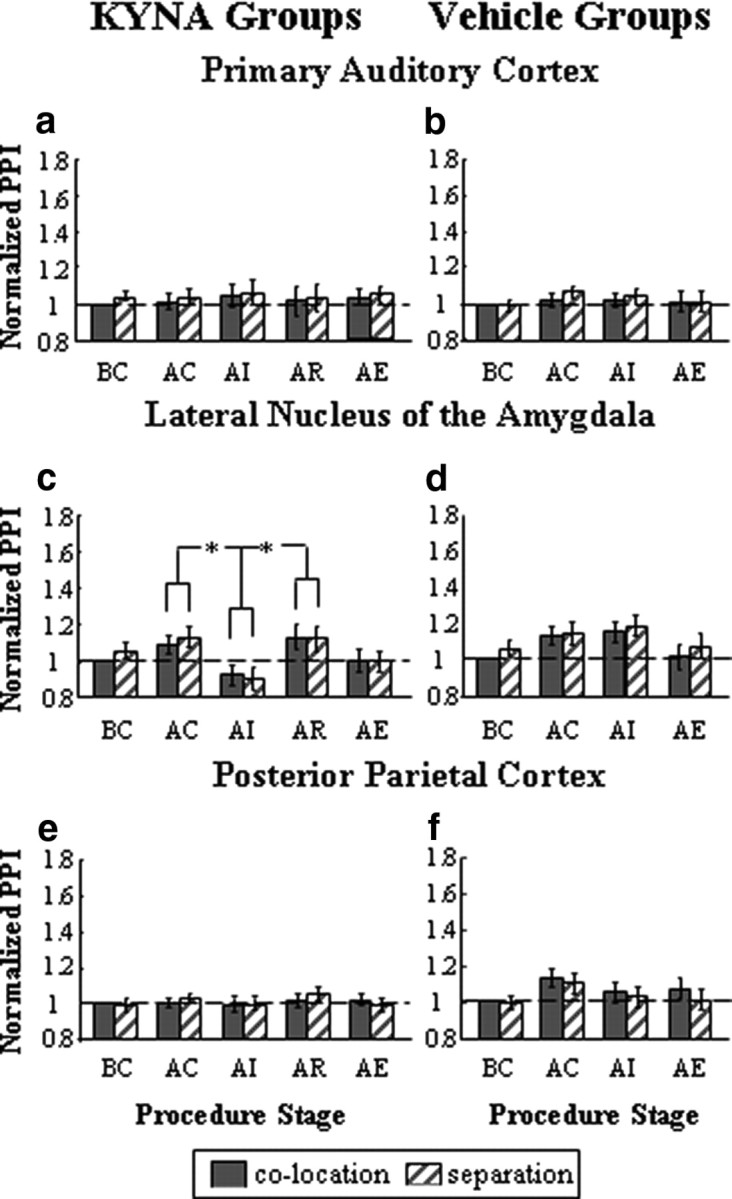
Normalized PPI elicited by the conditioning-control prepulse at different procedure stages in A1/KYNA group (n = 12) (a), A1/vehicle group (n = 12) (b), LA/KYNA group (n = 12) (c), LA/vehicle group (n = 12) (d), PPC/KYNA group (n = 12) (e), and PPC/vehicle group (n = 12) (f). See Figure 2 legend for the explanation of symbols and abbreviations. *p < 0.05 (by repeated-measures ANOVA and Bonferroni's pairwise comparisons).
For the three groups with the injection of KYNA, the conditioning-control manipulation did not cause any marked effects on PPI. For either the A1/KYNA group (Fig. 3a) or the PPC/KYNA group (Fig. 3e), injection of KYNA did not change PPI induced by the conditioning-control prepulse. A 5 (procedure stage: BC, AC, AI, AR, AE) × 2 (separation type) within-subject ANOVA confirms that the main effect of procedure stage and the main effect of separation type were not significant (all F < 4.7; p > 0.05), and the interaction between the two factors was not significant (both F(4,44) < 1.6; p > 0.05).
However, for the LA/KYNA group, injection of KYNA into the LA reduced the PPI level by 15.7% in the group mean under the colocation condition and 19.7% under the separation condition, and this PPI reduction disappeared 2 h after the injection (Fig. 3c). A 5 (procedure stage) × 2 (separation type) within-subject ANOVA shows that the main effect of procedure stage was significant (F(4,44) = 3.459; p < 0.05), the main effect of separation type was not significant (F(1,11) < 0.4; p > 0.05), and the interaction between the two factors was not significant (F(4,44) < 1.0; p > 0.05). Pairwise comparisons show that the PPI level at procedure stage AI was significantly smaller than those at procedure stage AC and procedure stage AR (p < 0.05).
Meanwhile, neither the conditioning-control manipulation nor injection of Locke's solution affected PPI in each of the three vehicle control groups (A1/vehicle, LA/vehicle, PPC/vehicle). For each group, a 4 (procedure stage: BC, AC, AI, AE) × 2 (separation type) within-subject ANOVA confirms that either the main effect of procedure stage or the main effect of separation type was not significant (all F < 4.4; p > 0.05), and the interaction between the two factors was not significant (all F < 1.4; p > 0.05).
Effects of blocking the S1BF area on PPI induced by conditioned prepulse
To examine the anatomical specificity of KYNA injection, PPI was tested in 10 rats with KYNA injection into the S1BF area. As Figure 4 shows, bilateral injection of 2 μl of KYNA into the S1BF did not significantly affect PPI under either the colocation or the separation condition, when PPI was induced by either the conditioned prepulse or the conditioning-control prepulse (all p > 0.05). Thus, the results confirm the anatomical specificity of the blocking effect of KYNA injection.
Figure 4.
Normalized PPI elicited by the conditioned prepulse (left panel) and conditioning-control prepulse (right panel) at different procedure stages in the S1BF/KYNA group (n = 10). See Figure 2 legend for the explanation of symbols and abbreviations. **p < 0.010 and *p < 0.05 (by repeated-measures ANOVA, Bonferroni's pairwise comparisons, and paired t tests).
Discussion
Two types of top-down enhancements of PPI
The PPI level depends on the salience and processing depth of the prepulse signal (Carlson and Willott, 1996; Ison et al., 1998; Röskam and Koch, 2006; Franklin et al., 2007; Huang et al., 2007; Zou et al., 2007; Li et al., 2008; Du et al., 2009b, 2010). Fear conditioning of the prepulse specifically improves the ecological salience of the conditioned prepulse stimulus and facilitates rats' attention to the “selected” prepulse, thereby enhancing PPI as revealed in this and previous studies (Huang et al., 2007; Zou et al., 2007; Li et al., 2008; Du et al., 2009b, 2010; Ishii et al., 2010). Also, the precedence effect-based perceived spatial separation between the masker and the conditioned prepulse (but not the conditioning-control prepulse) causes a further enhancement of PPI as revealed by this and previous studies (Du et al., 2009b, 2010). More importantly, this study for the first time reveals that the three forebrain structures, A1, LA, and PPC, contribute to the two types of PPI enhancements differently.
Contributions of the A1
Synthesized and released within the CNS, KYNA is the only known endogenous antagonist of excitatory amino acid (glutamate) receptors (Swartz et al., 1990). Using KYNA as a blocking means has several advantages over other chemically or physically blocking methods (such as local injection of anesthetics to block sodium channels and local cooling): First, due to its broad-spectrum nature in blocking glutamate receptors, KYNA blocks both non-NMDA and NMDA receptors (Stone and Connick, 1985; Kessler et al., 1989; Thomson et al., 1989). Thus, KYNA generally blocks glutamate receptor-mediated excitatory inputs to the injected area, reducing excitation of neurons in the area. In addition, since KYNA does not influence axonal conduction, activity of unrelated axons passing the injected area is not affected. Finally, the blocking effect of KYNA is reversible (Li and Kelly, 1992; Malmierca et al., 2003).
The results of this study show that both the conditioning-induced and perceptual separation-induced PPI enhancements were completely eliminated by reversibly blocking excitatory glutamate receptors in the A1 with KYNA, suggesting that the initial cortical processing of the conditioned-prepulse signals is critical for the formation of the two types of top-down enhancements of PPI.
It is known that the A1 is the primary cortical source for providing auditory signals to other cortical regions and forebrain subcortical structures including the PPC and amygdala (Romanski and LeDoux, 1993; Reep et al., 1994) (for review, see Wang et al., 2008). If some of the forebrain structures receiving auditory signals from the A1 are closely involved in the conditioning-induced and/or perceptual separation-induced PPI enhancements, blocking the A1 diminishes acoustically driven activities of these forebrain structures and eliminates the top-down modulations. In addition, by measuring regional cerebral blood flows (O'Leary et al., 1997; Hugdahl et al., 2000), neuromagnetic fields (Fujiwara et al., 1998; Poghosyan and Ioannides, 2008), hemodynamic responses (Jäncke et al., 1999; Krumbholz et al., 2007), or intracranial electrophysiological activities (Bidet-Caulet et al., 2007), studies using human participants suggest that the A1 is involved in auditory attention. Particularly, using the method of magnetoencephalography, our recent studies have shown that the human A1 plays a role in integrating both spectral (feature) cues and spatial cues to perceptually segregate co-occurring speech sounds in a complex listening environment (Du et al., 2011). Electrophysiological studies using laboratory animals have also shown that the A1 is important for mediating attention in rats (Polley et al., 2006; Jaramillo and Zador, 2011), ferrets (Fritz et al., 2007), and cats (Lee and Middlebrooks, 2011). Thus, blocking the A1 may impair both auditory object/feature-based attention and auditory spatial attention, leading to that attention-impaired rats do not exhibit any attentional modulations of PPI. Moreover, the A1 sends descending axonal projections to some important relay sites in the pathway mediating PPI, including the IC (Herbert et al., 1991; Druga et al., 1997; Coomes et al., 2005; Schofield, 2009), pedunculopontine tegmental nucleus (PPTg), and laterodorsal tegmental nucleus (Schofield and Motts, 2009; Schofield, 2010). Thus, the A1 may directly mediate the top-down modulations of PPI via its direct projections to the PPI pathway. Since blocking the A1 does not have any effects on PPI if the prepulse is not conditioned, the role of the A1 in modulating PPI occurs only when the prepulse stimulus becomes ethologically significant.
Contributions of the LA
In this study, the conditioning-induced but not the perceptual separation-induced PPI enhancement was eliminated by reversibly blocking the LA. It is known that the LA is important for the formation of fear conditioning (Romanski and LeDoux, 1992; Pitkänen et al., 1997), suggesting that it is the LA that establishes the association between the conditioned prepulse (CS) and the footshock (US). More importantly, since the amygdala mediates fear-related attention toward the most salient signal, such as a threat, under stressful circumstance (Meck and MacDonald, 2007), and the LA is the critical site for storing memories of the CS–US association (Blair et al., 2005; Schafe et al., 2005), the LA may play a role in retrieving the ecological meanings of the conditioned prepulse and allocating selective attention to the conditioned prepulse that signals a potential threat (the footshock). Thus, blocking the LA may impair expression of the memories of the prepulse–footshock association, and consequently, reduce the rat's attention to the conditioned prepulse. Since blocking the LA abolished conditioning-induced but not perceptual separation-induced PPI enhancement, the LA mainly mediates object/feature-based selective attention to the conditioned prepulse stimulus.
Based on our knowledge, there are two possible pathways for the top-down PPI modulations by amygdala: (1) the amygdala projects to the globus pallidus (Haber et al., 1985), which in turn sends inhibitory projections to the PPTg (Takahashi et al., 2007); (2) the amygdala projects to the deeper layers of the superior colliculus (Meloni and Davis, 2000), another important relay site in the PPI pathway (Fendt et al., 2001). Unlike blocking the A1, blocking the LA reduces the PPI level even when the prepulse is the conditioning-control tone complex, suggesting that the amygdala also provides conditioning-unrelated influences to the PPI pathway.
Since the reduction of the PPI induced by the conditioned prepulse was obviously larger than that of the PPI induced by the conditioning-control prepulse, the PPI reduction after blocking the LA when the prepulse was the conditioned tone complex cannot be explained by a general, conditioning-unrelated function of the LA in modulating PPI.
Contributions of the PPC
The results of this study also show that blocking the PPC mildly reduced the conditioning-induced PPI enhancement but completely abolished the perceptual separation-induced PPI enhancement, indicating that the PPC specifically contributes to spatial attention to the conditioned prepulse. It is known that, in humans, the PPC plays a role in spatial attention (Kim et al., 1999; Yantis et al., 2002; Greenberg et al., 2010). In rats, it mediates spatial orientation (Reep and Corwin, 2009), attentional set-shifting (Fox et al., 2003), long-term memory representation of spatial information (Kesner, 2009), sustained attention against competing distractors (Broussard and Givens, 2010), and incremental processing of conditioned stimuli (Bucci et al., 1998). Anatomically, the rat PPC has reciprocating neural connections with the auditory cortex and the medial prefrontal cortex (Reep et al., 1994), both of which send axonal projections to the amygdala (Romanski and LeDoux, 1993; McDonald et al., 1996). Thus, in the testing environment used in this study, the PPC mainly allocates spatial attention specifically to the conditioned prepulse.
Effects of the conditioning/conditioning-control manipulation on responses to the startling stimulus alone
Consistent with previous reports (Du et al., 2010), the results of this study show that, following the conditioning/conditioning-control manipulation (at the procedure stage AC), the startle amplitude to the startling stimulus alone became significantly larger in each of the rat groups (Table 1). Within a testing session, the trials with the startling stimulus alone intermixed with both those with the startling stimulus preceded by the conditioned prepulse and those with the startling stimulus preceded by the conditioning-control prepulse. Thus, the enhanced baseline startle response would be associated with sustained fear and/or anxiety without the prepulse specificity (Du et al., 2010). Note that fear-potentiated startle has been traditionally defined as an increase in startle amplitude in the presence versus the absence of the conditioned fear stimulus (i.e., CS) when the CS duration is usually set at a sufficiently long value (e.g., 3700 ms) and the CS ending is a few hundred milliseconds behind the offset of the startling stimulus (Kim et al., 1993; Walker and Davis, 1997). Thus, the design of this study was not specifically for investigating fear-induced potentiation of startle.
Moreover, in this study, injection of KYNA into the A1, LA, PPC, or S1BF did not affect the increase of the startle amplitude in the trials with the startling stimulus alone, suggesting that the potentiation of startle following the conditioning/conditioning-control manipulation was not mediated by the A1, LA, PPC, or S1BF. The results are generally consistent with the reports in the studies by Kim et al. (1993) and Walker and Davis (1997) showing that, after fear conditioning, the baseline startle amplitude (when only the startling stimulus was presented) was not substantially reduced by either injection of the non-NMDA-receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione into the basolateral amygdaloid nuclei (including the LA) (Kim et al., 1993) or injection of the specific AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline into one of the three brain regions: the basolateral amygdala, central nucleus of the amygdala, and bed nucleus of the stria terminalis (Walker and Davis, 1997).
New animal models for studying mental disorders
In patients with schizophrenia, impaired PPI that is induced by the attended prepulse, but not ignored prepulse, is more correlated with the severity of some critical symptoms (Dawson et al., 2000; Braff et al., 2001; Hazlett et al., 2007). However, the correlation between symptoms and PPI deficits cannot be detected in the passive-attention PPI paradigm (Swerdlow et al., 2006). Moreover, in children with attention deficit/hyperactivity disorder (ADHD), PPI induced by attended prepulse is reduced, but it is unaffected if children with ADHD are instructed to ignore the prepulse (Hawk et al., 2002, 2003). Since the impaired attentional modulation of PPI is more correlated with the two disorders than impaired baseline PPI, and particularly, the two disorders have their roots in dysfunctions of the A1 (Javitt et al., 1993; Bekker et al., 2005), the amygdala (Aleman and Kahn, 2005; Serene et al., 2007), and the PPC (Danckert et al., 2004; Curatolo et al., 2009), the top-down modulation of PPI in rats will be useful for establishing new animal models for studying the two mental disorders.
Furthermore, as shown by the results of this study, both the conditioning-induced and perceptual separation-induced PPI enhancements can be completely eliminated by the extinction manipulation. Thus, it is also of interest to know whether the extinction of PPI enhancement involves the forebrain structures, such as the auditory cortex, amygdala, and prefrontal cortex (Falls et al., 1992; Quirk et al., 1997; Milad and Quirk, 2002), and the extinction of top-down PPI enhancements in rats also becomes useful for studying posttraumatic stress disorder (PTSD) (Adamec, 1997).
Summary: differentially organized top-down modulations of PPI
Although the primary pathway that mediates PPI is located in the brainstem (Fendt et al., 2001; Li and Yue, 2002), PPI can be top-down modulated (Li et al., 2009). Previous studies have suggested that multiple forebrain structures are involved in regulating PPI (Bakshi and Geyer, 1998; Miller et al., 2010).
This study, for the first time, provides evidence that the three forebrain structures, A1, LA, and PPC, contribute to the conditioning-induced PPI enhancement and the perceptual separation-induced PPI enhancement differently. We conclude that the neural bases underlying attentional modulations of PPI are differentially organized: The PPC is mainly involved in the spatially attentional modulation, the LA is mainly involved in nonspatially attentional modulation, and the A1 is involved in both the spatially and nonspatially attentional modulations. Thus, the differentially organized top-down enhancements of PPI refine functions of PPI, making the gating process more flexible to complex environments. In the future, it is important to investigate whether the top-down enhancements of PPI are useful for studying mental disorders, such as schizophrenia, ADHD, and PTSD.
Footnotes
This work was supported by National Natural Science Foundation of China Grant 30950030, “973” National Basic Research Program of China Grant 2009CB320901, Chinese Ministry of Education Grant 20090001110050, and “985” grants from Peking University.
References
- Adamec R. Transmitter systems involved in neural plasticity underlying increased anxiety and defense—implications for understanding anxiety following traumatic stress. Neurosci Biobehav Rev. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker EM, Overtoom CC, Kooij JJ, Buitelaar JK, Verbaten MN, Kenemans JL. Disentangling deficits in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2005;62:1129–1136. doi: 10.1001/archpsyc.62.10.1129. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O. Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci. 2007;27:9252–9261. doi: 10.1523/JNEUROSCI.1402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Huynh VK, Vaz VT, Van J, Patel RR, Hiteshi AK, Lee JE, Tarpley JW. Unilateral storage of fear memories by the amygdala. J Neurosci. 2005;25:4198–4205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle, normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Broussard JI, Givens B. Low frequency oscillations in rat posterior parietal cortex are differentially activated by cues and distractors. Neurobiol Learn Mem. 2010;94:191–198. doi: 10.1016/j.nlm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- Carlson S, Willott JF. The behavioral salience of tones as indicated by prepulse inhibition of the startle response: relationship to hearing loss and central neural plasticity in C57BL/6J mice. Hear Res. 1996;99:168–175. doi: 10.1016/s0378-5955(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin Psychol Rev. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomes DL, Schofield RM, Schofield BR. Unilateral and bilateral projections from cortical cells to the inferior colliculus in guinea pigs. Brain Res. 2005;1042:62–72. doi: 10.1016/j.brainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Paloscia C, D'Agati E, Moavero R, Pasini A. The neurobiology of attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13:299–304. doi: 10.1016/j.ejpn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Danckert J, Saoud M, Maruff P. Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophr Res. 2004;70:241–261. doi: 10.1016/j.schres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman PM. Plasticity of the acoustic startle response in the acutely decerebrate rat. J Comp Physiol Psychol. 1977;91:549–563. doi: 10.1037/h0077345. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Res. 2000;96:187–197. doi: 10.1016/s0165-1781(00)00208-0. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J, Rajkowska G. Projections of auditory cortex onto the inferior colliculus in the rat. Physiol Res. 1997;46:215–222. [PubMed] [Google Scholar]
- Du Y, Huang Q, Wu X, Galbraith GC, Li L. Binaural unmasking of frequency-following responses in rat amygdala. J Neurophysiol. 2009a;101:1647–1659. doi: 10.1152/jn.91055.2008. [DOI] [PubMed] [Google Scholar]
- Du Y, Li J, Wu X, Li L. Precedence-effect-induced enhancement of prepulse inhibition in socially reared but not isolation-reared rats. Cogn Affect Behav Neurosci. 2009b;9:44–58. doi: 10.3758/CABN.9.1.44. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma T, Wang Q, Wu X, Li L. Two crossed axonal projections contribute to binaural unmasking of frequency-following responses in rat inferior colliculus. Eur J Neurosci. 2009c;30:1779–1789. doi: 10.1111/j.1460-9568.2009.06947.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Wu X, Li L. Emotional learning enhances stimulus-specific top-down modulation of sensorimotor gating in socially reared rats but not isolation-reared rats. Behav Brain Res. 2010;206:192–201. doi: 10.1016/j.bbr.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Du Y, He Y, Ross B, Bardouille T, Wu X, Li L, Alain C. Human auditory cortex activity shows additive effects of spectral and spatial cues during speech segregation. Cereb Cortex. 2011;21:698–707. doi: 10.1093/cercor/bhq136. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle—blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brainstem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Foss JA, Ison JR, Torre JP, Jr, Wansack S. The acoustic startle response and disruption of aiming: I. Effect of stimulus repetition, intensity, and intensity changes. Hum Factors. 1989;31:307–318. doi: 10.1177/001872088903100305. [DOI] [PubMed] [Google Scholar]
- Fox JE. Habituation and prestimulus inhibition of auditory startle reflex in decerebrate rats. Physiol Behav. 1979;23:291–297. doi: 10.1016/0031-9384(79)90370-6. [DOI] [PubMed] [Google Scholar]
- Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci. 2003;23:676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Moretti NA, Blumenthal TD. Impact of stimulus signal-to-noise ratio on prepulse inhibition of acoustic startle. Psychophysiology. 2007;44:339–342. doi: 10.1111/j.1469-8986.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J Neurophysiol. 2007;98:2337–2346. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Nagamine T, Imai M, Tanaka T, Shibasaki H. Role of the primary auditory cortex in auditory selective attention studied by whole-head neuromagnetometer. Brain Res Cogn Brain Res. 1998;7:99–109. doi: 10.1016/s0926-6410(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum, evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr, Redford JS, Baschnagel JS. Influence of a monetary incentive upon attentional modification of short-lead prepulse inhibition and long-lead prepulse facilitation of acoustic startle. Psychophysiology. 2002;39:674–677. [PubMed] [Google Scholar]
- Hawk LW, Jr, Yartz AR, Pelham WE, Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology. 2003;165:118–127. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Romero MJ, Haznedar MM, New AS, Goldstein KE, Newmark RE, Siever LJ, Buchsbaum MS. Deficient attentional modulation of startle eyeblink is associated with symptom severity in the schizophrenia spectrum. Schizophr Res. 2007;93:288–295. doi: 10.1016/j.schres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. J Comp Neurol. 1991;304:103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Overman W. Performance disruption by startle-eliciting acoustic stimuli. Psychol Sci. 1971;24:233–235. [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Huang J, Yang Z, Ping J, Liu X, Wu X, Li L. The influence of the perceptual or fear learning on rats' prepulse inhibition induced by changes in the correlation between two spatially separated noise sounds. Hear Res. 2007;223:1–10. doi: 10.1016/j.heares.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li J, Zou X, Qu T, Wu X, Mao L, Wu Y, Li L. Perceptual fusion tendency of speech sounds. J Cogn Neurosci. 2011;23:1003–1014. doi: 10.1162/jocn.2010.21470. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Law I, Kyllingsbaek S, Brønnick K, Gade A, Paulson OB. Effects of attention on dichotic listening: an 15O-PET study. Hum Brain Mapp. 2000;10:87–97. doi: 10.1002/(SICI)1097-0193(200006)10:2<87::AID-HBM50>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Fujita Y, Sutoh C, Ohtsuka H, Matsuda S, Kanahara N, Hashimoto K, Iyo M, Shimizu E. Enhancement of acoustic prepulse inhibition by contextual fear conditioning in mice is maintained even after contextual fear extinction. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:183–188. doi: 10.1016/j.pnpbp.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: a study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J Acoust Soc Am. 1998;104:1696–1704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- Jaramillo S, Zador AM. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat Neurosci. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr Impairment of early cortical processing in schizophrenia—an event-related potential confirmation study. Biol Psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- Kesner RP. The posterior parietal cortex and long-term memory representation of spatial information. Neurobiol Learn Mem. 2009;91:197–206. doi: 10.1016/j.nlm.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav Neural Biol. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Eickhoff SB, Fink GR. Feature- and object-based attentional modulation in the human auditory “where” pathway. J Cogn Neurosci. 2007;19:1721–1733. doi: 10.1162/jocn.2007.19.10.1721. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The startle pattern. New York: Farrar and Rinehart; 1939. [Google Scholar]
- Lee CC, Middlebrooks JC. Auditory cortex spatial sensitivity sharpens during task performance. Nat Neurosci. 2011;14:108–114. doi: 10.1038/nn.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Frost BJ. Azimuthal directional sensitivity of prepulse inhibition of the pinna startle reflex in decerebrate rats. Brain Res Bull. 2000;51:95–100. doi: 10.1016/s0361-9230(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Li L, Kelly JB. Inhibitory influence of the dorsal nucleus of the lateral lemniscus on binaural responses in the rat's inferior colliculus. J Neurosci. 1992;12:4530–4539. doi: 10.1523/JNEUROSCI.12-11-04530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yue Q. Auditory gating processes and binaural inhibition in the inferior colliculus. Hear Res. 2002;168:113–124. doi: 10.1016/s0378-5955(02)00356-8. [DOI] [PubMed] [Google Scholar]
- Li L, Korngut LM, Frost BJ, Beninger RJ. Prepulse inhibition following lesions of the inferior colliculus: prepulse intensity functions. Physiol Behav. 1998a;65:133–139. doi: 10.1016/s0031-9384(98)00143-7. [DOI] [PubMed] [Google Scholar]
- Li L, Priebe RP, Yeomans JS. Prepulse inhibition of acoustic or trigeminal startle of rats by unilateral electrical stimulation of the inferior colliculus. Behav Neurosci. 1998b;112:1187–1198. doi: 10.1037//0735-7044.112.5.1187. [DOI] [PubMed] [Google Scholar]
- Li L, Daneman M, Qi JG, Schneider BA. Does the information content of an irrelevant source differentially affect speech recognition in younger and older adults? J Exp Psychol Hum Percept Perform. 2004;30:1077–1091. doi: 10.1037/0096-1523.30.6.1077. [DOI] [PubMed] [Google Scholar]
- Li L, Qi JG, He Y, Alain C, Schneider BA. Attribute capture in the precedence effect for long-duration noise sounds. Hear Res. 2005;202:235–247. doi: 10.1016/j.heares.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Li N, Ping J, Wu R, Wang C, Wu X, Li L. Auditory fear conditioning modulates prepulse inhibition in socially-reared rats and isolation-reared rats. Behav Neurosci. 2008;122:107–118. doi: 10.1037/0735-7044.122.1.107. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoust Soc Am. 1999;106:1633–1654. doi: 10.1121/1.427914. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Hernández O, Falconi A, Lopez-Poveda EA, Merchán M, Rees A. The commissure of the inferior colliculus shapes frequency response areas in rat: an in vivo study using reversible blockade with microinjection of kynurenic acid. Exp Brain Res. 2003;153:522–529. doi: 10.1007/s00221-003-1615-1. [DOI] [PubMed] [Google Scholar]
- Maren S. The threatened brain. Science. 2007;317:1043–1044. doi: 10.1126/science.1147797. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Meck WH, MacDonald CJ. Amygdala inactivation reverses fear's ability to impair divided attention and make time stand still. Behav Neurosci. 2007;121:707–720. doi: 10.1037/0735-7044.121.4.707. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. GABA in the deep layers of the superior colliculus/mesencephalic reticular formation mediates the enhancement of startle by the dopamine D1 receptor agonist SKF 82958 in rats. J Neurosci. 2000;20:5374–5381. doi: 10.1523/JNEUROSCI.20-14-05374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Saint Marie LR, Breier MR, Swerdlow NR. Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience. 2010;165:601–611. doi: 10.1016/j.neuroscience.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, Arndt SV, Cizadlo TJ, Ponto LL, Watkins GL, Hichwa RD. Auditory and visual attention assessed with PET. Hum Brain Mapp. 1997;5:422–436. doi: 10.1002/(SICI)1097-0193(1997)5:6<422::AID-HBM3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Poghosyan V, Ioannides AA. Attention modulates earliest responses in the primary auditory and visual cortices. Neuron. 2008;58:802–813. doi: 10.1016/j.neuron.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Posterior parietal cortex as part of a neural network for directed attention in rats. Neurobiol Learn Mem. 2009;91:104–113. doi: 10.1016/j.nlm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex—topography of corticocortical and thalamic connections. Exp Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamoamygdala and thalamocorticoamygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Röskam S, Koch M. Enhanced prepulse inhibition of startle using salient prepulses in rats. Int J Psychophysiol. 2006;60:10–14. doi: 10.1016/j.ijpsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Doyère V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25:10010–10015. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR. Projections to the inferior colliculus from layer VI cells of auditory cortex. Neuroscience. 2009;159:246–258. doi: 10.1016/j.neuroscience.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR. Projections from auditory cortex to midbrain cholinergic neurons that project to the inferior colliculus. Neuroscience. 2010;166:231–240. doi: 10.1016/j.neuroscience.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Motts SD. Projections from auditory cortex to cholinergic cells in the midbrain tegmentum of guinea pigs. Brain Res Bull. 2009;80:163–170. doi: 10.1016/j.brainresbull.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serene JA, Ashtari M, Szeszko PR, Kumra S. Neuroimaging studies of children with serious emotional disturbances: a selective review. Can J Psychiatry. 2007;52:135–145. doi: 10.1177/070674370705200302. [DOI] [PubMed] [Google Scholar]
- Stone TW, Connick JH. Quinolinic and other kynurenines in the central nervous system. Neuroscience. 1985;15:597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogeous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J Pharmacol Exp Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat, current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Sverdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arc Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nagai T, Kamei H, Maeda K, Matsuya T, Arai S, Mizoguchi H, Yoneda Y, Nabeshima T, Takuma K, Yamada K. Neural circuits containing pallidotegmental GABAergic neurons are involved in the prepulse inhibition of the startle reflex in mice. Biol Psychiatry. 2007;62:148–157. doi: 10.1016/j.biopsych.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Walker VE, Flynn DM. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989;338:422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach H, Newman EB, Rosenzweig MR. The precedence effect in sound localization. Am J Psychol. 1949;62:315–336. [PubMed] [Google Scholar]
- Wang WJ, Wu XH, Li L. The dual-pathway model of auditory signal processing. Neurosci Bull. 2008;24:173–182. doi: 10.1007/s12264-008-1226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Zou D, Huang J, Wu X, Li L. Metabotropic glutamate subtype 5 receptors modulate fear-conditioning induced enhancement of prepulse inhibition in rats. Neuropharmacology. 2007;52:476–486. doi: 10.1016/j.neuropharm.2006.08.016. [DOI] [PubMed] [Google Scholar]