Abstract
In the last decade, research on error and conflict processing has become one of the most influential research areas in the domain of cognitive control. There is now converging evidence that a specific part of the posterior frontomedian cortex (pFMC), the rostral cingulate zone (RCZ), is crucially involved in the processing of errors and conflict. However, error-related research has focused primarily on a specific error type, namely, response errors. The aim of the present study was to investigate whether errors on the task level rely on the same neural and functional mechanisms. Here we report a dissociation of both error types in the pFMC: whereas response errors activate the RCZ, task errors activate the dorsal frontomedian cortex. Although this last region shows an overlap in activation for task and response errors on the group level, a closer inspection of the single-subject data is more in accordance with a functional anatomical dissociation. When investigating brain areas related to conflict on the task and response levels, a clear dissociation was perceived between areas associated with response conflict and with task conflict. Overall, our data support a dissociation between response and task levels of processing in the pFMC. In addition, we provide additional evidence for a dissociation between conflict and errors both at the response level and at the task level.
Introduction
There is a long-lasting debate regarding the functional organization of the posterior frontomedian cortex (pFMC) in adaptive control (Ridderinkhof et al., 2004; Ullsperger and von Cramon, 2004). The term pFMC refers to the part of the medial frontal cortex that extends from the presupplementary motor area (preSMA) anteriorly and dorsally from the anterior cingulate sulcus including parts of the anterior cingulate cortex (Ridderinkhof et al., 2004; Ullsperger and von Cramon, 2004). Several studies have reported a dorsal–ventral distinction in this region, with more ventral parts being involved in error processing and more dorsal parts involved in conflict resolution (Kiehl et al., 2000; Braver et al., 2001; Ullsperger and von Cramon, 2001; Wittfoth et al., 2008). From a neuroanatomical perspective, such a dissociation seems plausible because ventral parts of the pFMC are more related to the primary motor cortex and the spinal cord, whereas dorsal parts are connected to brain areas related to high-level motor cognition (Ullsperger and von Cramon, 2001, 2004). Since response errors require a response change, it is reasonable to assume that they rely on motor-related brain areas, whereas response conflict cannot be resolved by giving another response, and therefore should rely on brain areas involved in higher-level adaptive processes. So far, however, error and conflict research has focused primarily on the response level while greatly ignoring the more abstract task level. Interestingly, recent conflict research suggests that increasing the level of abstractness leads to a shift of conflict-related brain activity in the anterior direction. In particular, it seems that posterior parts of the pFMC are related to response conflict, whereas anterior parts are related to other forms of conflict such as conflict between decisions and strategies (Pochon et al., 2008; Kouneiher et al., 2009; Venkatraman et al., 2009).
The aim of the current study is to manipulate the level of abstractness both for errors and conflict by investigating errors and conflict on the task and the response levels. Task-related control processes have been investigated with the so-called task-switching paradigm (Monsell, 2003). Here participants have to alternate between different task representations. However, most task-switching research has focused exclusively on accurate performance. Only few studies have addressed erroneous performance or task errors (Steinhauser and Hübner, 2006, 2008; Steinhauser, 2010). By studying both errors and conflict at the response and task levels our design permits us to combine the dimension of abstractness (response level and task level) with the dimension of control (conflict and errors). This raises the interesting possibility to compare task related processing with response related processing for errors (task errors versus response errors) and conflict (task conflict versus response conflict). In addition, the design enables us to compare error with conflict-related processing within each level of abstractness (response errors versus response conflict/task errors versus task conflict).
Based on the above-mentioned literature, we expect to find dissociations between response conflict and response errors. Furthermore, we also expect a dissociation between response conflict and task conflict. However, with regard to the activity for task errors and its relation to response errors, two alternative hypotheses can be formulated. On the one hand, one can argue that both response and task errors signal the need for adaptive processes. From this perspective, both types of errors should activate similar brain regions. However, one can argue that the types of adaptive behavior required after each error are completely different. Whereas response errors require an adaptation of the motor output, task errors require more abstract adaptive processes. From this perspective, one would predict task and response errors to rely on different brain areas.
Method
Participants.
Twenty-one participants (16 females) participated in this study (mean age, 22.8 years; SD, 2.5 years). All were right handed as measured by the Edinburgh Inventory (Oldfield, 1971). Twenty-five euros could be earned in exchange for participation. All participants gave written informed consent and had no history of neurological disorders. Ethical approval was given by the Medical Ethical Review Board of the Ghent University Hospital.
Stimuli and tasks.
The experiment was implemented using Tscope software (Stevens et al., 2006). Stimuli were presented on a black background. The target stimulus was centered on the middle of the screen and consisted of a colored letter. The letter could be printed in green or in yellow and was either an L or an R. The word “color” or the word “letter” preceded target presentation and served as task cue for the color task or the letter task, respectively. The presentation of both cues was randomized over all trials. During the letter task, participants had to decide whether the letter on the screen was an L or and R. In the color task, the letter had to be classified as green or as yellow. In the task change condition, a secondary task cue could appear after target presentation and consisted of a vertical ellipse centered on the target. The cues and the ellipse were both presented in white.
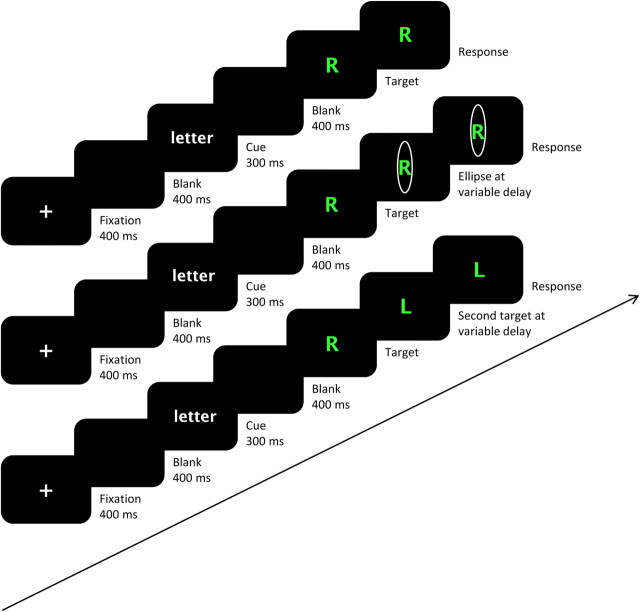
Three types of trials were presented. First, to induce task errors, a secondary task cue was presented after target presentation. This secondary task cue consisted of an ellipse and always indicated a task switch with respect to the primary task cue or the word cue. For example, a green R surrounded by an ellipse after the letter cue should be answered with green and not with R. Furthermore, we adjusted the timing of the presentation of the ellipse to a staircase procedure. On the first ellipse trial (task change trial) the ellipse appeared 250 ms after target presentation. If participants made a correct response, then the presentation of the ellipse on the next trial was delayed by 20 ms. If, however, participants made a task error on a task change trial, the ellipse was presented 20 ms earlier on the next trial. In this way the chance of making a task error on a task change trial was ∼50%. The earliest time point at which the ellipse could appear was 5 ms after target presentation. Second, to induce response errors, we exchanged the appeared target with a different target after a certain delay. The second target differed from the first one according to the relevant stimulus dimension. For example, under execution of the color task, a green R would change into a yellow R. To perform the trial correctly participants had to respond to the stimulus that appeared the latest. As in the task change trials, the timing of the target exchange was adjusted to a staircase algorithm. This means that after a correct response on a stimulus change trial, a target change on the next trial occurred 20 ms later, whereas after a response error on a stimulus change trial, the target change on the next stimulus change trial appeared 20 ms earlier. Again, the earliest time point at which the change could occur was 5 ms after the first target presentation, and the time interval between first and second presentation on the first trial was 250 ms. Note that this method resembles that of the stop-change paradigm. By varying the timing of the change signal delay (time between the target presentation and the change signal) the chance of correctly performing the second task can be manipulated (for review, see Verbruggen et al., 2008). Third, to reduce waiting strategies, we also offered catch trials. In these trials, no ellipses or changes of target were presented. For an overview of the different trial types, see Figure 1.
Figure 1.
Sequence of the three different trial types (from top to bottom, catch trials, task change trials, and stimulus change trials). Two different tasks had to be performed; classifying the color of the letter as green or as yellow and classifying the letter as an R or an L. All trial types start with the presentation of a task cue. This task cue indicates the color task (the word “color” appears) or the letter task (the word “letter” appears). Four response buttons were used. They were allocated to the index and middle fingers of the left and right hands. One task was allocated to one hand, and the L response was always allocated to the left finger of the corresponding hand. In catch trials, the task indicated by the task cue had to be applied on the stimulus at the moment of stimulus presentation. In task change trials, we tried to elicit task errors by presenting a task change signal after stimulus presentation. This task change signal indicates a task switch with respect to the primary cue. In the figure, the correct response for the task change trial is thus green and not R. The delay between the stimulus and the task change signal was adjusted to a staircase algorithm. As a result, participants should have made ∼50% task errors in the task change trials. In stimulus change trials, we tried to elicit response errors by presenting a stimulus change after the first stimulus presentation. Participants should have tried to respond to this changed stimulus and not to the first presented stimulus. In the figure, the correct response for the stimulus change trial is thus L and not R. We also adjusted the delay between the primary and secondary stimuli to a staircase algorithm so that the percentage of response errors would be ∼50%.
Responses were given by the index and the middle fingers of the left and the right hands by response button boxes that were placed on the right and left upper leg. Similar to Meiran and Daichman (2005), we used univalent stimulus–response mappings. This means that we mapped every possible response to one effector. More specifically, we allocated each task to a different hand and then allocated the index and the middle fingers of each hand to a different response. Furthermore, the L response always corresponded to the left finger, and the R response always corresponded to the right finger. The mapping of the hands to the tasks and of the colors to the fingers was balanced across participants. In this way, every possible response, and likewise every possible error, was mapped onto a different effector. This allows inferring from the subject's responses which type of error was made (Meiran and Daichman, 2005). A response error corresponds to a response with the correct hand but with the wrong finger of that hand. This means that the correct task is performed but that the wrong response is given. For example, the response yellow to a green letter under the execution of the color task would correspond to a response error. A task error corresponds to a response with the wrong hand but with the correct finger of that hand. This equals a correct response to the wrong task. For example, the response green to a green R under the execution of the letter task is considered a task error. Note that a response with the wrong hand is not automatically classified as a task error. One could also respond with the wrong hand and with the wrong finger. This would represent a combination of a response and a task error. For example, the response yellow to a green R under the execution of the letter task would correspond to this combined error.
Design and procedure.
In total, there were four blocks. Every block consisted of 80 experimental trials and eight null events. The null events consisted of a blank screen presented for 4500 ms. The 80 experimental trials were divided over 32 task change trials, 32 stimulus change trials, and 16 catch trials. The presentation of trials was randomized so that the amount of task repetitions and task switches between trials was the same over all trials.
Participants received a first training phase outside the scanner. This training phase was divided over four blocks. In the first three blocks, only catch trials, task change trials, and stimulus change trials, respectively, were offered. During the final block, all trial types were then intermixed. The second training phase took place in the scanner while the anatomical scan was taken. During this phase, a mixture of all trial types was immediately presented.
Each trial started with a variable jitter interval of 0, 500, 1000, or 1500 ms. Then a fixation cross was presented for 400 ms. After a blank screen presentation for 400 ms, the cue appeared. After a cue stimulus interval of 700 ms, the target appeared until participants responded or until the deadline of 3000 ms had passed. Between the presentation of the target and the response deadline, an ellipse or a changed stimulus could appear. Likewise, the target presentation, the ellipse, and the changed stimulus stayed on the screen until participants responded or until 3000 ms had passed. During the practice phase, feedback was provided after an erroneous answer [the word “FOUT” (wrong) appeared on the screen for 400 ms]; during the experiment itself, no feedback was provided. Only after too-slow responses the words “TE TRAAG” (too slow) appeared on the screen for 400 ms. The sequence of the different trial types is shown in Figure 1.
Functional magnetic resonance imaging methods.
The experiment was performed on a 3T scanner (Siemens Trio) using an eight-channel radiofrequency head coil. Subjects were positioned head first and supine in the magnet bore. First, 176 high-resolution anatomical images were acquired using a T1-weighted three-dimensional magnetization-prepared rapid-acquisition gradient echo sequence [repetition time (TR), 2530 ms; echo time (TE), 2.58 ms; image matrix, 256 × 256; field of view (FOV), 220 mm; flip angle, 7°; slice thickness, 0.90 mm; voxel size, 0.9 × 0.86 × 0.86 mm (resized to 1 × 1 × 1 mm)]. Whole-brain functional images were collected using a T2*-weighted echoplanar imaging (EPI) sequence, sensitive to blood oxygenation level-dependent contrast (TR, 2000 ms; TE, 35 ms; image matrix, 64 × 64; FOV, 224 mm; flip angle, 80°; slice thickness, 3.0 mm; distance factor, 17%; voxel size, 3.5 × 3.5 × 3 mm; 30 axial slices). A varying number of images were acquired per run because of the self-paced initiation of trials. All data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). To account for T1 relaxation effects, each EPI sequence started with two dummy scans. First, all functional images were spatially realigned using rigid body transformation. After the realignment they were slice-time corrected using the first slice as a reference. The structural image of each subject was coregistered with their mean functional image. Furthermore, all functional images were normalized to the Montreal Neurological Institute (MNI) T1 template. The images were resampled into 3.5 mm3 voxels and spatially smoothed with a Gaussian kernel of 8 mm (full-width at half-maximum). A high-pass filter of 128 Hz was applied during functional magnetic resonance imaging (fMRI) data analysis. To correct for multiple comparisons, we used the program AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). This program determines the probability of a false positive detection from the frequency count of cluster sizes using Monte Carlo simulations. The program determined that a cluster size of 22 contiguous voxels, considered that Z > 3.1 (p < 0.001, uncorrected), corresponded to a corrected p < 0.05 level. Consequently, in the results section we report only activated clusters of minimum 22 voxels. Statistical analyses were performed using the general linear model implemented in SPM5. We distinguished task errors, response errors, and correct trials for the three experimental conditions (catch trials, task change trials, and stimulus change trials), resulting in nine regressors. Because we wanted to dissociate different error types, the moment of response execution (correct response or error) was used as a main event of interest in the general linear model. Both a canonical hemodynamic response function (HRF) and the first time derivative were modeled on the moment of response for each trial. Six regressors defining head movement were also included in the model to account for residual movement effects. We computed contrast images by comparing the parameter estimates for the regressors containing the canonical HRF.
Results
Behavioral results
Two participants (two females) were excluded from the analyses. Their mean error rates (66 and 64%) differed more than two SDs from the overall mean.
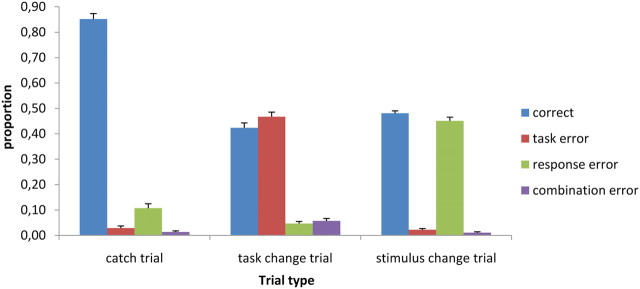
To investigate the effect of both staircase algorithms, we compared the percentage of errors and correct trials in the different trial types. In task change trials, we observed 42% correct trials (SD, 8), 47% task errors (SD, 8), 5% response errors (SD, 3), and 6% combination errors (SD, 4). In stimulus change trials, there were 48% correct trials (SD, 2), 2% task error trials (SD, 2), 46% response errors (SD, 6), and 1% combination errors (SD, 1). In catch trials, we observed 85% correct trials (SD, 9), 3% task error trials (SD, 4), 11% response errors (SD, 8), and 1% combination errors (SD, 2) (Fig. 2). Overall, the percentage of combination errors was very small (mean, 3%; SD, 3), and even in the task change trials the percentage rose to only 6%. This shows that participants did not just switch hands and guess one out of the two responses during task change trials; otherwise, the percentage of combination errors would have been higher.
Figure 2.
Proportion of correct answers, task errors, response errors, and combination errors on catch trials, task change trials, and stimulus change trials. Error bars represent SEs across subjects.
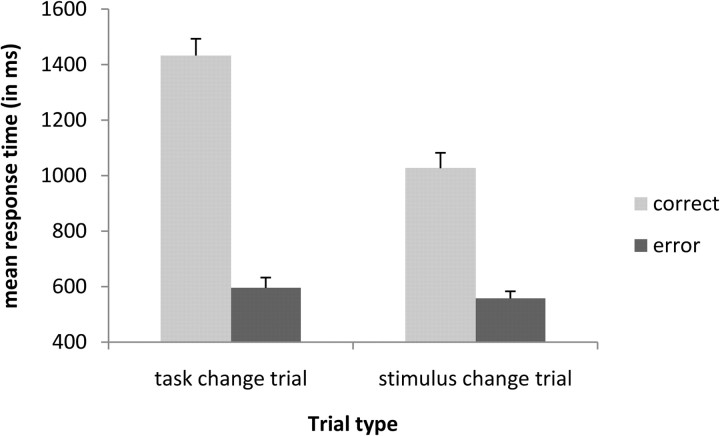
The mean reaction time over conditions and over participants was 754 ms (SD, 155). We performed a repeated-measures ANOVA analysis on the factors trial type (task change trials and stimulus change trials) by type of response (correct, error) on reaction times. Note that the error corresponds to task errors in the task change trials and to response errors in the stimulus change trials. The analysis showed that correct answers (1229 ms) were performed slower than errors (576 ms; F(1,18) = 263.61; p < 0.001) and that task change trials (1014 ms) were performed slower than stimulus change trials (792 ms; F(1,18) = 146.03; p < 0.001). These effects are logically explained by the fact that erroneous responses are a consequence of an insufficient processing of the secondary cue or secondary stimulus and are thus faster than correct responses where the secondary cue or stimulus is more deeply processed. More interestingly, the interaction between both factors also reached significance (F(1,18) = 79.49; p < 0.001). It seems that it is harder to perform a correct response under a task change trial (1432 ms) than a correct response under a stimulus change trial (1026 ms). In other words, overcoming task conflict seems to be more difficult than overcoming response conflict (Fig. 3).
Figure 3.
Interaction of trial type (task change trial, stimulus change trial) by type of response (correct, error) on mean response times in milliseconds. Error bars represent SEs across subjects.
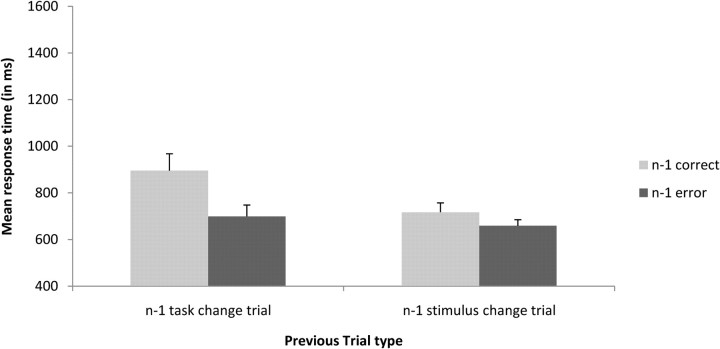
In the previous analysis, it is difficult to interpret reaction times because the staircase algorithm is applied on the investigated trials and therefore can interfere with the observed effects. In the following analyses, we investigated whether these effects remained on the subsequent trial. We performed a repeated-measures ANOVA analysis on the factors previous trial type (trial n − 1 task change trials and trial n − 1 stimulus change trials) by previous type of response (trial n − 1 correct, trial n − 1 error) on reaction times and error rates. Furthermore, we included only correct catch trials on the current trial. Thus, in these analyses, the staircase algorithm cannot interfere with the observed effects since there are no ellipses or changes of stimuli on catch trials. Participants were slower after correct trials (806 ms) than after erroneous trials (678 ms; F(1,18) = 11.87; p < 0.01). The phenomenon of post-error speeding has been reported before. Notebaert et al. (2009) showed that reaction times after an error are dependent on the proportion of errors in the experiment. In experiments where the frequency of occurrence of an error is high, and therefore not surprising (as in our experiment), post-error speeding instead of post-error slowing is observed (Notebaert et al., 2009). Furthermore, participants were slower after task change trials (797 ms) than after stimulus change trials (688 ms; F(1,18) = 10.81; p < 0.01). The two-way interaction between previous trial type (trial n − 1 task change trials and trial n − 1 stimulus change trials) and previous type of response (trial n − 1 correct, trial n − 1 error) was marginally significant (F(1,18) = 4.05; p = 0.06). The effects of the difficulty of overcoming task conflict (trial n − 1 correct task change trial, 895 ms) compared with response conflict (trial n − 1 correct stimulus change trial, 716 ms) seemed thus to persist in the next trial (Fig. 4). The same analysis on error rates revealed only a main effect of previous trial type (F(1,18) = 10.57; p = 0.004); participants made more errors after a task change trial (21%) than after a stimulus change trial (14%). The main effect of previous type of response and the interaction between both factors did not reach significance (F values <1).
Figure 4.
Interaction of previous trial type (trial n − 1 task change trial, trial n − 1 stimulus change trial) by previous type of response (trial n − 1 correct, trial n − 1 error) on mean response times in milliseconds on catch trials. Error bars represent SEs across subjects.
fMRI results
Error-related activation
The first part of the analyses concentrated on whole-brain contrasts revealing brain areas associated with response errors and brain areas associated with task errors. To this aim we selected task change trials to compute task error contrasts and stimulus change trials to compute response error contrasts. The logic underlying the composition of the contrasts was equal for both situation; we subtracted correct trials from error trials.
The first contrast subtracted task change correct trials from task change task error trials and should thus show areas associated with task errors. Brain activity in the pFMC was found. More precisely, the contrast revealed activation in a region located more dorsal and anterior than the rostral cingulate zone (RCZ) (as defined by Picard and Strick, 1996), which we will label as the dorsal frontomedian cortex (dFMC) (MNI coordinates, 6, 39, 54). Furthermore, we found activation in the right inferior parietal lobe (rIPL; MNI coordinates, 57, −48, 48).
The second contrast aimed at revealing response error regions and subtracted stimulus change correct trials from stimulus change response error trials. Again, we found pFMC activity. It seems that an area somewhat anterior to the RCZ (MNI coordinates, 6, 51, 30) was activated along with the dFMC (MNI coordinates, 6, 27, 54). Furthermore, we observed activity in the right middle temporal gyrus (rMTG; MNI coordinates, 57, −66, 3) and the right insula (rINS; MNI coordinates, 39, 21, −9) Brain activity related to task and response errors is shown in Figure 5(for an overview of all related activations per contrast, see Table 1).
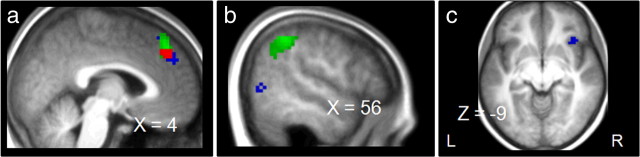
Figure 5.
Response-error-related (blue) and task-error-related (green) activation superimposed on anatomical slices averaged across subjects. Response error activation is related to the following contrast: stimulus change trials, response errors minus correct trials. Task error activation is related to the following contrast: task change trials, task errors minus correct trials. a, Activation for response and task errors in the pFMC (MNI coordinates of maximal random-effect Z scores, blue, x = 6, y = 51, z = 30, Z = 3.87, and x = 6, y = 27, z = 54, Z = 4.13; green, x = 6, y = 39, z = 54, Z = 4.45). Overlapping activation is presented in red (MNI coordinates of maximal random-effects Z scores, x = 3, y = 45, z = 39, Z = 3.43). b, Activation in the MTG for response errors and in the IPL for task errors (MNI coordinates of maximal random-effects Z scores, blue, x = 57, y = −66, z = 3, Z = 3.87; green, x = 57, y = −48, z = 48, Z = 4.56). c, Activation in the INS for response errors (MNI coordinates of maximal random-effects Z scores, x = 39, y = 21, z = −9, Z = 3.46).
Table 1.
MNI coordinates of whole brain contrasts
| Peak coordinates | Z-score | Extent | |
|---|---|---|---|
| Task error (task change trials: task error minus correct) | |||
| dFMC | 6, 39, 54 | 4.45 | 99 |
| IPL | 57, −48, 48 | 4.56 | 108 |
| Response error (stimulus change trials: response error minus correct) | |||
| dFMC | 6, 27, 54 | 4.13 | 32 |
| RCZ | 6, 51, 30 | 3.87 | 44 |
| INS | 39, 21, −9 | 3.46 | 22 |
| MTG | 57, −66, 3 | 3.87 | 39 |
| Conjunction task error and response error | |||
| dFMC | 3, 45, 39 | 3.43 | 28 |
| Response conflict (correct stimulus change trial minus correct catch trial) | |||
| IFJ | −45, 18, 27 | 3.52 | 69 |
| RCZ | −6, 30, 39 | 3.70 | 35 |
| MFG | 42, 27, 42 | 4.43 | 69 |
| FMS | −36, 54, 6 | 4.03 | 115 |
| Task conflict (correct task change trial minus correct catch trial) | |||
| preSMA | −9, 15, 57 | 4.03 | 56 |
| MFG | 42, 54, 18 | 4.09 | 49 |
| IFG | −51, 15, −3 | 4.02 | 22 |
| PM | −39, 9, 60 | 5.09 | 947 |
| IFJ | −39, 15, 24 | 4.75 | |
| DLPFC | −39, 45, 3 | 4.94 | |
| Conjunction task conflict and response conflict | |||
| MFG | −36, 51, 27 | 4.46 | 173 |
| IFJ | −39, 15, 24 | 3.62 | 69 |
IFG, Inferior frontal gyrus.
To answer one of the main questions of the research, namely, do task and response errors activate different or overlapping regions, we performed a conjunction analysis on the two above contrasts. This analysis shows activated voxels in the response error and in the task error contrast at a group level. As expected from the results described above, we observed a significant overlap in the pFMC (MNI coordinates, 3, 45, 39) after applying the conjunction analysis. In addition, we examined whether there were activations in the pFMC uniquely related to one type of error. Therefore we masked both error contrasts with each other at a threshold of p < 0.001. For example, to investigate unique activation for task errors, we took the task error contrast (task change task error minus task change correct) and masked it with the response error contrast (stimulus change response error minus stimulus change correct). The results showed that there was unique brain activity related to task errors in the dorsal part of the pFMC (MNI coordinates, 6, 36, 60), whereas unique brain activation related to response errors was located more ventral and more anterior in the pFMC (MNI coordinates, 3, 48, 33; cluster size, 19 voxels).
From the above analyses, it seems that there is an overlap in the pFMC between both error activations but that there is also unique activation belonging to response errors and unique activation belonging to task errors. In a final analysis, we investigated whether the overlap between both error activations could be identified for each participant individually. It could well be that the observed overlap is an artifact of the conjunction analysis. More precisely, in the conjunction analysis, the overlap in activation is defined as the overlap between the group contrast for response errors and the group contrast for task errors. Therefore, individual variation is no longer taken into account. It is thus possible that none of the subjects show an overlap in task and response errors, whereas at a group level this overlap is significant. Considering that the cingulate sulcus shows a lot of anatomical variability between subjects (Paus et al., 1996; Yücel et al., 2001; Pujol et al., 2002), this hypothesis seems plausible. To investigate overlapping and unique brain activation at an individual level, we first defined a region of interest in the pFMC. This region was defined as follows: we took the three peak voxels of the task and response error activation in the pFMC (MNI coordinates, response errors, 6, 51, 30; 6, 27, 54; task errors, 6, 39, 54) and drew a sphere of 20 mm around each of these coordinates. The range of the region stretched for the x-axis from −14 to 26, for the y-axis from 7 to 71, and for the z-axis from 10 to 74. Next, for each participant, a conjunction and two masking analyses were performed in this region of interest. The conjunction and masking analyses were equal to the ones described in the group results, namely, a conjunction between the task error and the response error contrast, a masking of the response error contrast with the task error contrast, and a masking of the task error contrast with the response error contrast. The threshold was set low (p = 0.05, uncorrected) to observe activation for every subject, and no smoothing was applied. To be able to compare the activations across participants, we will express the number of activated voxels in percentages for each participant. The percentage of activation for task errors was calculated by dividing the number of voxels that were uniquely activated by task errors by the total amount of error activation in the mask. The percentage of activation for response errors was calculated by dividing the number of uniquely activated voxels for response errors by the total amount of error activation in the mask. Finally, the percentage of overlapping activation was calculated by dividing the number of voxels activated in the conjunction analysis by the total amount of error activation in the mask. Overall, the mean percentage of unique task error activation was larger (55%) than the mean percentage of unique response error activation (37%), although not significantly (t(18) = 1.56; p = 0.14). If we take a closer look at Table 2, we see that 11 participants showed more activation for task errors than for response errors. More importantly, the overall percentage of overlap between both error types was quite small (8%). Furthermore, it seems that this figure was mainly based on a relatively strong overlap for only four participants (overlap of >15%) (Table 2). To check that the absence of overlap was not attributable to an inflation of noise, we replicated the above analysis with a higher threshold (p < 0.01). From Table 2, it is clear that we replicated the above findings; the overall amount of overlap was very small, 3%. Only one participant showed an overlap >15%. The percentage of unique task error activation (63%) was higher than the amount of unique response error activation (33%) (t(18) = 2.28; p = 0.04). Furthermore, 12 participants showed higher task error activation than response error activation. Although one participant showed low activation for task errors (2%), six participants showed a low activation for response errors (<15%).
Table 2.
Percentage of activation uniquely related to task errors, uniquely related to response errors and percentage of overlap of both errors for p = 0.05
| Participant | Total number of activated voxels | Task error (in %) | Response error (in %) | Overlap (in %) |
|---|---|---|---|---|
| 1 | 425 (93) | 43 (40) | 49 (58) | 8 (2) |
| 2 | 403 (179) | 90 (94) | 8 (5) | 2 (1) |
| 3 | 236 (68) | 86 (100) | 11 (0) | 3 (0) |
| 4 | 552 (170) | 3 (2) | 97 (98) | 0 (0) |
| 5 | 319 (67) | 36 (37) | 62 (63) | 3 (0) |
| 6 | 145 (23) | 54 (57) | 45 (43) | 1 (0) |
| 7 | 428 (173) | 80 (95) | 8 (3) | 12 (2) |
| 8 | 90 (12) | 81 (83) | 18 (17) | 1 (0) |
| 9 | 1204 (699) | 68 (83) | 13 (9) | 19 (8) |
| 10 | 223 (48) | 26 (21) | 73 (79) | 2 (0) |
| 11 | 227 (41) | 23 (51) | 73 (49) | 4 (0) |
| 12 | 340 (67) | 38 (49) | 56 (49) | 7 (1) |
| 13 | 147 (32) | 48 (44) | 49 (53) | 3 (3) |
| 14 | 655 (261) | 88 (92) | 5 (5) | 7 (3) |
| 15 | 694 (284) | 61 (75) | 21 (17) | 19 (8) |
| 16 | 78 (14) | 62 (86) | 38 (14) | 0 (0) |
| 17 | 222 (55) | 73 (80) | 22 (18) | 5 (2) |
| 18 | 948 (390) | 27 (34) | 45 (51) | 28 (15) |
| 19 | 1147 (792) | 61(80) | 3 (1) | 36 (19) |
Values in parentheses correspond to a p value of 0.01.
Conflict-related activation
In the second part of the analyses, we investigated brain areas related with response and task conflict. Both conflict contrasts (response and task conflict) were designed by contrasting correct conflict trials with correct no-conflict trials. More precisely, we subtracted correct catch trials from correctly executed stimulus change trials and correctly executed task change trials respectively for response and task conflict. Because these contrasts also subtract two different screen displays, a lot of sensory-related brain activity, for example in the occipital lobe, showed up. Therefore, we only report the activated frontal areas.
The first contrast aimed to look at response conflict. Therefore, we subtracted correct catch trials from correct stimulus change trials. An area slightly anterior to the left inferior frontal junction (lIFJ; MNI coordinates, −45, 18, 27), the RCZ (−6, 30, 39), the right middle frontal gyrus (rMFG; 42, 27, 42), and an area in the left frontopolar cortex, the frontomarginal sulcus (lFMS; −36, 54, 6) were activated.
The second contrast aimed at revealing regions related to task conflict. We subtracted correct catch trials from correct task change trials. The preSMA (MNI coordinates, −9, 15, 57) and the rMFG (42, 54, 18) were activated along with a region stretching from the premotor cortex (PM; −39, 9, 60) over an area somewhat anterior to the lIFJ (−39, 15, 24), ending in the left dorsolateral prefrontal cortex (lDLPFC; −39, 45, 3). Figure 6 shows brain activity related to response conflict and task conflict.
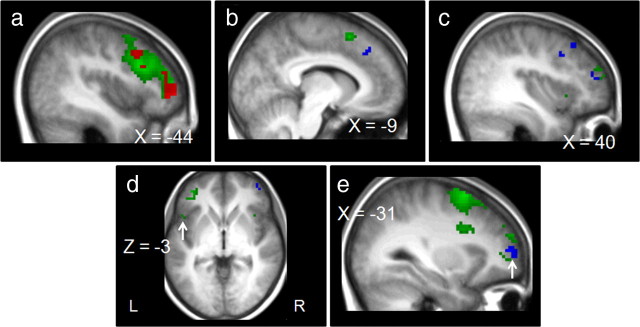
Figure 6.
Response-conflict-related (blue) and task-conflict-related (green) activation superimposed on anatomical slices averaged across participants. Response conflict is related to the contrast stimulus change correct trials minus catch correct trials. Task conflict is related to the contrast task change correct trials minus catch correct trials. a, Activation in the PM, IFJ, and DLPFC for task conflict (MNI coordinates of maximal random-effect Z scores, green, PM, x = −39, y = 9, z = 60, Z = 5.09; IFJ, x = −39, y = 15, z = 24, Z = 4.75; DLPFC, x = −39, y = 45, z = 3, Z = 4.94) and activation in the IFJ for response conflict (MNI coordinates of maximal random-effect Z scores, blue, x = −45, y = 18, z = 27, Z = 3.52). Overlapping activity is represented in red in the IFJ and the MFG (MNI coordinates of maximal random-effect Z scores, IFJ, x = −39, y = 15, z = 24, Z = 3.62; MFG, x = −36, y = 51, z = 27, Z = 4.46). b, Activation in the preSMA for task conflict and in the RCZ for response conflict (MNI coordinates of maximal random-effect Z scores, green, x = −9, y = 15, z = 57, Z = 4.03; blue, x = −6, y = 30, z = 39, Z = 3.70). c, Activation in the MFG for task conflict and for response conflict (MNI coordinates of maximal random-effect Z scores, green, x = 42, y = 54, z = 18, Z = 4.09; blue, x = 42, y = 27, z = 42, Z = 3.43). d, Activation in the inferior frontal gyrus for task conflict (MNI coordinates of maximal random-effect Z scores, x = −51, y = 15, z = −3, Z = 4.02). e, Activation in the frontopolar cortex for response conflict (MNI coordinates of maximal random-effect Z scores, x = −36, y = 54, z = 6, Z = 4.03).
Finally, we wanted to see whether response and task conflict subserved overlapping brain activation. Because task change trials and stimulus change trials comprise two completely different types of events, we will not directly compare them. However, as in the error contrasts, we performed a conjunction analysis on the two above contrasts (task and response conflict). This should also reveal which brain regions are commonly activated by response and task conflict. The conjunction analysis showed that the left middle frontal gyrus (lMFG; MNI coordinates, −36, 51, 27) and the lIFJ (−39, 15, 24) showed activation for both conflict levels.
Integrating conflict and errors
In the final part of the analyses, we will compare conflict and error activations for the two different levels of abstractness. In other words, we will relate response conflict with response error activation and task conflict with task error activation. To this aim, we performed two conjunction analyses.
First, we performed a conjunction analysis between the response error contrast and the response conflict contrast. No significant activations emerged. This means that in our study there was no significant overlap between response conflict activity and response error activity. If we take a closer look at the coordinates in the pFMC related to response conflict and response errors, we can conclude that response conflict triggers regions located more dorsal and more posterior than response errors. In addition, we performed this analysis for each participant separately. We defined a region of interest based on the peak coordinates for response errors (MNI coordinates, 6, 51, 30) and response conflict (−6, 30, 39) in the pFMC. We took the mean of both coordinates and drew a sphere of 15 mm around this center. As a result, the region extended for the x-axis from −15 to +15, for the y-axis from 25 to 55, and for the z-axis from 20 to 50. Similar to the individual analyses on task and response errors, we computed the percentage of brain activity uniquely related to response errors and uniquely related to response conflict, and the percentage of overlapping activation. The threshold was set at p < 0.05, and no smoothing was applied. The overall percentage of overlap was minimal (1%). The largest percentage of overlap was 3%. Furthermore, the percentages for response error and response conflict activity did not differ significantly (53 and 46%, respectively; t(18) < 1).
Second, we performed a conjunction analysis between the task error contrast and the task conflict contrast. As expected from the above task error contrast and task conflict contrast, the conjunction analysis showed no significant overlap. If we compare the regions associated with task conflict and task errors, we see that task conflict regions are located more posterior than task error regions.
See Figure 7 for an overview of the peak activations in the pFMC for task errors, task conflict, response errors, and response conflict.
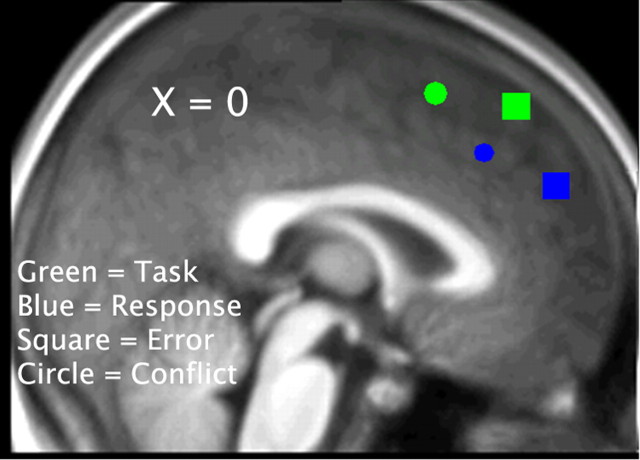
Figure 7.
Peak activations in the pFMC for task errors (MNI coordinates of maximal random-effect Z scores, x = 6, y = 39, z = 54, Z = 4.45), task conflict (MNI coordinates of maximal random-effect Z scores, x = −9, y = 15, z = 57, Z = 4.03), response errors (MNI coordinates of maximal random-effect Z scores, x = 6, y = 51, z = 30, Z = 3.87), and response conflict (MNI coordinates of maximal random-effect Z scores, x = −6, y = 30, z = 39, Z = 3.70). To represent all activations on the same figure, all activations were mapped on an x value of 0. Task-related activation is presented in green, and response-related activation is presented in blue. Error-related activation is presented as squares, and conflict-related activation is presented as circles.
Discussion
In this study, we investigated errors and conflict at two levels of abstractness. Our data show that task and response errors activate a region in the pFMC that stretches from the rostral cingulate zone to the dorsal frontomedian cortex. A conjunction analysis at the group level revealed an overlap in brain activation for both types of errors. However, an investigation at the individual level did not support the conclusion of a strong overlap. Furthermore, the differential pattern of brain activation outside the pFMC supports the hypothesis that task and response errors are related to different neural substrates. The second part of the analysis, comparing response and task conflict, also revealed a clear dissociation of the task and the response levels. It seems that task conflict activates regions more posteriorly located than response conflict. However, it seems that both conflict types also share some overlapping activations outside the frontomedian cortex such as the IFJ and the MFG. In the last part of the analysis, we compared conflict and error processing within each level of abstractness. For both levels it seems that there is no overlap between conflict and errors. In addition, error-activated regions seem to be located more anterior than conflict-related regions. Furthermore, this distinction is more salient at the task level than at the response level. A final point that supports the dissociation between the task and response levels is provided by the behavioral data. It seems that participants are slower on task than on response errors (p = 0.07). In addition, response times are higher on task conflict than on response conflict trials. Even on subsequent trials, the effect of task conflict is stronger than the effect of response conflict (p = 0.06), although it does not make a difference if a task error or a response error was made on the previous trial. In line with the fMRI data, this shows that the difference between task and response processing is more expressed at the conflict level than at the error level.
Dissociating task and response errors
In the present study, we found a distinction of task and response errors in the pFMC. From the individual analysis it is clear that most participants do not show an overlap in response and task error activation. The question now is whether we can dissociate both error regions anatomically. In accordance with our hypothesis, the data suggest that response errors rely on areas in the pFMC that have a strong link to the motor system, whereas task errors rely on areas in the pFMC that are associated with more abstract processing. This dissociation is in accordance with the existing literature on task-related and response-related processes. As mentioned in the introduction, response errors have been associated with ventral parts of the pFMC, especially the RCZ (Ridderinkhof et al., 2004; Ullsperger and von Cramon, 2004). However, in task-related studies, more dorsal regions such as the preSMA (Brass and von Cramon, 2002; Rushworth et al., 2002; Crone et al., 2006) seem to play an important role. The task error region that we described in the pFMC (dFMC) is more anterior to the preSMA and has been described in higher-level decision processes. It seems that this region is involved under conditions in which abstract rules have to be applied to a certain stimulus. For example, Volz et al. (2003, 2004) reported this region when participants where highly uncertain about their decisions compared to situations where participants could recollect their decisions from memory. Furthermore, in a study by Goel and Dolan (2000), the dFMC was active if participants had to derive a classification rule themselves, whereas there was no dFMC activity when participants had to classify stimuli based on predetermined features. In a similar experiment by Elliott and Dolan (1998), the dFMC was activated under situations of rule searching. Finally, Rushworth et al. (2002) reported the dFMC in situations where participants had to switch between different stimulus-response rules. In line with our results, this suggests that the task error region is related to abstract processing. However, one should be cautious of the fact that activation in the dFMC was also found for response errors. Although this activation was not as strong as for task errors and was more posteriorly located, we cannot conclude that task errors exclusively activate dorsal regions and response errors exclusively activate ventral regions. However, we can conclude that both error types activate different subregions in the pFMC.
From the individual analyses it seems that some participants show larger percentages for task errors whereas others show the opposite pattern. Such effects might be attributable to strategies in response selection. In previous research using univalent stimulus–response mappings, it was shown that some participants first select the correct hand and then the correct finger, whereas others first select the correct fingers (for example, both middle fingers) and then the correct hand (Bernstein et al., 1995). It was demonstrated that hand preference subjects (those who first select their hand) show larger error-related negativity to hand errors and vice versa. Mapped on our results, this could explain why some participants have larger activation for task errors and others have larger activation for response errors in the individual analyses (if task errors are considered as hand errors and response errors as finger errors). However, unlike these previous studies, where only one task with four possible responses was offered, we offered two different tasks, each with two possible responses. In addition, participants could already start preparing the task and the concurrent hand before information about the correct finger was forehand.
Additional evidence for a differentiation between task and response errors is provided by the different brain networks both errors activate. In addition to the prefrontal areas, we found activation in the rMTG and the rINS for response errors and activation in the rIPL for task errors. The rMTG has been related to color processing (Chao and Martin, 1999; Simmons et al., 2007). Presumably, this activation thus resulted from the changing color situations in the stimulus change trials. The activation of the rINS, however, is probably related to the response switches made during stimulus change trials, as this region has been related to response switching (Paulus et al., 2005). In regard to the rIPL activation, this region has been linked to the maintenance of current task goals and the processing of new task information (Singh-Curry and Husain, 2009). In the task error contrast, two task switching situations were subtracted from one another. Thus, like Singh-Curry and Husain (2009), we find support for the role of the IPL in flexible adaptive behavior.
It should be mentioned that the response errors in our study were evoked in a different way than is usually done in the literature. Usually, response errors are elicited by the use of an interference paradigm such as the flanker task. However, in our study we wanted to elicit both errors in the same way so that the differences between them were kept as small as possible. Therefore, response errors were also elicited by means of a stop-change signal. One could argue that in this way we elicit another type of error than the response errors usually described. Furthermore, the activation correlated with response errors was located more dorsal and more anterior in our study compared to the activation generally found for response errors. However, if we compared the response error activation in the pFMC to the cluster of performance-monitoring activation described by Ridderinkhof et al. (2004), our response error activation clusters were still situated in this zone. At first sight, the dFMC activation could be explained by the inhibition procedure we used. Similar dFMC activation, namely, was found in a stop-signal study (Li et al., 2006). In a follow-up study, stopping was impaired when this region was disrupted by transcranial magnetic stimulation (Chen et al., 2009). However, the fact that we found a distinction between the task level and the response level shows that we do not merely assess inhibition processes, but rather response- and task-related processes.
Dissociating task and response conflict
In addition to the error contrasts, we performed conflict contrasts. Whereas response conflict activated the RCZ, the lIFJ, the rMFG, and the lFMS, task conflict was associated with the preSMA, the PM, the lIFJ, the lDLPFC, and the rMFG. The activation of the left frontopolar cortex in the response conflict contrast is probably related to the fact that we compared changing stimuli. Pollmann (2000) and Pollmann et al. (2000) already associated left frontopolar cortex with changes in visual dimension. The RCZ is often reported as a response conflict region, whereas task conflict seems to be correlated with regions associated with more abstract cognitive processes such as the preSMA and the premotor cortex (Abe et al., 2007). This anterior–posterior dissociation is partly reproduced in our response- and task-conflict-related brain regions. However these effects should be treated with care since the conflict contrasts subtract two different trial types from each other; that is, we compared trials in which a secondary ellipse or stimulus is presented with trials where there are no secondary changes.
Integrating two dimensions
Overall, we found that depending on the level at which an error occurs different brain regions are activated. This suggests that an error is more than a general comparison between an intended and an actual outcome. In addition, the dissociation in brain activity between different levels of errors could point to the difference in adjustments they apply for. The same conclusion can be formed based on our conflict results; namely, different forms of conflict are correlated with different brain areas. This is in line with recent findings showing that the medial prefrontal cortex interacts with the lateral prefrontal cortex in a parallel hierarchical way to provide control (Kouneiher et al., 2009).
Finally, we replicated the dissociation between conflict and errors at the response level. It seems that response errors activate regions more ventral and more anterior than response conflict areas. In addition, we found that conflict and errors were even more pronouncedly dissociated at the task level.
Footnotes
This work was supported by Grant G.0630.08 from the Fonds Wetenschappelijk Onderzoek Vlaanderen and by the Belgian Science Policy, Interuniversity Attraction Poles program (P6/29). We acknowledge the support of Ghent University (Multidisciplinary Research Partnership “The integrative neuroscience of behavioral control”).
References
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H. Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci. 2007;27:3429–3438. doi: 10.1523/JNEUROSCI.4273-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Scheffers MK, Coles MG. Where did I go wrong—a psychophysiological analysis of error-detection. J Exp Psychol Hum Percept Perform. 1995;21:1312–1322. doi: 10.1037//0096-1523.21.6.1312. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Cortical regions associated with perceiving, naming, and knowing about colors. J Cogn Neurosci. 1999;11:25–35. doi: 10.1162/089892999563229. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJL, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage. 1998;8:17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Anatomical segregation of component processes in an inductive inference task. J Cogn Neurosci. 2000;12:110–119. doi: 10.1162/08989290051137639. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N, Daichman A. Advance task preparation reduces task error rate in the cuing task-switching paradigm. Mem Cognit. 2005;33:1272–1288. doi: 10.3758/bf03193228. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness—Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J Neurosci. 2008;28:3468–3473. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S. Conference on Action and Visuo-Spatial Attention—Neurobiological Bases and Disorders. Konigswinter, Germany: Academic; 2000. Switching between dimensions, locations, and responses: the role of the left frontopolar cortex; pp. S118–S124. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, von Cramon DY. A fronto-posterior network involved in visual dimension changes. J Cogn Neurosci. 2000;12:480–494. doi: 10.1162/089892900562156. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuiss S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser M. How to correct a task error: task-switch effects following different types of error correction. J Exp Psychol Learn Mem Cogn. 2010;36:1028–1035. doi: 10.1037/a0019340. [DOI] [PubMed] [Google Scholar]
- Steinhauser M, Hübner R. Response-based strengthening in task shifting: evidence from shift effects produced by errors. J Exp Psychol Hum Percept Perform. 2006;32:517–534. doi: 10.1037/0096-1523.32.3.517. [DOI] [PubMed] [Google Scholar]
- Steinhauser M, Hübner R. How task errors affect subsequent behavior: evidence from distributional analyses of task-switching effects. Mem Cognit. 2008;36:979–990. doi: 10.3758/mc.36.5.979. [DOI] [PubMed] [Google Scholar]
- Stevens M, Lammertyn J, Verbruggen F, Vandierendonck A. Tscope: a C library for programming cognitive experiments on the MS Windows platform. Behav Res Methods. 2006;38:280–286. doi: 10.3758/bf03192779. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci. 2009;29:13158–13164. doi: 10.1523/JNEUROSCI.2708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Schneider DW, Logan GD. How to stop and change a response: the role of goal activation in multitasking. J Exp Psychol Hum Percept Perform. 2008;34:1212–1228. doi: 10.1037/0096-1523.34.5.1212. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19:271–280. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage. 2004;21:848–857. doi: 10.1016/j.neuroimage.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Kustermann E, Fahle M, Herrmann M. The influence of response conflict on error processing: evidence from event-related fMRI. Brain Res. 2008;1194:118–129. doi: 10.1016/j.brainres.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]