Abstract
Animals facing conflicting sensory cues make a behavioral choice between competing alternatives through integration of the sensory cues. Here, we performed a genetic screen to identify genes important for the sensory integration of two conflicting cues, the attractive odorant diacetyl and the aversive stimulus Cu2+, and found that the membrane-bound guanylyl cyclase GCY-28 and the receptor tyrosine kinase SCD-2 regulate the behavioral choice between these alternatives in Caenorhabditis elegans. The gcy-28 mutants and scd-2 mutants show an abnormal bias in the behavioral choice between the cues, although their responses to each individual cue are similar to those in wild-type animals. Mutants in a gene encoding a cyclic nucleotide gated ion channel, cng-1, also exhibit the defect in sensory integration. Molecular genetic analyses suggested that GCY-28 and SCD-2 regulate sensory integration in AIA interneurons, where the conflicting sensory cues may converge. Genetic ablation or hyperpolarization of AIA interneurons showed nearly the same phenotype as gcy-28 or scd-2 mutants in the sensory integration, although this did not affect the sensory response to each individual cue. In gcy-28 or scd-2 mutants, activation of AIA interneurons is sufficient to restore normal sensory integration. These results suggest that the activity of AIA interneurons regulates the behavioral choice between the alternatives. We propose that GCY-28 and SCD-2 regulate sensory integration by modulating the activity of AIA interneurons.
Introduction
Multimodal sensory cues should interact properly in our brain. These kinds of interactions are important to enhance the precision of perception (Meredith, 2002). On the other hand, the interaction is required in order for animals to choose behaviors when they receive conflicting sensory signals simultaneously (Meredith, 2002). For example, copulating behavior is suppressed by the perceived risk of predation and vice versa (Svenssona et al., 2007). The behavioral choice reflects the relative preference between the contradictory sensory cues (Ishihara et al., 2002; Certel et al., 2007; Svenssona et al., 2007; Kristan, 2008); hence, the choice can be altered by an animal's status (Schall, 2001). Hunger increases the probability of food-seeking behavior, even at the cost of increased risk of becoming prey (Gillette et al., 2000). The trade-off between conflicting responses has been studied at the neural network level (Shaw and Kristan, 1997; Kristan, 2008). However, the dissection of behavioral choice through sensory integration at the molecular and cellular levels is often difficult in higher organisms because of their complex nervous systems.
Caenorhabditis elegans has a simple neural network composed of 302 neurons; the neuronal morphologies and their connections are well described (White et al., 1986), which enables us to assign functional and molecular properties to a specific neuron. For instance, sensory neurons called AWA, AWC, and ASE are involved in responses to an attractant (Bargmann et al., 1993); whereas, sensory neurons called ADL, ASH, and AWB function in the sensing and avoidance of repellents (Sambongi et al., 1999). Furthermore, calcium imaging studies have shown which neurons are activated by the presentation or removal of sensory stimuli (Suzuki et al., 2003, 2008; Kimura et al., 2004; Hilliard et al., 2005) and revealed information processing in local neural networks of C. elegans (Chalasani et al., 2007, 2010).
In a previous study, we studied the response to two conflicting sensory signals, an attractive odorant diacetyl sensed by AWA neurons (Bargmann et al., 1993) and an aversive copper ion sensed by ASH and ADL neurons (Sambongi et al., 1999). We identified a secretory protein with a low-density lipoprotein (LDL) receptor motif, HEN-1, which regulates a sensory integration process non-cell autonomously in the mature neuronal circuit (Ishihara et al., 2002). Here, we describe additional regulators and neurons required for the sensory integration. We screened for mutants defective in the sensory integration and identified gcy-28, which encodes a receptor guanylyl cyclase. The receptor tyrosine kinase SCD-2 also regulates the sensory integration, probably as a receptor for HEN-1. Interestingly, both GCY-28 and SCD-2 regulate the sensory integration in a pair of interneurons called AIA, in which the conflicting sensory cues could converge. These studies not only reveal the mechanisms modulating sensory integration at the molecular and cellular levels, but also indicate that the activity of the integrative interneurons regulate the behavioral choice in the context of sensory integration.
Materials and Methods
Strains and culture.
The strains used in this study were as follows: wild-type strain N2, gcy-28(qj4), gcy-28(tm2411), gcy-28(ky713), gcy-28(ky887), gcy-28(tm3028), hen-1(tm501), glc-3(ok321), scd-2(sa249), cng-1(jh111), cng-1(ok3292), CX9734 (gcy-28(ky713); kyEx2138 [podr-3:t01a4.1d::gfp]), CX10586 (gcy-28(ky713); kyEx2642 [pstr-2::t01a4.1d::gfp]), EG1470(oxEx229 [Mos1 Substrate, pmyo-2::gfp]), EG2762 (oxEx166 [HSP::MosTRANSPOSASE, lin-15(+), punc-122::gfp]).
All strains were cultured on NGM plates with E. coli strain OP-50 under standard conditions (Brenner, 1974). EG1470 and EG2762 were grown at 25°C. The other strains were grown at 20°C.
Screening for the mutants.
Mos1-mediated insertional mutagenesis was performed as described previously (Boulin and Bessereau, 2007) with some modifications. Wild-type worms with both oxEx229 and oxEx166 were subjected to heat shock at 35°C for 75 min. From the F1 progeny from eggs laid 12–16 h after the heat shock, 2520 F1 worms with oxEx229 were collected and allowed to lay F2 worms. The adult F2 worms were subjected to two rounds of interaction assays, using a 1:100 dilution of diacetyl and 30 mm Cu2+, to select mutants defective in the integration process. After the assays were finished, worms that did not cross the Cu2+ barrier were subjected to a chemotaxis assay to diacetyl (1:200 dilution) to exclude mutants defective in chemotaxis to diacetyl. In the chemotaxis assay, the worms that migrated to the spot of diacetyl were allowed to lay F3 worms, which were subjected to the two interaction assays and one chemotaxis assay according to the same protocol. After this set of behavioral assays was repeated a few times, animals defective in the interaction assay but not in chemotaxis were chosen as candidates for mutants defective in sensory integration. From these candidates, worms with Mos1 insertion in the genome were selected for further analysis of their ability to perform chemotaxis and sensory integration. The Mos1 insertion of mutant qj4 was located in the fourth exon of gcy-28.
Behavioral assays.
All data points are averages of more than six assays with >50 worms in each assay. The assays were performed on at least 3 different days. The asterisks indicate statistically significant differences (p < 0.01) relative to control. Statistical significance was determined by Student's t test for the comparison between wild types and mutants and for the rescue experiments.
The interaction assay between chemotaxis to diacetyl and avoidance of Cu2+ ion was performed as described previously (Ishihara et al., 2002) with some modifications. Ten adult worms were put on a 6 cm NGM plate and allowed to lay eggs for 4 h. After cultivation for 4 d, adult worms were collected using S-basal buffer (100 mm NaCl, 50 mm potassium phosphate, pH 6, 0.02% gelatin). The washed worms were placed on one side of the interaction assay plate, on which a Cu2+ ion gradient had been formed at 25°C for 18–22 h by spreading 30 μl of copper acetate solution on the midline of the assay plate. The assay was started by spotting 2 μl of diluted diacetyl on the other side of the plate (see Fig. 1A). In this assay paradigm, to reach diacetyl, animals should cross the Cu2+ barrier. After 45 min, the numbers of the worms on each side of the plate were counted. To evaluate the tendency to cross the Cu2+ barrier to reach diacetyl, the index was defined as the number of worms on the side of the diacetyl divided by the total number of the worms on the plate. In the assay, 1 μl of 1 m sodium azide was placed on the attractant area. Unless otherwise indicated, 1:100 diluted diacetyl and 30 mm copper acetate were used in the interaction assay.
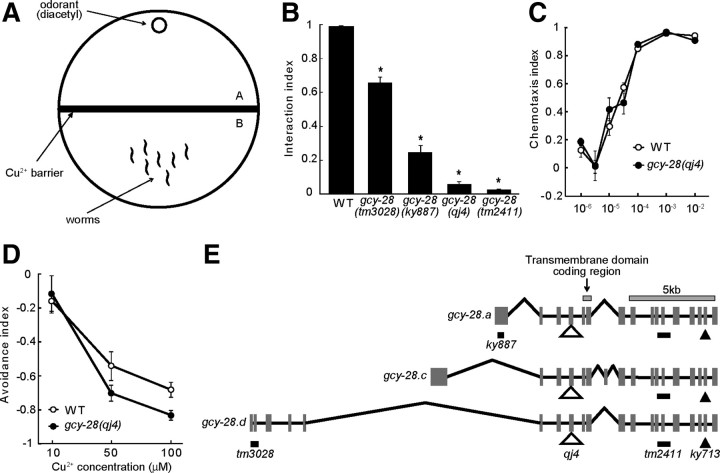
Figure 1.
gcy-28 mutants show behavioral defects in the interaction of the two sensory signals. A, Schematic diagram for an assay to test the interaction of two sensory signals, called the “interaction assay.” Worms must cross aversive Cu2+ barrier spread on the midline of the assay plate to reach the spot containing the attractive odorant diacetyl. Forty-five minutes after the start of the assay, the numbers of worms on the original side (A) and on the odorant side (B) were scored. The index was calculated as A/(A+B). B, Phenotypes of gcy-28 mutants in the interaction assay. Error bars in B–D indicate SEM. *p < 0.01. C, Chemotaxis toward various concentrations of diacetyl of gcy-28(qj4) was analyzed by the conventional chemotaxis assay. D, Avoidance of various concentrations of Cu2+ of gcy-28(qj4) was analyzed by the quadrant assay. In all points, the differences were not significant (p > 0.01). E, Gene structure and mutations of gcy-28. Three alternative splice forms (gcy-28.a, gcy-28.c, gcy-28.d) contain partly different 5′ exons encoding the extracellular domain. Black bars indicate deletions, and a filled triangle indicates a missense mutation. An open triangle indicates Mos1 insertion. ky713 missense mutation results in substitution of an amino acid in the conserved cyclase domain (Tsunozaki et al., 2008). The alleles tm3028, ky887, and tm2411 are deletions predicted to cause a frameshift. Mos1 transposon insertion (qj4) is located in the fourth exon (I:4499749..4499750) and would result in premature termination of GCY-28 protein at the extracellular domain. WT, Wild type.
Chemotaxis toward the attractive odorants was based on Bargmann et al. (1993) except that the assay plate contained 50 mm NaCl. Chemotaxis index was calculated as described previously (Bargmann et al., 1993).
Avoidance of Cu2+ ion was investigated as described previously (Wicks et al., 2000) with some modifications. The worms washed four times with S-basal buffer and once with water were placed on the center of the quadrant assay plate (50 mm NaCl, 10 mm HEPES-Na, pH 7, 1 mm MgSO4, 1 mm CaCl2, 2% agar), which was partitioned into four parts with or without Cu2+ ion. After 30 min, the numbers of worms over the repellent area and the control area were counted. The index was calculated as described previously (Wicks et al., 2000). It was defined as the number of the worms over the repellent area minus the number of the worms over the control area, divided by the total number of the worms on the plate.
Heat shock experiments.
gcy-28(qj4) mutants carrying phsp16.2::gcy-28 were cultivated at 20°C to young adulthood, and shifted to 33°C for 2 h. The worms were cultured again at 20°C for 2 h and subjected to the interaction assay.
DNA constructs and germline transformation.
gcy-28.c cDNA amplified by PCR was subcloned into the pPD49.26 vector. gcy-28.d cDNA, pkc-1(gf) cDNA, and gcy-28 promoters were as described previously (Tsunozaki et al., 2008). glc-3 cDNA was PCR amplified as described previously (Chalasani et al., 2007). For cell-specific RNA interference (RNAi), cng-1.b cDNA amplified by PCR was inserted into Invitrogen Gateway destination vector in the sense orientation or in the antisense orientation. The gcy-28.d promoter was used for the AIA-specific expression. For the expression analysis of cng-1, the 4.6 kb upstream promoter region of cng-1 gene amplified by PCR was inserted into pPD95.77 to generate pcng-1::gfp construct. For the construction of genomic scd-2::gfp fusion gene, green fluorescent protein (GFP) was fused just before the stop codon of the scd-2 genomic fragment. The genomic fragment including ∼4 kb of upstream regulatory region was cloned into pMW118. The promoters were inserted into the upstream multicloning site of each plasmid. Transgenic strains were generated by microinjecting the DNA to be tested at a concentration of 10–75 ng/μl along with the 5 ng/μl cotransformation marker pmyo-3::gfp or plin-44::gfp and 0–85 ng/μl carrier DNA, as described by Mello and Fire (1995).
The fluorescence image of adult-stage hermaphrodites harboring pcng-1::gfp, genomic scd-2::gfp, or pgcy-28.d::gcy-28.d::gfp was obtained with a LSM 510 laser-scanning confocal microscope (Zeiss) or an Axioplan 2 microscope (Zeiss).
Calcium imaging.
To generate an AWA imaging line, Cameleon YC 3.60 (Nagai et al., 2004) was expressed under the odr-10 promoter (Sengupta et al., 1996). We confirmed that the expression of Cameleon in this line did not change the chemotactic behavior in response to diacetyl. Calcium imaging was performed using the microfluidic “olfactory” chip, as described previously (Chronis et al., 2007). Briefly, an animal was immobilized by trapping in a microchannel, and the nose of the animal was exposed to a flowing stream of diacetyl-containing or odor-free solutions. Fluorescent images of the calcium indicator were obtained using a Zeiss Axioplan2 microscope equipped with 40× objective and a 3 CCD digital camera C7780 (Hamamatsu). All images were collected at 200 or 400 ms exposure time with no intervening interval, for 2–3 min total duration. Using the AquaCosmos software (version 2.6, Hamamatsu), the time stacks were analyzed for the yellow fluorescent protein (YFP) intensity/cyan fluorescent protein (CFP) intensity emission ratio in the AWA cell bodies. R/R0 was calculated as the change in the YFP/CFP ratio (R) relative to the mean basal ratio (R0) during 12 s preceding the first application of diacetyl.
Results
GCY-28 regulates the integration of two sensory signals
To investigate sensory integration, we used an interaction assay, in which an attractive odorant, diacetyl, and an aversive stimulus, Cu2+ ion, were used as conflicting sensory cues (Fig. 1A) (Ishihara et al., 2002). In this assay paradigm, animals must cross the Cu2+ barrier to reach the diacetyl; the percentage of animals that reach the diacetyl changes depending on the concentration of both Cu2+ and diacetyl. Therefore, by using this interaction assay, we can evaluate the sensory integration quantitatively (Ishihara et al., 2002). To identify genes regulating the sensory integration, we screened for mutants defective in the ability to cross the barrier in the interaction assay. Mutants were generated using the Mos1 transposon as a mutagen (Bessereau et al., 2001). The screen identified an allele, qj4, which contains Mos1 insertion in the gcy-28 gene. gcy-28 mutants crossed the copper ion barrier in the interaction assay at much lower frequency than the wild type (Fig. 1B), although it appeared healthy. This phenotype could be explained by defects in the sensory integration, weaker response to diacetyl, or enhanced response to Cu2+. To distinguish among these possibilities, we analyzed the sensitivity of gcy-28(qj4) animals to diacetyl and to Cu2+, respectively. Since gcy-28(qj4) animals are devoid of three primal alternative splicing isoforms (a, c, and d) (Fig. 1E) and show a stronger phenotype than other gcy-28 mutants, the role of all of the isoforms in the sensation of diacetyl and Cu2+ could be examined. First, we analyzed chemotaxis in response to various concentrations of diacetyl and found that the response of gcy-28(qj4) animals to diacetyl was very similar to that of wild-type animals (Fig. 1C). Next, we examined the responses to Cu2+ in wild-type animals and gcy-28(qj4) and found that the avoidance response to Cu2+ in gcy-28(qj4) animals was nearly the same as that in wild-type animals (Fig. 1D). These results suggested that gcy-28(qj4) animals have a defect in the integration of the two sensory signals.
GCY-28 acts in the mature AIA interneurons
gcy-28 encodes a receptor-type guanylyl cyclase with an extracellular ligand-binding domain, a transmembrane domain, a protein kinase-like domain, and a guanylyl cyclase domain. Previous studies showed that GCY-28 regulates odor preferences in AWC sensory neurons (Tsunozaki et al., 2008). In addition, these studies reported that three splice isoforms (a, c, and d), which share the intracellular domain but have distinct extracellular domains (Fig. 1E), are expressed in various tissues including the nervous system. Ortiz et al. (2006) analyzed the expression of gcy-28.c using a gfp fusion gene and found that gcy-28.c is broadly expressed in the nervous system. We analyzed the expression of gcy-28.c and gcy-28.d using gfp fusion genes. Although the pgcy-28.c::gcy-28.c::gfp fusion gene was expressed in many neurons, pgcy-28.d::gcy-28.d::gfp fusion gene was expressed specifically in a pair of interneurons, AIA (Fig. 2A).
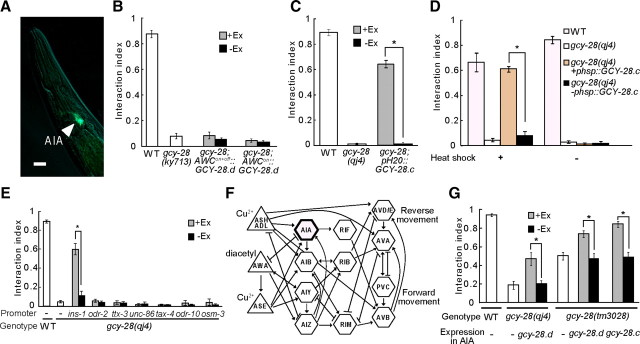
Figure 2.
GCY-28 in the AIA interneurons is important for the interaction of two signals. A, The expression of the gcy-28.d::gfp fusion gene under control of the gcy-28.d promoter at the adult stage. The site of expression is indicated by an arrowhead. Scale bar, 10 μm. B, The phenotypes of gcy-28 mutant expressing gcy-28.d cDNA in AWCON+OFF neurons or AWCON were analyzed by the interaction assay. Worms carrying the transgene (+Ex, gray columns) and worms not carrying the transgene (−Ex, black columns) were reared on the same culture plate and subjected to the assay on the same assay plate to compare the indices. White columns indicate nontransgenic worms reared on separate culture plates and subjected to the assay on separate assay plates. C–E, The phenotypes of gcy-28 mutants expressing gcy-28.c cDNA by various promoters were analyzed by the interaction assay. The expression of H20 promoter: pan-neuron. The expression of ins-1 promoter: neurons including AIA. The expression of unc-86 promoter: several neurons including AIZ. The expression of ttx-3 promoter: AIY. The expression of odr-2 promoter: neurons including AIB. The expression of tax-4, odr-10, or osm-3 promoter: sensory neurons including ADL, ASE, ASH, AWA, and AWC. gcy-28(qj4) animals carrying phsp::gcy-28.c were heat shocked at young adult stage. F, The schematic diagram for the putative neural network for the interaction of two sensory signals, extracted from the complete circuit (White et al., 1986). Sensory neurons are shown as triangles and interneurons as hexagons. Arrows indicate chemical synapses, and lines with T-shaped ends indicate gap junctions. The AIA neurons expressing GCY-28.d are emphasized. G, gcy-28 mutants expressing gcy-28 cDNA in AIA interneurons by gcy-28.d promoter were analyzed in the interaction assay. Open bars show nontransgenic animals for comparison. B–E, G, +Ex and −Ex worms were compared. Error bars in B–E and G indicate SEM. *p < 0.01. WT, Wild type.
To examine whether GCY-28 in AWC sensory neurons regulates not only odor preference but also the behavioral choice, we analyzed the behavior of the gcy-28 mutant expressing GCY-28.d in AWC in the interaction assay, because the expression of GCY-28.d in AWC can completely rescue the phenotype in the odor preference (Tsunozaki et al., 2008). gcy-28 animals carrying podr-3::gcy-28.d or pstr-2::gcy-28.d, both of which express GCY-28.d in AWCON+OFF or AWCON, respectively, showed similar phenotypes as gcy-28 animals in the interaction assay, indicating that the odor preference in AWC does not affect the sensory integration between diacetyl and Cu2+ (Fig. 2B).
To identify neurons in which GCY-28 regulates sensory integration, we analyzed the sensory integration phenotype of gcy-28 animals expressing GCY-28 in various subsets of neurons by using the interaction assay. A gcy-28.c cDNA expressed by H20 pan-neuronal promoter rescued the defect of gcy-28(qj4) in the interaction assay (Fig. 2C), suggesting that gcy-28 regulates the sensory integration in the nervous system. Moreover, the expression of gcy-28.c cDNA in adults under the control of a heat shock promoter rescued the defect of gcy-28(qj4), indicating that the function of gcy-28 at adulthood is sufficient for normal sensory integration (Fig. 2D). To further investigate where gcy-28 acts in the nervous system, we analyzed the phenotypes of gcy-28 animals expressing gcy-28.c cDNA in various sets of neurons. These analyses showed that the defect in sensory integration could not be restored by the expression of gcy-28.c cDNA in sensory neurons for diacetyl (AWA) or for Cu2+ ion (ASH, ADL) (Fig. 2E,F). These results also supported the idea that gcy-28 mutants show a defect in the integration of the two sensory signals, rather than in the perception of those signals individually. On the other hand, gcy-28 animals that expressed gcy-28.c cDNA under control of ins-1 promoter, which results in expression in multiple neurons including AIA interneurons (Tomioka et al., 2006), showed almost the same phenotype as wild-type animals in the interaction assay (Fig. 2E). These results suggested that specific neurons are involved in the sensory integration.
As GCY-28.d is mainly expressed in AIA interneurons, we analyzed the phenotypes of gcy-28(tm3028), in which gcy-28.d-specific exons are deleted and the deletion would lead to null mutation in gcy-28.d, in the interaction assay. We found that gcy-28(tm3028) showed the defect in the sensory integration (Fig. 1B). Although the defect in gcy-28(tm3028) was weaker than in the other gcy-28 mutants, these results raised a possibility that GCY-28.d in AIA interneurons is involved in the regulation of sensory integration. To examine this possibility, we investigated the behavior of gcy-28(tm3028) mutants that express gcy-28 cDNA in AIA. The expression of gcy-28.d cDNA in AIA can partially rescue the defect of gcy-28(qj4) mutants devoid of all GCY-28 isoforms, and significantly rescue that of gcy-28(tm3028) mutants devoid of the GCY-28.d isoform (Fig. 2G). Moreover, the expression of gcy-28.c cDNA in AIA also rescued the defect of gcy-28(tm3028). These results suggested that GCY-28 in AIA interneurons is important for sensory integration.
CNG-1, a cGMP-gated ion channel, regulates the sensory integration
To further investigate the molecular mechanisms of the sensory integration in AIA interneurons, we used the interaction assay to analyze the phenotype of various mutants that are defective in the cGMP signaling pathway. These experiments were motivated by the finding that the mutation ky713 in the guanylyl cyclase domain of GCY-28 caused a defect in the sensory integration (Fig. 2B). Among those mutants, cng-1, a mutant defective in a cGMP-gated ion channel, showed a defect in sensory integration. Like gcy-28 mutants, cng-1 animals showed a weaker tendency to cross the copper ion barrier than wild-type animals in the interaction assay (Fig. 3A), whereas their individual responses to diacetyl and Cu2+ are similar to that of wild-type animals (Fig. 3B,C). To determine the regulatory pathway of the sensory integration, the phenotype of gcy-28;cng-1 double-null mutant animals was analyzed. For the generation of the double mutant, gcy-28(tm3028) allele was used to investigate genetic interaction between gcy-28 in AIA and cng-1, because cng-1 may function in AIA like GCY-28.d (see next paragraph). gcy-28;cng-1 double-mutant animals showed nearly the same phenotype as cng-1 single-mutant animals (Fig. 3D). Therefore, gcy-28 and cng-1 may operate in the same genetic pathway to regulate sensory integration.
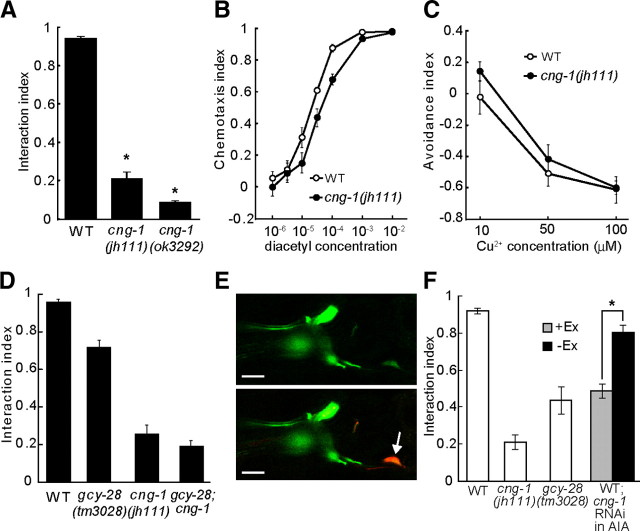
Figure 3.
gcy-28 and cng-1 regulate the interaction of two sensory signals in the same genetic pathway. A, Phenotypes of cng-1 mutants in the interaction assay. Error bars in A–D and F indicate SEM. *p < 0.01. B, Chemotaxis toward various concentrations of diacetyl of cng-1 was analyzed by the conventional chemotaxis assay. C, Avoidance of various concentrations of Cu2+ of cng-1 was analyzed by the quadrant assay. D, The genetic interaction between cng-1 and gcy-28 in the interaction assay (15 mm copper acetate was used as Cu2+ barrier). E, Confocal views of wild-type animals expressing GFP under the control of the cng-1 promoter at the adult stage. The expression of GFP was colocalized with monomeric red fluorescent protein expressed in AIA by ins-1 promoter (bottom, arrow). Scale bars, 10 μm. F, RNAi knockdown of cng-1 in AIA interneurons of wild-type animals; their behavior was analyzed in the interaction assay. Worms carrying the transgene (+Ex) and worms not carrying the transgene (−Ex) were compared. WT, Wild type.
A cng-1::gfp fusion gene is expressed in subsets of head neurons (Cho et al., 2005). To further characterize the expression pattern of CNG-1, we examined the expression pattern of the transcriptional reporter fusion. Expression was observed in subsets of head neurons including AIA interneurons (Fig. 3E), suggesting the role of CNG-1 in sensory integration within AIA interneurons. To determine whether cng-1 regulates sensory integration in AIA interneurons, we knocked down cng-1 in AIA interneurons by using cell-specific RNAi (Esposito et al., 2007). Transgenic worms expressing sense and antisense RNAs corresponding to cng-1 only in AIA crossed the Cu2+ barrier at lower frequency than wild type (Fig. 3F). The defect of the transgenic worms was comparable to that of gcy-28(tm3028) mutants, in which gcy-28.d-specific exons are deleted. These results suggested that CNG-1 in AIA interneurons is important for sensory integration like GCY-28.
HEN-1/SCD-2 pathway regulates the integration of two sensory signals in AIA interneurons in parallel to GCY-28
We previously identified HEN-1 as a regulator of sensory integration by using the interaction assay (Ishihara et al., 2002). hen-1 encodes a secretory protein with an LDLa motif similar to that of Jeb protein in Drosophila. Jeb was reported to regulate development of visceral mesodermal cells via the tyrosine kinase receptor DAlk (Lee et al., 2003). The worm genome contains only one gene for a tyrosine kinase receptor similar to DAlk, scd-2 (Reiner et al., 2008). Furthermore, hen-1 and scd-2 mutants showed nearly the same phenotype in dauer formation, suggesting that SCD-2 is a receptor for HEN-1 (Reiner et al., 2008). Therefore, we explored the function of SCD-2 in sensory integration. We found that scd-2(sa249)-null mutants tended not to cross the copper ion barrier in the interaction assay, just as with hen-1(tm501)-null mutants (Fig. 4A). The responses of scd-2 animals to diacetyl or copper ions individually were indistinguishable from those of wild-type animals (Fig. 4B,C). Next, we analyzed the phenotype of scd-2;hen-1 double-mutant animals in the interaction assay and found that scd-2;hen-1 double-mutant animals showed a phenotype similar to each of the single mutants, suggesting that hen-1 and scd-2 act in the same genetic pathway (Fig. 4A), as they do to regulate dauer formation (Reiner et al., 2008).
Figure 4.
SCD-2 in AIA interneurons is important for the interaction of two sensory signals. A, The genetic interaction between hen-1 and scd-2 in the interaction assay. Error bars in A–C, E, and F indicate SEM. B, Chemotaxis toward various concentrations of diacetyl of scd-2 was analyzed by the conventional chemotaxis assay. C, Avoidance of various concentrations of Cu2+ of scd-2 was analyzed by the quadrant assay. D, Confocal view of wild-type animal expressing the functional scd-2::gfp fusion gene under the control of its own promoter at the adult stage. The colocalization of GFP with monomeric red fluorescent protein expressed in AIA by ins-1 promoter is indicated by arrows. Scale bar, 10 μm. E, The phenotype of scd-2 mutants expressing scd-2 cDNA by gcy-28.d promoter was analyzed by the interaction assay. Worms carrying the transgene (+Ex) and worms not carrying the transgene (−Ex) were compared. *p < 0.01. F, Analysis of gcy-28;scd-2 double mutants in the interaction assay. *p < 0.01. WT, Wild type.
To elucidate the expression pattern of SCD-2, we analyzed the expression pattern of the functional gfp fusion gene and found that SCD-2 was expressed in many neurons including AIA interneurons (Fig. 4D). To determine whether the expression of SCD-2 in AIA is sufficient for the proper sensory integration, we analyzed scd-2 mutant animals expressing scd-2 cDNA under the control of the gcy-28.d promoter and found that the expression of SCD-2 in AIA significantly restored the phenotype of scd-2 in the interaction assay (Fig. 4E). On the other hand, expression of this gene in other neurons was unable to restore the phenotype (data not shown). These results suggest that SCD-2 in AIA interneurons is important for the regulation of sensory integration behavior.
As both gcy-28 and scd-2 function in AIA interneurons, these molecules could act in the same genetic pathway. To examine this possibility, we constructed the gcy-28(tm3028);scd-2(sa249) double-null mutant and analyzed its behavior in the interaction assay. gcy-28;scd-2 double-mutant animals showed a more severe defect than single-mutant animals in the interaction assay, suggesting that GCY-28 and SCD-2 function in distinct pathways (Fig. 4F).
AIA interneurons are necessary and sufficient for sensory integration
To determine the role of the AIA interneurons in the sensory integration, we analyzed the behavior of worms lacking AIA interneurons in the interaction assay. The loss of AIA interneurons would cause a behavioral change in the interaction assay. To disrupt AIA interneurons, we used a hyperactive form of MEC-4, called MEC-4(d). MEC-4 is a DEG/ENaC sodium channel, and the expression of MEC-4(d) in neurons causes neuronal toxicity in C. elegans (Harbinder et al., 1997). Most worms expressing MEC-4(d) exhibited degenerating AIA neurons (25/32) and did not cross Cu2+ barrier as much as wild-type animals in the interaction assay (Fig. 5A), whereas those animals show normal responses to the each individual cue (Fig. 5B,C). These results suggested that AIA interneurons are required for the proper regulation of the sensory integration.
Figure 5.
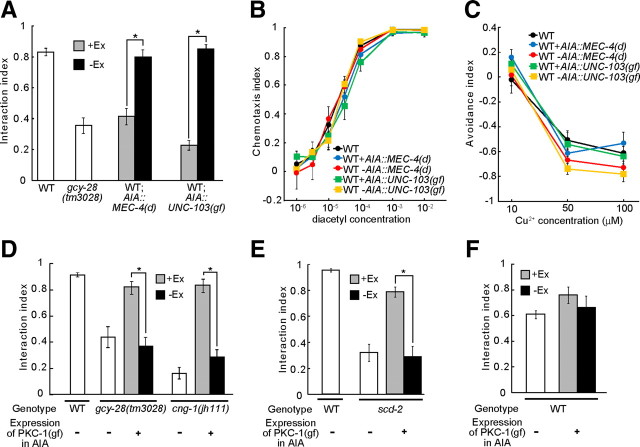
The AIA interneurons regulate sensory integration. A, The functions of AIA interneurons were inhibited by the expression of MEC-4(d) or constitutively active UNC-103 in wild-type animals; their behavior was analyzed in the interaction assay. B, Chemotaxis toward various concentrations of diacetyl of worms expressing MEC-4(d) or UNC-103(gf) in AIA was analyzed by the conventional chemotaxis assay. C, Avoidance of various concentrations of Cu2+ of worms expressing MEC-4(d) or UNC-103(gf) in AIA was analyzed by the quadrant assay. All points showed no significant differences from wild type (p > 0.01). D, E, The expression of constitutively active PKC-1 in AIA interneurons could rescue the defects of gcy-28 mutants, scd-2 mutants, and cng-1 mutants in the interaction assay. F, The effect of the expression of constitutively active PKC-1 in AIA interneurons on the phenotype of wild-type worms in the interaction assay (100 mm copper acetate was used as Cu2+ barrier). A–F, Worms carrying the transgene (+Ex) and worms not carrying the transgene (−Ex) were compared. Error bars in A–F indicate SEM. *p < 0.01. WT, Wild type.
We next examined whether inactivation of AIA interneurons affects the sensory integration behavior. To this end, we used the expression of UNC-103[gain of function (gf)], a constitutively active form of the ERG-like potassium channel UNC-103, which could hyperpolarize the neurons and reduce their ability to transmit signals (Reiner et al., 2006). Inactivation of the AIA interneurons by expressing UNC-103(gf) in AIA caused a defect in the sensory integration; animals expressing UNC-103(gf) in AIA neurons showed a weaker tendency to cross the Cu2+ barrier like gcy-28 animals (Fig. 5A), although the responses to each individual cue in these animals are indistinguishable from those of wild-type animals (Fig. 5B,C). These results suggested that the activity of AIA is important for proper regulation of the sensory integration and does not affect the sensory responses.
We then asked whether the activity of the AIA interneurons is important for the regulation of the sensory integration. The expression of constitutively active protein kinase C [PKC-1(gf)], which may positively regulate synaptic transmission and neuropeptide release (Sieburth et al., 2005, 2007), can activate neurons in C. elegans (Okochi et al., 2005; Macosko et al., 2009). We found that the expression of PKC-1(gf) in AIA interneurons is sufficient to restore the phenotype of gcy-28 animals and cng-1 animals in the interaction assay (Fig. 5D). Furthermore, the expression of PKC-1(gf) can also restore the phenotype of scd-2 (Fig. 5E). These results suggested that activation of synaptic release by AIA interneurons can bypass regulatory defects in sensory integration. On the other hand, the expression of PKC-1(gf) did not affect the behavior of wild-type worms in the interaction assay (Fig. 5F). Therefore, the expression of PKC-1(gf) may activate functions of the AIA interneurons and, hence, can restore the phenotypes. This implicates the possibility that activation of the AIA interneurons induces animals to cross the Cu2+ barrier to reach diacetyl.
The activity of AIA interneurons might be regulated by the balance between two conflicting sensory cues, diacetyl and Cu2+
The signals for the two conflicting sensory cues used in the interaction assay could converge in AIA interneurons. AIA interneurons connect with the AWA sensory neurons, which are involved in the sensation of diacetyl (Bargmann et al., 1993), via gap junctions; in contrast, AIA interneurons receive chemical synapses from the ASH/ADL sensory neurons, which are involved in the sensation of the Cu2+ ion (Fig. 2F) (White et al., 1986; Sambongi et al., 1999).
Previous studies indicated that the AWA neurons express the receptor for diacetyl, ODR-10 (Sengupta et al., 1996; Dwyer et al., 1998), but the response of the AWA sensory neurons remains unknown. To study this, we used the genetically encoded calcium indicator Cameleon YC3.60, which changes the ratio of YFP/CFP fluorescence upon calcium binding (Miyawaki et al., 1997). We found that intracellular calcium concentration in AWA neurons is transiently increased by stimulation with diacetyl (Fig. 6A). This result suggests that the AIA interneurons could be activated through gap junctions between AIA and AWA upon exposure to diacetyl.
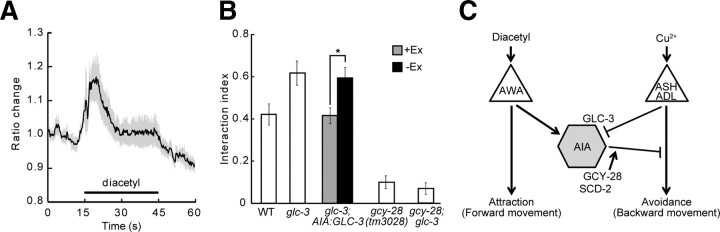
Figure 6.
AIA interneurons receive excitatory and inhibitory inputs from distinct sensory neurons. A, Calcium responses of AWA sensory neurons to diacetyl measured by the Ca2+-sensitive fluorescent indicator Cameleon in wild-type animals. The black line indicates the average trace, and the shaded areas around the trace represent SEM (n = 3 animals). The black bar indicates the presence of diacetyl. B, The effect of mutations in glutamate-gated chloride channel GLC-3 on the behavior of wild-type animals and gcy-28 mutants in the interaction assay (100 mm copper acetate was used as Cu2+ barrier). Worms carrying the transgene (+Ex) and worms not carrying the transgene (−Ex) were compared. Error bars indicate SEM. *p < 0.01. C, A neuronal circuit model for the interaction of two sensory signals. Sensory neurons are shown as triangles, and interneurons, as hexagons. Arrows indicate excitatory connections, and lines with T-shaped ends indicate inhibitory connections. The activity of AIA interneurons would be regulated by the balance between excitatory inputs from AWA through gap junction and inhibitory inputs from ADL/ASH through GLC-3. Activated AIA interneurons would increase the relative strength of the signals for diacetyl by inhibiting the signals for Cu2+. GCY-28 and SCD-2 may enhance the inhibitory synaptic outputs from AIA.
In ASH neurons, intracellular calcium ion increases as a function of stimulation by Cu2+ (Hilliard et al., 2005), but the synaptic property between the ASH neurons and the AIA interneurons has been unknown. To examine the property, as ASH sensory neurons are known to be glutamatergic, and the l-glutamate-gated chloride 3 (GLC-3) channel acts in the AIA interneurons (Chalasani et al., 2010), we analyzed the phenotype of the glc-3 mutant. glc-3 mutants crossed the barrier more frequently than wild-type animals (Fig. 6B). Furthermore, animals expressing GLC-3 in AIA interneurons could rescue the phenotype of the glc-3 mutant (Fig. 6B). These results suggested that GLC-3 mediates the inhibitory synaptic inputs from the ASH sensory neurons to the AIA interneurons. In contrast, loss of glc-3 function in the gcy-28 mutant did not cause the worms to cross more frequently than the gcy-28 mutant (Fig. 6B), suggesting that GCY-28 acts downstream of GLC-3 in the sensory integration. This result is consistent with the idea that GCY-28 regulates the neuronal activity of AIA interneurons. In conclusion, the AIA interneurons could regulate the neuronal activity and the synaptic output, depending on the balance between the signals from the AWA sensory neurons and those from the ASH sensory neurons (Fig. 6C).
Discussion
Regulators of the sensory integration
Sensory integration is a simple way of processing sensory information, in which two sensory inputs are processed into one output signal. Proper sensory integration is important for proper behavioral choices. To elucidate the mechanisms of sensory integration at the molecular and neuronal levels, we studied the behavior of C. elegans exposed to both an attractive odorant, diacetyl, and an aversive stimulus, Cu2+ ion. Molecular genetic analyses of the sensory integration revealed that two genetically distinct pathways modify the integration of the conflicting sensory cues, the GCY-28/CNG-1 pathway and HEN-1/SCD-2 pathway. We also found that GCY-28.d and SCD-2 function in a pair of interneurons, AIA, in which sensory signals from AWA sensory neurons (responding to diacetyl) and from ADL/ASH sensory neurons (responding to Cu2+ ion) converge. Our results suggested that the neuronal activity of AIA interneurons regulates sensory integration to generate the appropriate behavioral choice.
GCY-28 may modulate a neuronal function in the nervous system. Most of the 34 guanylyl cyclases identified in C. elegans are expressed in sensory neurons, and are thought to function as chemoreceptors or downstream signaling molecules in the sensing of substances in the environment (Inada et al., 2006; Ortiz et al., 2006, 2009; Zimmer et al., 2009). However, GCY-28 functions as a sensory regulator but not as a sensory receptor in AWC sensory neurons (Tsunozaki et al., 2008). gcy-28 encodes the guanylyl cyclase that is most similar to natriuretic peptide receptor-A (NPR-A) and -B. In mammals, NPR-A and -B modulate neurotransmission as receptors for natriuretic peptides, which are synthesized and secreted depending on the context (Herring and Paterson, 2009). We found that GCY-28 is expressed and functions in interneurons, where it may regulate sensory integration by binding an unknown ligand. Domain-swap experiments also suggest that the guanylyl cyclase of GCY-28 can be activated by endogenous peptides (Baude et al., 1997). While there are no obvious homologs of natriuretic peptides in the C. elegans genome, an unknown ligand secreted in specific contexts may enable worms to change their behavioral choices on the basis of previous experience and current status.
scd-2 encodes the C. elegans homolog of an anaplastic lymphoma kinase (ALK). Mammalian ALK has been postulated to function in the nervous system because of its expression in the adult brain (Iwahara et al., 1997; Bilsland et al., 2008; Palmer et al., 2009). A putative ligand for ALK plays a role in synaptic plasticity (Amet et al., 2001; Pavlov et al., 2006). In C. elegans, the HEN-1/SCD-2 pathway modulates dauer larva formation, which is induced by harsh conditions such as high temperature, food deprivation, and crowding (Reiner et al., 2008). Food supply reduces the frequency of dauer formation induced by high population density of worms (Golden and Riddle, 1984). The entry into dauer larva stage happens under conditions of a low ratio of food to population density, implying that worms integrate environmental stimuli in the nervous system to choose their developmental fate. As the HEN-1/SCD-2 pathway regulates the sensory integration for the behavioral choice, it also might participate in the regulation of the developmental choice between forming a dauer or an L3 larva.
GCY-28 and SCD-2 are expressed in many neurons. Our results suggested that GCY-28 and SCD-2 regulate integrative behavior by modulating the activity of AIA. Killing AIA interneurons caused a defect not only in integration but also in salt chemotaxis learning (Tomioka et al., 2006), suggesting that AIA interneurons are important for behavioral plasticity. However, it is likely that GCY-28 and SCD-2 also modulate the function of other interneurons that integrate sensory signals other than Cu2+ and diacetyl. Upon thermotaxis, opposing cryophilic and thermophilic driving signals are integrated in RIA interneurons (Mori et al., 2007). Mutations in gcy-28 or hen-1 encoding a ligand for SCD-2 individually cause a defect in food-associated thermotactic behavioral plasticity (Ishihara et al., 2002; Kodama et al., 2006; Tsunozaki et al., 2008), consistent with an important role for GCY-28 and SCD-2 in modulating neural circuits involved in integrative behaviors.
AIA interneurons integrate multiple sensory cues to adjust behavioral choices
Our results suggested that AIA interneurons are important for proper sensory integration. Because these interneurons receive synaptic inputs from multiple sensory neurons, the AIA interneurons can act as an integrator for such inputs. AWA sensory neurons, which are activated by diacetyl, make gap junctions with AIA interneurons (White et al., 1986); hence, AIA interneurons could be activated by diacetyl. On the other hand, ASH and ADL sensory neurons, which are activated by Cu2+ (Sambongi et al., 1999; Hilliard et al., 2005), may inhibit AIA interneurons through the glutamate-gated chloride channel GLC-3. We propose that, depending on the balance of these conflicting signals from AWA and ASH/ADL, the synaptic transmission from AIA is regulated by the HEN-1/SCD-2 pathway and by GCY-28 pathway for the proper behavioral choice. In gcy-28 mutants, the activity of AIA neurons tends to be lower than that in wild-type animals, thereby decreasing synaptic transmission. Therefore, the mutants may behave in the interaction assay as if the worms sensed a higher ratio of Cu2+ to diacetyl than wild-type animals (Fig. 6C). In mammals, cyclic nucleotide-gated ion channels regulate the synaptic transmission and the excitability of olfactory receptor neurons (ORNs) (Murphy and Isaacson, 2003). By the expression of a constitutively active form of PKC-1, the activation of AIA rescued the defects of gcy-28 mutants and cng-1 mutants in the sensory integration. This observation is consistent with the hypothesis that GCY-28 activates AIA interneurons to facilitate the synaptic transmission.
The neural circuit for the sensory integration
AIA interneurons, in which GCY-28/CNG-1 and HEN-1/SCD-2 regulate the sensory integration, are amphid interneurons. How do AIA interneurons regulate the sensory integration? In the interaction assay, the percentages of animals that reach the diacetyl change depend on the concentration of both Cu2+ and diacetyl (Ishihara et al., 2002), suggesting that the signals for the two conflicting cues suppress each other. Here, we demonstrated that gcy-28 and scd-2 mutants showed normal responses to Cu2+ or diacetyl individually. Furthermore, the genetic ablation or inactivation of AIA interneurons of wild-type animals caused behavior similar to gcy-28 or scd-2 mutants in the interaction assay, although this did not affect the sensory responses to each individual cue. Therefore, we propose that the AIA interneurons, where GCY-28 and SCD-2 modulate activity, are important for mutual inhibition of conflicting cues, but not for transduction of each sensory cue.
Our results suggested that the AIA interneurons are important to regulate the behavioral choice between crossing the Cu2+ barrier to reach diacetyl and not crossing the barrier (Fig. 5). Although AIA interneurons send synaptic outputs onto many neurons, the major synaptic target of AIA is AIB interneurons (White et al., 1986), which regulate reversal movement; removal of odors sensed by AWC neurons activates AIB interneurons and induces a reversal movement (Chalasani et al., 2007). On the other hand, the presentation of such odors causes activation of AIA interneurons and forward movement (Chalasani et al., 2010). Therefore, AIA and AIB exhibit opposite functions on behavior, suggesting that AIA sends inhibitory synapses onto AIB. Inhibitory connectivity from AIA to AIB is also predicted by the laser ablation studies (Wakabayashi et al., 2004). AIB interneurons receive synaptic inputs from ASH and ADL neurons that sense aversive chemicals (White et al., 1986), suggesting that AIB transmits aversive signals. Activated AIA interneurons may induce worms to cross the Cu2+ barrier to reach diacetyl by inhibiting AIB interneurons. Alternatively, AIA interneurons may release neuropeptides to modulate the neuronal circuit, as AWC sensory neurons release NLP-1 (Chalasani et al., 2010).
Interneurons play an important role in the processing of sensory information. In vertebrates, amacrine cells in the retina receive excitatory inputs from bipolar cells and mutually inhibit other bipolar cells (Cook and McReynolds, 1998). Furthermore, in Drosophila, interneurons receive many inputs from ORNs and make synapses onto the presynaptic terminals of ORNs, playing the role of gain control in the modulation of sensory sensitivity (Olsen and Wilson, 2008). These interneurons allow lateral interaction among signals sensed by distinct neurons, like AIA interneurons do in the sensory integration. Mammalian NPRs and their ligands are expressed in the layers of retina, including the amacrine cells (Blute et al., 2000; Abdelalim et al., 2008). The function of NPR-A and -B in these neurons could be analogous to that of GCY-28 in C. elegans.
In the future, if electrophysiological analyses can be applied in this system, we can understand more precise mechanisms of the sensory integration. Further analyses on the relationship between the neuronal properties of AIA interneurons and molecules that regulate the sensory integration may shed light on the fundamental neural mechanisms of sensory integration for behavioral choice in invertebrates and decision making in vertebrates.
Footnotes
This work was supported by a Grant-in-Aid for Scientific Research (T.I. and Y.I.), by Asahi Glass Foundation (T.I.), and by the Naito Foundation (T.I.). We thank A. Miyawaki, L. Garcia, M. Driscoll, and A. Fire for YC3.60, and unc-103(gf) cDNA, mec-4(d) cDNA, and plasmids, respectively. We also thank S. Mitani for gcy-28 strains, D. Reiner for scd-2 strains and DNAs, the Caenorhabditis Genetic Center for strains, and Y. Kohara for gcy-28 cDNA.
References
- Abdelalim EM, Masuda C, Tooyama I. Expression of natriuretic peptide-activated guanylate cyclases by cholinergic and dopaminergic amacrine cells of the rat retina. Peptides. 2008;29:622–628. doi: 10.1016/j.peptides.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Amet LE, Lauri SE, Hienola A, Croll SD, Lu Y, Levorse JM, Prabhakaran B, Taira T, Rauvala H, Vogt TF. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol Cell Neurosci. 2001;17:1014–1024. doi: 10.1006/mcne.2001.0998. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Baude EJ, Arora VK, Yu S, Garbers DL, Wedel BJ. The cloning of a Caenorhabditis elegans guanylyl cyclase and the construction of a ligand-sensitive mammalian/nematode chimeric receptor. J Biol Chem. 1997;272:16035–16039. doi: 10.1074/jbc.272.25.16035. [DOI] [PubMed] [Google Scholar]
- Bessereau JL, Wright A, Williams DC, Schuske K, Davis MW, Jorgensen EM. Mobilization of a Drosophila transposon in the Caenorhabditis elegans germ line. Nature. 2001;413:70–74. doi: 10.1038/35092567. [DOI] [PubMed] [Google Scholar]
- Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, Thakur M, Beaumont V, Bonnert TP, Heavens R, Whiting P, McAllister G, Munoz-Sanjuan I. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- Blute TA, Lee HK, Huffmaster T, Haverkamp S, Eldred WD. Localization of natriuretic peptides and their activation of particulate guanylate cyclase and nitric oxide synthase in the retina. J Comp Neurol. 2000;424:689–700. [PubMed] [Google Scholar]
- Boulin T, Bessereau JL. Mos1-mediated insertional mutagenesis in Caenorhabditis elegans. Nat Protoc. 2007;2:1276–1287. doi: 10.1038/nprot.2007.192. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel SJ, Savella MG, Schlegel DC, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci U S A. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Cho JH, Song HO, Park CS. Identification and characterization of a putative cyclic nucleotide-gated channel, CNG-1, in C. elegans. Mol Cells. 2005;19:149–154. [PubMed] [Google Scholar]
- Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci. 1998;1:714–719. doi: 10.1038/3714. [DOI] [PubMed] [Google Scholar]
- Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–466. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, Bazzicalupo P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene. 2007;395:170–176. doi: 10.1016/j.gene.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci U S A. 2000;97:3585–3590. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1997;94:13128–13133. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell. 2002;109:639–649. doi: 10.1016/s0092-8674(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Miyawaki A, Matsumoto K, Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14:1291–1295. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, Iino Y, Mori I. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20:2955–2960. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan WB. Neuronal decision-making circuits. Curr Biol. 2008;18:R928–R932. doi: 10.1016/j.cub.2008.07.081. [DOI] [PubMed] [Google Scholar]
- Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425:507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Meredith MA. On the neuronal basis for multisensory convergence: a brief overview. Brain Res Cogn Brain Res. 2002;14:31–40. doi: 10.1016/s0926-6410(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mori I, Sasakura H, Kuhara A. Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol. 2007;17:712–719. doi: 10.1016/j.conb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Isaacson JS. Presynaptic cyclic nucleotide-gated ion channels modulate neurotransmission in the mammalian olfactory bulb. Neuron. 2003;37:639–647. doi: 10.1016/s0896-6273(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–2137. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz CO, Etchberger JF, Posy SL, Frøkjaer-Jensen C, Lockery S, Honig B, Hobert O. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz CO, Faumont S, Takayama J, Ahmed HK, Goldsmith AD, Pocock R, McCormick KE, Kunimoto H, Iino Y, Lockery S, Hobert O. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Rauvala H, Taira T. Enhanced hippocampal GABAergic inhibition in mice overexpressing heparin-binding growth-associated molecule. Neuroscience. 2006;139:505–511. doi: 10.1016/j.neuroscience.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Tian H, Thomas JH, Nishiwaki K, Miwa J, Gruninger T, Leboeuf B, Garcia LR. Behavioral genetics of Caenorhabditis elegans unc-103-encoded erg-like K+ channel. J Neurogenet. 2006;20:41–66. doi: 10.1080/01677060600788826. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Ailion M, Thomas JH, Meyer BJ. C. elegans anaplastic lymphoma kinase ortholog SCD-2 controls dauer formation by modulating TGF-beta signaling. Curr Biol. 2008;18:1101–1109. doi: 10.1016/j.cub.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999;10:753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Shaw BK, Kristan WB., Jr The neuronal basis of the behavioral choice between swimming and shortening in the leech: control is not selectively exercised at higher circuit levels. J Neurosci. 1997;17:786–795. doi: 10.1523/JNEUROSCI.17-02-00786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Ch'ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, Ruvkun G, Kaplan JM. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kerr R, Bianchi L, Frøkjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenssona GP, Löfstedt C, Skals N. Listening in pheromone plumes: disruption of olfactory-guided mate attraction in a moth by a bat-like ultrasound. J Insect Sci. 2007;7:59. doi: 10.1673/031.007.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron. 2006;51:613–625. doi: 10.1016/j.neuron.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tsunozaki M, Chalasani SH, Bargmann CI. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–971. doi: 10.1016/j.neuron.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, Kitagawa I, Shingai R. Neurons regulating the duration of forward locomotion in Caenorhabditis elegans. Neurosci Res. 2004;50:103–111. doi: 10.1016/j.neures.2004.06.005. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HG, Plasterk RH. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, Marletta MA, Hudson ML, Morton DB, Chronis N, Bargmann CI. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]