Abstract
In the brain, neuronal activation triggers a local increase in cerebral blood flow, a response named functional hyperemia. The extent to which functional hyperemia faithfully reports brain activation, spatially or temporally, remains a matter of debate. Here, we used the olfactory bulb glomerulus as a neurovascular model and two-photon microscopy imaging to investigate the correlation between calcium signals in glutamatergic terminals of olfactory sensory neurons and local vascular responses. We find that, depending on odor stimulation intensity, vascular responses are differently coupled to calcium signals. Upon moderate odor stimulation, glomerular vascular responses increase accordingly with calcium signals. In contrast, in silent glomeruli neighboring strongly activated ones and in glomeruli adapting upon high odor stimulation, vascular responses are independent of or negatively coupled to presynaptic calcium signals, respectively. Hence, functional hyperemia, a key signal used in functional imaging, can be, at times, an unreliable marker of local brain activation.
Introduction
In humans, most noninvasive brain imaging techniques assess neuronal activation from measurement of hemodynamic changes. However, if functional hyperemia is indeed tightly correlated to neuronal activation, the molecular and cellular mechanisms underlying this neurovascular coupling are not fully understood (for review, see Gordon et al., 2007; Iadecola and Nedergaard, 2007; Attwell et al., 2010). There is now a general consensus that neurons increase cerebral blood flow (CBF) using a feedforward mechanism in which input activity leads to the release of glutamate and adenosine triphosphate, followed by the activation of several signaling pathways involving astrocytes and neurons, and controlling the arteriolar tone. The multiplicity of these signaling pathways raises the question of the extent to which CBF changes and blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) signals reflect local neuronal activity. Both signals are best correlated to local field potential (LFP) responses rather than to spiking (Logothetis, 2008) and are maintained in conditions where spiking is strongly reduced (Mathiesen et al., 1998; Rauch et al., 2008). Still, LFP responses themselves are complex and involve EPSPs, IPSPs, neuronal intrinsic membrane properties, and occasionally volume-conducted LFP responses from neighboring activated networks. Therefore, it is questionable that LFP responses always faithfully reflect the level of glutamatergic input activation, which should be best represented by measurements of glutamate release.
The functional organization of olfactory bulb glomeruli make them ideal functional modules to investigate neurovascular coupling and functional hyperemia (Shepherd and Charpak, 2008). During odor stimulation, LFP responses have several components; one of them, the LFP rapid negativities, reflects neuronal activation restricted to a single glomerulus. These negativities are odor-specific, locked to the respiration frequency [which is driving air past the olfactory receptor neurons (ORNs)], show sharp transitions in space (laterally and in depth), are tightly correlated to mitral cell postsynaptic activity, and are abolished by intraglomerular application of glutamate ionotropic receptor antagonists (Chaigneau, 2007). LFP negativities thus reflect the summation of EPSP in glomeruli. More recently, we have labeled ORN terminals with the calcium sensor, calcium green 1 (Lecoq et al., 2009), and shown that LFP rapid negativities are tightly correlated to presynaptic calcium (Ca2+) signals detected in ORN terminals converging into the same glomerulus. This correlation is surprisingly strong and independent of odor stimulation intensity, suggesting that presynaptic Ca2+ signals could be considered as solid markers of the amount of glutamate released onto the glomerular dendrites that generate LFP negativities. Because odors also trigger blood flow increases in glomeruli, a response termed glomerular functional hyperemia (Kida et al., 2002; Chaigneau et al., 2003, 2007; Petzold et al., 2008; Shepherd and Charpak, 2008), we examined whether this functional hyperemia is correlated to presynaptic Ca2+ signals in ORN terminals under three conditions of neuronal activation: in the absence or presence of peripheral adaptation and below the ORN activation threshold.
Materials and Methods
Animal preparation and two-photon imaging.
Wistar rats, 30–60 d old, were anesthetized with an intraperitoneal bolus injection of ketamine and xylazine (90 and 10 mg/kg, respectively) followed by a continuous intravenous injection (25–30% of the bolus/h). The animal was held in a standard stereotaxic apparatus with ear bars and a craniotomy was performed above the two olfactory bulb hemispheres, the posterior cisterna was drained, and the dura was removed. A 100-μm-thick glass coverslip was placed over the bulb, fixed on the cranium and the space below filled with a 3% Agar solution. The temperature of the animal was monitored with a rectal thermometer and maintained at 37°C with a feedback controlled heating blanket (Harvard Apparatus). For all experiments, breathing frequency was monitored through a pneumogram transducer (BIOPAC Systems). Heart rate, oxygen saturation, and pulse distension were monitored through a pulse oxymeter located on the rat hindpaw (STARR Life Sciences). Animals continuously breathed filtered air enriched with oxygen (20–30%). Olfactory nerve terminals were labeled with Ca2+ green 1 dextran, 10 kDa (Invitrogen), 2–8 d before experiments, as described previously (Lecoq et al., 2009). To label vessels, a bolus of 70 kDa Texas Red dextran was injected intravenously through a catheter placed in the femoral vein. Ca2+ measurements were obtained either from images comprising the entire glomerulus and acquired at rates up to 20 frames per second (using a custom-built two-photon laser-scanning microscope) or from line scans running through a capillary and the neighboring neuropil (Chaigneau et al., 2007). Ca2+ changes and red blood cell (RBC) velocity were measured as previously described (Chaigneau et al., 2007). Note that, because Ca2+ changes (ΔF/F) obtained with movies and line scans were similar, all data are pooled in the statistics. The initial Ca2+ peak or the overall Ca2+ signal (area) was used for quantification, as indicated in the text. For the entire study, average values are expressed as mean ± SEM.
Odor delivery.
Odorants were applied for 4 s with a custom-built olfactometer. The olfactometer delivered a constant flow of humidified air in a small Teflon reservoir surrounding the rat snout. All tubes connecting the odorant reservoir to the snout were made of Teflon to minimize odorant contamination. Odorants used were aldehydes and esters. Two flow meters (Aalborg), one in the clean air path and one before the odorant reservoir, allowed us to determine precisely the odorant concentration. To reveal adaptation, high odorant concentrations were used, i.e., from 3–100% when expressed in percentage of saturated air. Odor applications were repeated at least three times (with 3–4 min intertrial intervals) at each odorant concentration.
Results
We first determined that, in glomeruli that do not show peripheral adaptation during a sustained odor stimulation (4 s), the overall presynaptic Ca2+ signals and vascular responses increased accordingly with the odor stimulation intensity (Fig. 1 A,B). This was expected from our previous quantifications based on the summed LFP peaks (ΣLFP) (Chaigneau et al., 2007) and is in accordance with what has been reported in many brain regions upon moderate glutamatergic input activation (Iadecola et al., 1996; Mathiesen et al., 1998; Norup Nielsen and Lauritzen, 2001; Logothetis et al., 2001). In contrast, the correlation between presynaptic Ca2+ signals and vascular responses markedly differed in more sensitive glomeruli showing peripheral ORN adaptation.
Figure 1.
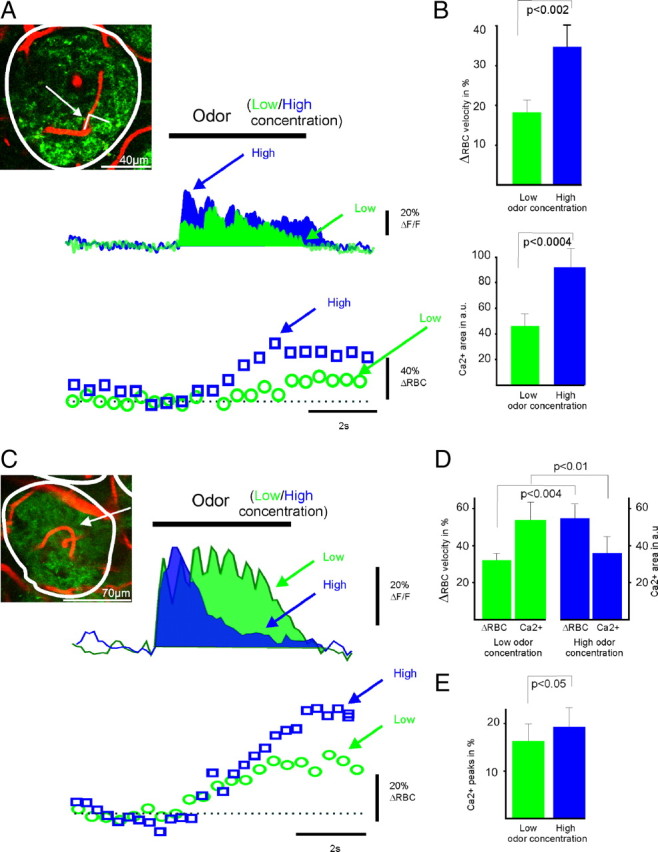
Functional hyperemia and glutamate release by ORN terminals in the absence and presence of peripheral odor adaptation. A, Neuronal and vascular responses in the absence of odor adaptation. Top left, Glomerulus with boundaries outlined by ORN terminals labeled with calcium (Ca2+) green dextran, and a capillary (arrow) in which RBC velocity was monitored. Line scans were acquired on a segment going along the longitudinal axis of the capillary for its first part and through the neighboring neuropil for its second part. RBC velocity measurements (the first part of the line scan) were acquired on one detector and Ca2+ on a second one. Top scan, At low concentration, ethyl butyrate (50%; green trace) induced a presynaptic Ca2+ signal, which overall response (the area below the curve, in a.u.) increased with concentration (high = 75%; blue trace). Bottom scan, The vascular response increased accordingly with the odor concentration. B, Summary graphs showing that functional hyperemia is positively coupled to the overall Ca2+ signal (n = 9 capillaries in seven animals). C, Neuronal and vascular responses in the presence of peripheral adaptation. Top left, The recorded glomerulus and capillary. Ca2+ responses were measured over the entire glomerulus at a rate of 9 image/s. Top scan, At low odorant concentration (heptaldehyde, 17%), the presynaptic Ca2+ response displayed a large initial Ca2+ signal peak followed by smaller ones which were phase-locked to respiration and maintained during the entire stimulus duration. At higher odorant concentration (83%), the initial Ca2+ peak was slightly larger; however, the overall Ca2+ response markedly decreased (blue trace). Bottom scan, The vascular response was larger at high than low odor concentration. D, E, Summary graphs showing that during odor adaptation (n = 9 animals), functional hyperemia is positively coupled to the initial presynaptic Ca2+ signal, i.e., glutamate released during the first inhalation but negatively coupled to the overall Ca2+ signal, i.e., glutamate continuously released during the sustained stimulation. Averages of three to six odor applications at each odor concentration.
Such adaptation is triggered by Ca2+ influx in ORNs in the epithelium, and involves several negative feedback processes, among which is a sensitivity decrease of cyclic nucleotide-gated channels or the hydrolysis of cAMP (for review, see Kleene, 2008). We have recently shown that peripheral adaptation can also be detected centrally in glomeruli, with LFP responses decreasing upon strong odorant stimulation (Lecoq et al., 2009). This phenomenon did not involve postsynaptic processing but resulted from a decrease of presynaptic Ca2+ signals in ORN terminals and thus a decrease of the amount of glutamate released by these terminals. Because presynaptic GABA B receptors (Aroniadou-Anderjaska et al., 2000; Murphy and Isaacson, 2003; Vucinic et al., 2006; Pírez and Wachowiak, 2008), dopamine receptors (Hsia et al., 1999), or purinergic receptors (our unpublished observations) were not involved in the phenomenon (Lecoq et al., 2009), we could conclude that presynaptic Ca2+ signal adaptation indirectly reflected peripheral adaptation. Figure 1, C and D, illustrates that, during such peripheral adaptation, the overall presynaptic Ca2+ signal from ORNs and the vascular response uncoupled; during strong odor stimulation (Fig. 1 C, blue), the local vascular response was larger, whereas the overall presynaptic Ca2+ signal was smaller than during weak stimulation (Fig. 1 C, green). In nine animals (Fig. 1 D), the mean change in RBC velocity increased from 31 ± 3% to 54 ± 7% when the odor concentration increased, leading to peripheral adaptation, whereas the overall presynaptic Ca2+ signal decreased from 53 ± 9 [arbitrary units (a.u.)] to 35 ± 9 (a.u.). However (Fig. 1 E), a positive correlation was maintained between the initial Ca2+ peak and vascular responses during strong stimulation. These data show that during peripheral adaptation, functional hyperemia is coupled in a complex manner to locally presynaptic Ca2+. During sustained odor stimulation, the vascular response is correlated in a concentration-dependent manner to the first Ca2+ transient, i.e., the first inhalation, but then becomes progressively independent of, or even negatively coupled to, the over Ca2+ rise. We propose that during peripheral adaptation, functional hyperemia reports the regional and not local glomerular activation (see Discussion, below).
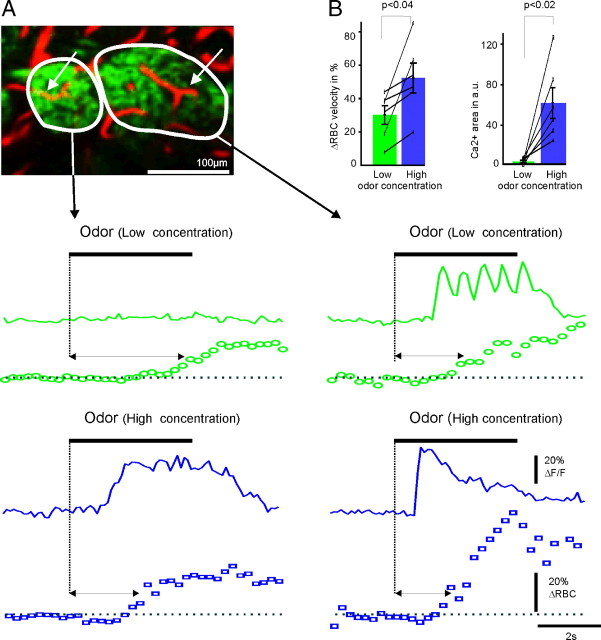
In a second series of experiments, we investigated whether functional hyperemia could be detected in the absence of glutamate release, i.e., below the ORN activation threshold. Glomeruli were precisely chosen, with one weakly sensitive, responding only during strong stimulation and adjacent to a second glomerulus, which responded during weak stimulation and eventually adapted at higher odor concentration. Figure 2 shows that in the weak glomerulus immediately adjacent to the strongly responsive glomerulus and in the absence of a presynaptic Ca2+ increase, vascular responses, although delayed, could be recorded (mean ΔRBC velocity increase, 30 ± 6%). Note that in the weak glomerulus, increasing the odor concentration generated specific and local Ca2+ increases (mean ΔCa2+ of 62 ± 16 a.u.) and vascular responses (mean ΔRBC velocity increase, 52 ± 9%), demonstrating that ORN terminals were functional. Note that the vascular response latency decreased at higher odor concentration (from 3.9 ± 0.6 to 2.0 ± 0.3 s; n = 6, p = 0.045). These results show that strongly activated glomeruli may influence blood flow in neighboring inactive glomeruli, demonstrating that due to the loose vascular tree organization (e.g., in the presence of capillaries crossing directly from one glomerulus to another), the spatial resolution of vascular responses is not as sharp as that of neuronal responses.
Figure 2.
Functional hyperemia below the ORN activation threshold. A, Top left, Two glomeruli in which presynaptic Ca2+ responses were simultaneously imaged and in which RBC velocity was sequentially measured in capillaries indicated by the arrows. Middle left, At low concentration, heptaldehyde (16%) did not cause any Ca2+ response; however, it triggered a delayed RBC velocity increase. The horizontal double-headed arrow indicates the delay between the odor onset and the vascular response. Middle right, In contrast, the same concentration induced typical presynaptic Ca2+ and vascular responses in the adjacent glomerulus. Bottom, At higher concentrations (100%), heptaldehyde stimulated both glomeruli. Note that the vascular delay of the left glomerulus significantly decreased. Averages of three or four odor applications at each odor concentration. B, Summary graphs showing that, in glomeruli adjacent to strongly responsive glomeruli, functional hyperemia occurs in the absence of presynaptic Ca2+ signal, i.e., in the absence of glutamate locally released (mean ± SEM and responses of individual capillaries, n = 6 animals).
Discussion
Glomeruli are particularly well defined neuronal networks. However, even in such a simple neuronal model, the relationship between functional hyperemia and neuronal activation is complex. Under moderate odor stimulation, i.e., in the absence of adaptation, vascular responses and postsynaptic responses (ΣLFP) (Chaigneau et al., 2007) or presynaptic Ca2+ signals are positively correlated. This is in agreement with numerous studies reporting strong correlations between LFP and BOLD fRMI or laser Doppler signals (for review, see Logothetis, 2008). However, at a higher odor concentration, functional hyperemia no longer reflects the level of local sensory stimulation measured from markers such as ΣLFP peaks or the overall presynaptic Ca2+ responses. Note that such mismatch between vascular responses and presynaptic Ca2+ would have been similarly observed if glomerular adaptation was due to presynaptic inhibition and not to ORN adaptation. Finally, the presence of a delayed nonspecific vascular response in glomeruli adjacent to activated glomeruli also stresses that the vascular tree organization does not allow vascular signals to perfectly match neuronal activity.
Can we extend our findings, exclusively obtained in olfactory glomeruli, to other brain regions? The strength of the glomerular model, as opposed to other regions where some neurovascular uncoupling has been suggested (Norup Nielsen and Lauritzen, 2001; Kim et al., 2004), is that it is a unique structure in which glutamate release from ORN terminals can be inferred from presynaptic Ca2 and modulated physiologically and in a quantified manner. The strong correlation between LFP peaks and Ca2+ transients justify such a hypothesis, in particular since in vitro, the relationship between ORN transmitter release and extracellular calcium is almost linear (Murphy et al., 2004). In fact, the overall Ca2 is probably the best marker of glutamate release, as opposed to the overall LFP response that comprises nonsynaptic and volume-conducted components. We conclude that if vascular responses are to be generally considered well correlated to glutamate release and subsequent neuronal activation, credence should be paid to their presence or absence in particular conditions of neuronal network activation such as adaptation of any glutamatergic inputs or presynaptic inhibition. Thus, functional hyperemia and BOLD fMRI signals, under all kinds of brain activity, are not a priori read outs of local neuronal activation.
Footnotes
This work was supported by the Leducq Fundation, the Fondation Bettancourt Schueller, the Human Frontier Science Program Organization, the European Commission FP6 (LSHM-CT-2007-037765), and the Fondation pour la Recherche Médicale. We thank Jonathan Bradley and Etienne Audinat for their critical comments.
References
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Li J, Xu S, Yang G. Neural mechanisms of blood flow regulation during synaptic activity in cerebellar cortex. J Neurophysiol. 1996;75:940–950. doi: 10.1152/jn.1996.75.2.940. [DOI] [PubMed] [Google Scholar]
- Kida I, Xu F, Shulman RG, Hyder F. Mapping at glomerular resolution: fMRI of rat olfactory bulb. Magn Reson Med. 2002;48:570–576. doi: 10.1002/mrm.10248. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ronen I, Olman C, Kim SG, Ugurbil K, Toth LJ. Spatial relationship between neuronal activity and BOLD functional MRI. Neuroimage. 2004;21:876–885. doi: 10.1016/j.neuroimage.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33:839–859. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Tiret P, Charpak S. Peripheral adaptation codes for high odor concentration in glomeruli. J Neurosci. 2009;29:3067–3072. doi: 10.1523/JNEUROSCI.6187-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgören N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Isaacson JS. Presynaptic cyclic nucleotide-gated ion channels modulate neurotransmission in the mammalian olfactory bulb. Neuron. 2003;37:639–647. doi: 10.1016/s0896-6273(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci. 2004;24:3023–3030. doi: 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pírez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci. 2008;28:6360–6371. doi: 10.1523/JNEUROSCI.0793-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Charpak S. The olfactory glomerulus: a model for neuro-glio-vascular biology. Neuron. 2008;58:827–829. doi: 10.1016/j.neuron.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucinić D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]