Abstract
In the human brain, homologous regions of the primary motor cortices (M1s) are connected through transcallosal fibers. Interhemispheric communication between the two M1s plays a major role in the control of unimanual hand movements, and the strength of this connection seems to be dependent on arm activity. For instance, a lesion in the M1 can induce an increase in the excitability of the intact M1 and an abnormal high inhibitory influence onto the damaged M1. This can be attributable to either the disuse of the affected limb or the overuse of the unaffected one. Here, to directly investigate cortical modifications induced by an abnormal asymmetric use of the two limbs, we studied both the excitability of the two M1s and transcallosal interaction between them in healthy subjects whose right hand was immobilized for 10 h. The left “not-immobilized” arm was completely free to move in one group of participants (G1) and limited in the other one (G2). We found that the non-use reduced the excitability of the left M1 and decreased the inhibitory influence onto the right hemisphere in the two groups. However, an increase in the excitability of right M1 and a deeper inhibitory interaction onto the left hemisphere were evident only in G1. Thus, modifications in the right M1 were not directly produced by the non-use but would depend on the overuse of the “not-immobilized” arm. Our findings suggest that the balance between the two M1s is strongly use dependent.
Introduction
In the human brain, homologous regions of the primary motor cortices (M1s) are connected through transcallosal fibers (Wahl et al., 2007). These connections seem to be mainly of the inhibitory type and may be measured assessing interhemispheric inhibition (IHI) via transcranial magnetic stimulation (TMS) (Ferbert et al., 1992). Precisely, transcallosal inhibition presumably involves GABAergic inhibitory interneurons, because the corpus callosum itself consists of glutamatergic excitatory fibers (Asanuma and Okuda, 1962; Jenny, 1979). Several evidences suggested that the interhemispheric communication between M1s plays a major role in the control of unimanual hand movements and that the strength of these connections are dependent on the arm use. Indeed, during the execution of unimanual finger movements, the contralateral M1 inhibits deeper the ipsilateral one through the transcallosal pathway (Duque et al., 2007), inducing a decrease of ipsilateral M1 excitability (Liepert et al., 2001). Furthermore, studies in patients with stroke in motor areas reported an increase of activity in the intact M1 (Liepert et al., 2000) and an abnormally high IHI from the intact to the damaged M1, more prominent in cases with greater motor impairment (Murase et al., 2004). Whether these modifications in cortical excitability are attributable to the disuse of the affected hand or to a compensatory overuse of the unaffected hand (Liepert et al., 2000; Hummel and Cohen, 2006) is not clear. Altogether, these findings raised the hypothesis that reduction of excitability in the intact hemisphere may contribute to improvements of motor function in stroke (Hummel and Cohen, 2006). Accordingly, previous studies in healthy volunteers showed that downregulation of excitability in one motor cortex, by repetitive TMS (rTMS) on that area, results in an increased excitability of the opposite motor cortex and an improved motor performance of the ipsilateral hand (Gilio et al., 2003; Kobayashi et al., 2004; Avanzino et al., 2008). Here, to directly investigate cortical modifications induced by an abnormal asymmetric use of the two limbs, we propose an experimental approach based on short-term hand immobilization in healthy volunteers. Indeed, recent studies demonstrated that even a short period of upper limb immobilization leads to a reduction of cortical activity of the contralateral M1 (Huber et al., 2006). However, whether hand non-use influences the ipsilateral M1 excitability and the interhemispheric interaction has not been evaluated. Furthermore, to what extent cortical changes may also be attributable to the use of the not-immobilized arm has to be estimated. Toward this goal, we investigated both the excitability of the two M1s and transcallosal interaction between them in healthy subjects whose right hand was immobilized and whose left not-immobilized arm was completely free to move in one group of participants (G1) and limited in the other one (G2). We expected that short-term non-use might induce, in addition to downregulation of the contralateral M1, upregulation of the ipsilateral one and modifications of interhemispheric interactions between the two M1s. Furthermore, if the use of the “not-immobilized” arm influences these changes, different effects should be observed in the two groups of subjects.
Materials and Methods
Subjects.
Nineteen subjects were recruited for the study and were divided in two groups (G1, 12 subjects; G2, 7 subjects). The two groups were matched for age and gender (G1, mean age, 25.4 ± 3.0 years, six females; G2, mean age, 25.0 ± 2.0 years, three females). All participants were right handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). All subjects were naive to the purpose of the experiment. They reported no previous history of neurological disorders or orthopedic problems for the right hand. Subjects had no contraindication to TMS, and they participated in this study after giving an informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Immobilization procedure.
All subjects were instructed not to move their right hand for 10 h from the morning (8:00 A.M.) to the evening (6:00 P.M.). To prevent any right hand movement, subjects wore a soft bandage, typically used in everyday clinical practice by physiotherapists. Also a cotton support was given to the participants to hold the arm and keep it in a comfortable way during the 10 h of hand immobilization.
Monitoring left-arm activity.
Participants were divided in two groups regarding the activity of the left not-immobilized arm. In the first group (G1), participants received no instructions concerning the left-arm use, which was completely free to move. In the other group (G2), we asked the subjects to limit the left-arm movements, trying to use it as usual. In the day before immobilization, all participants were completely free to move. They spent both days in the laboratory doing similar daily life activities (i.e., reading or working at the computer).
To quantitatively check whether participants were able to respect the given instructions concerning the not-immobilized limb, we monitored the physical activity duration of the left arm. To this aim, subjects wore an accelerometer set up in a multisensor actigraph (InnerView Professional, SenseWear PRO Armband) on their left forearm for 2 d, 1 d before and during the immobilization, from approximately 8:00 A.M. to 6:00 P.M. every day. The multisensor actigraph records the cumulative amount of time spent (in minutes) during physical activity at a certain level of energy expenditure by means of mathematical algorithms. Data were sampled at 32 Hz. The energy cost of an activity can be measured in units called METs (metabolic equivalent). Using the definition for 1 MET as the ratio of work metabolic rate to a standard resting metabolic rate, 1 MET is considered a resting metabolic rate obtained during quiet sitting. Here, to record physical activity duration, we set a threshold level of 1.5 METs that is usually the energy expenditure during deskwork (Ainsworth et al., 2000).
Electromyography recording.
Electromyography (EMG) was recorded with silver disc surface electrodes placed in a tendon belly arrangement over the bulk of the first dorsal interosseus (FDI) muscle and the first metacarpophalangeal joint bilaterally. The ground electrode was placed at the elbow. EMG signals were amplified and filtered (20 Hz to 1 kHz) with a D360 amplifier (Digitimer). The signals were sampled at 5000 Hz, digitized using a laboratory interface (Power1401; Cambridge Electronics Design), and stored on a personal computer for display and later offline data analysis. Each recording epoch lasted 400 ms, of which 100 ms preceded the TMS. Trials with background EMG activity were excluded from analysis.
Transcranial magnetic stimulation.
We tested cortical excitability of left and right M1s by means of recruitment curve (RC) and interhemispheric communication between the two M1s assessing IHI. TMS procedure was performed 1 d before and immediately after 10 h of hand non-use, always at approximately 6:00 P.M.
For RC study, TMS was performed with a single Magstim 200 magnetic stimulator (Magstim Company) connected with a figure-of-eight coil with wing diameters of 70 mm. For IHI study, TMS was given through two Magstim 200 stimulators, one connected to a figure-of-eight coil with wing diameters of 70 mm (test stimulus) and the other connected to a small figure-of-eight coil with wing diameters of 50 mm (conditioning stimulus) (Magstim Company). The coils were placed tangentially to the scalp with the handle pointing backward and laterally at a 45° angle to the sagittal plane inducing a posteroanterior current in the brain. This orientation was chosen based on the findings that the lowest motor threshold is achieved when the induced electrical current flows approximately perpendicular to the line of the central sulcus (Werhahn et al., 1994). We determined the optimal position for activation of the left and right FDI muscles by moving the coil in 0.5 cm steps around the presumed motor hand area. Resting motor threshold (RMT), defined as the minimum stimulus intensity that produced a MEP of at least 0.05 mV in 5 of 10 consecutive trials, was found and expressed as a percentage of maximum stimulator output (MSO).
RCs were tested in all the subjects of G1 and G2, while IHI was studied in 9 subjects of G1 and in all subjects of G2. Three subjects of G1 were excluded from the study of interhemispheric connectivity because before immobilization we did not find an inhibition of at least 50% and therefore it might be difficult to evaluate possible changes in IHI after immobilization.
Recruitment curve (RC).
RC was examined by measuring peak-to-peak amplitude (expressed in mV) of motor evoked potentials (MEPs) elicited at stimulus intensities of 5, 10, 15, 20, and 25% of MSO above RMT (calculated on the values of the individual RMT obtained each day). Ten trials were recorded at each stimulus intensity, and the average MEP amplitude was taken as MEP size.
Interhemispheric inhibition.
IHI both from left to right (LtoR) and from right to left (RtoL) M1s was tested after a randomized conditioning-test design reported previously (Ferbert et al., 1992). A suprathreshold conditioning stimulus (CS) was given to one hemisphere before a test stimulus (TS) delivered to the other side. The TS was adjusted to produce an MEP of ∼1 mV peak-to-peak amplitude. The CS was set at 130% of RMT. IHI was measured at five interstimulus intervals (ISIs): the CS was given 6, 7, 8, 9, and 10 ms before the TS. Stimuli were randomly delivered in one set of 70 trials: 50 conditioned, 10 for each ISI, and 20 unconditioned. IHI was expressed as the ratio between the mean peak-to-peak MEP amplitude in conditioned versus unconditioned trials. In each day, CS was adjusted on the basis of the individual RMT found in that session, and TS was adjusted to obtain a 1 mV MEP amplitude.
Statistical analysis.
To check that the experimental instructions about the use of the left arm during the period of hand non-use were respected by the two groups of subjects, we compared the left-arm physical activity duration measured the day before and during immobilization by means of repeated-measures (RM) ANOVA with group (G1 and G2) as between-subjects and time (before and during immobilization) as within-subjects factors.
To evaluate the effect of immobilization on cortical excitability, RMT of left and right hemisphere was compared before and after immobilization by means of a paired t test in G1 and G2 separately. TMS recruitment curve data were analyzed, separately for each group and separately for each hemisphere by means of an RM-ANOVA with time (before and after immobilization) and intensity (+5, +10, +15, +20, and +25% of MSO) as within-subjects factors. Similarly, to assess the effect of immobilization on interhemispheric inhibition, LtoR IHI and RtoL IHI of G1 and G2 were subjected separately to an RM-ANOVA with time and ISI (6, 7, 8, 9, and 10 ms) as within-subjects factors. Then, to evaluate whether there was any difference in the effect of immobilization on the basis of the use of the left arm, for each parameter (RC left M1, RC right M1, LtoR IHI, and RtoL IHI), we ran an RM-ANOVA with the within-subjects factors described previously (i.e., time and intensity for RC; time and ISI for IHI) and with group (G1 and G2) as between-subjects factor. Significance threshold was set at p < 0.05. If ANOVA showed a significant interaction effect, we performed post hoc comparisons using the least significance difference (Fisher's) test to directly compare the experimental factors. All statistical analyses were performed by using SPSS 13.0. Data are presented as mean ± SE.
Results
Preliminary note
All subjects tolerated TMS procedure and were able to comply with the given instructions regarding the left-arm use as confirmed by the physical activity duration recorded through the accelerometer. Accordingly, statistical analysis revealed a significant interaction of group × time (F(1,17) = 11.89, p = 0.005), and post hoc analysis showed that in G1 the physical activity duration of the left arm increased during immobilization (p = 0.005), whereas in G2 it did not change (p = 0.20).
Cortical excitability in G1and G2
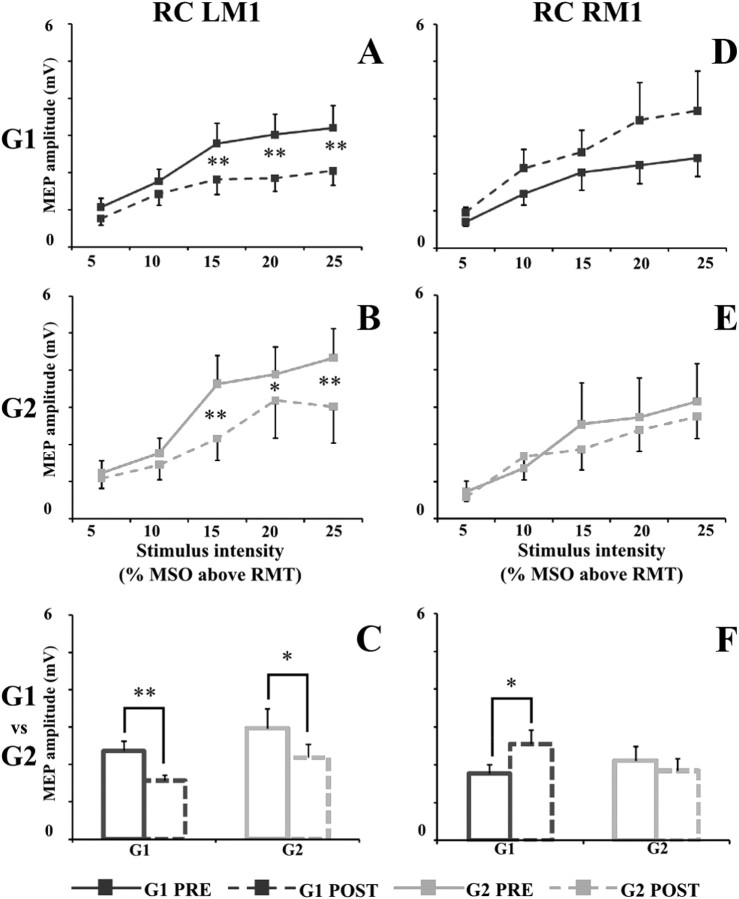
In G1, after immobilization, cortical excitability of the left and right M1changed (Fig. 1A,D). Resting motor threshold of left M1 significantly increased (from a value of 36.3 ± 1.7 to a value of 37.7 ± 1.7; p = 0.006). Cortical excitability of left M1 strongly decreased after immobilization (time × intensity, F(4,44) = 3.40, p = 0.017), and post hoc analysis revealed that MEP size recorded in “post” at +15, +20, and +25% of MSO was significantly lower than MEP size recorded in “pre” (+15%, p = 0.00099; +20%, p = 0.00021; +25%, p = 0.00022) (Fig. 1A). Differently, RMT of the right hemisphere did not change (from a value of 38.1 ± 1.2 to a value of 37.1 ± 1.3; p = 0.13), whereas cortical excitability of the right M1, when tested with RC, increased after immobilization at all the stimulation intensities (time, F(1,11) = 6.51, p = 0.026; time × intensity, F(4,44) = 2.01, p = 0.11) (Fig. 1D).
Figure 1.
Left and right motor cortex RC in G1 (dark gray square) and G2 (light gray square) before (PRE; solid line) and after (POST; dashed line) immobilization. A (G1) and B (G2) illustrate the MEP recruitment curve (peak-to-peak amplitude, in millivolts, on the ordinate) of left motor cortex (LM1), whereas D (G1) and E (G2) illustrate the MEP recruitment curve of right motor cortex (RM1). On the abscissa, the stimulus intensities are shown (5, 10, 15, 20, and 25% of MSO above RMT). On C and F, the mean values of MEP sizes in G1 (dark gray) and G2 (light gray) before (solid line) and after (dashed line) immobilization are shown. Data are represented as mean values ± SE. In A, B, D, and E, asterisks indicate significant difference between pre and post values when interaction of time × intensity was statistically significant. In C and F, asterisks indicate the main effect of time. *p < 0.05, **p < 0.01.
In G2, after immobilization, left M1 RMT increased (from 37.7 ± 1.2 to 39.5 ± 1.2; p = 0.05), and cortical excitability, when tested with RC, significantly decreased after immobilization (time × intensity (F(4,24) = 3.77, p = 0.016). Post hoc analysis revealed that MEP size recorded in post at +15, +20, and +25% of MSO was significantly lower than MEP size recorded in pre (+15%, p = 0.00006; +20%, p = 0.03; +25%, p = 0.0002) (Fig. 1B). Differently from G1, in G2, neither the RMT (from a value of 37.1 ± 1.3 to a value of 36.6 ± 1.0; p = 0.51) nor cortical excitability of the right M1, when tested with RC, changed after immobilization (time, F(1,6) = 0.40, p = 0.55; time × intensity, F(4,24) = 0.73, p = 0.58) (Fig. 1E).
Figure 1, C and F, shows the mean values of MEPs across the different stimulation intensities in G1 and G2 before and after immobilization.
Interhemispheric inhibition in G1and G2
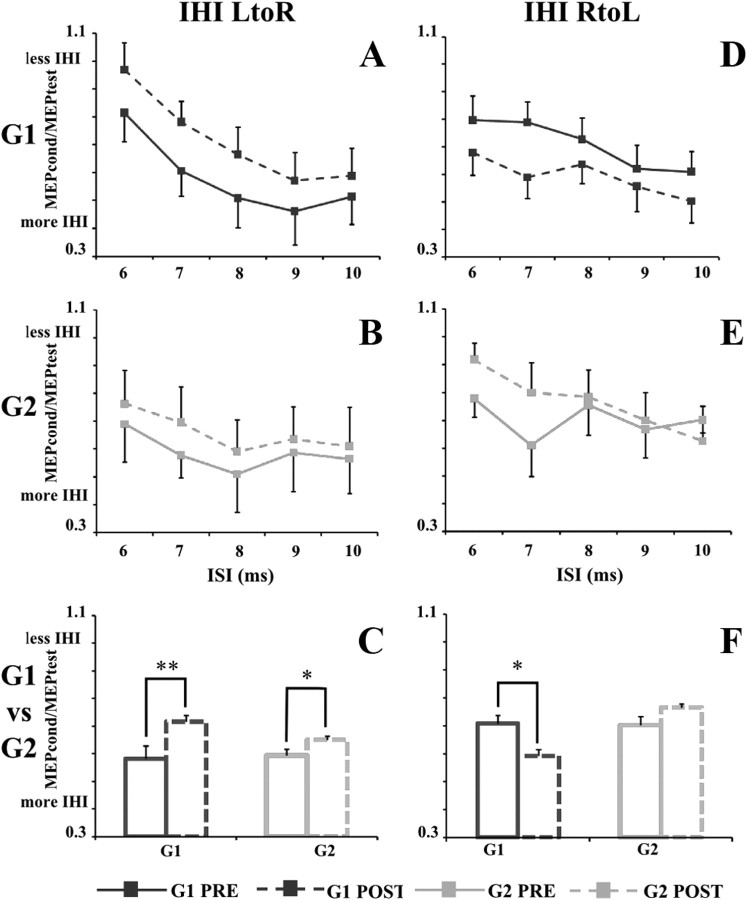
In G1, after immobilization, interhemispheric inhibition between the two hemispheres (LtoR and RtoL) changed (Fig. 2A,D). LtoR IHI was markedly attenuated (time, F(1,8) = 11.8, p = 0.008). The decrease in LtoR IHI was consistently observed at all the ISIs evaluated with no interaction between ISI and time (F(4,32) = 0.33, p = 0.85). On the contrary, RtoL IHI increased (main effect of time, F(1,8) = 7.72, p = 0.024). Again, the increase in RtoL IHI was consistently observed at all the ISIs evaluated with no interaction between ISI and time (F(4,32) = 0.79, p = 0.54).
Figure 2.
IHI from LtoR and RtoL hemispheres in G1 (dark gray square) and G2 (light gray square) before (PRE, solid line) and after (POST, dashed line) immobilization. A (G1) and B (G2) illustrate LtoR IHI expressed as the ratio between the mean peak-to-peak MEP amplitude in conditioned versus unconditioned trials (MEPcond/MEPtest on the ordinate), whereas D (G1) and E (G2) illustrate the RtoL IHI. On the abscissa, the ISIs (6, 7, 8, 9, and 10 ms) are shown. On C and F, the mean values of IHI in G1 (dark gray) and G2 (light gray) before (solid line) and after (dashed line) immobilization are shown. Data are represented as mean values ± SE. In A, B, D, and E, asterisks indicate significant difference between pre and post values when interaction of time × ISI was statistically significant. In C and F, asterisks indicate the main effect of time. *p < 0.05, **p < 0.01.
In G2, after immobilization, only LtoR IHI changed, whereas RtoL IHI remained stable (Fig. 2B,E). LtoR IHI was attenuated (time, F(1,6) = 9.45, p = 0.02). The decrease in LtoR IHI was consistently observed at all the ISIs evaluated, with no interaction between ISI and time (F(4,24) = 0.37, p = 0.83). Differently, RtoL IHI did not change after immobilization as revealed by RM-ANOVA (time, F(1,6) = 1.21, p = 0.31; time × ISI, F(4,24) = 0.56, p = 0.67).
Figure 2, C and F, shows the mean values of IHI across the different ISIs in G1 and G2 before and after immobilization.
Comparison between groups: RC and IHI in G1 and G2
When comparing RC of G1 and G2, statistical analysis revealed that cortical excitability of the left M1 was similarly modified by immobilization in the two groups (Fig. 1C) (group, F(1,17) = 0.95, p = 0.34; group × time, F(1,17) = 0.000001, p = 0.99; group × time × intensity, F(4,68) = 0.88, p = 0.48). Differently, because in G2 RC of the right M1 did not change after immobilization, RM-ANOVA showed a significant interaction of group × time (F(1,17) = 4.19, p = 0.045). Post hoc analysis showed that, whereas in G1 MEP size recorded in post was significantly higher than that measured in pre (p = 0.02), in G2 MEP size was similar in the two testing days (p = 0.54) (Fig. 1F).
Concerning interhemispheric inhibition, statistical analysis revealed that LtoR IHI followed the same trend in the two groups after immobilization (group, F(1,14) = 0.04, p = 0.85; group × time, F(1,14) = 1.49, p = 0.24; group × time × ISI, F(4,56) = 0.06, p = 0.99) (Fig. 2C). Differently, because immobilization did not induce any change in RtoL IHI in G2, RM-ANOVA showed a significant interaction group × time (F(1,14) = 7.12, p = 0.018). Post hoc analysis showed that, whereas in G1 RtoL IHI significantly increased after immobilization (p = 0.02), in G2 there was no difference in RtoL IHI between the two testing days (p = 0.27) (Fig. 2F).
Discussion
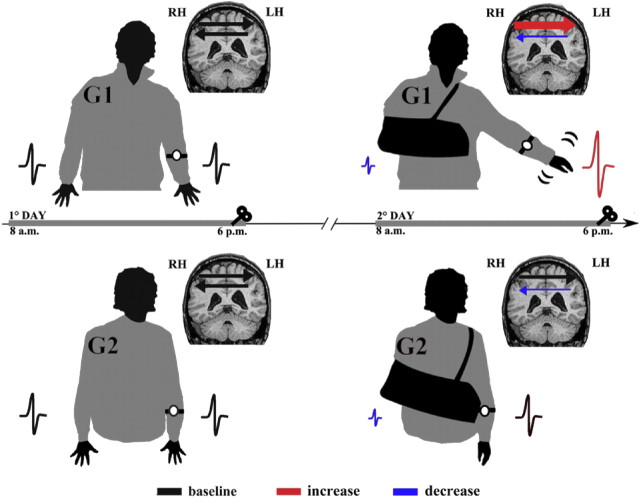
In the present study, we showed that 10 h of right-hand non-use induced a decrease in cortical excitability of the left M1 and a reduction of IHI from the left hemisphere to the right one. Furthermore, we found that changes in cortical excitability of right M1 and IHI from the right to left hemisphere were dependent on the use of the left, not-immobilized, arm. In fact, only in the group of subjects free to move the left arm (i.e., G1), we observed an increase in cortical excitability of the right M1 and a deeper IHI from the right to the left M1 after immobilization. A summary of these findings is graphically shown in Figure 3.
Figure 3.
A schematic view of the experimental design and main results. All participants in G1 and G2 were instructed not to use the dominant (right) hand for 10 h, wearing a soft bandage. The use of the left, not-immobilized arm was monitored by means of an actigraph for 10 h during the period of immobilization (2° day) and 1 d before (1° day). MEP size decreased in the left hemisphere (reduced MEP in blue, compare with black one) in both G1 and G2, whereas it increased in the right hemisphere only in G1 (increased MEP in red, compare with black one). LtoR IHI was reduced in both G1 and G2 (thin arrow in blue, compare with black one), whereas RtoL IHI was deeper only in G1(thick arrow in red, compare with black one).
Immobilization effect
TMS of the motor cortex has been used to study plastic changes in cortical excitability associated with immobilization (Liepert et al., 1995; Zanette et al., 1997, 2004; Facchini et al., 2002; Huber et al., 2006). Although these studies yielded conflicting findings, a likely result was that even a short-term non-use is able to induce a decrease of cortical excitability in the motor area related to the restricted muscles (Facchini et al., 2002; Huber et al., 2006), probably because of the induction of local synaptic depression (Huber et al., 2006).
To our knowledge, this is the first study showing that transcallosal interaction can be modulated by short-term hand non-use. Recent works showed that the activity of transcallosal and corticospinal neurons are modulated by similar interneuron population (Trompetto et al., 2004; Avanzino et al., 2007). Therefore, a possible neurophysiological explanation of short-term non-use effect on transcallosal interaction might deal with changes in the excitability of the population of interneurons that controls both transcallosal and corticospinal neurons, thus inducing similar effects in the two neural systems. In agreement with this hypothesis, it has been shown that the administration of an inhibitory protocol on left M1 by means of rTMS was able to decrease the corticospinal excitability of left M1 and transcallosal pathway activity (i.e., IHI from left to right M1s) (Pal et al., 2005). Furthermore, a reduction in transcallosal pathways activity (LtoR IHI) induced by a corticocortical associative stimulation protocol elicited an increase of the right M1 corticospinal excitability (Rizzo et al., 2009, 2011). Behaviorally, the reduction of the inhibitory control from the left to the right motor cortex may be instrumental to release the right motor cortex to facilitate unilateral movements in the left hand.
All these findings are in accordance with previous studies based on short-term deprivation of sensory inputs by ischemic nerve block. In fact, an acute upper limb deafferentation induced a focal increase in the excitability of the non-deafferented hand motor cortex (Werhahn et al., 2002a; Floel et al., 2008) together with improvements in the non-deafferented hand in either tactile spatial acuity in healthy subjects (Werhahn et al., 2002b) and in hand motor performance in patients with stroke (Floel et al., 2008).
Use-dependent effects
A remarkable result was that only when subjects were free to move the left hand (G1) we observed significant changes in right M1 excitability, in either the corticospinal and transcallosal pathways. Indeed, these subjects showed an increase in the quantity of movement of the left arm during the immobilization period (i.e., overuse), together with an increased excitability of the corticospinal and transcallosal neurons of right M1. Differently, participants of G2, instructed to limit the left-arm activity during immobilization, despite the similar changes in LtoR IHI as observed in G1, showed no changes in the right M1 excitability. Therefore, the modifications observed in the right M1 are likely to be dependent on the left-arm use and not only on the modification of the LtoR IHI observed in both groups. This hypothesis is in accordance with recent results by Granert et al. (2011) showing that, in patients affected by writer's cramp, 4 weeks of right-hand immobilization induced a mirrored pattern of gray matter volume changes in left (decrease) and right (increase) M1. Modifications in right M1 has been interpreted by the authors as linked to the “forced” use of the not-immobilized hand for daily manual skills during immobilization. Furthermore, on the basis of recent works on the role of GABA in practice-dependent plasticity (Ziemann et al., 2001), we hypothesize that a fundamental role in modulating right M1 excitability is played by the interaction between the “forced” use of the left arm and the changes in LtoR IHI induced by right-hand immobilization. Accordingly, in literature, it has been shown that plasticity is facilitated by a decrease of GABA-related inhibition (Ziemann et al., 1998) and that motor practice performed without a previous downregulation of the inhibitory GABAergic activity induced only mild or no changes in cortical excitability (Ziemann et al., 2001). Here, we can assume that, after immobilization, the disinhibition of right M1 likely attributable to the reduced activity of transcallosal fibers from left M1 onto intracortical GABAergic interneurons in right M1 could act together with the engagement of the sensorimotor system induced by the increase of left-arm use. This interaction might induce a positive aftereffect on right M1 excitability through an Hebbian potentiation mechanism (Hebb, 1949; Avanzino et al., 2009). However, an investigation of the changes in GABA-related intracortical inhibition induced by hand immobilization on the right M1 should be addressed in future studies to confirm this hypothesis.
Motor cortex activity and proprioception
We can suppose that the effect on M1 activity observed in the present work was not only attributable to the absence of voluntary movement during hand non-use but also to the reduction of proprioceptive information from the immobilized hand. Indeed, using EEG recording, Huber et al. (2006) described a significant reduction of the amplitude of the P45 component after a short-term arm immobilization. P45 classically represents the proprioceptive information processing within the sensorimotor areas (Allison et al., 1992). These findings could suggest that the reduction in proprioceptive information processing recorded after short-term immobilization could be mainly attributable to a decrease of the proprioceptive input inflow from right-arm muscle spindles. Presumably, Ia input, which is related to perceived arm motion, could be here the mostly affected (i.e., dynamic proprioception) (Burke et al., 1976). More, in animal models, it has been demonstrated that this input is thought to have direct access to contralateral sensory and motor cortical areas (Heath et al., 1976; Hore et al., 1976). Furthermore, recent work by Naito et al. (2002) showed that, in humans, M1 neurons react also to proprioceptive stimuli (i.e., hidden sensory neurons). This suggests a fundamental link between dynamic proprioceptive information and the excitability of M1 in humans. Therefore, some investigators (Rosenkranz and Rothwell, 2003; Swayne et al., 2006) demonstrated that the vibration of hand and forearm muscles, activating Ia fibers, can modify the excitability of both M1s as well as the transcallosal interaction between them. This finding underlies the effect of proprioceptive input on that population of interneurons modulating both corticospinal and transcallosal neurons. In the same vein, the increased excitability recorded in G1 in the right M1 could be attributable to the enhancement of proprioceptive input produced by the left-arm overuse.
Relevance of the present findings
In conclusion, we demonstrated that a brief period of right-hand non-use decreased the excitability of left M1 and reduced interhemispheric inhibition from left-to-right hemisphere. Moreover, right-hand non-use induced an increase of the excitability of right M1 and a deeper IHI from right-to-left hemisphere only in those subjects who overused the left arm. This motor behavior probably had the aim to compensate for the right-hand non-use attributable to immobilization procedure. Together, these results confirm the hypothesis of a fundamental role played by voluntary movement in the balance between the two M1s. Indeed, arm immobilization creates an important imbalance between the use of the two arms. An alternative approach in investigating this topic could deal with short-term period of left-hand overuse without any immobilization of right hand. Nevertheless, the immobilization procedure used in the present work was also designed to reproduce in healthy subjects, for a short period, an already developed neuro-rehabilitative protocol, such as the constraint-induced movement therapy (CIMT). CIMT is a current approach to stroke rehabilitation that implies the massed practice of the affected arm by restraining, through immobilization, the unaffected limb (Langhorne et al., 2009; Sirtori et al., 2009). Stroke is a classical model of an unbalanced activity of the two M1s supported by an abnormal stronger interhemispheric communication from the intact to the lesioned hemisphere (Hummel and Cohen, 2006). In stroke patients, CIMT has been demonstrated to induce an enhanced neuronal excitability in the damaged hemisphere (Liepert et al., 1998) functionally relevant in motor recovery.
Taking advantage of this study, we can speculate that CIMT might improve motor function in stroke, restoring a normal interhemispheric communication from intact to lesioned hemisphere, and that the amount of work done with the affected limb is crucial in determining the positive rehabilitation outcome.
Footnotes
We thank Dr. Alessandro Giannini for help with the collection of TMS data.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC. The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalogr Clin Neurophysiol. 1992;84:301–314. doi: 10.1016/0168-5597(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Bove M, Trompetto C, Tacchino A, Ogliastro C, Abbruzzese G. 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci. 2008;27:1285–1291. doi: 10.1111/j.1460-9568.2008.06086.x. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Bove M, Tacchino A, Trompetto C, Ogliastro C, Abbruzzese G. Interaction between finger opposition movements and aftereffects of 1Hz-rTMS on ipsilateral motor cortex. J Neurophysiol. 2009;101:1690–1694. doi: 10.1152/jn.90428.2008. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Facchini S, Romani M, Tinazzi M, Aglioti SM. Time-related changes of excitability of the human motor system contingent upon immobilisation of the ring and little fingers. Clin Neurophysiol. 2002;113:367–375. doi: 10.1016/s1388-2457(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, Deuschl G, Zeuner KE, Siebner HR. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage. 2011;54:32–41. doi: 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Hore J, Phillips CG. Inputs from low threshold muscle and cutaneous afferents of hand and forearm to areas 3a and 3b of baboon's cerebral cortex. J Physiol. 1976;257:199–227. doi: 10.1113/jphysiol.1976.sp011364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Hore J, Preston JB, Cheney PD. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hind limb muscles in the baboon. J Neurophysiol. 1976;39:484–500. doi: 10.1152/jn.1976.39.3.484. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–145. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron. 2002;36:979–988. doi: 10.1016/s0896-6273(02)00980-7. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pal PK, Hanajima R, Gunraj CA, Li JY, Wagle-Shukla A, Morgante F, Chen R. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J Neurophysiol. 2005;94:1668–1675. doi: 10.1152/jn.01306.2004. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex. 2009;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Bove M, Naro A, Tacchino A, Mastroeni C, Avanzino L, Crupi D, Morgante F, Siebner HR, Quartarone A. Associative cortico-cortical plasticity may affect ipsilateral finger opposition movements. Behav Brain Res. 2011;216:433–439. doi: 10.1016/j.bbr.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirtori V, Corbetta D, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in stroke patients. Cochrane Database Syst Rev. 2009;7:CD004433. doi: 10.1002/14651858.CD004433.pub2. [DOI] [PubMed] [Google Scholar]
- Swayne O, Rothwell J, Rosenkranz K. Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol. 2006;117:855–863. doi: 10.1016/j.clinph.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–140. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002a;125:1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002b;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol. 1997;105:269–279. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–1275. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced short-term plasticity in human motor cortex. J Neurosci. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]