Abstract
Presynaptic compartments are formed through the recruitment of preassembled clusters of proteins to points of cell—cell contact, however, the molecular mechanism(s) underlying this process remains unclear. We demonstrate that clusters of polymerized actin can recruit and maintain synaptic vesicles to discrete sites along the axon, and that cadherin/β-catenin/scribble/β-pix complexes play an important role in this event. Previous work has demonstrated that β-catenin and scribble are important for the clustering of vesicles at synapses. We demonstrate that β-pix, a Rac/Cdc42 guanine nucleotide exchange factor (GEF), forms a complex with cadherin, β-catenin, and scribble at synapses and enhances localized actin polymerization in rat hippocampal neurons. In cells expressing β-pix siRNA or dominant-negative β-pix that lacks its GEF activity, actin polymerization at synapses is dramatically reduced, and synaptic vesicle localization is disrupted. This β-pix phenotype can be rescued by cortactin overexpression, suggesting that β-pix-mediated actin polymerization at synapses regulates vesicle localization.
Introduction
Synapse formation begins with the formation of incipient contacts and culminates with the recruitment of synaptic proteins to points of contact. Presynaptic proteins are transported in preassembled clusters along axons in at least two types of transport packets; large dense-core vesicles containing active zone components, and synaptic vesicle transport packets, pleiomorphic vesicles containing synaptic vesicle-associated proteins as well as proteins critical for exocytosis and endocytosis (McAllister, 2007). It is still unclear what signals mediate the accumulation of these transport packets at developing synapses.
Recent work has resurrected the concept that artificial cell contact is sufficient to induce the clustering of synaptic vesicles (SVs) and that actin polymerization is required in this process. Indeed, vesicles accumulate at points of contact between axons and beads coated with poly-d-lysine, poly-l-lysine, or growth factors (Burry and Hayes, 1986; Burry et al., 1986; Lee and Peng, 2006; Lucido et al., 2009). Actin polymerization at contact points precedes vesicle clustering, and treatment with latrunculin A abolishes the accumulation of vesicles at discrete sites along the axon (Kuromi and Kidokoro, 1998; Zhang and Benson, 2001; Lee and Peng, 2006; Lucido et al., 2009). This suggests, but does not directly demonstrate, a role for actin in SV clustering. Indeed, although gross actin depolymerization disrupts vesicle clustering, this can be attributed to a variety of factors including the weakening of strong cell—cell adhesion. It also remains unclear what signals enhance the preferential polymerization of actin at sites of cell—cell contact.
The cadherin/β-catenin adhesion complex has been shown to play an important role in clustering SVs at synapses (Iwai et al., 2002; Togashi et al., 2002; Bamji et al., 2003, 2006; Lee et al., 2008). Perturbation of intercellular cadherin interactions (Togashi et al., 2002) or ablation of β-catenin (Bamji et al., 2003) dramatically impairs the accumulation of SVs at contact sites. We have recently demonstrated that cadherin/β-catenin complexes localize SVs to contact sites by recruiting the PDZ protein, scribble, to developing synapses, and that the mislocalization of SVs is phenocopied in scribble knockdown cells (Sun et al., 2009).
In this study, we identify a molecular pathway through which cell—cell contact can translate to the recruitment and localization of SVs to incipient synapses. We first demonstrate a positive role for actin in clustering SVs at synapses. We next demonstrate that β-pix, a Rac/Cdc42-specific guanine nucleotide exchange factor (GEF), can enhance actin polymerization at synapses and can recruit SVs to synapses. Knockdown of β-pix results in the mislocalization of SVs along the axon, which can be rescued by enhancing actin polymerization through cortactin overexpression. This directly implicates actin in mediating the effects of β-pix on SV clustering. Finally, we show that β-pix forms a complex with cadherin, β-catenin, and scribble at synapses, and that scribble is important for the localization of β-pix at synapses. Together, our data suggest that cadherin/β-catenin/scribble complexes recruit β-pix to sites of cell—cell contact, and that this enhances the local polymerization of actin, which can “trap” SVs as they translocate along the axon.
Materials and Methods
Recombinant DNAs and siRNAs
To suppress expression of endogenous scribble, two short hairpin RNAs (shRNAs) corresponding to mouse scribble (GenBank accession no. NM_134089) nucleotides 3396–3416 (shRNA1) and 1280–1300 (shRNA2) were transiently transfected into mouse hippocampal neurons (Sun et al., 2009). An shRNA specifically against human scribble (GenBank accession no. NM_015356) nucleotides 1842–1862 was used as a control (shRNA control) (Dow et al., 2007; Sun et al., 2009). To suppress expression of endogenous β-pix, three interfering RNA oligonucleotides against rat β-pix (Invitrogen) were transiently transfected into rat hippocampal neurons. Sequences of siRNA-1 and siRNA-2 correspond to rat β-pix (GenBank accession no. NM_053740) nucleotides 21–45 and 1779–1803, respectively. A mixture of siRNA-1 and siRNA-2 (siRNA-M) was further used as a control for off-target effects. siRNA-negative control duplexes (siRNA-C) were purchased from Invitrogen. To knockdown expression of N-cadherin, we transfected neurons with a previously published N-cadherin siRNA (N-cad siRNA) (Aiga et al., 2011). As controls, cells were transfected with scrambled N-cadherin siRNA (N-cad siRNA-C), or cotransfected with N-cad siRNA plus siRNA-resistant N-cadherin-CFP (N-cad-CFP) (Aiga et al., 2011).
GFP-actin, GFP-β-pix, HA-β-pix, dominant-negative β-pix (DN-β-pix), HA-tagged cortactin (Cort-HA), synaptophysin-RFP (Syn-RFP), synaptophysin-GFP (Syn-GFP), and the calponin homology domain of utrophin tagged with RFP (UtrCH-RFP) constructs were kind gifts from Michael Colicos (University of Calgary, Calgary, Canada), Eunjoon Kim (Korea Advanced Institute of Science and Technology, South Korea), Rick Horwitz (University of Virginia, Charlottesville, VA), Lorraine Santy (Penn State, University Park, PA), Tim O'Connor (University of British Columbia, Vancouver, Canada), Louis Reichardt (University of California, San Francisco, San Francisco, CA), Tadashi Nakata (RIKEN Institute of Physical and Chemical Research, Saitama, Japan), and William M. Bement (University of Wisconsin, Madison, WI), respectively.
Neuron cultures
Hippocampi from embryonic day 18 (E18) rat and mouse of either sex were prepared as previously described (Xie et al., 2000) and plated at a density of 130 cells/mm2 and 170 cells/mm2, respectively. Neurons were transfected using Lipofectamine 2000 (Invitrogen) at 6–8 DIV according to manufacturer's recommendations and imaged at 8–10 DIV. Neurons were treated with 20 μm ALLN (Calbiochem) for 24 h at 7 DIV.
Immunohistochemistry
Neuron cultures were fixed in 4% paraformaldehyde/4% sucrose for 10 min, permeabilized in 0.1% Triton-X for 10 min, and blocked in 10% goat serum for 1 h at room temperature. Primary antibodies were diluted in 1% goat serum overnight at 4°C and secondary antibodies were diluted in 1% goat serum for 1 h at room temperature. Primary antibodies were as follows: rabbit anti-synaptophysin (Abcam), mouse anti-PSD-95 (Affinity BioReagents), guinea pig anti-bassoon (Synaptic Systems), rabbit anti-bassoon (Synaptic Systems), mouse anti-HA (Cedarlane Laboratories), and rabbit anti-β-pix (Santa Cruz Biotechnology). Secondary antibodies were as follows: Alexa 488, Alexa 633, Cy5 and Texas Red-conjugated goat anti-mouse, anti-rabbit or anti-guinea pig (Invitrogen).
FM 4-64 analyses
FM 4-64 experiments were done as previously described (Gerrow et al., 2006). Briefly, 15 μm FM 4-64 (Invitrogen) was loaded for 30 s into presynaptic terminals using a hyperkalemic solution of 90 mm KCl in modified HBSS, where equimolar NaCl was omitted for final osmolarity of 310 mOsm. Neurons were rinsed three times and maintained in HBSS without Ca2+ for imaging. ADVESAP-7 (1 mm; Sigma) was added to quench nonspecific signals. Three images were captured every 30 s to confirm that the positive FM 4-64 sites were stationary presynaptic terminals. Unloading was done using the hyperkalemic solution described above and neurons were rinsed three times with NeuroBasal media for continued imaging.
Immunoblot analysis
Preparation of protein lysates.
Brain tissues or cultured cells were homogenized in ∼4 vols (w/v) of lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1.0% NP-40, and 10% glycerol) and centrifuged at 14,000 × g for 30 min at 4°C.
Crude synaptosomal fraction.
Crude synaptosomal fraction (P2) fractions were prepared as previously described with minor modifications (Becher et al., 1999; Oh et al., 2010). Briefly, adult rat brains were homogenized in ice-cold buffer (320 mm sucrose, 4 mm HEPES, and 1 mm EGTA) using a homogenizer (Canadian Laboratory Supplies), six strokes at 2300 rpm. The homogenate was centrifuged at 1312 × g for 10 min to remove nuclei and cell debris. The resulting supernatant was centrifuged at 14,481 × g for 15 min to remove small cell fragments and total soluble proteins. The resulting pellet was resuspended in homogenization buffer and centrifuged at 17,522 × g for 15 min to obtain the crude synaptosomal fraction, P2. P2 was resuspended in the lysis buffer described above and used for immunoprecipitation assays.
Immunoprecipitation.
P2 synaptosomal preparations were incubated overnight at 4°C with either anti-β-catenin (Santa Cruz Biotechnology), anti-scribble (Santa Cruz Biotechnology), or anti-β-pix or preimmune serum. Protein A/G–Sepharose (50 μl; GE Healthcare) was added to the synaptosomal fractions, and the bead-bound immunocomplexes were recovered after 2 h, washed four times with lysis buffer, solubilized with loading buffer, separated by SDS—PAGE, and analyzed by immunoblotting with antibodies against cadherin, β-catenin, scribble, β-pix, or synaptophysin.
Western blot analysis.
Proteins were visualized using enhanced chemiluminescence (Pierce Biotechnology) on a Bio-Rad Versadoc 4000 (Bio-Rad Laboratories). The brightness and contrast of entire images was moderately adjusted using Photoshop (Adobe Systems) after recommended, scientifically acceptable procedures, and no information was obscured or eliminated from the original (Rossner and Yamada, 2004).
Confocal imaging.
Neurons were imaged using an Olympus Fluoview 1000 confocal microscope (60×/1.4 Oil Plan-Apochromat). For time-lapse imaging, neurons were imaged every 12 s for 10 min. To avoid bias, all transfected neurons (except analysis of GFP-β-pix) on each coverslip were imaged and quantified blindly to conditions. All images in a given experiment were captured and analyzed with the same exposure time and conditions. To analyze the localization of GFP-β-pix, weakly transfected cells exhibiting a comparable pattern of expression to endogenous β-pix were chosen blind to the conditions being analyzed. Image acquisition was optimized based on this cell and every transfected cell in the dish was imaged using the same parameters. All cells that exhibited areas of saturation in the look up tables were discarded from the analysis.
Image analysis and quantification
Density analyses.
To determine the density of puncta along axons, axon length was measured using ImageJ and density expressed as the average number of puncta per 100 μm of axon length.
Analysis of puncta area and Integrated Density.
Images were thresholded using ImageJ based on a subjective evaluation of “real” clusters compared with background noise. Once the threshold was set for a given experiment, the same threshold was used throughout the analyses. Puncta area and Integrated Density (IntDen; the product of an area and the average gray value within that area) were then determined using ImageJ. To examine the IntDen at synapses, puncta associated with synaptic markers were identified, and the IntDen measured using ImageJ.
Integrated Density of Syn-GFP fluorescence at synapses.
To quantify the IntDen of Syn-GFP at synapses, a “synaptic mask” was made of regions of colocalization between PSD-95 and bassoon (see Fig. 6) using ImageJ with a colocalization plugin downloaded from the program's website (http://rsb.info.nih.gov/ij/plugins/colocalization.html). Points of colocalization were defined as regions >4 pixels in size where the intensity ratio of the two channels was >50. The IntDen of Syn-GFP fluorescence within this mask was then determined. In Figure 7, masks were made of PSD-95 immunoreactive signals.
Figure 6.

The localization of synaptic vesicles at synapses is disrupted in neurons with reduced β-pix activity. A–D, Confocal images of 9 DIV hippocampal neurons transfected with Syn-GFP plus the indicated β-pix construct and immunolabeled for PSD-95 and bassoon. In control neurons (A) and those expressing FL-β-pix (C), Syn-GFP exhibited a punctate distribution and colocalized with PSD-95 and bassoon (open arrowheads). In contrast, neurons expressing β-pix siRNA-1 (B) or DN-β-pix (D) exhibited a more diffuse pattern of Syn-GFP distribution, and the coverage of Syn-GFP (the sum of the length of Syn-GFP fluorescence signal per 10 μm axon length ± SE) was increased (B, D, E). A significant decrease in the IntDen of Syn-GFP fluorescence at synapses was observed in β-pix siRNA-expressing neurons and those expressing DN-β-pix (F). The area (G) and density (H) of PSD-95 and bassoon were similar in control and siRNA-expressing neurons. N = 18–29 cells per condition from 3 separate cultures. *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA with Tukey post hoc. I, Neurons transfected with Syn-GFP plus either control or β-pix siRNA-1 were loaded with FM 4-64. The density of FM 4-64 puncta was reduced in cells expressing β-pix siRNA-1. N = 30–31 cells per condition from 3 separate cultures. ***p < 0.001; Student's t test. Scale bars, 10 μm.
Figure 7.

Cortactin expression rescues the disruption in synaptic vesicle localization in β-pix knockdown cells. A, B, The IntDen of synaptic GFP-actin clusters (A) and synaptic UtrCH-RFP clusters (B) was decreased in neurons expressing β-pix siRNA, but unchanged in neurons expressing β-pix siRNA plus Cort-HA compared with control. N = 12–20 cells per condition from 2 separate cultures. C–E, Confocal images of 9 DIV hippocampal neurons transfected with Syn-GFP and β-pix siRNA plus or minus Cort-HA and immunolabeled for PSD-95. D, F, β-Pix siRNA-1-expressing neurons exhibited a more diffuse localization of Syn-GFP (D) and Syn-GFP coverage was increased compared with control (F). In neurons expressing both β-pix siRNA-1 and Cort-HA, discrete Syn-GFP clusters were observed at PSD-95 sites (E, open arrowheads). Syn-GFP coverage in these neurons was significantly decreased compared with cells expressing β-pix siRNA-1 alone, however, Syn-GFP coverage was not completely rescued to control levels (F). G, The IntDen of Syn-GFP fluorescence (within masks of PSD-95 clusters) was significantly decreased in β-pix siRNA-1-expressing neurons, and unchanged in neurons expressing siRNA-1 plus Cort-HA compared with control. N = 13–20 cells per condition from 2 separate cultures. *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA with Tukey post hoc. Scale bar, 10 μm.
Coverage of Syn-GFP fluorescence.
To quantify the length of Syn-GFP fluorescence signal, the length of the major axis of Syn-GFP fluorescence signal (Feret's diameter) was analyzed using ImageJ. Syn-GFP fluorescence coverage represents the sum of the Feret's diameter of Syn-GFP fluorescence signal per 10 μm axon length.
Localization of β-pix at synapses.
To examine the localization of β-pix at synapses, a “mask” was made of regions of colocalization between bassoon and PSD-95 using the colocalization plugin, and synaptic β-pix defined as β-pix puncta that associated with bassoon/PSD-95 clusters. The number of β-pix puncta that localized to synapses and the number of synapses that associate with β-pix was expressed as a percentage of the number of total puncta. All puncta in a field were analyzed.
Time-lapse imaging of GFP-actin and Syn-RFP.
To determine the distribution of Syn-RFP clusters, and its association with GFP-actin clusters, puncta were divided into stable and mobile categories. Puncta that remained stationary for the duration of the 10 min imaging period were identified as stable puncta, and were otherwise classified as mobile puncta.
Results
Increased actin polymerization enhances SV clustering
To examine the role of actin in clustering SVs at synapses, fluorescently tagged actin and synaptophysin were used to determine the distribution of these proteins. Fluorescently tagged actin has been widely used to determine the localization of actin at both presynaptic and postsynaptic terminals. Tagged actin constructs have proved to be particularly useful when focusing on presynaptic actin, and alleviate the eclipsing effect of postsynaptic actin observed using conventional immunostaining or phalloidin dyes (Fischer et al., 1998; Morales et al., 2000; Colicos et al., 2001; Sankaranarayanan et al., 2003; Saneyoshi et al., 2008; Lucido et al., 2009). In this study, GFP-actin driven by the PDGF promoter to minimize actin overexpression was used to examine the distribution of actin along the axon (Colicos et al., 2001). When expressed in neurons, GFP-actin was expressed in a punctate pattern along axons and largely colocalized with the postsynaptic marker, PSD-95, and the presynaptic marker, bassoon, suggesting an enrichment of GFP-actin at synapses as previously reported (Fig. 1A,A') (Fischer et al., 1998; Morales et al., 2000; Colicos et al., 2001; Sankaranarayanan et al., 2003). Thus, GFP-actin is a faithful marker for assaying the localization of endogenous actin. GFP-actin that was not associated with bassoon and PSD-95 may represent populations at inhibitory synapses or at nonsynaptic sites.
Figure 1.
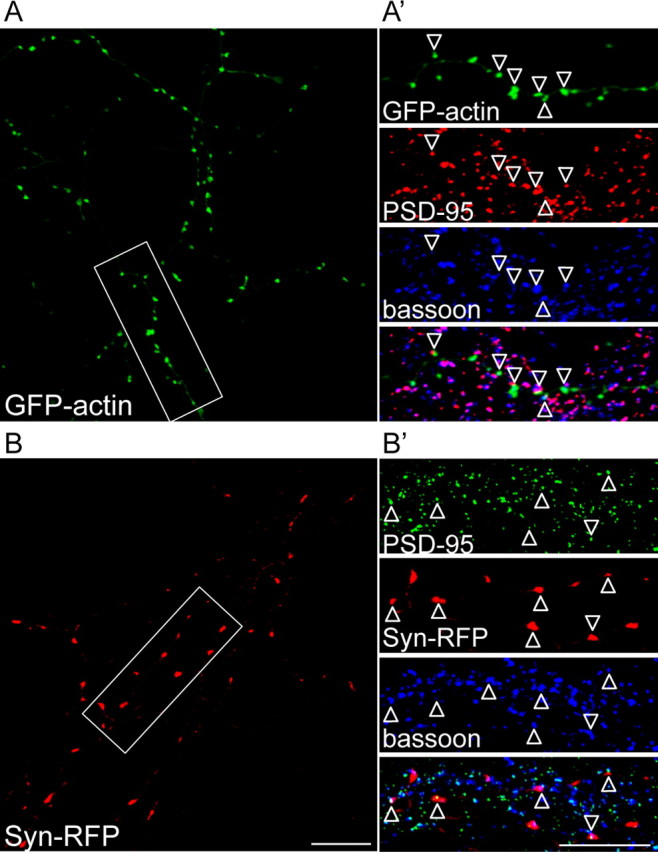
GFP-actin and Syn-RFP are localized to synapses. Confocal images of 10 DIV hippocampal neurons transfected with GFP-actin (A) or Syn-RFP (B) and immunolabeled with synaptic markers, PSD-95 and bassoon. Bassoon and PSD-95 immunopositive puncta that are not colocalized with GFP-actin or Syn-RFP may represent puncta on untransfected cells. Higher magnifications of the insets from A and B are shown in A' and B', respectively. GFP-actin and Syn-RFP were highly colocalized with PSD-95/bassoon (open arrowheads). Scale bars, 10 μm.
Fluorescently tagged SV marker proteins have been widely used to mark presynaptic sites in vertebrates, and do not compromise the secretory physiology of the synapse (Sankaranarayanan et al., 2003). Our work has previously demonstrated that the pattern of Syn-GFP expression is comparable to that of endogenous SV proteins (Bamji et al., 2003; Sun et al., 2009), and we observed appropriate Syn-RFP colocalization with PSD-95 and bassoon at synapses (Fig. 1B,B').
Recent work has demonstrated that repressing calpain protease activity using the calpain inhibitor, ALLN, enhances actin polymerization at discrete sites along neurites (Mingorance-Le Meur and O'Connor, 2009). To globally enhance actin polymerization in hippocampal cultures, cells were treated with 20 μm ALLN. To quantify actin clusters, we measured the IntDen of each puncta. This is the product of the area and mean gray value of each puncta, and most accurately represents the amount of protein per puncta. The density and IntDen of GFP-actin clusters were significantly increased 24 h following ALLN treatment (Fig. 2A–D). The density and IntDen of Syn-RFP clusters that were associated with GFP-actin clusters were specifically increased, whereas the density and IntDen of Syn-RFP clusters that were not associated with GFP-actin clusters remained unchanged (Fig. 2A–D). The increase in SV clustering was not attributable to increased synaptophysin expression, as Western blot analysis revealed no change in synaptophysin protein levels following ALLN treatment (Fig. 2E). Instead, the increase in SV density and IntDen was likely attributable to the accumulation of small SV clusters that were originally below the imaging threshold. Together, these results demonstrate that ALLN treatment enhances the accumulation of actin at discrete sites along the axon and mediates the recruitment of SVs to these sites. Despite the increase in SV cluster density, there was no significant change in the density of synapses as determined by the density of colocalized Syn-RFP and PSD-95 puncta, 24 h following ALLN treatment (Fig. 2A–C).
Figure 2.
The density and IntDen of GFP-actin and Syn-RFP clusters are increased following ALLN treatment. A, B, Confocal images of 8 DIV hippocampal neurons transfected with GFP-actin and Syn-RFP and immunolabeled with PSD-95. Neurons were treated either with 20 μm ALLN or with DMSO vehicle 24 h before fixation. Open arrowheads in A and B indicate synapses as defined by colocalized Syn-RFP/GFP-actin/PSD-95 clusters, whereas closed arrowheads indicate sites of colocalization between GFP-actin and Syn-RFP, but not PSD-95. ALLN treatment increased the density (A, B, open plus closed arrowheads; C) and IntDen (D) of GFP-actin clusters. C, D, The density and IntDen of Syn-RFP clusters that were associated with GFP-actin were specifically increased. Arrows indicate Syn-RFP clusters that were not associated with GFP-actin clusters. N = 23–33 cells per condition from 3 separate cultures. *p < 0.05, **p < 0.01; Student's t test. Scale bar, 10 μm. E, Synaptophysin protein levels were similar in control and ALLN-treated cells (α-tubulin was used as a loading control). N = 3 different blots from 3 separate cultures.
We suggest that bath treatment of neurons with ALLN regulates vesicle localization through its effects on calpain and actin polymerization; however, it is impossible to discount off target effects or regulation of other pathways downstream of calpain. Moreover, ALLN can enhance actin polymerization at both presynaptic and postsynaptic compartments, and may enhance SV density by transsynaptic mechanisms. Therefore, to more definitively test whether presynaptic actin recruits SVs in a cell-autonomous manner, actin polymerization was enhanced in individual neurons through expression of Cort-HA. Cortactin is an actin-binding protein that promotes actin polymerization by stabilizing Arp2/3 complexes (Cosen-Binker and Kapus, 2006). In neurons, cortactin is proteolysed by calpain, resulting in the suppression of membrane protrusion (Mingorance-Le Meur and O'Connor, 2009). Cort-HA was distributed in a punctate pattern along the axon, and was highly colocalized with GFP-actin clusters (Fig. 3B). Overexpression of Cort-HA significantly increased the density of GFP-actin clusters (Fig. 3A–C). Interestingly, GFP-actin clusters were specifically added to nonsynaptic sites and the number of actin clusters associated with either the excitatory or inhibitory postsynaptic markers, PSD-95 and gephyrin, respectively, was unchanged (Fig. 3D). The IntDen of actin was also increased (Fig. 3G). Similar to that observed in ALLN-treated cells, the density and IntDen of Syn-RFP clusters that were associated with GFP-actin clusters were specifically increased, whereas the density and IntDen of Syn-RFP clusters that were not associated with GFP-actin clusters remained unchanged (Fig. 3A–C,G).
Figure 3.
The density and IntDen of GFP-actin, Syn-RFP, and UtrCH-RFP clusters are increased in neurons expressing cortactin. A, B, Confocal images of 8 DIV hippocampal neurons cotransfected with GFP-actin and Syn-RFP plus either empty vector or Cort-HA and immunolabeled for HA. GFP-actin, Syn-RFP, and Cort-HA were highly colocalized (B, open arrowheads). In neurons expressing Cort-HA, the density and IntDen of GFP-actin clusters and Syn-RFP clusters associated with GFP-actin clusters were increased (A, B, open arrowheads; C, G), whereas the density and IntDen of Syn-RFP clusters that were not associated with GFP-actin clusters were not significantly changed (A, B, closed arrowheads; C, G). N = 23–31 cells per condition from 3 separate cultures. D, The density of GFP-actin clusters associated with PSD-95 and gephyrin remained unchanged, whereas the number of GFF-actin clusters that were not colocalized with either marker was increased in cells expressing Cort-HA. N = 14–16 cells per condition from 2 separate cultures. E, F, Confocal images of 8 DIV hippocampal neurons cotransfected with GFP-actin and UtrCH-RFP plus either empty vector or Cort-HA and immunolabeled for HA. Utrophin-RFP clusters were completely colocalized with GFP-actin clusters (E, F, open arrowheads), and the IntDen of UtrCH-RFP clusters was significantly increased in cells expressing Cort-HA (H). N = 19 cells per condition from 3 separate cultures. H, The IntDen of UtrCH-RFP at synapses, defined by UtrCH-RFP clusters colocalized with PSD-95, was increased in cells expressing Cort-HA. N = 14–15 cells per condition from 2 separate cultures. I, J, Confocal images of 8 DIV hippocampal neurons cotransfected with Syn-GFP plus either empty vector or Cort-HA and immunolabeled for PSD-95 and HA. Open arrowheads indicate synapses, which are defined as sites of colocalization between Syn-GFP and PSD-95. A subset of Cort-HA clusters localized to synapses (J, open arrowheads). The IntDen of Syn-GFP puncta in these cells was significantly increased at synapses associated with Cort-HA clusters (+Cort-HA), but unaffected at synapses not associated with Cort-HA (−Cort-HA) (I, K). N = 19–24 cells per condition from 3 separate cultures. *p < 0.05; one-way ANOVA with Dunnett's post hoc. L, Time-lapse analysis demonstrated an increase in the density of stable Syn-RFP clusters in cells expressing Cort-HA. N = 16–17 cells per condition from 3 separate cultures. *p < 0.05, **p < 0.01, ***p < 0.001; Student's t test. Scale bars, 10 μm.
To specifically examine the effect of cortactin expression on actin polymerization, neurons were transfected with UtrCH-RFP, which specifically binds polymerized F-actin (Burkel et al., 2007). UtrCH-RFP is a faithful marker of polymerized actin in living and fixed cells, and does not affect actin dynamics (Burkel et al., 2007). UtrCH-RFP clusters completely colocalized with GFP-actin clusters, and the IntDen of these clusters was significantly increased in cells expressing Cort-HA (Fig. 3E,F,H). More specifically, the IntDen of UtrCH-RFP clusters at synapses, defined by the UtrCH-RFP clusters colocalized with PSD-95, was significantly increased (Fig. 3H). These results demonstrate that overexpression of cortactin enhances actin polymerization at synapses, and induces the polymerization of new actin clusters at nonsynaptic sites.
The majority of synapses along transfected axons were associated with Cort-HA clusters (87.4 ± 4.3% of synapses contained Cort-HA, n = 21 cells from three separate cultures; Fig. 3J) in accord with previous work demonstrating the enrichment of endogenous cortactin at synapses (Boeckers et al., 1999; Naisbitt et al., 1999). While those studies focused on cortactin at postsynaptic sites, our study demonstrates that cortactin is also localized presynaptically. To examine how increased actin polymerization at synapses impacts the localization of SVs, the IntDen of Syn-GFP at synapses was measured. The IntDen of Syn-GFP was specifically increased at synapses that were associated with Cort-HA clusters, whereas there was no significant change in the IntDen of Syn-GFP at synapses not associated with Cort-HA clusters (Fig. 3I–K).
Two pools of SVs have previously been identified by time-lapse analyses; a relatively stable pool that remains stationary for hours and localizes primarily at presynaptic boutons, and a mobile pool that translocates along the axon in a saltatory manner (Kraszewski et al., 1995; Dai and Peng, 1996; Nakata et al., 1998; Ahmari et al., 2000; Bresler et al., 2004). To determine whether enhanced actin polymerization increases the density of stable or mobile SV clusters, neurons cotransfected with GFP-actin and Syn-RFP plus or minus Cort-HA were imaged using time-lapse microscopy. The density of total SV clusters was increased in cells expressing Cort-HA, which was attributable to a specific increase in the density of stable Syn-RFP clusters (Fig. 3L). Moreover, the density of stably colocalized GFP-actin/Syn-RFP clusters was threefold greater in cells expressing Cort-HA than in control cells (control: 3.21 ± 0.52 clusters per 100 μm; Cort-HA: 9.16 ± 0.56 clusters per 100 μm; p < 0.001 Student's t test, n = 16–17 neurons per condition from three cultures). Together, these results demonstrate that clusters of polymerized actin can recruit SVs and stably localize them at discrete sites. We next examined how cell–cell contact enhances the local polymerization of actin and enhances the localization of SVs to nascent synapses.
β-pix interacts with cadherin/β-catenin/scribble complexes at synapses
We have previously shown that β-catenin/scribble complexes are important for clustering SVs at developing synapses (Bamji et al., 2003; Sun et al., 2009). To further elucidate the mechanism through which this occurs, and to determine whether this complex is involved in the regulation of actin polymerization, we took a candidate approach to search for the protein(s) that associates with β-catenin/scribble complexes to localize SVs. In mouse brains, scribble forms a complex with β-pix, a Rac/Cdc42 GEF, which promotes actin polymerization via regulation of Rac/Cdc42 activity (Audebert et al., 2004). We hypothesized that β-catenin/scribble complexes act as scaffolds to localize β-pix to developing synapses and that β-pix, in turn, enhances the local polymerization of actin and the subsequent accumulation of vesicles at these sites.
Endogenous β-pix was distributed in a punctate pattern in neurons and highly colocalized with the presynaptic and postsynaptic markers, bassoon and PSD-95, respectively (Fig. 4A; 70.7 ± 2.9% of β-pix clusters colocalized with bassoon/PSD-95 clusters, n = 12 images from two separate cultures). This is in accord with previous studies showing that β-pix immunoprecipitates with the presynaptic scaffold protein, Piccolo, and localizes to spines (Kim et al., 2003; Park et al., 2003; Zhang et al., 2003). β-Pix clusters that were not associated with PSD-95 and bassoon may represent populations at inhibitory synapses or at nonsynaptic sites.
Figure 4.
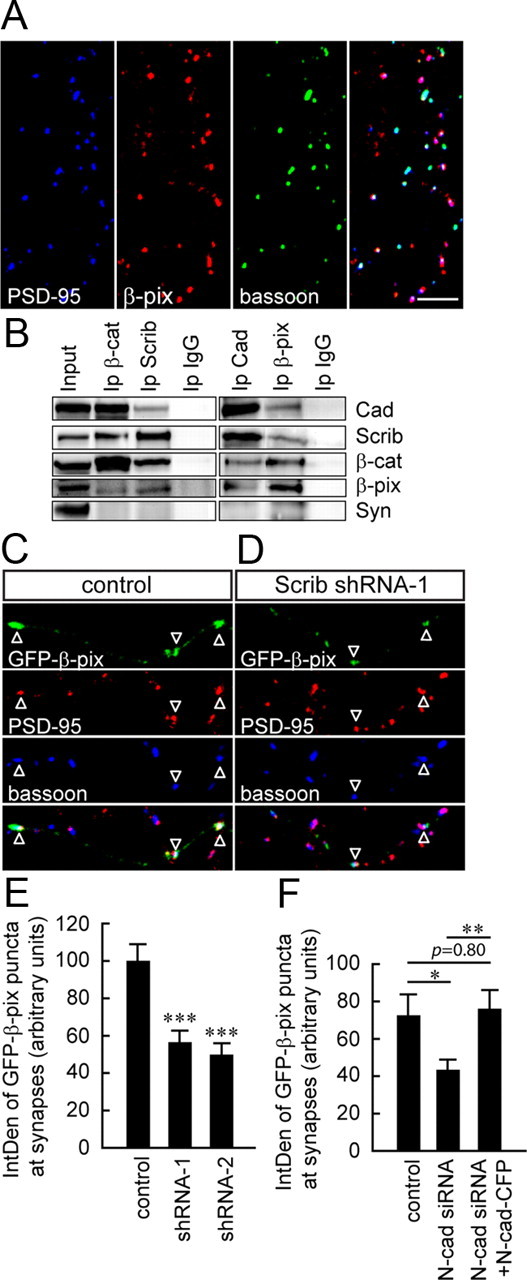
β-Pix interacts with cadherin, β-catenin, and scribble at synapses. A, Confocal images of 10 DIV hippocampal cultures immunolabeled for β-pix and synaptic markers, PSD-95 and bassoon. β-Pix clusters are highly colocalized with PSD-95/bassoon in neurons. B, Synaptosomal fractions from adult brains were immunoprecipitated with antibodies against cadherin, β-pix, β-catenin, or scribble and separated by SDS-PAGE. β-Pix, scribble, β-catenin, and cadherin coimmunoprecipitated with one another, but not with the synaptic protein, synaptophysin. The input lane corresponds to the crude synaptosomal fraction, P2. Rabbit IgG was used as a control. N = 3 different blots from 2 separate preparations. C, D, Confocal images of 8 DIV hippocampal neurons transfected with GFP-β-pix plus either shRNA control or Scrib shRNAs and immunolabeled for PSD-95 and bassoon. The IntDen of synaptic GFP-β-pix clusters, defined by GFP-β-pix at sites of colocalization between PSD-95 and bassoon, was smaller in cells expressing Scrib shRNA (C, D, open arrowheads; E, N = 14–27 cells per condition from 3 separate cultures) or N-cadherin siRNA (F, N = 15–18 cells per condition from 2 separate cultures). *p < 0.05, **p < 0.01; one-way ANOVA with Tukey post hoc. ***p < 0.001; one-way ANOVA with Dunnett's post hoc. Scale bars, 10 μm.
To examine whether β-pix interacts with β-catenin/scribble complexes at synapses, crude synaptosomal fractions from adult brains were prepared and immunoprecipitated with antibodies against cadherin, scribble, β-catenin, and β-pix. Coimmunoprecipitation assays demonstrated that these four proteins associate with one another, but not with synaptophysin at synapses (Fig. 4B).
Localization of β-pix at synapses is decreased in N-cadherin and scribble knockdown cells
To examine whether scribble is involved in localizing β-pix to presynaptic sites, cells were transfected with two short hairpin RNAs against scribble (Scrib shRNA-1 and -2). We have previously used these shRNAs to attenuate scribble protein levels in hippocampal neurons (Sun et al., 2009). Previous work has demonstrated that β-pix localizes to both presynaptic and postsynaptic compartments (Kim et al., 2003; Park et al., 2003; Zhang et al., 2003). To alleviate the potential eclipsing effect of postsynaptic β-pix observed using conventional immunostaining, we used a GFP-tagged β-pix to specifically examine how scribble knockdown impacts β-pix localization to presynaptic compartments. GFP-β-pix is diffusely localized when expressed at high levels (Zhang et al., 2003), therefore only weakly transfected cells exhibiting a punctate pattern of GFP-β-pix, that was comparable to endogenous β-pix, were analyzed (see Materials and Methods). Image acquisition was done blind to the conditions being analyzed. We assessed the localization of GFP-β-pix at synapses by analyzing the IntDen of those GFP-β-pix clusters specifically associated with synapses (defined as points of colocalization between PSD-95/bassoon). The IntDen of synaptic GFP-β-pix clusters was significantly decreased in cells expressing Scrib shRNAs (Fig. 4C–E). These results suggest that scribble is involved in localizing β-pix to presynaptic compartments.
We also checked the effect of scribble knockdown on endogenous β-pix and, as expected, postsynaptic β-pix eclipsed the magnitude of the presynaptic β-pix mislocalization. Indeed, no significant difference was observed in the IntDen and density of β-pix immunoreactive puncta in scribble knockdown cells (IntDen, shRNA-C: 33.8 ± 1.82, and shRNA-1: 31.2 ± 1.39 arbitrary units, p = 0.27 Student's t test; density, shRNA-C: 28.6 ± 1.83, and shRNA-1: 28.8 ± 1.85 clusters per 100 μm, p = 0.92 Student's t test; n = 15–16 neurons per condition from two cultures). This further validates our use of GFP-β-pix to specifically determine the localization of presynaptic or postsynaptic β-pix.
To further demonstrate that β-pix is recruited to nascent synapses via cadherin/β-catenin/scribble complexes, we also quantified the IntDen of GFP-β-pix at synapses in N-cadherin knockdown cells. To modify N-cadherin levels, cells were transfected with either N-cadherin siRNA (N-cad siRNA), scrambled siRNA (N-cad siRNA-C), or N-cad siRNA plus RNAi-resistant N-cadherin-CFP (N-cad-CFP), as previously published (Aiga et al., 2011). The IntDen of GFP-β-pix at synapses was significantly decreased in N-cadherin knockdown cells (Fig. 4F).
β-pix GEF activity mediates localized actin polymerization at synapses
To study the function of β-pix at synapses, β-pix protein levels were attenuated in neurons using RNA interference. To minimize possible off-target effects, two short interfering RNA oligonucleotides (siRNA-1 and siRNA-2) against rat β-pix were used. SiRNA-negative control duplexes were used as a control (siRNA-C). Given the low transfection efficiency of primary cultured hippocampal neurons, the efficacy of β-pix siRNAs was examined in the HEK 293 cell line using Western blot analysis. β-Pix siRNAs significantly attenuated the expression of GFP-β-pix (Fig. 5A). A mixture of siRNA-1 and siRNA-2 was also used to further control for off-target effects (siRNA-M).
Figure 5.

The IntDen of synaptic GFP-actin clusters is decreased in neurons with reduced β-pix activity. A, GFP-β-pix expression in HEK 293 cells is decreased in cells expressing β-pix siRNA. N = 2 different blots from 2 separate experiments. B, C, Confocal images of 8 DIV hippocampal neurons transfected with GFP-actin plus scrambled or β-pix siRNAs and immunolabeled for PSD-95 and bassoon. D, The density of GFP-actin clusters was unaltered in β-pix siRNAs expressing neurons. However, the IntDen of synaptic GFP-actin clusters (GFP-actin at sites of PSD-95 and bassoon colocalization) was decreased in cells expressing β-pix siRNAs (B, C, open arrowheads; E). N = 16–24 cells per condition from 2 separate cultures. *p < 0.05, ***p < 0.001; one-way ANOVA with Dunnett's post hoc. F, The IntDen of synaptic GFP-actin clusters was significantly attenuated in neurons expressing DN-β-pix. N = 11–15 cells per condition from 2 separate cultures. ***p < 0.001; one-way ANOVA with Tukey post hoc. G, H, Confocal images of 8 DIV hippocampal neurons transfected with UtrCH-RFP plus control or β-pix siRNA-1 and immunolabeled for PSD-95. I, The IntDen of synaptic UtrCH-RFP clusters (UtrCH-RFP clusters that were colocalized with PSD-95) was significantly decreased in neurons expressing β-pix siRNA-1. N = 12–16 cells per condition from 2 separate cultures. *p < 0.05; Student's t test. Scale bars, 10 μm.
To determine the effects of β-pix knockdown on actin, cells were cotransfected with GFP-actin plus β-pix siRNAs. The density of GFP-actin was unaltered in neurons expressing β-pix siRNAs (Fig. 5B–D); however, the IntDen of synaptic GFP-actin and UtrCH-RFP (puncta associated with PSD-95/bassoon coclusters) was decreased in these cells (Fig. 5B,C,E,G–I).
To determine whether the GEF activity of β-pix regulates actin clusters at synapses, cells were cotransfected with GFP-actin plus either HA-tagged full-length β-pix (FL-β-pix) or a DN-β-pix that contains two mutations in the Dbl homology domain (L238R and L239S) and lacks GEF activity (Manser et al., 1998; Zhang et al., 2003). FL-β-pix did not alter GFP-actin at synapses; however, the IntDen of synaptic GFP-actin clusters in cells expressing DN-β-pix was significantly reduced (Fig. 5F). These results suggest that β-pix GEF activity is important for actin polymerization at synapses.
Perturbation of β-pix activity disrupts the localization of SVs at synapses
To test whether β-pix is involved in the clustering of SVs at synapses, Syn-GFP localization was examined in β-pix knockdown neurons. In control cells, Syn-GFP was distributed in a punctate pattern (Fig. 6A). In contrast, the distribution of Syn-GFP fluorescence in siRNA-1-expressing neurons was relatively uniform along the axon, with only few discrete Syn-GFP clusters observed (Fig. 6B).
Variability in the “punctate-ness” of Syn-GFP was observed in siRNA-expressing neurons. To determine the distribution of vesicles along the axon, the Feret's diameter (defined as the greatest distance possible between any two points along the boundary of a region of interest, hereafter called the “length”) of the Syn-GFP fluorescence signal was first measured and Syn-GFP florescence coverage (the sum of the length of Syn-GFP fluorescence signal per 10 μm axon length) was then determined as previously described (Bamji et al., 2003; Lee et al., 2008; Sun et al., 2009). To avoid bias, all transfected neurons on each coverslip were imaged and quantified. There was no significant difference in the coverage of Syn-GFP fluorescence in neurons expressing Syn-GFP alone and Syn-GFP plus siRNA-C (Fig. 6E). In contrast, in siRNA-expressing neurons the coverage of Syn-GFP fluorescence along the axon was ∼2-fold greater than controls (Fig. 6E). In addition, the IntDen of Syn-GFP fluorescence at synapses (within masks made of regions of overlap between PSD-95/bassoon) was significantly reduced in siRNA-expressing cells compared with controls, further demonstrating that the clustering of SVs at synapses is dependent on β-pix (Fig. 6F). The decreased IntDen of Syn-GFP fluorescence at synapses could not be attributed to changes in the density or area of bassoon and PSD-95 clusters, which were similar in wild-type and β-pix knockdown cells (Fig. 6G,H).
To determine whether the GEF activity of β-pix is required for the appropriate clustering of vesicles at synapses, cells were cotransfected with Syn-GFP plus either FL-β-pix or DN-β-pix. Expression of FL-β-pix did not alter Syn-GFP fluorescence coverage or the IntDen of Syn-GFP at synapses compared with control cells (Fig. 6C,E,F). In contrast, neurons expressing DN-β-pix exhibited a diffuse pattern of Syn-GFP expression, which was reflected in an increased coverage of Syn-GFP fluorescence along the axon and an attenuated IntDen of Syn-GFP fluorescence at synapses (Fig. 6D–F).Together, these results suggest that β-pix regulates vesicle localization through its GEF activity.
To determine whether deficits in vesicle clustering in β-pix knockdown cells are associated with impaired presynaptic function, the efficiency of vesicle recycling was studied by stimulating neurons with a high-K+ solution in the presence of FM 4-64, a fluorescent dye that marks sites of endocytosis. In cells expressing β-pix siRNA-1, this uptake was strongly suppressed, with greatly reduced density of FM 4-64-positive puncta compared with control cells (Fig. 6I).
Cortactin overexpression rescues the SV clustering phenotype observed in β-pix knockdown cells
To further test whether the mislocalization of SVs in β-pix knockdown cells is attributable to decreased polymerization of actin at synapses, we determined whether this phenotype could be rescued by overexpressing cortactin. The IntDen of GFP-actin and UtrCH-RFP clusters at synapses (defined as those clusters that were associated with PSD-95) were restored to wild-type levels in β-pix knockdown cells overexpressing Cort-HA (Fig. 7A,B). Overexpression of cortactin in β-pix knockdown cells also rescued the mislocalization of SVs, resulting in an increased clustering of SVs along the axon. Indeed, in neurons coexpressing β-pix siRNA-1 plus Cort-HA, discrete Syn-GFP clusters that colocalized with PSD-95 were observed, similar to those observed in control cells (Fig. 7C,E). Both the Syn-GFP fluorescence coverage (Fig. 7F), and the IntDen of Syn-GFP at synapses (Fig. 7G) were significantly rescued by cortactin overexpression. Together, these observations strongly suggest a model by which cadherin/β-catenin/scribble complexes localize β-pix to nascent synapses where β-pix promotes actin polymerization through its GEF activity, and enhances the recruitment of SV clusters to these areas of actin polymerization.
Discussion
Transsynaptic adhesion molecules have been shown to stabilize transient, dynamic axodendritic contacts and activate intracellular signals that recruit synaptic proteins (McAllister, 2007). The cadherin adhesion complex has previously been shown to play a large role in localizing SVs to developing synapses. In N-cadherin knock-out cultures (Stan et al., 2010), or cultures treated with peptides that block intercellular cadherin interactions (Togashi et al., 2002), SVs do not accumulate at contact points. We have shown that presynaptically localized cadherin can mediate this effect through its association with β-catenin (Bamji et al., 2003, 2006; Lee et al., 2008) and scribble (Sun et al., 2009). Recently, it has also been shown that cadherins can mediate SV localization transsynaptically through the recruitment of neuroligin-1 to postsynaptic compartments (Stan et al., 2010; Aiga et al., 2011). In this study, we further examine the effects of presynaptic cadherin adhesion complexes in recruiting SVs to sites of contact. We demonstrate that scribble is important for the recruitment of β-pix to developing synapses, and that scribble and β-pix form complexes with cadherin and β-catenin. Moreover, we show that β-pix-mediated enhancement of actin polymerization is important for the localization of SVs to discrete sites along the axon.
Actin localizes synaptic vesicles to developing synapses
SVs do not cluster appropriately at developing synapses in the presence of actin depolymerizing agents (Kuromi and Kidokoro, 1998; Zhang and Benson, 2001). Although this suggests a role for actin in SV clustering, it is also plausible that this is a secondary effect to reduced cell—cell adhesion, and it was unclear whether actin actively localizes SVs to nascent synapses. Utilizing gain-of-function assays, we demonstrate that enhancing actin polymerization increases the clustering of SVs. Indeed, there was a direct correlation between the density and IntDen of actin and SV clusters in all conditions analyzed. Increasing the polymerization of actin by itself is not sufficient to induce the formation of new synapses. Synapse formation requires intercellular communication and coordination of developing presynaptic and postsynaptic compartments. Enhanced actin polymerization along axons can therefore cluster SVs, but not induce clustering of postsynaptic proteins at these sites.
A conventional way to enhance actin polymerization is to stabilize polymerized actin using jasplakinolide (Sankaranarayanan et al., 2003; Darcy et al., 2006; Lucido et al., 2009). However, this method compromises dynamic actin reorganization, which is essential for the mobilization of SVs. Indeed, inhibiting actin turnover using jasplakinolide greatly reduces the transport of SVs along the axon (Darcy et al., 2006) and nearly abolishes the accumulation of SVs at poly-d-lysine bead-induced contact sites (Lucido et al., 2009). We therefore promoted actin polymerization by treating cells with the calpain protease inhibitor, ALLN. Calpain maintains neurite consolidation by suppressing actin polymerization along neurites, and inhibiting calpain activity enhances actin polymerization (Mingorance-Le Meur and O'Connor, 2009). There are a number of substrates for calpain including cortactin, cadherin, and β-catenin (Perrin et al., 2006; Abe and Takeichi, 2007; Jang et al., 2009). As calpain inhibition and cortactin overexpression can enhance GFP-actin cluster density and IntDen, we concluded that inhibition of calpain enhances SV localization through its effects on actin. SV clustering is unlikely to be attributable to elevated levels of cadherin and β-catenin, because overexpression of these proteins does not increase SV density (Scheiffele et al., 2000; Bamji et al., 2003; Sara et al., 2005; Latefi et al., 2009; Linhoff et al., 2009).
How does polymerized actin “trap” SVs that are being transported along microtubules? It has been known for some time that the actin motor, myosin-V, can form a complex with the microtubule motor, kinesin, and that this heteromotor complex enables vesicles to be transported on both microtubules and actin filaments. The direct interaction of motors from both filament systems may represent the mechanism by which the transition of vesicles from microtubules to actin filaments is regulated (Langford, 2002).
Scribble regulates β-pix localization at synapses
β-Pix has previously been shown to coimmunoprecipitate with the presynaptic protein, Piccolo, and to colocalize with synaptic markers at dendritic spines (Kim et al., 2003; Park et al., 2003; Zhang et al., 2003). Shank (a postsynaptic scaffolding protein) and GIT1 (G protein-coupled receptor kinase-interacting protein 1) have been shown to be important for the localization of β-pix to postsynaptic compartments (Park et al., 2003; Zhang et al., 2003). In the present study, we show that the scaffold protein, scribble, is important for the localization of β-pix at presynaptic compartments. Cadherin and scribble are also essential for the appropriate localization of β-pix in other cell types. For instance, in epithelial cells, β-pix is recruited to points of cell adhesion in an E-cadherin-dependent manner (Liu et al., 2010). Moreover, scribble can localize β-pix to the cell membrane in PC12 cells (Audebert et al., 2004). As the presynaptic localization of β-pix was not completely abolished upon scribble knockdown, it is possible that other proteins such as GIT1, which is expressed both presynaptically and postsynaptically (Kim et al., 2003; Zhang et al., 2003), may also play a role in this event.
β-Pix regulates actin polymerization at synapses
β-Pix activates Rac and Cdc42 (Bagrodia et al., 1998; Manser et al., 1998), which are known regulators of actin remodeling. Rac/Cdc42 can promote actin polymerization through the Scar/WASP/Arp2/3 and PAK/LIMK-1/ADF/cofilin pathways (Rodriguez et al., 2003). Previous work has demonstrated that β-pix can regulate spine formation through modulation of actin (Parnas et al., 2001; Park et al., 2003; Zhang et al., 2003; Zhang et al., 2005; Saneyoshi et al., 2008). Here, we suggest that β-pix exerts a similar role in regulating actin polymerization at the presynaptic compartment. Indeed, actin polymerization at presynaptic terminals was significantly decreased in cells lacking β-pix and in cells lacking the β-pix GEF activity.
Dynamic regulation of cadherin adhesion complexes and SV localization
Previous work has shown that the cadherin adhesion complex can be dynamically regulated, and that this may be a mechanism to regulate presynaptic plasticity. Phosphorylation of β-catenin on tyrosine residue 654 can rapidly uncouple cadherin from β-catenin and result in the enhanced mobility of SVs (Bamji et al., 2006). The phosphorylation and dephosphorylation of β-catenin can be mediated by the tyrosine kinase, Fer, and the phosphatase, SHP2. These proteins have been shown to modulate SV localization through their regulation of β-catenin (Lee et al., 2008). β-Catenin phosphorylation can also be regulated by leukocyte antigen related (LAR) tyrosine phosphatase (Kypta et al., 1996). Interestingly, the synaptic localization of LAR depends on liprin-α1 (Hoogenraad et al., 2007), the vertebrate homolog of Sad-2. Sad-2 is important for the clustering of SVs, and Sad-2 mutants display a diffuse localization of SVs along the axon (Zhen and Jin, 1999). It is possible that liprin-α1 modulates vesicle localization by recruiting LAR tyrosine phosphatase to nascent synapses where it maintains the integrity of the cadherin-β-catenin complex by dephosphorylating β-catenin.
Temporal effects of cadherin complexes at synapses
We believe that enhanced local polymerization of actin by cadherin/β-catenin/scribble/β-pix complexes plays an important role in synapse development and SV localization. This is based on a number of reasons. First, cadherin is one of the first components recruited to points of cell—cell contact, before the assembly of functional synapses (Zhai et al., 2001). Second, actin polymerization at contact points precedes vesicle clustering on poly-d-lysine coated beads (Lucido et al., 2009). Third, cadherin, β-catenin, scribble, and β-pix colocalize together at synapses in young cultures. Finally, perturbation of cadherin, β-catenin, scribble or β-pix, or disruption of actin polymerization using latrunculin, all result in the mislocalization of SVs in young cultures (5–10 DIV) (Zhang and Benson, 2001; Togashi et al., 2002; Bamji et al., 2003; Sun et al., 2009).
This pathway may also be involved in synapse unsilencing during neuronal development. Indeed, Yao et al. (2006) demonstrate that stabilizing actin filaments using jasplakinolide in young neurons (7–11 DIV) increases the number of active boutons labeled by the FM 1-43 dye. This suggests that actin polymerization is important for the conversion of silent boutons into functional ones. It is attractive to speculate that transsynaptic signaling by cadherins is involved in the coordination of synapse unsilencing.
It is unclear how important this pathway is during the maintenance of synapses. In older cultures (18–20 DIV), the clustering of SVs and SV recycling were largely resistant to F-actin depolymerization (Zhang and Benson, 2001). This correlated with an increase in the localization of synaptic scaffold proteins. In contrast, knocking down β-catenin levels at postnatal day 17 in vivo significantly decreased the number of SV per synapse, indicating a role for this protein in the maintenance of the reserve pool of vesicles at these points of contact (Bamji et al., 2003).
Our results suggest that synaptically localized cadherin/β-catenin/scribble/β-pix complexes enhance actin polymerization at points of cell—cell contact, and that polymerized actin traps SVs as they translocate along the axon. These findings provide a mechanism by which cell—cell contract leads to the assembly of synaptic components.
Footnotes
This work was supported by grants from Canadian Institutes of Health Research MOP-81158 (S.X.B.), the Pacific Alzheimer's Research Foundation OP06-09 (S.X.B.), and the American Alzheimer's Association NIRG-07-58917 (S.X.B.).
References
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Aiga M, Levinson JN, Bamji SX. N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J Biol Chem. 2011;286:851–858. doi: 10.1074/jbc.M110.176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lécine P, Bellaiche Y, Dupont JL, Premont RT, Sempéré C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher A, Drenckhahn A, Pahner I, Margittai M, Jahn R, Ahnert-Hilger G. The synaptophysin-synaptobrevin complex: a hallmark of synaptic vesicle maturation. J Neurosci. 1999;19:1922–1931. doi: 10.1523/JNEUROSCI.19-06-01922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckers TM, Winter C, Smalla KH, Kreutz MR, Bockmann J, Seidenbecher C, Garner CC, Gundelfinger ED. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun. 1999;264:247–252. doi: 10.1006/bbrc.1999.1489. [DOI] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci. 2004;24:1507–1520. doi: 10.1523/JNEUROSCI.3819-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burry RW, Hayes DM. Development and elimination of presynaptic elements on polylysine-coated beads implanted in neonatal rat cerebellum. J Neurosci Res. 1986;15:67–78. doi: 10.1002/jnr.490150107. [DOI] [PubMed] [Google Scholar]
- Burry RW, Ho RH, Matthew WD. Presynaptic elements formed on polylysine-coated beads contain synaptic vesicle antigens. J Neurocytol. 1986;15:409–419. doi: 10.1007/BF01611725. [DOI] [PubMed] [Google Scholar]
- Colicos MA, Collins BE, Sailor MJ, Goda Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 2001;107:605–616. doi: 10.1016/s0092-8674(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- Dai Z, Peng HB. Dynamics of synaptic vesicles in cultured spinal cord neurons in relationship to synaptogenesis. Mol Cell Neurosci. 1996;7:443–452. doi: 10.1006/mcne.1996.0032. [DOI] [PubMed] [Google Scholar]
- Darcy KJ, Staras K, Collinson LM, Goda Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci. 2006;9:315–321. doi: 10.1038/nn1640. [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Feliu-Mojer MI, Spangler SA, Milstein AD, Dunah AW, Hung AY, Sheng M. Liprinalpha1 degradation by calcium/calmodulin-dependent protein kinase II regulates LAR receptor tyrosine phosphatase distribution and dendrite development. Dev Cell. 2007;12:587–602. doi: 10.1016/j.devcel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Hirota Y, Ozaki K, Okano H, Takeichi M, Uemura T. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol Cell Neurosci. 2002;19:375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- Jang YN, Jung YS, Lee SH, Moon CH, Kim CH, Baik EJ. Calpain-mediated N-cadherin proteolytic processing in brain injury. J Neurosci. 2009;29:5974–5984. doi: 10.1523/JNEUROSCI.6178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ko J, Shin H, Lee JR, Lim C, Han JH, Altrock WD, Garner CC, Gundelfinger ED, Premont RT, Kaang BK, Kim E. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J Biol Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford GM. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–865. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- Latefi NS, Pedraza L, Schohl A, Li Z, Ruthazer ES. N-cadherin prodomain cleavage regulates synapse formation in vivo. Dev Neurobiol. 2009;69:518–529. doi: 10.1002/dneu.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Peng HB. Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol. 2006;66:522–536. doi: 10.1002/neu.20245. [DOI] [PubMed] [Google Scholar]
- Lee SH, Peng IF, Ng YG, Yanagisawa M, Bamji SX, Elia LP, Balsamo J, Lilien J, Anastasiadis PZ, Ullian EM, Reichardt LF. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and beta-catenin. J Cell Biol. 2008;183:893–908. doi: 10.1083/jcb.200807188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhoff MW, Laurén J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Jia L, Thompson-Baine AM, Puglise JM, Ter Beest MB, Zegers MM. Cadherins and Pak1 control contact inhibition of proliferation by Pak1-betaPIX-GIT complex-dependent regulation of cell-matrix signaling. Mol Cell Biol. 2010;30:1971–1983. doi: 10.1128/MCB.01247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucido AL, Suarez Sanchez F, Thostrup P, Kwiatkowski AV, Leal-Ortiz S, Gopalakrishnan G, Liazoghli D, Belkaid W, Lennox RB, Grutter P, Garner CC, Colman DR. Rapid assembly of functional presynaptic boutons triggered by adhesive contacts. J Neurosci. 2009;29:12449–12466. doi: 10.1523/JNEUROSCI.1381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, O'Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–260. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–674. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D, Han S, Seo J, Lee JR, Choi J, Groffen J, Kim K, Cho YS, Choi HS, Shin H, Woo J, Won H, Park SK, Kim SY, Jo J, Whitcomb DJ, Cho K, Kim H, Bae YC, Heisterkamp N, et al. Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins. J Neurosci. 2010;30:14134–14144. doi: 10.1523/JNEUROSCI.1711-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Na M, Choi J, Kim S, Lee JR, Yoon J, Park D, Sheng M, Kim E. The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the beta PIX guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem. 2003;278:19220–19229. doi: 10.1074/jbc.M301052200. [DOI] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Perrin BJ, Amann KJ, Huttenlocher A. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol Biol Cell. 2006;17:239–250. doi: 10.1091/mbc.E05-06-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Rossner M, Yamada KM. What's in a picture? The temptation of image manipulation. J Cell Biol. 2004;166:11–15. doi: 10.1083/jcb.200406019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Südhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci U S A. 2010;107:11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20:3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Xie C, Markesbery WR, Lovell MA. Survival of hippocampal and cortical neurons in a mixture of MEM+ and B27-supplemented neurobasal medium. Free Radic Biol Med. 2000;28:665–672. doi: 10.1016/s0891-5849(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–8147. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Horwitz AF. Synapse formation is regulated by the signaling adaptor GIT1. J Cell Biol. 2003;161:131–142. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]




