Abstract
Nutritional deprivation or malnutrition suppresses immune function in humans and animals, thereby conferring higher susceptibility to infectious diseases. Indeed, nutritional deprivation induces atrophy of lymphoid tissues such as thymus and spleen and decreases the number of circulating lymphocytes. Leptin, a major adipocytokine, is exclusively produced in the adipose tissue in response to the nutritional status and acts on the hypothalamus, thereby regulating energy homeostasis. Although leptin plays a critical role in the starvation-induced T-cell-mediated immunosuppression, little is known about its role in B-cell homeostasis under starvation conditions. Here we show the alteration of B-cell development in the bone marrow of fasted mice, characterized by decrease in pro-B, pre-B, and immature B cells and increase in mature B cells. Interestingly, intracerebroventricular leptin injection was sufficient to prevent the alteration of B-cell development of fasted mice. The alteration of B lineage cells in the bone marrow of fasted mice was markedly prevented by oral administration of glucocorticoid receptor antagonist RU486 (11β-[p-(dimethylamino)phenyl]-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one). It was also effectively prevented by intracerebroventricular injection of neuropeptide Y Y1 receptor antagonist BIBP3226 [(2R)-5-(diaminomethylideneamino)-2-[(2,2-diphenylacetyl)amino]-N-[(4-hydroxyphenyl)methyl]pentanamide], along with suppression of the otherwise increased serum corticosterone concentrations. This study provides the first in vivo evidence for the role of central leptin signaling in the starvation-induced alteration of B-cell development. The data of this study suggest that the CNS, which is inherent to integrate information from throughout the organism, is able to control immune function.
Introduction
Nutritional deprivation or malnutrition results in neuroendocrine dysfunction, decrease in reproductive and thyroid hormones, and activation of the hypothalamus–pituitary–adrenal axis (Ahima et al., 1996; Chan and Mantzoros, 2005). Such neuroendocrine alteration represents an adaptive response to energy deficiency. It is also known that nutritional deprivation or malnutrition suppresses immune function in humans and animals, thereby conferring higher susceptibility to infectious diseases (Cason et al., 1986; Chandra, 1991). For instance, nutritional deprivation induces atrophy of lymphoid tissues such as thymus and spleen and decreases the number of circulating T and B cells (Chandra, 1991; Lord et al., 1998; Chan et al., 2006).
Leptin, a major adipocyte-derived bioactive substance, is produced in response to the nutritional status and acts directly on the hypothalamus, thereby regulating food intake and energy expenditure (Friedman and Halaas, 1998; Ogawa et al., 1999; Cone, 2006). The leptin receptor is expressed not only in the hypothalamus but also in a variety of peripheral tissues (Friedman and Halaas, 1998). There is considerable evidence that leptin plays a role in the pathophysiology of acute and chronic inflammatory diseases, such as bacterial infection, atherosclerosis, and tissue fibrosis (La Cava and Matarese, 2004; Bodary et al., 2007; Tanaka et al., 2010).
It is known that leptin-deficient ob/ob mice exhibit the immunological changes similar to those induced by starvation, i.e., thymic atrophy and decreased maturation of T and B cells (Howard et al., 1999; Claycombe et al., 2008; Lam et al., 2010). Because serum leptin concentrations are rapidly lowered by starvation (Ahima et al., 1996), it is conceivable that leptin plays a role in the starvation-induced immunodeficiency. Indeed, leptin prevents the starvation-induced immunosuppression, which is mediated by T cells (Lord et al., 1998). There is also in vitro evidence suggesting that leptin can activate cytokine signaling pathway in T cells, thereby regulating their proliferation and apoptosis (Lord et al., 1998; Palmer et al., 2006). Conversely, little is known about the role of leptin in B-cell development and function. In this regard, ob/ob mice exhibit impaired humoral as well as cellular immune responses to experimental arthritis (Busso et al., 2002). ob/ob mice also show impaired B lymphopoiesis in the bone marrow, which is reversed by intraperitoneal injection of leptin, suggesting the role of leptin in B-cell development (Claycombe et al., 2008). However, it is currently unknown whether leptin signaling is involved in B lymphopoiesis under starvation conditions and, if so, how leptin regulates B-cell development in vivo.
Here, we show the alteration of B-cell development in the bone marrow of both fasted mice and ob/ob mice. Notably, such alterations are reversed by central leptin administration. Our data also suggest that corticosterone is involved in the starvation-induced alteration of B-cell development. This study highlights the role of central leptin signaling in B-cell development under starvation conditions, thereby suggesting that the CNS, which is inherent to integrate information from throughout the organism, is able to control immune function.
Materials and Methods
Reagents.
All reagents were purchased from Sigma-Aldrich or Nacalai Tesque unless otherwise noted. Allophycocyanin (APC)-conjugated anti-B220 (clone RA3–6B2) and biotin-conjugated anti-IgD (clone 11-26) antibodies and phycoerythrin (PE)-conjugated streptavidin were purchased from eBioscience. PE-conjugated anti-CD43 (clone S7) and FITC-conjugated anti-IgM (clone R6-60.2) were purchased from BD Biosciences Pharmingen.
Animals.
Eight-week-old male C57BL/6J-ob/ob and wild-type mice were purchased from Charles River Japan. They were maintained in a temperature-, humidity-, and light-controlled room (12 h light/dark cycles) and allowed ad libitum access to water and standard chow (CE-2; CLEA Japan) unless otherwise noted. All animal experiments were conducted according to the guidelines of the Tokyo Medical and Dental University Committee on Animal Research (publications 0090058, 100098, and 0110003A).
Intraperitoneal injection experiments.
Eight-week-old male C57BL/6J wild-type mice were divided into three groups. One group was allowed ad libitum access to chow and received an intraperitoneal injection of 0.2 ml of PBS at 10:00 A.M. and 7:00 P.M. for 48 h. Two groups were fasted for 48 h, when they received an intraperitoneal injection of 0.2 ml of recombinant mouse leptin (1 μg/g initial body weight) or PBS at 10:00 A.M. and 7:00 P.M.
Intracerebroventricular injection experiments.
Eight-week-old male C57BL/6J wild-type mice were anesthetized with isoflurane, and a cannula was implanted into the lateral ventricle (C315GS-2; Plastics One). Five days after the intracerebroventricular cannulation, mice were divided into three groups. One group was allowed ad libitum access to chow and received an intracerebroventricular injection of 0.5 μl of artificial CSF (aCSF) at 10:00 A.M. and 7:00 P.M. for 48 h. Two groups were fasted for 48 h, when they received an intracerebroventricular injection of 0.5 μl of recombinant mouse leptin (0.5 μg), neuropeptide Y Y1 receptor (NPY Y1R) antagonist BIBP3226 [(2R)-5-(diaminomethylideneamino)-2-[(2,2-diphenylacetyl)amino]-N-[(4-hydroxyphenyl)methyl]pentanamide] (10 nmol; Tocris Bioscience), or aCSF at 10:00 A.M. and 7:00 P.M. for 48 h.
Intracerebroventricular injection of leptin to ob/ob mice was performed as described (Satoh et al., 1998; Tanaka et al., 2010). In brief, mice were anesthetized with isoflurane, and a cannula was implanted into the lateral ventricle (Brain infusion kit 3; Durect Corporation). The cannula was connected to the micro-osmotic pump (model 1002; Durect Corporation) placed in the dorsal subcutaneous space of mice. The rate of delivery was 5 ng/h recombinant mouse leptin or aCSF. Seven days after the intracerebroventricular cannulation, mice were subjected to additional analysis.
Adrenalectomy and implantation of corticosterone pellet.
Eight-week-old male C57BL/6J wild-type mice were anesthetized with isoflurane, and a cannula was implanted into the lateral ventricle. One week after the intracerebroventricular cannulation, mice were anesthetized with pentobarbital (30 mg/kg) and adrenalectomized and implanted with a 7.5 mg corticosterone pellet (Innovation Research of America) or were sham operated. One week after the operation, the mice were subjected to additional experiments.
Blood glucose and serum analysis.
Blood was sampled from the tail vein before being killed. Blood glucose concentrations were determined by the blood glucose test meter (Glutest PRO R; Sanwa-Kagaku). Serum leptin and corticosterone concentrations were determined by the commercially available enzyme-linked immunoassay kits (R & D Systems and Assaypro, respectively).
Flow cytometry analysis.
Flow cytometry analysis was performed to evaluate the B lineage cells in the bone marrow, mononuclear cells in the circulating blood, and splenocytes as described (Hashimoto et al., 2007; Kim-Saijo et al., 2008) using a FACSCalibur HG (BD Biosciences). The data were analyzed with BD CellQuest Pro (BD Biosciences). B cells were identified by anti-B220–APC, and their subpopulations were determined as follows: pro-B (CD43+, IgM−), pre-B (CD43−, IgM−), immature B (IgM+, IgDlow), and mature B (IgM+, IgDhigh) cells (Claycombe et al., 2008).
Quantitative real-time PCR.
Total RNA was extracted from the bone using Sepasol reagent, and quantitative real-time PCR was performed with an ABI Prism 7000 Sequence Detection System using PCR Master Mix Reagent (Applied Biosystems) as described (Suganami et al., 2005; Tanaka et al., 2010). Primers used to detect mRNAs are as follows: interleukin-7 (IL-7), forward, 5′-GGCACACAAACACTGGTGAACT-3′ and reverse, 5′-TGCATCATTCTTTTTCTGTTCCTT-3′; stromal cell-derived factor-1 (SDF-1), forward, 5′-AGCCAACGTCAAGCATCTGA-3′ and reverse, 5′-TCGGGTCAATGCACACTTGT-3′; adiponectin, forward, 5′-ATGGCAGAGATGGCACTCCT-3′ and reverse, 5′-CCTTCAGCTCCTGTCATTCCA-3′; and 36B4, forward, 5′-GGCCCTGCACTCTCGCTTTC-3′ and reverse, 5′-TGCCAGGACGCGCTTGT-3′. Levels of mRNA were normalized to those of 36B4 mRNA.
Statistical analysis.
Data are presented as the mean ± SEM, and p < 0.05 and p < 0.01 were considered statistically significant. Statistical analysis was performed using one-way ANOVA followed by Tukey–Kramer test.
Results
Starvation-induced alteration of B-cell development in the bone marrow
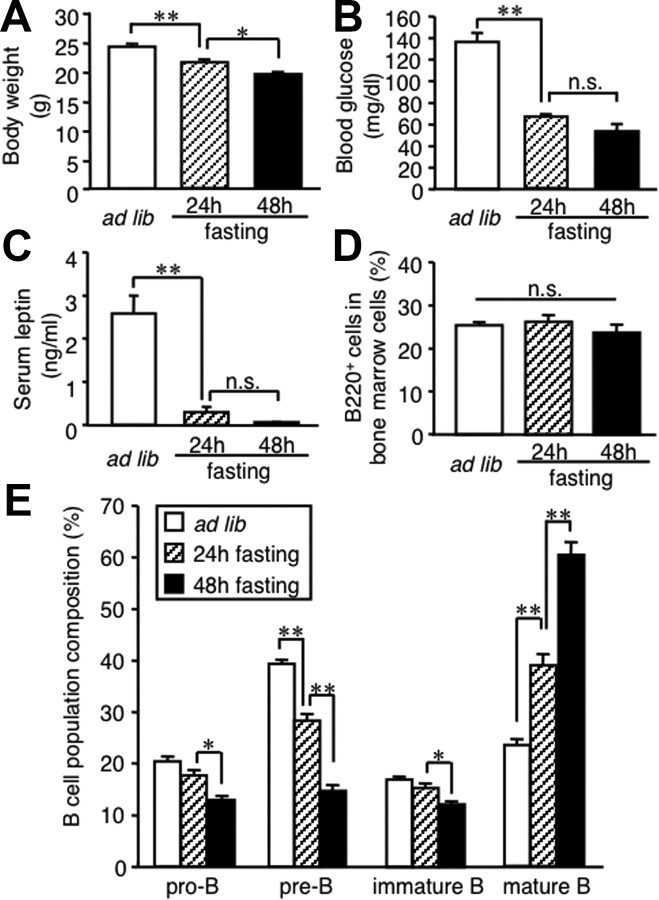
To investigate the effect of nutritional deprivation on B-cell development, we analyzed B lineage cells in the bone marrow of fasted mice. Food deprivation for up to 48 h significantly reduced body weight and blood glucose concentrations in C57BL/6J mice (Fig. 1A,B). Serum leptin concentrations were markedly reduced after 24 h of food deprivation and almost undetectable at 48 h (Fig. 1C). FACS analysis revealed that the proportion of B220-positive cells (the whole B-cell compartment) in bone marrow cells tends to be decreased at 48 h, but this trend did not reach statistical significance (Fig. 1D). In parallel with the decrease in serum leptin concentrations, the proportion of B lineage cells were markedly altered in the bone marrow of fasted mice; pro-B, pre-B, and immature B cells were decreased, whereas mature B cells were increased (Fig. 1E). The proportion of B220-positive cells (mostly bone marrow-derived mature B cells) in splenocytes and mononuclear cells of the circulating blood tended to be decreased in fasted mice relative to ad libitum fed mice (Table 1). These observations together suggest that nutritional deprivation leads to the alteration of B-cell development in the bone marrow.
Figure 1.
Effect of starvation on B lineage cells in the bone marrow. Mice were deprived of chow for up to 48 h. A–C, The effect of starvation on body weight (A) and blood glucose (B) and serum leptin (C) concentrations. D, E, FACS analysis on the whole B-cell compartment (D) and B lineage cells (E) in the bone marrow. Data in all graphs are mean ± SEM. n.s., Not significant. *p < 0.05, **p < 0.01; n = 5.
Table 1.
The proportion of B cells in the spleen and circulating blood of fasted mice and ob/ob mice
| Proportion of B220-positive cells |
||
|---|---|---|
| % of splenocytes | % of mononuclear cells of the circulating blood | |
| Wild type | ||
| Ad libitum | 56.6 ± 1.2 | 54.0 ± 1.0 |
| Fasting | 50.4 ± 2.1 | 49.0 ± 4.7 |
| ob/ob | ||
| Ad libitum | 44.7 ± 0.9* | 42.3 ± 2.7* |
The proportion of the B220-positive cells in splenocytes and mononuclear cells of the circulating blood of ad libitum-fed wild-type, fasted wild-type, and ad libitum-fed ob/ob mice. Data are mean ± SEM.
*p < 0.05 versus ad libitum-fed wild-type mice; n = 4.
Peripheral leptin administration prevents the starvation-induced alteration of B-cell development
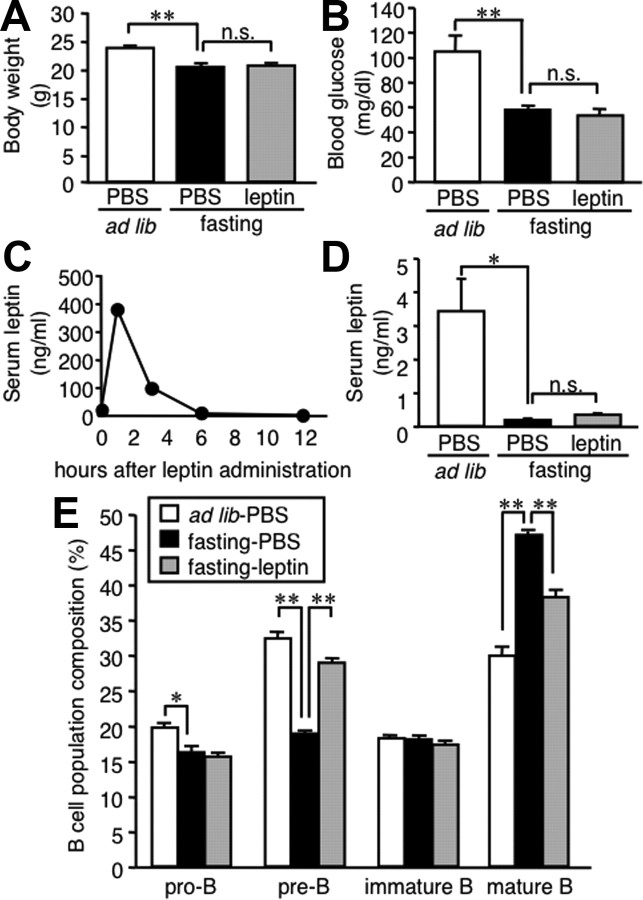
To explore the role of hypoleptinemia in the starvation-induced alteration of B-cell development in the bone marrow, C57BL/6J mice were administered intraperitoneally with recombinant mouse leptin twice daily during the starvation period. Intraperitoneal injection of leptin did not affect body weight and tended to reduce blood glucose concentrations in fasted mice (Fig. 2A,B). In this study, serum leptin concentrations peaked at 1 h after the intraperitoneal injection of leptin and thereafter went down to the levels equivalent to those in mice fed ad libitum (Fig. 2C). There was no significant difference in serum leptin concentrations at 48 h between the fasted mice receiving leptin and PBS (Fig. 2D). The alteration of B lineage cells in the bone marrow of fasted mice was markedly prevented by peripheral leptin administration (Fig. 2E). These observations suggest that leptin plays a critical role in the starvation-induced alteration of B-cell development in the bone marrow.
Figure 2.
Effect of peripheral leptin administration on B lineage cells in the bone marrow of fasted mice. Mice were divided into three groups: one group was allowed ad libitum access to chow with twice-a-day intraperitoneal injection of PBS, and two groups were deprived of chow for 48 h with twice-a-day intraperitoneal injection of recombinant mouse leptin (1 μg/g initial body weight) or PBS. A, B, Body weight (A) and blood glucose concentrations (B) at 48 h. C, Time course of serum leptin concentrations after intraperitoneal injection of recombinant mouse leptin. D, Serum leptin concentrations at the time the animals were killed. E, FACS analysis on the B lineage cells in the bone marrow. Data in all graphs are mean ± SEM. n.s., Not significant. *p < 0.05, **p < 0.01; n = 6–8.
Central leptin administration prevents the starvation-induced alteration of B-cell development
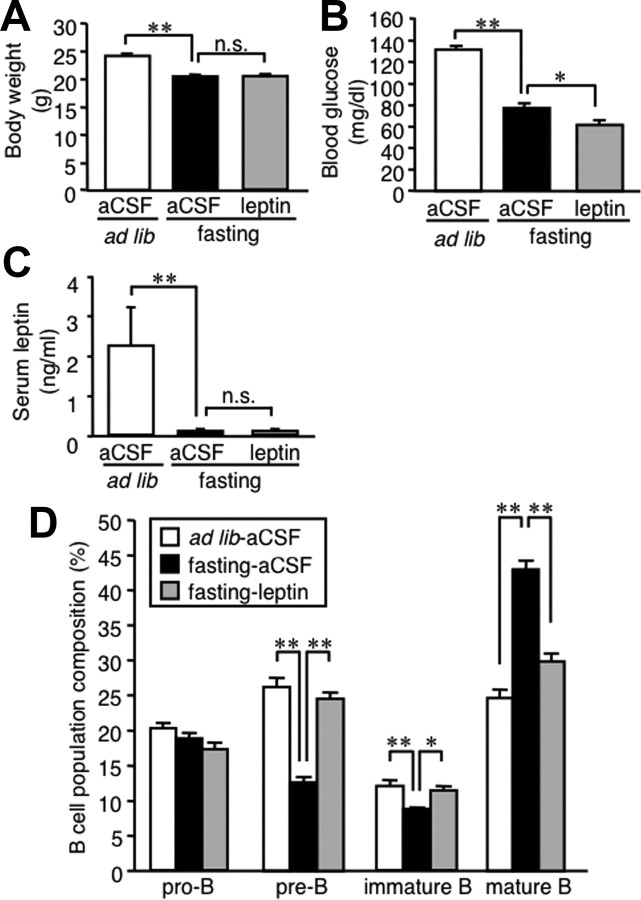
Leptin is produced in the adipose tissue and is considered to regulate food intake and energy expenditure through the CNS, although the leptin receptor is expressed in a variety of peripheral tissues, including B cells (Papathanassoglou et al., 2006). To address whether leptin is capable of regulating B-cell development in the bone marrow via a central mechanism, we examined the effect of intracerebroventricular injection of recombinant mouse leptin on B lineage cells in the bone marrow. Intracerebroventricular injection of leptin did not affect body weight and significantly reduced blood glucose concentrations in fasted mice (Fig. 3A,B). Serum leptin concentrations were unaffected by intracerebroventricular injection of leptin (Fig. 3C), suggesting no substantial leakage of exogenous leptin into the systemic circulation. Importantly, the alteration of B lineage cells in the bone marrow of fasted mice was markedly prevented by central leptin administration (Fig. 3D). These observations suggest that leptin is capable of affecting B-cell development in the bone marrow via a central mechanism.
Figure 3.
Effect of central leptin administration on B lineage cells in the bone marrow of fasted mice. Mice were divided into three groups: one group was allowed ad libitum access to chow with twice-a-day intracerebroventricular injection of aCSF, and two groups were deprived of chow for 48 h with twice-a-day intracerebroventricular injection of recombinant mouse leptin (0.5 μg/0.5 μl) or aCSF. A–C, Body weight (A) and blood glucose (B) and serum leptin (C) concentrations at the time the animals were killed. D, FACS analysis on the B lineage cells in the bone marrow. Data in all graphs are mean ± SEM. n.s., Not significant. *p < 0.05, **p < 0.01; n = 6–8.
Central leptin administration reverses the alteration of B-cell development in ob/ob mice
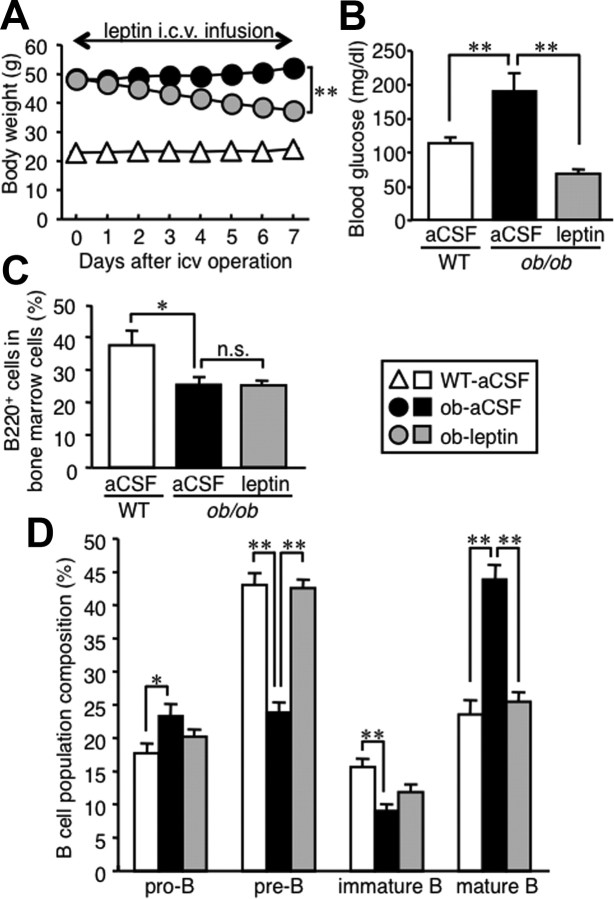
To elucidate the long-term effect of leptin on B-cell development, we used leptin-deficient ob/ob mice. As reported previously (Friedman and Halaas, 1998), 8-week-old male ob/ob mice exhibited marked obesity (50.3 ± 0.2 g) and mild hyperglycemia (266.5 ± 22.4 mg/dl) (n = 4). In this study, the proportion of B220-positive cells in the splenocytes and circulating mononuclear cells was significantly reduced in ob/ob mice relative to wild-type C57BL/6J mice (Table 1).
We next infused recombinant mouse leptin intracerebroventricularly to ob/ob mice for 7 d using a micro-osmotic pump to examine the effect of central leptin administration on B lineage cells in the bone marrow. As reported previously (Friedman and Halaas, 1998), intracerebroventricular injection of leptin effectively decreased body weight and blood glucose concentrations in ob/ob mice (Fig. 4A,B). The proportion of B220-positive cells in the bone marrow was significantly decreased in ob/ob mice relative to wild-type mice (Fig. 4C). In this study, ob/ob mice exhibited the alteration of B lineage cell proportion in the bone marrow, which is similar to that in fasted wild-type mice (Figs. 1E, 4D). Such alteration in ob/ob mice was almost completely reversed by intracerebroventricular injection of leptin (Fig. 4D), when there was no significant difference in the proportion of B220-positive cells between the ob/ob mice receiving leptin and aCSF (Fig. 4C). These observations together suggest that central leptin administration reverses the alteration of B-cell development in the bone marrow of leptin-deficient ob/ob mice.
Figure 4.
Effect of central leptin administration on B lineage cells in the bone marrow of ob/ob mice. Mice received continuous intracerebroventricular (icv) injection with leptin (5 ng/h) or aCSF for 7 d. A, Time course of body weight change. B, Blood glucose concentrations at day 7. C, D, FACS analysis on the whole B-cell compartment (C) and B lineage cells (D) in the bone marrow. Data in all graphs are mean ± SEM. WT, Wild type; n.s., not significant. *p < 0.05, **p < 0.01; n = 6.
IL-7, SDF-1, and adiponectin do not play a major role in the starvation-induced alteration of B-cell development
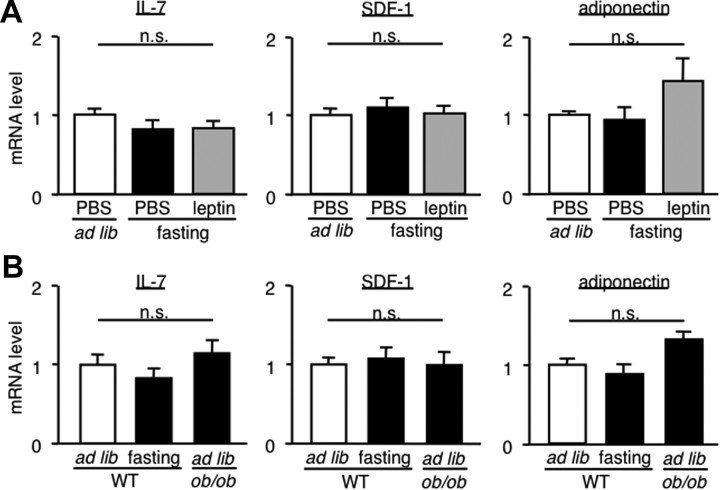
To obtain insight into the mechanism underlying the starvation-induced alteration of B-cell development in the bone marrow, we examined mRNA expression of IL-7, SDF-1 (also called CXCL12), and adiponectin in the bone. IL-7 and SDF-1 are known to be derived from osteoblasts, thereby regulating B-cell development in the bone marrow (Tokoyoda et al., 2004). It is also known that adiponectin, a major adipocytokine, potently inhibits B-cell development (Yokota et al., 2003). In this study, we did not observe significant differences in IL-7, SDF-1, and adiponectin mRNA expression between ad libitum-fed wild-type, fasted wild-type, and fasted wild-type receiving intraperitoneal injection of leptin (Fig. 5A). We also observed no significant differences in IL-7, SDF-1, and adiponectin mRNA expression among ad libitum-fed wild-type, fasted wild-type, and ad libitum-fed ob/ob mice (Fig. 5B).
Figure 5.
IL-7, SDF-1, and adiponectin mRNA expression in the bone of fasted mice and ob/ob mice. A, Effect of intraperitoneal leptin injection on IL-7, SDF-1, and adiponectin mRNA expression of in the bone of wild-type mice. B, IL-7, SDF-1, and adiponectin mRNA expression of in the bone of ob/ob and wild-type mice. Mice were deprived of chow for 48 h or fed ad libitum. Data in all graphs are mean ± SEM. WT, Wild type; n.s., not significant. n = 4.
Corticosterone plays a role in the starvation-induced alteration of B-cell development
Because starvation increases corticosterone production as a physiologic adaptation to energy deprivation through the activation of the hypothalamus–pituitary–adrenal axis (Ahima et al., 1996; Chan and Mantzoros, 2005), we examined the role of corticosterone in the starvation-induced alteration of B-cell development. In this study, intracerebroventricular injection of leptin significantly prevented the increase in serum corticosterone concentrations in fasted mice and ob/ob mice (Fig. 6A,B). To explore the functional significance of corticosterone, we examined the effect of RU486 (11β-[p-(dimethylamino)phenyl]-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one), a glucocorticoid receptor antagonist, on the starvation-induced alteration of B-cell development. Oral administration of RU486 did not affect body weight and blood glucose and serum corticosterone concentrations during the starvation period (Fig. 6C–E). Similar to the previous report that RU486 administration suppresses the otherwise increased phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression in the liver of db/db mice with leptin receptor defect (Liu et al., 2005), the starvation-induced PEPCK mRNA expression was effectively suppressed by RU486 (Fig. 6F). In this setting, the alteration of B lineage cell proportion in the bone marrow of fasted mice was markedly prevented by RU486 administration (Fig. 6G).
Figure 6.
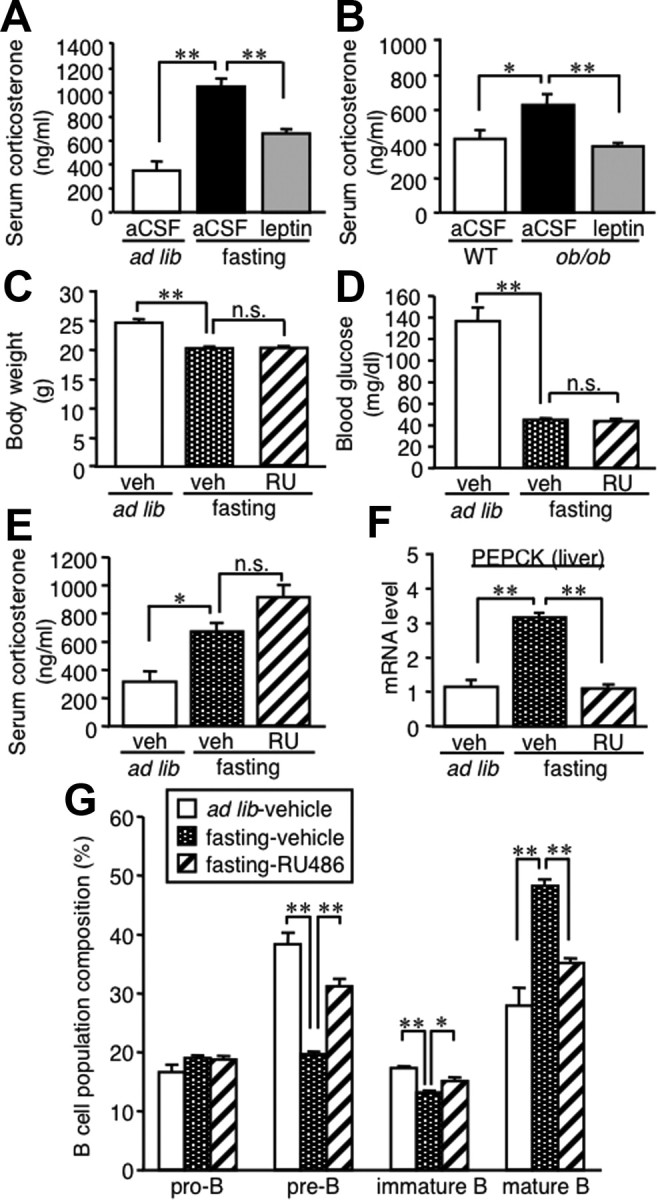
Role of corticosterone in the starvation-induced alteration of B-cell development in the bone marrow. A, B, Effect of intracerebroventricular leptin injection on serum corticosterone concentrations in fasted mice (A) and ob/ob mice (B). Mice were deprived of chow for 48 h with twice-a-day intracerebroventricular injection of recombinant mouse leptin (0.5 μg/0.5 μl) or aCSF (A). Mice received continuous intracerebroventricular injection with leptin (5 ng/h) or aCSF for 7 d (B). C–G, Effect of RU486, a glucocorticoid receptor antagonist, on B lineage cells in the bone marrow of fasted mice. Mice were deprived of chow for 48 h with twice-a-day oral administration of RU486 (25 μg/g initial body weight) or vehicle. C, Body weight; D, blood glucose concentration; E, serum corticosterone concentrations. F, PEPCK mRNA expression in the liver. G, FACS analysis on B lineage cells in the bone marrow. WT, Wild type; veh, vehicle; RU, RU486; n.s., not significant. Data in all graphs are mean ± SEM. *p < 0.05, **p < 0.01; n = 4–6.
We further examined the connection between central leptin signaling, corticosterone, and B-cell development using adrenalectomized mice with implantation of corticosterone pellet (Fig. 7A). In this study, serum corticosterone concentrations were mostly comparable with those in fasted mice (Figs. 6A,E, 7B). Starvation and central leptin administration did not affect serum corticosterone and blood glucose concentrations and body weight (Fig. 7B–D). In this setting, the adrenalectomized mice showed B lineage cell proportion in the bone marrow, which is similar to those in fasted mice (Figs. 1E, 7E). Moreover, we observed no significant difference in B lineage cells among the three groups with adrenalectomy: ad libitum-fed mice, fasted mice, and fasted mice with intracerebroventricular leptin injection. These observations together suggest that corticosterone plays an important role in the regulation of B-cell development under starvation conditions.
Figure 7.
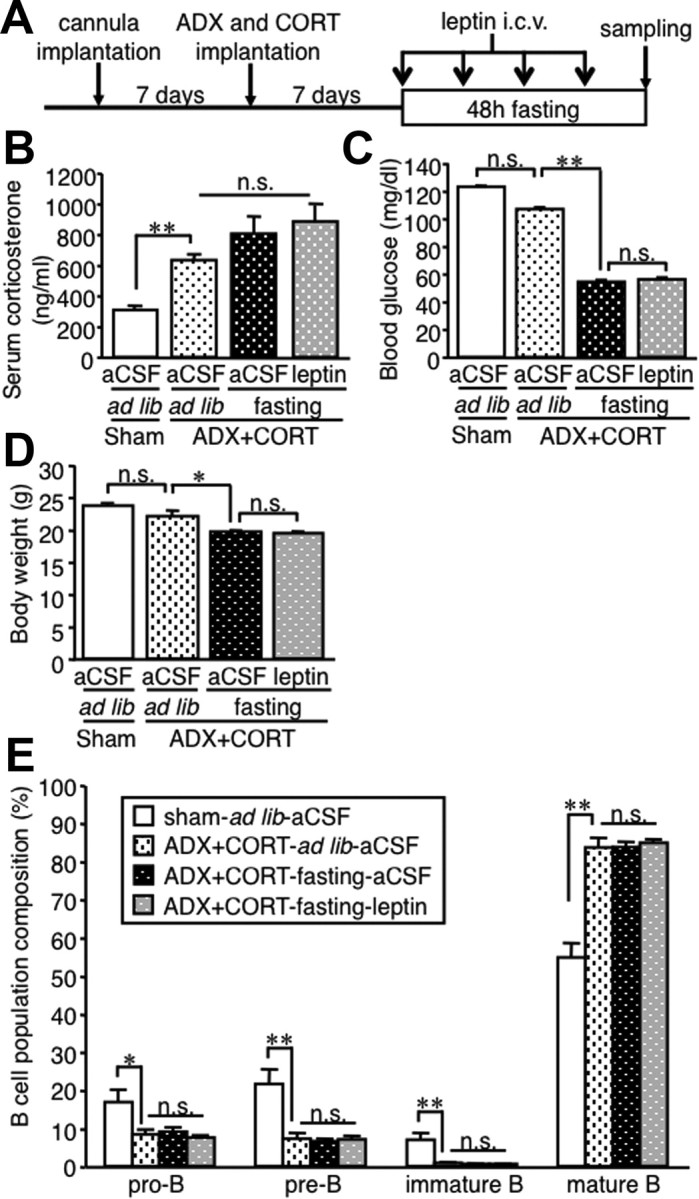
Effect of leptin in the starvation-induced alteration of B-cell development in the bone marrow of adrenalectomized mice. A, Experimental protocol. Adrenalectomized mice with subcutaneous implantation of corticosterone pellet (7.5 mg/pellet per mouse) were deprived of chow for 48 h with twice-a-day intracerebroventricular injection of recombinant mouse leptin (0.5 μg/0.5 μl) or aCSF. B–D, Serum corticosterone (B) and blood glucose (C) concentrations and body weight (D) at the time the animals were killed. E, FACS analysis on the B lineage cells in the bone marrow. ADX, Adrenalectomy; CORT, corticosterone pellet; icv, intracerebroventricular; n.s., not significant. Data in all graphs are mean ± SEM. *p < 0.05, **p < 0.01; n = 4–5.
NPY plays a role in the starvation-induced alteration of B-cell development
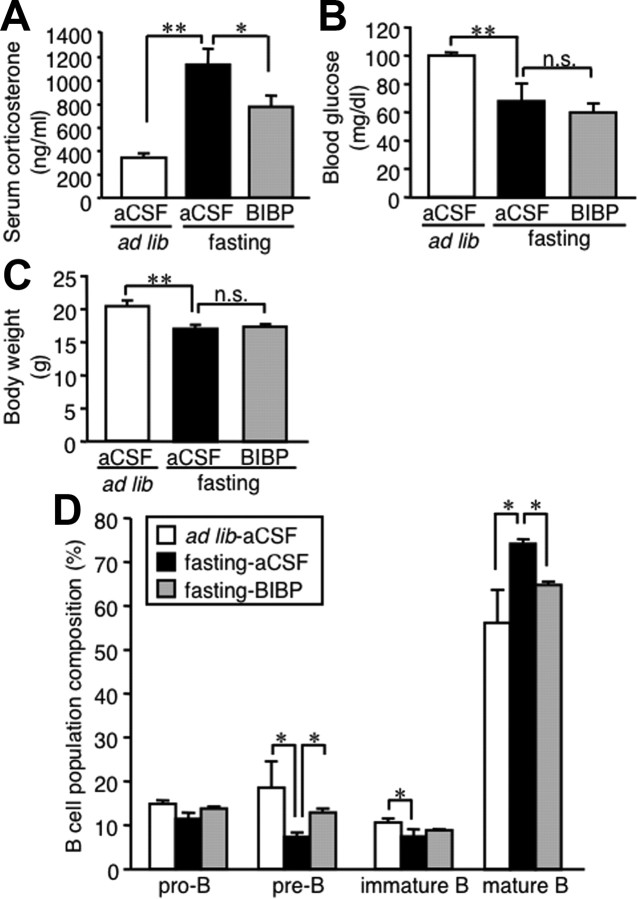
Because it is known that NPY, when increased in the hypothalamus under starvation conditions, contributes to increased corticosterone production (Luque et al., 2007), we next examined the effect of BIBP3226, a Y1R antagonist, on the regulation of B-cell development. Intracerebroventricular injection of BIBP3226 significantly reduced serum corticosterone concentrations in fasted mice, whereas there were no significant changes in blood glucose concentrations and body weight (Fig. 8A–C). In this setting, the alteration of B lineage cell proportion in the bone marrow of fasted mice was markedly prevented by intracerebroventricular BIBP3226 injection (Fig. 8D). These observations suggest that the NPY–Y1R pathway plays a role in the starvation-induced hypercorticosteronemia and alteration of B-cell development.
Figure 8.
Effect of central administration of Y1R antagonist on B lineage cells in the bone marrow of fasted mice. Mice were deprived of chow for 48 h with twice-a-day intracerebroventricular injection of the Y1R antagonist BIBP3226 (10 nmol/0.5 μl) or aCSF. A–C, Serum corticosterone (A) and blood glucose (B) concentrations and body weight (C) at the time the animals were killed. D, FACS analysis on the B lineage cells in the bone marrow. BIBP, BIBP3226; n.s., not significant. Data in all graphs are mean ± SEM. *p < 0.05, **p < 0.01; n = 4–5.
Discussion
It is known that nutritional deprivation or malnutrition leads to immune suppression in humans and animals (Cason et al., 1986; Chandra, 1991; Lord et al., 1998; Chan et al., 2006). Here, we provided evidence that B-cell development in the bone marrow is altered by nutritional deprivation in parallel with decreased serum leptin concentrations. Importantly, intraperitoneal injection of recombinant leptin effectively prevented the alteration of B-cell development in the bone marrow of fasted mice. In this regard, Claycombe et al. (2008) reported that ob/ob mice exhibit impaired B lymphopoiesis in the bone marrow relative to wild-type mice. These observations together suggest that hypoleptinemia plays a critical role in the starvation-induced alteration of B-cell development. This discussion is consistent with the previous report by Chan et al. (2006) that 72 h fasting significantly reduces the B-cell population in the circulating blood of healthy humans, which is partly prevented by subcutaneous injection of recombinant human leptin (Chan et al., 2006). Unlike fasted mice, ob/ob mice exhibited marked obesity and high blood glucose concentrations, although they both developed the alteration of B-cell development. Moreover, leptin did not affect body weight and blood glucose concentrations in fasted mice, which is consistent with a previous report (Ahima et al., 1996). These observations suggest that leptin regulates B-cell development and energy metabolism via different mechanisms. Conversely, evidence has suggested the role of leptin in the starvation-induced thymic atrophy and T-cell apoptosis (Lord et al., 1998; Howard et al., 1999). Because leptin is produced in the adipose tissue in response to the nutritional change, the data of this study support the concept that leptin acts as a nutritional sensor to regulate the acquired immune system. Thus, the leptin-mediated alteration of B-cell development may be relevant to the immune dysfunction in energy-deprivation states, such as anorexia nervosa, malnutrition, or cachexia.
Because leptin receptor is expressed in a variety of central and peripheral tissues and cells including T and B cells, it is important to know whether leptin can regulate B-cell development in the bone marrow via central and/or peripheral mechanisms. It is reported that B cells from mouse spleen are protected from Fas-mediated apoptosis in vitro when treated with recombinant leptin (Palmer et al., 2006). Leptin also promotes survival of B cells from the spleen via induction of B-cell CLL/lymphoma (Bcl-2) and cyclin D1 (Lam et al., 2010). Conversely, there is considerable in vivo evidence that central leptin signaling is sufficient for its effect on energy and glucose homeostasis and even locomotor activity (Coppari et al., 2005; de Luca et al., 2005). In this study, we demonstrated for the first time that central leptin administration effectively prevents the alteration of B-cell development in the bone marrow of fasted mice and ob/ob mice. This is reminiscent of our recent observation that central leptin administration in ob/ob mice reverses the otherwise reduced macrophage infiltration in a mouse model of renal injury (Tanaka et al., 2010). Similarly, Tschöp et al. (2010) reported that central leptin administration increases survival during sepsis in ob/ob mice as well as wild-type mice. Although our data do not rule out the possibility that leptin acts directly on B cells to regulate their development, it is likely that central leptin signaling plays a major role in B-cell development in the bone marrow in vivo under starvation conditions.
It is also important to understand how central leptin signaling regulates B-cell development. Leptin plays a critical role in the neuroendocrine response to fasting such as the hypothalamus–pituitary–adrenal axis (Ahima et al., 1996; Chan and Mantzoros, 2005). Laakko and Fraker (2002) previously reported that subcutaneous implantation of corticosterone pellets into wild-type mice results in the alteration of B-cell development in the bone marrow, which is similar to that in fasted mice. These findings led us to speculate the role of corticosterone in the starvation-induced alteration of B-cell development. In this study, we observed that serum corticosterone concentrations are markedly increased short and long term of leptin deficiency (corresponding to fasted wild-type mice and ob/ob mice, respectively), which is effectively suppressed by central leptin administration as reported previously (Ahima et al., 1996; Huang et al., 1998). Importantly, administration of a glucocorticoid receptor antagonist to fasted mice markedly prevented the alteration of B-cell development in the bone marrow. Moreover, we confirmed that the proportion of B lineage cells in the bone marrow is not affected by starvation and central leptin administration in adrenalectomized mice, when serum corticosterone concentrations are unaltered. These observations support the concept that corticosterone plays an important role in the alteration of B-cell development under starvation conditions. Although central leptin signaling regulates bone formation via the sympathetic nervous system, in which β2-adrenargic receptor (adrb2) expressed on osteoblasts may play an important role (Elefteriou et al., 2005), we observed no significant difference in B lineage cells in the bone marrow between adrb2-deficient and wild-type mice (M. Tanaka, T. Soganami, and Y. Ogawa, unpublished observations). Additional studies are required to elucidate the involvement of humoral and neuronal mechanisms in the alteration of B-cell development in the bone marrow under starvation conditions.
It is known that NPY, when increased in the hypothalamus under starvation conditions, stimulates potently food intake mainly through Y1R (Pedrazzini et al., 1998; Kanatani et al., 2000; Lin et al., 2004). The NPY–Y1R pathway in the CNS also plays a role in the regulation of blood pressure and the hypothalamus–pituitary–adrenal axis (Pedrazzini et al., 1998; Dimitrov et al., 2007). In this study, we demonstrated that intracerebroventricular injection of a Y1R antagonist significantly prevents the starvation-induced hypercorticosteronemia and alteration of B-cell development in the bone marrow. Of note, the starvation-induced upregulation of NPY in the hypothalamus is effectively suppressed by leptin injection (Ahima et al., 1999; Shimizu-Albergine et al., 2001; Jovanovic et al., 2010). It is, therefore, conceivable that the NPY–Y1R pathway is involved in the leptin-mediated regulation of B-cell development in the bone marrow, at least partly through increased production of corticosterone. Additional studies are required to elucidate the role of the NPY–Y1R pathway in B-cell development independent of the hypothalamus–pituitary–adrenal axis. In this study, the proportion of B cells in splenocytes and circulating blood mononuclear cells was not statistically decreased after 48 h fasting, which may be, at least in part, attributable to the long (5–6 weeks) half-life of mature B cells (Abbas and Lichtman, 2003). Indeed, ob/ob mice exhibited a marked decrease in the proportion of B cells in the spleen and circulating blood relative to wild-type mice, suggesting that the long-term nutritional deprivation leads to the decrease in the B-cell population in the spleen and circulating blood. It is known that early B-cell development (pro-B, pre-B, and immature B cells) is confined to the bone marrow (Hardy et al., 1991). Some immature B cells then leave the bone marrow to mature in the spleen, whereas others remain in the bone marrow for maturation (Cariappa et al., 2007). It is also known that mature B cells in the spleen recirculate into the bone marrow (Cariappa et al., 2005; Cinamon et al., 2008). In this study, we observed that the proportion of mature B cells in the bone marrow is significantly elevated in fasted mice and ob/ob mice, which is in contrast to that in spleen. Because mature B cells in the bone marrow can participate in humoral immune responses to microbes similar to those in the spleen (Cariappa et al., 2005), it would be interesting to know the functional difference of B cells between the bone marrow and spleen under leptin-deficient conditions.
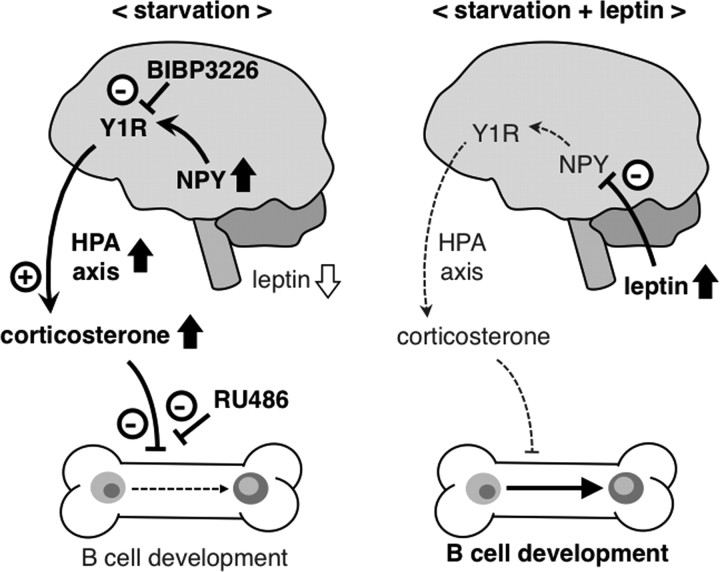
In conclusion, this study provides the first in vivo evidence for the role of central leptin signaling in the starvation-induced alteration of B-cell development in the bone marrow (Fig. 9). The data of this study suggest that the CNS, which is inherent to integrate information from throughout the organism, is able to control immune function.
Figure 9.
Potential mechanism of the leptin-mediated regulation of B-cell development in the bone marrow under starvation conditions. Under starvation conditions, activation of the NPY–Y1R pathway in the CNS as a result of hypoleptinemia plays an important role in the alteration of B-cell development in the bone marrow, at least partly through increased production of corticosterone. Central leptin administration effectively reverses the starvation-induced alteration of B-cell development, probably through suppressing the NPY–Y1R pathway and corticosterone production. HPA axis, Hypothalamus–pituitary–adrenal axis.
Footnotes
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health, Labor, and Welfare of Japan, and research grants from Mitsubishi Parma Research Foundation, Kao Research Council for the Study of Healthcare Science, and Takeda Science Foundation. M.Ta. was supported by Mishima Kaiun Memorial Foundation (2005-2006) and Tokyo Biochemical Research Foundation (2008). We thank Dr. Goro Katsuura for technical advice and Ai Togo for secretarial assistance.
The authors declare no competing financial interests.
References
- Abbas AK, Lichtman AH. Cellular and molecular immunology. Ed 5. Philadelphia: Saunders; 2003. pp. 16–39. [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–76. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- Busso N, So A, Chobaz-Péclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, Gabay C. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- Cason J, Ainley CC, Wolstencroft RA, Norton KR, Thompson RP. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64:370–375. [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra RK. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr. 1991;53:1087–1101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A. 2008;105:2017–2021. doi: 10.1073/pnas.0712053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148:3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fujimoto T, Shimoda Y, Huang X, Sakamoto H, Ogawa M. Distinct hemogenic potential of endothelial cells and CD41+ cells in mouse embryos. Dev Growth Differ. 2007;49:287–300. doi: 10.1111/j.1440-169X.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Rivest R, Richard D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology. 1998;139:1524–1532. doi: 10.1210/endo.139.4.5889. [DOI] [PubMed] [Google Scholar]
- Jovanovic Z, Tung YC, Lam BY, O'Rahilly S, Yeo GS. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J Neuroendocrinol. 2010;22:915–925. doi: 10.1111/j.1365-2826.2010.02026.x. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH, Saga Y, Nishimura S, Ihara M. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- Kim-Saijo M, Janssen EM, Sugie K. CD4 cell-secreted, posttranslationally modified cytokine GIF suppresses Th2 responses by inhibiting the initiation of IL-4 production. Proc Natl Acad Sci U S A. 2008;105:19402–19407. doi: 10.1073/pnas.0810035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakko T, Fraker P. Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology. 2002;105:111–119. doi: 10.1046/j.1365-2567.2002.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lam QL, Wang S, Ko OK, Kincade PW, Lu L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc Natl Acad Sci U S A. 2010;107:13812–13817. doi: 10.1073/pnas.1004185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Sakurai R, Tripathi PV, Lutfy K, Friedman TC. Increased glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes. 2005;54:32–40. doi: 10.2337/diabetes.54.1.32. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology. 2007;148:300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, Odaka H, Kasuga H, Fujisawa Y, Inoue G, Nishimura H, Yoshimasa Y, Nakao K. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. doi: 10.2337/diabetes.48.9.1822. [DOI] [PubMed] [Google Scholar]
- Palmer G, Aurrand-Lions M, Contassot E, Talabot-Ayer D, Ducrest-Gay D, Vesin C, Chobaz-Péclat V, Busso N, Gabay C. Indirect effects of leptin receptor deficiency on lymphocyte populations and immune response in db/db mice. J Immunol. 2006;177:2899–2907. doi: 10.4049/jimmunol.177.5.2899. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Seydoux J, Künstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett. 1998;249:107–110. doi: 10.1016/s0304-3940(98)00401-7. [DOI] [PubMed] [Google Scholar]
- Shimizu-Albergine M, Ippolito DL, Beavo JA. Downregulation of fasting-induced cAMP response element-mediated gene induction by leptin in neuropeptide Y neurons of the arcuate nucleus. J Neurosci. 2001;21:1238–1246. doi: 10.1523/JNEUROSCI.21-04-01238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Suganami T, Sugita S, Shimoda Y, Kasahara M, Aoe S, Takeya M, Takeda S, Kamei Y, Ogawa Y. Role of central leptin signaling in renal macrophage infiltration. Endocr J. 2010;57:61–72. doi: 10.1507/endocrj.k09e-296. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tschöp J, Nogueiras R, Haas-Lockie S, Kasten KR, Castañeda TR, Huber N, Guanciale K, Perez-Tilve D, Habegger K, Ottaway N, Woods SC, Oldfield B, Clarke I, Chua S, Jr, Farooqi IS, O'Rahilly S, Caldwell CC, Tschöp MH. leptin action modulates immune response and survival in sepsis. J Neurosci. 2010;30:6036–6047. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091–5099. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]