Abstract
Classical conditioning of a motor response such as eyeblink is associated with the development of a pause in cerebellar Purkinje cell firing that is an important driver of the overt response. This conditioned Purkinje cell response is adaptively timed and has a specific temporal profile that probably explains the time course of the overt behavior. It is generally assumed that the temporal properties of the conditioned Purkinje cell response are determined by the temporal pattern of the parallel fiber impulses generated by the conditioned stimulus at the time of the conditioned response. We show here in the decerebrate ferret preparation that a very brief conditioned stimulus, consisting of only one or two impulses in the mossy fibers, can be sufficient to elicit a full conditioned Purkinje cell response with normal time course. The finding suggests that parallel fiber input to the Purkinje cell influences the firing rate several hundred milliseconds later. It poses a serious challenge to the standard view of the role of parallel fiber impulses in response timing.
Introduction
In the standard “delay” paradigm in classical conditioning of eyeblink, the conditioned stimulus (CS) is followed by and overlaps with the unconditioned stimulus (US). Paired CS–US stimulation results in a conditioned response (CR) that is adaptively timed. The CR reaches its peak amplitude at the end of the interstimulus interval (ISI), that is, at the time of the expected onset of the US. If the ISI is changed, additional training will lead to a corresponding change in CR latency (Kehoe and Macrae, 2002; Ivry and Spencer, 2004; Mauk and Buonomano, 2004).
It is known that the CS is carried by mossy and parallel fibers and the US by climbing fibers (Hesslow and Yeo, 2002). All current models of conditioning in the cerebellum assume that the behavioral CR is controlled by a learned inhibitory response to the CS in blink-controlling Purkinje cells (PCs) (Buonomano and Mauk, 1994; Hesslow and Yeo, 2002; Yamazaki and Tanaka, 2009). There is plenty of evidence to support this assumption. During training with a standard conditioning protocol, Purkinje cells that control the eyelid and specifically the blink CR (Hesslow, 1994a,b) reliably develop a pause response to the CS, a “Purkinje cell CR” (Hesslow and Ivarsson, 1994; Jirenhed et al., 2007). This is extinguished by unpaired stimulation and reappears with substantial savings when paired stimulation is resumed (Jirenhed et al., 2007). The response is adaptively timed and manipulations that change the latency of the behavioral CR have parallel effects on the Purkinje cell CR (Svensson et al., 2010; Jirenhed and Hesslow, 2011). Thus, although it cannot be excluded that some properties of the overt blink CR may be modulated in the pathway from Purkinje cells to eyelid, it is a reasonable assumption that the time course of the blink CR mainly reflects that of the Purkinje cell CR.
It is usually assumed that the time course of the Purkinje cell CR is determined by the temporal pattern of activity in CS-activated parallel fibers (Yamazaki and Tanaka, 2009). It is known from the behavioral literature that normal conditioning can occur with very brief CSs (“trace conditioning”) (Kehoe and Macrae, 2002). It is of course possible that the time course of the mossy fiber activity is quite different from the CS input. It has been shown by Woody et al. (1999) that a brief click sound, which can function as a CS, can elicit prolonged activity in cerebellar units. However, we have previously shown that when direct stimulation of mossy fibers is used as a CS, a very brief CS is sufficient to elicit normal behavioral CRs (Svensson and Ivarsson, 1999).
This raises the question whether a normal Purkinje cell CR requires a sustained mossy fiber input or whether it is possible to elicit Purkinje cell CRs with very short CSs and if so, how short. The answer to this question is highly relevant to understanding the role of the temporal pattern of the parallel fiber signal in determining the time course of the Purkinje cell CR.
Materials and Methods
The experimental procedures are described only briefly. For details, see Jirenhed et al. (2007), Svensson et al. (2010), and Jirenhed and Hesslow (2011). The experimental setup is illustrated in Figure 1A. The subjects were seven male ferrets, anesthetized with isoflurane and propofol and then decerebrated by aspiration of most of the forebrain and by sectioning the brainstem just rostral to the red nucleus. They were immobilized with curare and put on artificial respiration.
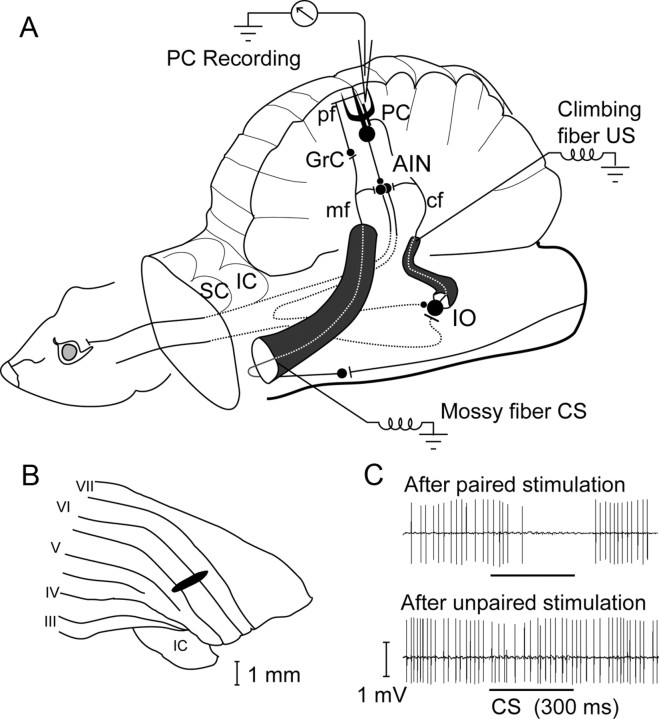
Figure 1.
Experimental setup. A, Experimental setup and cerebellar wiring diagram, showing the recording site, the CS and US stimulation sites, the deep cerebellar nuclei, and the inferior olive. cf, Climbing fiber; GrC, granule cell; mf, mossy fiber; pf, parallel fiber; AIN, anterior interpositus nucleus; IO, inferior olive; SC, superior colliculus; IC, inferior colliculus. B, Recording site. Area in the C3 zone with short-latency climbing fiber input from the periocular area. C, Sample records of typical Purkinje cell CR (upper trace) that disappears after unpaired stimulation (lower trace).
Stimulation of cerebellar afferents during training followed a protocol analogous to delay eyeblink conditioning. The ISI was 200 or 300 ms. The CS was always at least as long as the ISI, but it sometimes outlasted this by several hundred milliseconds. The CS was a 50 Hz stimulus train with a duration between 300 and 800 ms applied to mossy fibers in the middle cerebellar peduncle (90–100 μA, 0.1 ms pulse duration). The US consisted of two 10 ms stimulus trains delivered to the climbing fibers in the inferior cerebellar peduncle (50–800 μA), each train consisting of five square 0.1 ms pulses at 500 Hz. During paired stimulation, the ISIs between CS and US onsets were 200–400 ms. The intertrial interval was 15 s.
Extracellular single-unit recordings were made from Purkinje cells with glass-coated platinum-tungsten microelectrodes (Thomas Recording) located in the blink-controlling area in the C3 zone (cf. Fig. 1B), identified by criteria described by Hesslow (1994a,b).
Results
As described previously, a Purkinje cell CR developed to the CS in the Purkinje cell (cf. Fig. 1C) during training. After a robust Purkinje cell CR had been obtained, we tested the effects of presenting very brief CSs, consisting of only one to five stimuli (1–80 ms pulse trains). We tested short CS presentations after conditioning in a total of seven cells in seven animals. We observed the effect described here in four cells.
The main results are shown in Figure 2. For each cell, we show a raster plot and a peristimulus time histogram (PSTH) from 40 CS-alone trials with the long CS used during training and from trials with testing the effects of short CSs. In all cases, the short CSs consisting of one or two pulses (corresponding to stimulus durations of 1 and 20 ms) elicited a characteristic Purkinje cell CR, consistent with a previous report from our laboratory on behavioral CRs (Svensson and Ivarsson, 1999). Although the precise topography of the CRs varied between cells, they all showed pause responses with appropriate latencies to both onset and offset.
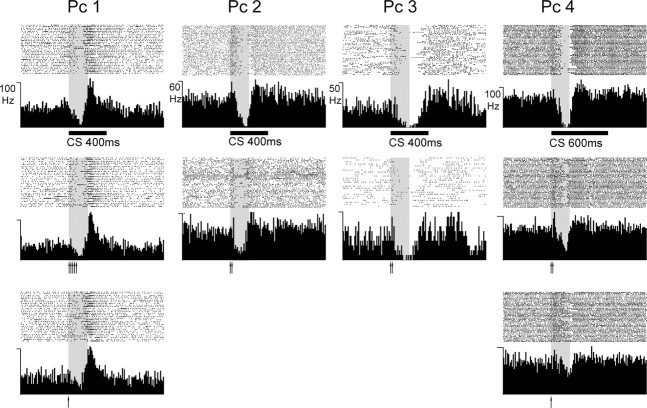
Figure 2.
Conditioned Purkinje cell responses. CRs from four different Purkinje cells (Pc 1–Pc 4) displayed as raster plots and PSTHs. The CS consisted of 50 Hz trains of single pulses to the mossy fibers. Upper row shows the average response to CS-alone stimulation after conditioning with a 200 ms CS–US interval (gray shading) using different CS durations (bar below graph). Each graph is based on 40 trials. The duration is 1.5 s. The second and third rows show CRs elicited with short CSs. Each CS pulse is indicated by an arrow (interval between stimuli was 20 ms).
Not only did the short CSs elicit appropriately timed CRs, but the topographies of the responses to long and short CSs were virtually identical. For instance, in PC 1, the 400 ms CS elicited CRs that were characterized by a gradual decrease in simple spike firing and a strong increase to more than twice the background level just after the ISI. These features were remarkably similar when the CS consisted of five pulses or even just a single pulse. The initial increase in simple spike firing in PC 2 was preserved when the CS consisted of two pulses. In PC 3, the CR had a longer duration than the other cells (continuing beyond the ISI), and this was also the case when the CS consisted of only two stimulus pulses.
In the illustrated cases, the ISI was 200 ms, but a similar observation was made when a 300 ms ISI was used. The cell shown as PC 1 was first trained with a 200 ms ISI and 400 ms CS. A short CS elicited a Purkinje cell CR (cf. Fig. 2, Pc 1). The ISI was then increased to 300 ms until a (later) CR was elicited by the CS. A short CS now also elicited a (later) CR (not illustrated).
Inspection of the PSTHs suggests that the Purkinje cell CRs elicited by the short CSs were slightly smaller than those elicited by the long CSs. This is particularly clear in PC 4. To some extent, this reflected smaller individual CRs. In some cells, however, the CR topographies were actually quite similar when the CS was short, but CRs had a lower probability of occurring. Close inspection of the raster diagrams, particularly in PC 1 and PC 2, shows that the cells failed to pause on a larger number of trials when the CS consisted of one or two pulses but that the individual CRs, when they occurred, looked much the same. Notice also that the offset of the Purkinje cell CRs was almost exactly the same whether the CS was 1 or 600 ms. The offset was thus independent of the termination of the CS.
The results described above raise a puzzling question. If all aspects of the CR topography, including the onset and offset latencies, are determined by the first few milliseconds of the CS, why does the cell not respond to the later parts of the CS? One possible reason could be that the granule cells do not follow the mossy fiber input throughout the whole CS period but only respond to the first impulses. This question was addressed by looking at the responses to individual mossy fiber stimuli during a long CS train.
One such case is shown in Figure 3. This Purkinje cell was trained with an 800 ms CS (50 Hz train of pulses to the mossy fibers) and a 200 ms ISI. Figure 3A shows a sample record after training from a CS-alone trial and Figure 3B shows a raster plot and PSTH over 40 trials. Apart from the suppression of simple spike firing during the CR, from ∼50 to ∼250 ms, the firing rate of the cell was not much different from background. However, the mossy fiber pulses did increase the probability of simple spikes in the Purkinje cell, with a latency of ∼2 ms. This can be seen in the raster diagram as blurred vertical “stripes” and more clearly in the poststimulus time histograms in Figure 3C. These show the spike probability over 40 trials after pulses 1, 10, 30, and 40 in the CS both before and after training. With the exception of the 10th pulse (which occurred in the middle of the CR), the mossy fiber stimuli were followed by an increased firing probability at ∼2 ms followed by a depression of firing. The Purkinje cell response to the mossy fiber impulses remained similar throughout the CS, and it is clear that the cell continued to receive parallel fiber input during the whole 800 ms period both before and after training.
Figure 3.
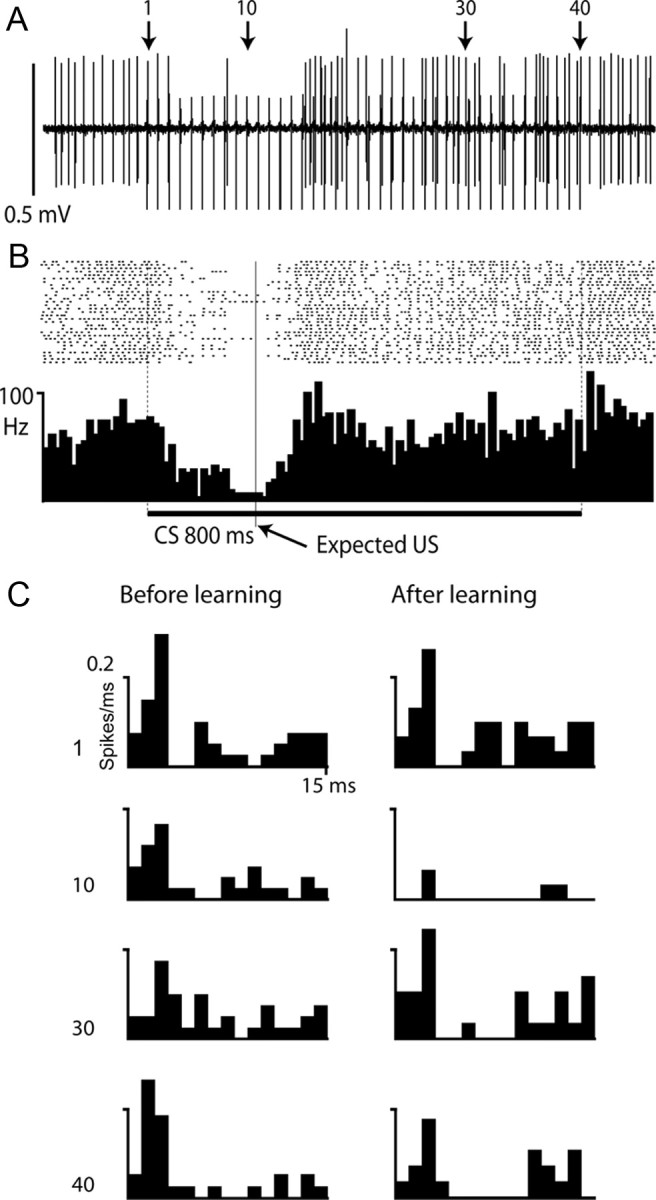
Purkinje cell response to 800 ms train of mossy fiber stimuli. A, B, Sample record (A) and raster plot and PSTH (B) showing simple spike firing in Purkinje cell on CS-alone trials. The cell was trained with a 200 ms ISI and 800 ms CS consisting of a 50 Hz train of stimuli to the mossy fibers. Shock artifacts can be seen in A as 40 vertical lines extending below the simple spikes. C, Poststimulus time histograms of responses over 40 trials to the mossy fiber stimuli 1, 10, 30, and 40, indicated by arrows in A, before (left) and after (right) training. Each bin is 1 ms and the y-axis indicates probability of firing.
Discussion
The results clearly demonstrate that a very brief mossy fiber input, sometimes consisting of a single impulse, can elicit a Purkinje cell CR with normal onset and offset latencies. The simple spike suppression during CRs is slightly weaker in some cases, but in some cells, this was due to a lower probability of occurring rather than a difference in the temporal profile of the individual Purkinje cell CR. The number of cells is small (n = 4 of 7), but notice that we are not claiming that our data are representative of all Purkinje cells. The question we addressed was as follows: What is the shortest possible mossy fiber CS that can elicit a normal CR? The results clearly indicate that CSs longer than one or two pulses are not necessary for eliciting a normally timed Purkinje cell CR. This phenomenon is not exceptional, however. Short CSs consisting of two or three pulses could elicit CRs in all four included cells, and a single pulse was sufficient in two of them. This conclusion is strengthened by the observation, in a previous study by Svensson and Ivarsson (1999), that normal behavioral CRs could be elicited by CSs consisting of one or two mossy fibers stimuli.
In two previous studies, we have shown that increasing the frequency of a CS train can shorten the latencies of both the overt and the Purkinje cell CR (Svensson et al., 1997, 2010). The present results suggest that it is only the initial part of that high-frequency CS that is important.
It is worth noticing that the animals were trained with a delay paradigm in which the CS continues throughout the ISI. We are only claiming that a single pulse is all that is necessary to elicit a CR after it has already been learned. A sustained CS signal might still be necessary for learning. We do not know whether short CSs can elicit CRs when the ISI is longer than 300 ms. It was shown recently that acquisition of a blink CR in rabbits, when the ISI is 500 ms or longer, requires a sustained mossy fiber signal (Kalmbach et al., 2009), but this is a requirement of learning, not necessarily of eliciting a response that has already been learned.
These results are important because they throw considerable doubt on some common assumptions concerning the timing of CRs as well as the synaptic learning mechanisms involved. As pointed out in a recent review by Yamazaki and Tanaka (2009), most theories of response timing in the cerebellum assume that the temporal profile of the CR is determined by the temporal pattern in the parallel fiber input to the Purkinje cells controlling the blink (Desmond and Moore, 1988; Bullock et al., 1994; Buonomano and Mauk, 1994; Dean and Porrill, 2008; D'Angelo and De Zeeuw, 2009). For additional examples, see Yamazaki and Tanaka (2009). Simply put, different parallel fibers are supposed to fire with different temporal patterns and will therefore have different temporal relationships with the US-elicited climbing fiber input. Those parallel fibers, which fire with the right temporal relationship with the climbing fibers, will have their synapses modified by long-term depression (LTD). This combination of temporal delays and LTD would account for the ability of the Purkinje cell to learn a specific time course. This view is very difficult to reconcile with the results described above.
First, a single impulse in the mossy fibers has effects hundreds of milliseconds later. Purkinje cell CRs with perfectly normal topography were elicited by very brief mossy fiber CSs. In many cases, a single pulse was sufficient to cause a delayed suppression of simple spikes that may take ∼100 ms to reach its maximum and a subsequent return to normal firing. Direct recordings from granule cells indicate that mossy fiber impulses cause only brief bursts of firing, lasting no more than 10–20 ms (Jörntell and Ekerot, 2006). Even if these figures might be open to discussion, it would seem unlikely that a single mossy fiber impulse can generate an appreciable change in granule cell activity after several hundred milliseconds. The suppression and recovery of simple spike firing during a Purkinje cell CR cannot be a result of parallel fiber input at that time but must result from parallel fiber input at the beginning of the CS, which may be hundreds of milliseconds earlier.
Second, later mossy fiber impulses, at the time of the CR or later, do not seem to have any effect on its topography. The CR is turned off at the appropriate time even if the CS continues for several hundred milliseconds after the US. As shown in Figure 3, the mossy fiber impulses can continue to generate parallel fiber input that looks much the same even after 800 ms. The repetitive mossy fiber stimulation probably activates the same granule cells and parallel fibers over and over again. From the Purkinje cell's point of view, the repeatedly incoming parallel fiber signals will all be identical, assuming that the time period between mossy fiber pulses is sufficient to normalize the granule cell response between incoming pulses (i.e., that the granule cell reaches resting potential and that there is no synaptic adaptation or facilitation). The data of Jörntell and Ekerot (2006) and the observed short latency simple spikes exemplified in Figure 3, B and C, suggest that this is indeed the case. Yet, this input has no effect on the topography of the Purkinje cell CR.
We conclude that the time course of the Purkinje cell CR is determined by the initial part of the CS and is independent of the temporal pattern of parallel fiber activity during all but the very first few milliseconds. The results suggest that the initial part of the CS signal triggers a mechanism for reducing the Purkinje cell firing with a specific, but adjustable, delay and duration, and that once this mechanism is activated, it runs its course relatively independent of further input. This also presents a challenge to the view that acquisition of a Purkinje cell CR can be explained fully by standard learning mechanisms such as strengthening or weakening of the parallel fiber to Purkinje cell synapse. This theory assumes that the simple spike firing at a particular point in time is controlled by the immediately preceding parallel fiber input, but our results suggest that this is not the case.
Footnotes
This work was supported by grants from the Swedish Research Council to The Linnaeus Centre for Cognition, Communication and Learning (349-2007-8695) at Lund University and to G.H. (09899) and from the Söderberg and Åhlen Foundations.
References
- Bullock D, Fiala JC, Grossberg S. A neural model of timed response learning in the cerebellum. Neural Netw. 1994;7:1101–1114. [Google Scholar]
- Buonomano DV, Mauk BD. Neural network model of the cerebellum: temporal discrimination and the timing of motor responses. Neural Comput. 1994;6:38–55. [Google Scholar]
- D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J. Adaptive-filter models of the cerebellum: computational analysis. Cerebellum. 2008;7:567–571. doi: 10.1007/s12311-008-0067-3. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Moore JW. Adaptive timing in neural networks: the conditioned response. Biol Cybern. 1988;58:405–415. doi: 10.1007/BF00361347. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol. 1994a;476:229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. J Physiol. 1994b;476:245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Ivarsson M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. Neuroreport. 1994;5:649–652. doi: 10.1097/00001756-199401000-00030. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Yeo CH. The functional anatomy of skeletal conditioning. In: Moore JW, editor. A neuroscientist's guide to classical conditioning. New York: Springer; 2002. pp. 86–146. [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jirenhed D-A, Hesslow G. Learning stimulus intervals—adaptive timing of conditioned Purkinje cell responses. Cerebellum. 2011 doi: 10.1007/s12311-011-0264-3. [DOI] [PubMed] [Google Scholar]
- Jirenhed D-A, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26:11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem. 2009;16:86–95. doi: 10.1101/lm.1178309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe EJ, Macrae M. Fundamental behavioral methods and findings in classical conditioning. In: Moore JW, editor. A neuroscientist's guide to classical conditioning. New York: Springer; 2002. pp. 171–231. [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Svensson P, Ivarsson M. Short-lasting conditioned stimulus applied to the middle cerebellar peduncle elicits delayed conditioned eye blink responses in the decerebrate ferret. Eur J Neurosci. 1999;11:4333–4340. doi: 10.1046/j.1460-9568.1999.00862.x. [DOI] [PubMed] [Google Scholar]
- Svensson P, Ivarsson M, Hesslow G. Effect of varying the intensity and train frequency of forelimb and cerebellar mossy fiber conditioned stimuli on the latency of conditioned eye-blink responses in decerebrate ferrets. Learn Mem. 1997;4:105–115. doi: 10.1101/lm.4.1.105. [DOI] [PubMed] [Google Scholar]
- Svensson P, Jirenhed DA, Bengtsson F, Hesslow G. Effect of conditioned stimulus parameters on timing of conditioned Purkinje cell responses. J Neurophysiol. 2010;103:1329–1336. doi: 10.1152/jn.00524.2009. [DOI] [PubMed] [Google Scholar]
- Woody CD, Nahvi A, Palermo G, Wan J, Wang XF, Gruen E. Differences in responses to 70 dB clicks of cerebellar units with simple versus complex spike activity: (i) in medial and lateral ansiform lobes and flocculus; and (ii) before and after conditioning blink conditioned responses with clicks as conditioned stimuli. Neuroscience. 1999;90:1227–1241. doi: 10.1016/s0306-4522(98)00558-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Tanaka S. Computational models of timing mechanisms in the cerebellar granular layer. Cerebellum. 2009;8:423–432. doi: 10.1007/s12311-009-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]