Abstract
Background:
Ciclopirox (CPX) has been used as an antifungal agent in various formulations to treat superficial fungal infection for decades. Its effectiveness and safety in treatments have been demonstrated by multiple studies.
Methods:
Here we briefly summarize the pharmacological and toxicological properties of CPX as an antifungal agent, the new medical uses of CPX, as well as the correspondent molecular mechanisms.
Results:
Increasing evidence has demonstrated that CPX is able to inhibit tumor growth, ameliorate diabetes and its complications, prevent human immunodeficiency virus (HIV) infection, and improve age-associated cardiovascular defects. Interestingly, its antifungal activity and all those newly observed effects are more or less related to its capability of chelating iron and interfering with the related signaling pathways. Mechanistically, CPX is capable of modulating the activities of certain enzymes or signaling pathways, such as ribonucleotide reductase (RR), deoxyhypusine hydroxylase (DOHH)/eukaryotic translation initiation factor 5A (eIF5A), Wnt/β-catenin, hypoxia-inducible factor-1α (HIF-1 α)/vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 3 (VEGFR-3)/extracellular signal-regulated protein kinases 1/2, mammalian target of rapamycin, and cyclin dependent kinases (CDKs). Most of these activities are related to its chelation of iron.
Conclusion:
CPX, as an antifungal agent, may be repositioned for treatment of cancer and other human diseases.
Keywords: Ciclopirox, iron chelator, fungicide, cancer, diabetes, HIV
1. INTRODUCTION
Ciclopirox (CPX) is a synthetic antifungal agent, which was introduced into market in the early 1980s and has been often used as an olamine salt, ciclopirox olamine (also called Batrafen, Loprox, Mycoster, Penlac, and Stieprox) [1]. The chemical name of CPX is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone (CAS Number: 29342-05-0) (Fig. 1), with a molecular formula of C12H17NO2, and a molecular weight of 207.27. CPX is a white or light yellow powder, with a melting point of 128–130°C [2]. It is hardly soluble in water, but very soluble in methanol, ethanol and dimethylsulfoxide [3].
Fig. (1).
Chemical structure of ciclopirox olamine.
As a family member of hydroxypyridone, both CPX and its olamine salt possess a broad-spectrum of antifungal activity [4], inhibiting the growth of most pathogenic yeasts, moulds, and dermatophytes [1]. There is no difference in pharmacological actions between CPX and its olamine salt [1]. The antifungal activity of 0.77% ciclopirox is roughly equal to that of 1% ciclopirox olamine, because the olamine entity has no antifungal activity [4].
Most of topical antimycotic drugs inhibit fungal growth primarily by impairing the function and the integrity of cell membrane [5]. Although CPX has been clinically used as a fungicide for decades, its antifungal mechanism is still not well understood. It has been proposed that CPX inhibits the growth of fungi by suppressing the function of certain enzymes (e.g. catalase and peroxidase) and other components of cellular metabolism [6], and by disrupting DNA repair, cell division, and structures (mitotic spindles) [7]. Of importance, CPX is able to chelate metal ions (such as Fe3+) [1]. However, the underlying linkage between the metal chelation ability and the antifungal activity of CPX remains to be elucidated.
While introduction of many new drugs did not prevent the occurrence of drug resistance in antifungal therapy [8], CPX was among the minority exempted from being detoxified by pathogens after it had been applied to clinical treatment of superficial mycoses for about three decades [1]. This unusual exemption was proposed to stem from the unique antifungal mechanism of CPX, which also contributes to its broad antifungal activity [9].
Currently, various formulations (suspension, cream, gel, and shampoo) of CPX are commercially available for antimycotic purpose [1]. Also, CPX is used in lacquers for topical treatment of onychomycosis (fungal infections of the nails) [1].
Of great interest, CPX has recently been found to have considerable potential to act against many other diseases including cancer [10–12], diabetes [13], acquired immune deficiency syndrome (AIDS) [14], cardiovascular disorders [15, 16], inflammation [4], and bacterial infection [4]. These findings implicate that CPX is a very promising agent in treatment and prevention of multiple diseases. Here we briefly summarize the pharmacological and toxicological properties of CPX as an antifungal agent, the therapeutic potential of CPX for cancer and other human diseases, as well as the correspondent molecular mechanisms.
2. PHARMACOLOGY AND TOXICOLOGY OF CPX
Studies have shown that when 1% ciclopirox olamine cream was topically applied to human skin, only approximately 1.3% of the dose was systematically absorbed [17]. Such absorption was through epidermis and hairfollies [18]. Following the topical use, the peak serum concentration (10 μg/L) of CPX appeared in 6 hours [17]. The majority (94–98%) of absorbed CPX bound to proteins, and the concentration of protein-binding CPX in serum was 0.01–11 μg/mL [18]. The biological half-life of CPX was about 1.7 hours [18]. Detected by utilizing 14C-labeled CPX, the excretion of both topically and vaginally administrated CPX was through urine and feces in dogs, primarily as a glucuronide [18], indicating that glucuronidation is the primary metabolizing route for CPX in the body.
In rats and mice, LD50 values of ciclopirox olamine are orally between 2,500 and 1,700 mg/kg, subcutaneously between 2,500 and 1,700 mg/kg, intraperitoneally between 172 and 83 mg/kg, and intravenously between 79 and 71 mg/kg [19]. In addition, oral administration of ciclopirox olamine did not affect body temperature, urine excretion, and blood coagulation in dogs [20]. Furthermore, oral administration of ciclopirox olamine at 30 mg/kg for 4 weeks or at 10 mg/kg for 3 months did not exhibit any toxic symptom (e.g. gross organ toxicity and body weight loss), revealing a favorable therapeutic index of CPX [20]. More convincingly, up to 10 μM serum concentrations of CPX were achievable after repeated administration of the compound to rats and dogs, and were not toxic [11].
According to the document from the US Food and Drug Administration [21], when CPX (1% and 5% solutions in polyethylene glycol 400) was topically given to female mice twice per week for 50 weeks, followed by a 6-month drug-free observation period prior to necropsy, no evidence of tumors was observed at the application sites, indicating that CPX is not carcinogenic. Besides, in vitro (human A549 cells and BALB/c 3T3 cells) and in vivo (Chinese hamster bone marrow) gene mutation assays also revealed that CPX is negative in mutagenicity [3]. Furthermore, the results from the studies in mice, rats, rabbits, and monkeys after oral or topical administration of CPX did not show any significant fetal malformations, suggesting that CPX does not cause teratogenicity. In addition, no reports have shown embryo toxicity or reproductive toxicity in human [20]. However, nursing women are suggested to consult their doctors before use, since it is not clear whether CPX passes into human milk [22]. Taken together, the pharmacological and toxicological profiles reveal that CPX is an effective and safe antifungal agent.
3. CPX AS AN ANTIFUNGAL AGENT
3.1. Application and Effectiveness
Superficial fungal infection is the most prevalent form of mycoses, affecting at least one fifth of the world’s population [23]. Dermatophytes are the most common pathogens that cause the superficial infections [24]. In general, these infections only deteriorate the quality of life [25]. However, while the hosts are immune-compromised, certain pathogens, like Candida family members that are limited to superficial infection in normal population, can cause systematic infection, which may be life-threatening [23, 25].
The history of CPX as a topical antifungal agent can be traced back to early 1970s [26]. The antifungal spectrum of CPX covers most of clinically identified fungal pathogens including yeasts, molds, and dermatophytes [1]. Its minimum inhibitory concentration range against these pathogens is commonly from 0.9 to 3.9 μg/mL, except from 1.9 to 15.6 μg/mL against molds [1].
There are five major formulations of CPX used in the United States including 0.77% cream, 0.77% suspension, 0.77% gel, 1% shampoo, and 8% lacquer [4]. While the 1% ciclopirox olamine cream is generally used for treatment of skin fungal infections, the 1% shampoo and 8% lacquer of CPX are applied to treat dermatitis and onychomycosis individually [1].
Topical application of 1% ciclopirox olamine cream can achieve inhibitory and fungicidal effects by 93% and 98% in a pig skin model, respectively, which is considerably better than those (<50%) of other topical fungicides [27]. This has been substantiated by the other report that the antifungal activity of 1% ciclopirox olamine cream is higher than that of other antifungal creams at the same concentration [28]. A survey including 991 cases of various skin infections has shown that administration of 1% ciclopirox olamine cream has a cure rate of 96% within 3 weeks [1].
Besides, shampoo containing 1% CPX has an excellent effect against various dermatitis [1]. A double blinded experiment of 102 patients with scalp seborrheic dermatitis has revealed that the improvement and the clear rate in CPX-treated group are significantly higher than that in the placebo-treated group [29]. Another study has compared the anti-dermatitis effects between different concentrations of CPX shampoo, and found that 1% CPX shampoo is much superior to both 0.3% and 0.1% shampoo, which share the same response rate as the vehicle [30]. Consistent with European reports, an American study including 499 patients has revealed that comparing to vehicle treatment, treatment with 1% CPX shampoo for 4 weeks can significantly improve seborrheic dermatitis and relieve the symptom [31]. The local tolerability of CPX is even better than that of vehicle, and less adverse events are identified in CPX-treated group than in the vehicle-treated group [31], implying the satisfactory safety of 1% CPX shampoo in topical use.
In addition to 1% ciclopirox olamine cream and 1% CPX shampoo, the 8% CPX lacquer has also been demonstrated to be effective against nail onychomycosis [32]. Although the effect of CPX on treating onychomycosis is generally not as good as that of the systematic antifungal agents [33], the pharmacoeconomic investigation has shown that the cost-effective figure of CPX in treating nail onychomycosis is greatly superior to that of the systematic drugs such as terbinafine, itraconazole, and fluconazole [34]. Moreover, the systemic antifungal agents have some serious adverse effects (e.g. agranulocytosis) in treatment of onychomycosis [1]. The key limitation on the application of topical agents as anti-onychomycosis drugs is the barrier effect of keratinized structure, which prevents topical agents from permeating to the deep layer of the nail [35]. The newly emerged 8% CPX hydrosoluble lacquer, P-3051, exhibits an excellent anti-onychomycosis effect [36], which is associated with improved efficacy in penetrating into the nail structure [37].
3.2. Adverse Effects of Topical Use of CPX
Topical use of CPX is considerably safe. Less than 5% patients have adverse effects, which are generally limited to local rash, itching, and burning, resulting in redness or pain [1]. Other adverse effects include headache, erythema, nail disorder, pruritus, alopecia, dry skin, facial oedema, and contact dermatitis [19]. Recently, it has been reported that in elderly patients treated with acenocoumarol, an anticoagulant that inhibits vitamin K epoxide reductase, topical use of CPX can increase International Normalized Ratio or cause rectal bleeding [38]. This has been speculated to be due to a possible interaction between CPX with acenocoumarol [38].
3.3. Antifungal Mechanism of CPX
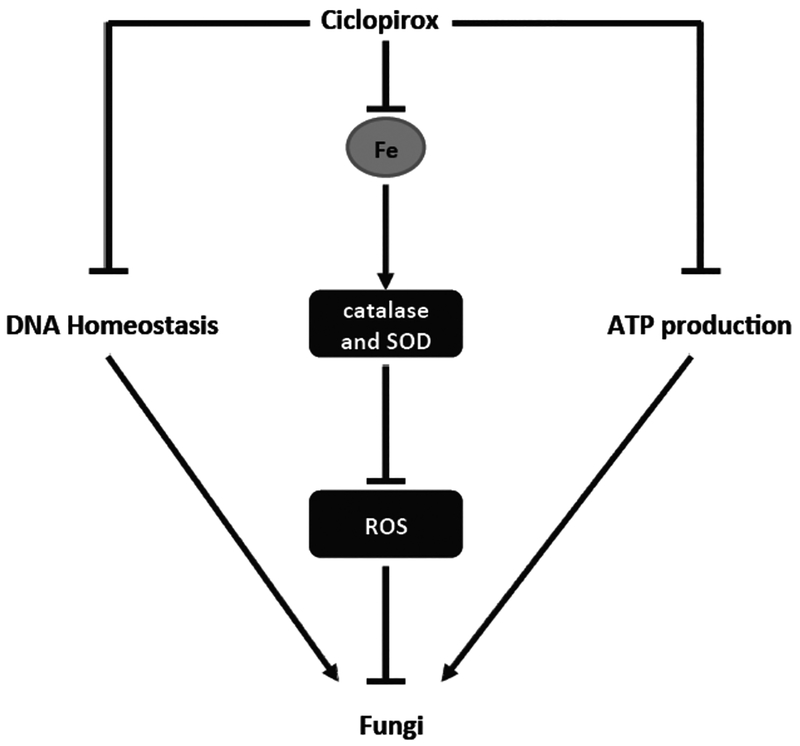
The activities of most topical antifungal agents depend on either disrupting the biosynthesis of ergosterol or interfering with the proper function of ergosterol, an essential component of fungal cell membrane [5]. However, the antifungal mechanism of CPX is still not well understood, although CPX has been clinically used for topical fungal infection for decades. It has been proposed that CPX inhibits the fungal growth by chelating iron and suppressing the enzyme activity responsible for metabolism of reactive oxygen species (ROS) and other components [6], by disrupting DNA repair, cell division, and structures (mitotic spindles) [7], as shown in Fig. (2).
Fig. (2).
Proposed antifungal mechanisms of ciclopirox.
An early study has shown that CPX does not influence the permeability barriers of protoplasts or lecithin liposomes in C. albicans, but inhibits energy (ATP) production, thereby blocking up-take of certain components such as amino acids, as well as potassium and phosphate ions, which are essential for cell growth and survival [39]. Furthermore, CPX disrupts the membrane integrity at high concentrations causing leakage of intracellular materials [9]. However, the above findings remain controversial, as CPX has also been found to neither reduce intracellular ATP level nor do damage to cell membrane in C. albicans [6]. Interestingly, it has been consistently found that CPX can mimic the effect of bipyridine, a well-known iron chelator and also an antifungal agent [3], upregulating the expression of certain genes, such as the high-affinity iron permease gene FTR1 and the low-affinity iron permease gene FTR2, which are essential for iron metabolism [9]. In particular, addition of iron ions (e.g. Fe2+ and Fe3+), but not other metal ions (e.g. Ca2+, Mg2+ and Mn2+), attenuates the antifungal activity of CPX [1], suggesting that the antifungal activity of CPX is attributed to iron chelation.
Further studies have shown that CPX depletion of iron impairs the activities of catalase and superoxide dismutase, two iron-dependent enzymes, which are responsible for scavenging intracellular ROS [6]. Besides, CPX also inhibits the activity of other enzymes related to ROS metabolism, such as glucose-6-phosphate-dehydrogenase and cytochrome c peroxidase [6]. Importantly, C. albicans cells pre-treated with hydrogen peroxide or menadione, which induces the expression of the enzymes involved in detoxification of ROS, are more resistant to CPX [6]. In contrast, the cells pre-cultured under poor oxygen conditions, which decreases the activities of the enzymes for ROS clearance, are more sensitive to CPX [6]. These data strongly support the notion that CPX acts as a fungicide via chelating iron, leading to induction of ROS.
It has also been proposed that the iron chelation capability of CPX not only contributes to its broad spectrum of antifungal activity, but also minimizes the possibility of drug-resistance [9]. In C. albicans, cerebellar degeneration-related protein 1 and 2 (CDR1 and CDR2) are two well-characterized drug-resistant genes [40]. Although CPX induces the expression of CDR1 and CDR2 genes in C. albicans, the pathogen does not show elevated resistance to the compound [9]. More impressively, even after incubation with a sub-inhibitory concentration (0.6 μg/mL) of ciclopirox olamine for 6 months, C. albicans does not develop tolerance to the antifungal agent [9], implying that it is hard for the microbials to develop a proper tactic to bypass the iron-limiting stress. However, the underlying linkage between the iron chelation ability and the antifungal activity of CPX remains to be elucidated.
4. CPX AS AN ANTICANCER AGENT
4.1. The Antitumor Activity of CPX
In early 1990s, CPX was first found to be capable of inhibiting DNA replication [41]. Further studies have revealed that CPX can arrest cell cycle at G1/S transition and induce apoptosis in HeLa cells [42]. Since cancer cells are characterized with hyperproliferation [43], deregulated cell cycle progression [44, 45], and resistance to apoptosis [46], CPX has been considered to be a promising anticancer agent.
Recently, two preclinical studies have further independently demonstrated the antitumor activity of CPX in animal models [11, 12]. Eberhard et al. have shown that oral administration of ciclopirox olamine (20–25 mg/day) inhibits primary acute myeloid leukemia xenograft growth in NOD/SCID mice, but does not exhibit obvious weight loss or gross organ toxicity [11]. Similarly, Zhou et al. have also revealed that oral administration of ciclopirox olamine (25 mg/day) inhibits breast cancer (MDA-MB-231) xenograft growth by 75% in BALB/c nu/nu mice comparing to control group [12]. The antitumor activity of ciclopirox olamine is attributed to inhibition of cell proliferation and induction of cell death [11, 12]. A recent phase I clinical trial of CPX in patients with advanced hematologic malignancies is a major breakthrough in this field [47]. Oral administration of CPX at a dose of 40 mg/m2 daily is able to achieve either hematologic improvement or disease stabilization in 2/3 patients, but does not have obvious toxicity in patients [47].
In addition, CPX can inhibit cell proliferation and angiogenesis in human umbilical vein endothelial cells by inhibiting expression of vascular endothelial growth factor [10], although this is controversial [48]. Recently, CPX has also been found to inhibit the tube formation of lymphatic endothelial cells [49], suggesting inhibition of lymphangiogenesis. As angiogenesis and lymphangiogenesis are critical for tumorigenesis and metastasis [50, 51], these findings further highlight the potential of CPX for cancer prevention and treatment.
4.2. The Anticancer Mechanism of CPX
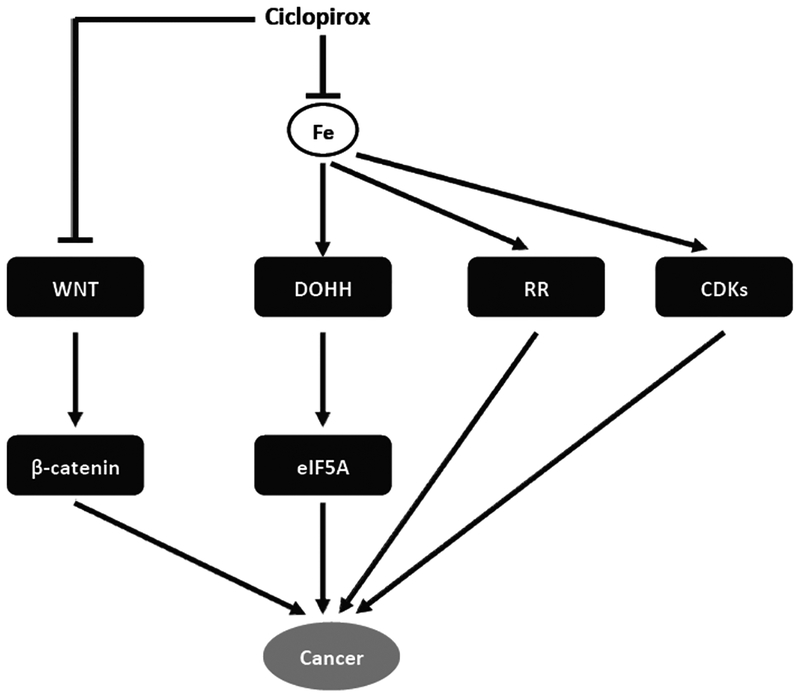
As described above, CPX is a newly identified anticancer agent. Although the anticancer mechanism of CPX is still not well understood, some molecular targets have been identified (Fig. 3).
Fig. (3).
The molecular targets of ciclopirox as an anticancer agent.
A recent study has shown that CPX inhibits cell proliferation and induces cell death in leukemia and myeloma cells by inhibiting ribonucleotide reductase (RR) [11]. RR is an iron-dependent enzyme, which catalyzes ribonucleotides to deoxyribonucleotides, an element for DNA synthesis [52]. Therefore, CPX inhibition of RR blocks DNA synthesis, resulting in cell cycle arrest at G1/S phase [11, 52]. Overexpression of M2 subunit of RR is able to attenuate CPX-induced apoptosis [22], implying that inhibition of RR also contributes to the cytotoxicity of CPX. These data strongly suggest that the antitumor activity of CPX is associated with inhibition of RR.
In addition, studies have demonstrated that CPX inhibits deoxyhypusine hydroxylase (DOHH). Like RR, DOHH is also an iron-dependent enzyme [53]. DOHH catalyzes deoxyhypusine to hypusine, which is essential for the maturation of eukaryotic translation initiation factor 5A (eIF5A) [54], an important factor of translation elongation [55]. Two isoforms of eIF5A, eIF5A-1 and eIF5A-2, have been identified (43). eIF5A-1 is constitutively expressed in most tissues, whereas eIF5A-2 is only highly expressed in certain cancer cell lines [55, 56]. As overexpression of eIF5A-2 is associated with cellular transformation, eIF5A-2 has been suggested as a potential oncogene [57]. Since either inhibition of DOHH or depletion of eIF5A is able to arrest cell cycle at G1/S [58, 59], the maturation of eIF5A has been proposed to be associated with cell proliferation [59]. CPX inhibits DOHH activity, bio-synthesis of hypusine, and G1/S cell cycle progression [10, 61], implying that targeting DOHH-eIF5A axis may also contribute to the anticancer effect of CPX. This is further supported by the observation that CPX synergistically inhibits proliferation of BCR-ABL-positive leukemia cells treated with imatinib, a selective inhibitor of the tyrosine kinase, BCR-ABL, which locates upstream of eIF5A [62].
Wnt/β-catenin pathway plays an important role in tumor development [63]. More recently, CPX has been found to effectively block Wnt/β-catenin pathway by promoting degradation of β-catenin [64]. CPX also inhibits the expression of β-catenin targeted genes in the samples from AML patients [64]. As a result, CPX induces apoptosis in lymphoma cells [65]. Similarly, CPX reduces cell survival in renal cell carcinoma and human pancreatic cancer cells by targeting Wnt/β-catenin [66, 67]. These results indicate that Wnt/β-catenin pathway is another target of CPX.
While the anti-angiogenic effect of CPX is disputable [10, 48], recent studies have demonstrated that CPX inhibits in vitro lymphangiogenesis by reducing expression of vascular endothelial growth factor receptor 3 (VEGFR-3) and VEGFR-3-mediated phosphorylation of extracellular signal-regulated protein kinases 1/2 (Erk1/2) [49]. CPX does not affect the mRNA level of VEGFR-3, but inhibits the protein synthesis rate of VEGFR-3 and promotes its turnover [49]. Further research is required to address whether this is related to iron-chelation.
It has been described that depletion of iron with pyridoxal isonicotinoyl hydrazine arrests cells at G1/S transition by reducing cyclin dependent kinases (CDK2 and CDK4), and cyclin D1 expression, and induces apoptosis by reducing Bcl-2 family member expression, increasing pro-apoptotic BAX expression, and elevating caspase activity [68]. In consistence with these findings, CPX downregulates expression of cyclin D1, cyclin E, and CDK2, and upregulates expression of CDK inhibitor, p21Cip1, leading to hypo-phosphorylation of retinoblastoma protein (Rb) and G1 cell cycle arrest in breast cancer and rhabdomyosarcoma cells [12]. In addition, CPX inhibits expression of Bcl-xL and survivin, activates caspases 3/7, and induces cleaved PARP, resulting in apoptosis in the tumor cells [12]. Moreover, CPX induces ROS, which activates c-Jun N-terminal kinase, leading to autophagy in rhabdomyosarcoma cells [69].
A recent study has reported that CPX enhances parthenolide-induced cell death in leukemic cells, by inhibiting the phosphorylation of ribosomal p70 S6 kinase 1 on Thr389, implying inhibition of mammalian target of rapamycin (mTOR) [70]. mTOR is a central controller of cell growth, proliferation, survival, motility, angiogenesis and lymphangiogenesis, which plays an important role in the tumorigenesis and metastasis [71, 72]. Whether CPX inhibition of mTOR plays a critical role in its anticancer action remains to be determined.
Taken together, CPX has multiple molecular targets, such as RR, DOHH/eIF5A, Wnt/β-catenin, VEGFR-3/Erk1/2, CDKs, Bcl-2 family members, mTOR, etc. Studies have implicated that most of the effects of CPX on these targets are linked to chelation of intracellular iron. Given the fact that cancer cells consume more iron to maintain their much higher proliferation rate than normal cells, and iron chelation generally has a greater impact on the cancer cells [73], it is expected that CPX may be exploited for tumor selective treatment.
4.3. The Safety of Systematic Administration of CPX
Unlike treating superficial fungal infections, treating either solid tumor or leukemia requires systematic administration of CPX. Animal studies have shown that the LD50 of ciclopirox olamine is 1,700–3,290 mg/kg in various animals including mice, rats, and rabbits, although the toxicity of systematic administration of ciclopirox olamine in human is still not available [19]. A serum concentration of 10 μM of CPX is achievable when the compound is administered orally at doses of 20–25 mg/kg in rats and dogs [11]. Recently, two groups have demonstrated that ciclopirox olamine, given to nude mice at 20–25 mg/kg/day, potently inhibit tumor growth, but does not display obvious toxicity [11, 12]. In a recent phase I clinical trial, oral administration of CPX at a dose of 40 mg/m2 does not cause obvious toxicity in patients [47]. Collectively, these findings suggest that ciclopirox olamine may be used systematically for cancer prevention and treatment.
5. CPX AND OTHER DISEASES
5.1. Diabetes
Diabetes mellitus, or simply diabetes, is a kind of metabolic disease in which one has abnormally high levels of blood sugar over a prolonged period. Diabetes can be classified into two major types: Type I (insufficient insulin produced in the body), and Type II (insensitivity of cells to insulin) [74]. The hypo-insulin status stems from the malfunction or even loss of islet β cells [74], which are responsible for secreting insulin [75]. The pro-inflammatory cytokine induces expression of inducible nitric oxide synthase, which, in turn, produces nitric oxide leading to blockage of ATP production, inhibition of insulin secretion, and induction of β cell death [75]. It has been described that eIF5A promotes Nos2 gene (encoding inducible nitric oxide synthase) translation by shuttling Nos2 mRNA from the nucleus to the cytoplasm [76]. Hypusination of eIF5A is essential for eIF5A-mediated Nos2 mRNA transportation and pathogenesis of islet β cells [76]. Since DOHH, an indispensible enzyme for hypusination of eIF5A, can be inhibited by ciclopirox olamine with IC50 around 5 μM [10], it is promising to apply ciclopirox olamine for treatment of Type I diabetes (Fig. 4).
Fig. (4).
The molecular targets of ciclopirox in diabetes, cardiovascular disease and HIV.
One of the most serious complications of diabetes is diabetic foot ulcers (DFUs), which leads to pain in low legs and even amputations [75]. DFUs results from the abnormal wound healing process attributing to multiple factors [77], of which the deficiency of angiogenesis is highly associated [78]. This is supported by the finding that the production of VEGF, a key factor in regulating angiogenesis [79], drops in diabetic patients and animal models [80, 81]. In addition, the response to hypoxia, which leads to elevated VEGF expression in normal cells [82, 83], is impaired in the diabetic fibroblasts [80]. It has been described that hyperglycemia-associated high iron level [84, 85] promotes the formation of methyglyoxal, which disrupts the interaction between hypoxiainducible factor-1α (HIF-1α) and its transcriptional cofactor P300, leading to the reduction of VEGF expression under hypoxia [85]. Therefore, topical administration of certain iron chelators has become a practical strategy to manage DFUs [13, 85]. Ciclopirox olamine has potent capability of iron chelation and confirmed safety for topical administration [1]. Undoubtedly, ciclopirox olamine is a very good candidate for treating DFUs (Fig. 4). This is further supported by the findings that ciclopirox olamine is able to induce HIF-1α and VEGF expression in a mouse skin wound model [48], promote angiogenesis both in vitro and in vivo [48], as well as improve wound healing in diabetic mice [13].
5.2. Acquired Immune Deficiency Syndrome (AIDS)
AIDS, a disease caused by human immunodeficiency virus (HIV), accounts for more than two million human death each year [86]. Current strategy in treatment of AIDS is to interrupt HIV life cycle by targeting viral proteins associated with infection such as reverse transcriptase and protease [87]. However, the high recombination rates of the virus lead to the emergence of new strains, which are resistant to current drugs [14, 86, 88]. Therefore, the Achilles’ heel of HIV might be the highly conserved host protein(s) essential for HIV’s infection and life cycle. eIF5A, expressed in host cells, is involved in HIV replication and nucleocytoplasmic transportation of viral mRNA [56]. Interference of hypusine formation on eIF5A by suppressing DOHH inhibits HIV replication [88]. With a potent inhibitory effect on DOHH-eIF5A axis [10, 61], ciclopirox olamine has been proposed to have a great potential for treatment and prevention of AIDS [14] (Fig. 4). This has been attested by a recent finding that CPX suppresses HIV replication in human peripheral blood mononuclear cells [14]. Also, CPX inhibits the expression of viral genes at least partially by impairing the maturation of eIF5A [14]. More interestingly, CPX blocks HIV gene expression also through an unique mode, targeting 5’-untranslated regions of HIV [14], which is not only essential for HIV replication, but also for the most conserved section of HIV genome [90]. Thus, CPX may have the ability to combat the drug resistance rooted from the variation nature of HIV.
5.3. Cardiovascular Diseases
The age-associated cardiovascular diseases are the major causes of mortality of the patients over 65 years old [91]. Since aging is correlated with reduced response to inotropic stimulation, which contributes to hypertension, heart failure, and hypertrophic cardiomyopathy [15], improving inotropic response should have benefits to those old patients. Recent studies have shown that CPX is able to attenuate the decreased response to inotropic stimulation in old myocytes through induction of HIF-1 [15]. Also, CPX-induced HIF-1α contributes to the elevated expression of urocortin 2, which has been identified to increase cardiac output and myocardial contractility, decrease peripheral resistance, and attenuate the aftermath of ischemia in rat hearts [16] (Fig. 4). Therefore, CPX may have some benefits to patients with certain cardiovascular diseases.
5.4. Other Potentials
Unlike other antifungal compounds, CPX also has antibacterial activities on both Gram-negative and Gram-positive strains [4], with minimum inhibitory concentrations ranging from 0.06 to 2 μg/mL [1]. This is clinically significant, particularly to the bacterially complicated fungal infection [4]. Besides, CPX is capable of inhibiting inflammation, which is frequently accompanied by fungal infection [4]. Interestingly, a new study has shown that CPX is able to inhibit the growth of an antibiotic-resistant E. coli strain, and the inhibition can be attenuated by iron addition [92], implying that the antibacterial activity of CPX is also attributed to its iron chelation capability.
CONCLUSION
CPX has been clinically used as a very effective antifungal agent to treat superficial fungal infections for decades. Besides, CPX is capable of affecting the activities of certain enzymes or signaling pathways, such as RR, DOHH/eIF5A, Wnt/β-catenin, HIF-1/VEGF, VEGFR-3/Erk1/2, mTOR, and CDKs. Most of these activities are related to its chelation of iron. As such, CPX has been found to possess new potentials, including suppressing tumor growth, mitigating diabetes and its complications, blocking HIV infection, and improving age-associated cardiovascular defects. However, the underlying mechanisms of its actions are only at the beginning to be unveiled. With further studies, more activities of CPX may be discovered.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health (CA115414; S. Huang), American Cancer Society (RSG-08-135-01-CNE; S. Huang), and Carroll-Feist Predoctoral Fellowship Award, Louisiana State University Health Sciences Center-Shreveport (T. Shen).
ABBREVIATIONS
- AIDS
acquired immune deficiency syndrome
- CDK
cyclin dependent kinase
- CPX
ciclopirox
- DFUs
diabetic foot ulcers
- DOHH
deoxyhypusine hydroxylase
- eIF5A
eukaryotic translation initiation factor 5A
- Erk1/2
extracellular signal-regulated protein kinases 1/2
- HIF-1α
hypoxia-inducible factor-1α
- HIV
human immunodeficiency virus
- mTOR
mammalian target of rapamycin
- ROS
reaction oxidative species
- RR
ribonucleotide reductase
- VEGF
vascular endothelial growth factor
- VEGFR-3
vascular endothelial growth factor receptor 3
Shile Huang

Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Subissi A, Monti D, Togni G, et al. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs 2010; 70(16): 2133–52. [DOI] [PubMed] [Google Scholar]
- [2].Sehgal V Ciclopirox: a new topical pyrodonium antimycotic agent. A double-blind study in superficial dermatomycoses. Br J Dermatol 1976; 95(1): 83–8. [DOI] [PubMed] [Google Scholar]
- [3].Korting H, Grundmann-Kollman M. The hydroxypyridones: a class of antimycotics of its own. Mycoses 1997; 40(7–8): 243–7. [DOI] [PubMed] [Google Scholar]
- [4].Gupta A, Plott T. Ciclopirox: a broad-spectrum antifungal with antibacterial and anti-inflammatory properties. Int J Dermatol 2004; 43 Suppl 1: 3–8. [DOI] [PubMed] [Google Scholar]
- [5].Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res 2009; 9(7): 1029–50. [DOI] [PubMed] [Google Scholar]
- [6].Sigle H-C, Thewes S, Niewerth M, et al. Oxygen accessibility and iron levels are critical factors for the antifungal action of ciclopirox against Candida albicans. J Antimicrob Chemother 2005; 55(5): 663–73. [DOI] [PubMed] [Google Scholar]
- [7].Leem S, Park J, Kim I, et al. The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae. Mol Cells 2003; 15(1): 55–61. [PubMed] [Google Scholar]
- [8].Pfaller M Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 2012; 125(1 Suppl): S3–13. [DOI] [PubMed] [Google Scholar]
- [9].Niewerth M, Kunze D, Seibold M, et al. Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistancefactors. Antimicrob Agents Chemother 2003; 47(6): 1805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clement P, Hanauske-Abel H, Wolff E, et al. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer 2002; 100(4): 491–8. [DOI] [PubMed] [Google Scholar]
- [11].Eberhard Y, McDermott SP, Wang X, et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009; 114(14): 3064–73. [DOI] [PubMed] [Google Scholar]
- [12].Zhou H, Shen T, Luo Y, et al. The antitumor activity of the fungicide ciclopirox. Int J Cancer 2010; 127(10): 2467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ko S, Nauta A, Morrison S, et al. Antimycotic ciclopirox olamine in the diabetic environment promotes angiogenesis and enhances wound healing. PLoS One 2011; 6(11): e27844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoque M, Hanauske-Abel H, Palumbo P, et al. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology 2009; 6: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tan T, Mar n-Garc a J, Damle S, et al. Hypoxia-inducible factor-1 improves inotropic responses of cardiac myocytes in ageing heart without affecting mitochondrial activity. Exp Physiol 2010; 95(6): 712–22. [DOI] [PubMed] [Google Scholar]
- [16].Buhler K, Plaisance I, Dieterle T, et al. The human urocortin 2 gene is regulated by hypoxia: identification of a hypoxia-responsive element in the 3’-flanking region. Biochem J 2009; 424(1): 119–27. [DOI] [PubMed] [Google Scholar]
- [17].Jue S, Dawson G, Brogden R. Ciclopirox olamine 1% cream. A preliminary review of its antimicrobial activity and therapeutic use. Drugs 1985; 29(4): 330–41. [DOI] [PubMed] [Google Scholar]
- [18].Kellner H, Arnold C, Christ O, et al. Pharmacokinetics and biotransformation of the antimycotic drug ciclopiroxolamine in animals and man after topical and systemic administration. Arzneimittelforschung 1981; 31(8A): 1337–53. [PubMed] [Google Scholar]
- [19].Weir S, Patton L, Castle K, et al. The repositioning of the antifungal agent ciclopirox olamine as a novel therapeutic agent for the treatment of haematologic malignancy. J Clin Pharm Ther 2011; 36(2): 128–34. [DOI] [PubMed] [Google Scholar]
- [20].Alpermann H, Schutz E. Studies on the pharmacology and toxicology of ciclopiroxolamine (author’s transl). Arzneimittelforschung 1981; 31(8A): 1328–32. [PubMed] [Google Scholar]
- [21].Review and evaluation of pharmacology/toxicology data. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-022_penlac%20nail%20lacquer%20tropical%20solution_pharmr.pdf. [Accessed October 06, 2013] [Google Scholar]
- [22].Gupta A Ciclopirox: an overview. Int J Dermatol 2001; 40(5): 305–10. [DOI] [PubMed] [Google Scholar]
- [23].Havlickova B, Czaika V, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008; 51 (Suppl 4): 2–15. [DOI] [PubMed] [Google Scholar]
- [24].Criado P, Oliveira C, Dantas K, et al. Superficial mycosis and the immune response elements. An Bras Dermatol 2011; 86(4): 726–31. [DOI] [PubMed] [Google Scholar]
- [25].Garber G An overview of fungal infections. Drugs. 2001; 61 (Suppl 1): 1–12. [DOI] [PubMed] [Google Scholar]
- [26].Dittmar W, Lohaus G. HOE 296, a new antimycotic compound with a broad antimicrobial spectrum. Laboratory results. Arzneimittelforschung 1973; 23(5): 670–4. [PubMed] [Google Scholar]
- [27].Aly R, Maibach H, Bagatell F, et al. Ciclopirox olamine lotion 1%: bioequivalence to ciclopirox olamine cream 1% and clinical efficacy in tinea pedis. Clin Ther 1989; 11(3): 290–303. [PubMed] [Google Scholar]
- [28].Abrams B, Hanel H, Hoehler T. Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol 1991; 9(4): 471–7. [DOI] [PubMed] [Google Scholar]
- [29].Gupta A, Bluhm R. Ciclopirox shampoo for treating seborrheic dermatitis. Skin Therapy Lett 2004; 9(6): 4–5. [PubMed] [Google Scholar]
- [30].Altmeyer P, Hoffmann K. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double-blind, vehicle-controlled trial. Int J Dermatol 2004; 43 (Suppl 1): 9–12. [DOI] [PubMed] [Google Scholar]
- [31].Lebwohl M, Plott T. Safety and efficacy of ciclopirox 1% shampoofor the treatment of seborrheic dermatitis of the scalp in the US population: results of a double-blind, vehicle-controlled trial. Int J Dermatol 2004; 43 (Suppl 1): 17–20. [DOI] [PubMed] [Google Scholar]
- [32].Gupta A, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol 2000; 43(4 Suppl): S70–80. [DOI] [PubMed] [Google Scholar]
- [33].Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol 2010; 28(2): 151–9. [DOI] [PubMed] [Google Scholar]
- [34].Gupta A Pharmacoeconomic analysis of ciclopirox nail lacquer solution 8% and the new oral antifungal agents used to treat dermatophyte toe onychomycosis in the United States. J Am Acad Dermatol 2000; 43(4 Suppl): S81–95. [DOI] [PubMed] [Google Scholar]
- [35].Monti D, Saccomani L, Chetoni P, et al. Hydrosoluble medicated nail lacquers: in vitro drug permeation and corresponding antimycotic activity. Br J Dermatol 2010; 162(2): 311–7. [DOI] [PubMed] [Google Scholar]
- [36].Baran R, Tosti A, Hartmane I, et al. An innovative water-soluble biopolymer improves efficacy of ciclopirox nail lacquer in the management of onychomycosis. J Eur Acad Dermatol Venereol 2009; 23(7): 773–81. [DOI] [PubMed] [Google Scholar]
- [37].Monti D, Herranz U, Dal Bo L, et al. Nail penetration and predicted mycological efficacy of an innovative hydrosoluble ciclopirox nail lacquer vs. a standard amorolfine lacquer in healthy subjects. J Eur Acad Dermatol Venereol 2013; 27(2): e153–8. [DOI] [PubMed] [Google Scholar]
- [38].Morales-Molina JA, Pérez-Moyano R, Fayet-Pérez A, et al. Interaction between ciclopirox and acenocoumarol. Eur J Clin Pharmacol 2013; 69(3): 727–8. [DOI] [PubMed] [Google Scholar]
- [39].Iwata K, Yamaguchi H. Studies on the mechanism of antifungal action of ciclopiroxolamine/Inhibition of transmembrane transport of amino acid, K+ and phosphate in Candida albicans cells (author’s transl). Arzneimittelforschung 1981; 31(8A): 1323–7. [PubMed] [Google Scholar]
- [40].Cowen LE, Sanglard D, Calabrese D, et al. Evolution of drug resistance in experimental populations of Candida albicans. J Bacteriol 2000; 182(6): 1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Levenson V, Hamlin JL. A general protocol for evaluating the specific effects of DNA replication inhibitors. Nucleic Acids Res 1993; 21(17): 3997–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Urbani L, Sherwood S, Schimke R. Dissociation of nuclear and cytoplasmic cell cycle progression by drugs employed in cell synchronization. Exp Cell Res 1995; 219(1): 159–68. [DOI] [PubMed] [Google Scholar]
- [43].Croce C Oncogenes and cancer. N Engl J Med 2008; 358(5): 502–11. [DOI] [PubMed] [Google Scholar]
- [44].Golias C, Charalabopoulos A, Charalabopoulos K. Cell proliferation and cell cycle control: a mini review. Int J Clin Pract 2004; 58(12): 1134–41. [DOI] [PubMed] [Google Scholar]
- [45].Vermeulen K, Van Bockstaele D, Berneman Z. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 2003; 36(3): 131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Evan G, Vousden K. Proliferation, cell cycle and apoptosis in cancer. Nature 2001; 411(6835): 342–8. [DOI] [PubMed] [Google Scholar]
- [47].Minden MD, Hogge DE, Weir SJ, et al. Oral ciclopirox olamine displays biological activity in a phase I study in patients with advanced hematologic malignancies. Am J Hematol 2014; 89(4): 363–8. [DOI] [PubMed] [Google Scholar]
- [48].Linden T, Katschinski DM, Eckhardt K, et al. The antimycotic ciclopirox olamine induces HIF-1α stability, VEGF expression, and angiogenesis. FASEB J 2003; 17(6): 761–3. [DOI] [PubMed] [Google Scholar]
- [49].Luo Y, Zhou H, Liu L, et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene 2011; 30(18): 2098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Folkman J Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29(6 Suppl 16): 15–8. [DOI] [PubMed] [Google Scholar]
- [51].Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol 2007; 25(27): 4298–307. [DOI] [PubMed] [Google Scholar]
- [52].Cerqueira N, Fernandes P, Ramos M. Ribonucleotide reductase: a critical enzyme for cancer chemotherapy and antiviral agents. Recent Pat Anticancer Drug Discov 2007; 2(1): 11–29. [DOI] [PubMed] [Google Scholar]
- [53].Linden T, Wenger R. Iron chelation, angiogenesis and tumor therapy. Int J Cancer 2003; 106(3): 458–9. [DOI] [PubMed] [Google Scholar]
- [54].Kim YS, Kang KR, Wolff EC, et al. Deoxyhypusine hydroxylase is an Fe(II)-dependent, heat-repeat enzyme. Identification of amino acid residues critical for Fe(II) binding and catalysis. J Biol Chem 2006; 281(19): 13217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wolff E, Kang K, Kim Y, et al. Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids 2007; 33(2): 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kaiser A Translational control of eIF5A in various diseases. Amino Acids 2012; 42(2–3): 679–84. [DOI] [PubMed] [Google Scholar]
- [57].Park M, Nishimura K, Zanelli C, et al. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 2010; 38(2): 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Guan X-Y, Fung JM-W, Ma N-F, et al. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res 2004; 64(12): 4197–200. [DOI] [PubMed] [Google Scholar]
- [59].Hanauske-Abel H, Park M, Hanauske A, et al. Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta 1994; 1221(2): 115–24. [DOI] [PubMed] [Google Scholar]
- [60].Kang HA, Hershey JW. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem 1994; 269(6): 3934–40. [PubMed] [Google Scholar]
- [61].Csonga R, Ettmayer P, Auer M, et al. Evaluation of the metal ion requirement of the human deoxyhypusine hydroxylase from HeLa cells using a novel enzyme assay. FEBS Lett 1996; 380(3): 209–14. [DOI] [PubMed] [Google Scholar]
- [62].Balabanov S, Gontarewicz A, Ziegler P, et al. Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood 2007; 109(4): 1701–11. [DOI] [PubMed] [Google Scholar]
- [63].Clevers H Wnt/β-catenin signaling in development and disease. Cell 2006; 127(3): 469–80. [DOI] [PubMed] [Google Scholar]
- [64].Song S, Christova T, Perusini S, et al. Wnt inhibitor screen reveals iron dependence of β-catenin signaling in cancers. Cancer Res 2011; 71(24): 7628–39. [DOI] [PubMed] [Google Scholar]
- [65].Kim Y, Schmidt M, Endo T, et al. Targeting the Wnt/β-catenin pathway with the antifungal agent ciclopirox olamine in a murine myeloma model. In Vivo 2011; 25(6): 887–93. [PubMed] [Google Scholar]
- [66].Wall I, Schmidt-Wolf IG. Effect of Wnt inhibitors in pancreatic cancer. Anticancer Res 2014; 34(10): 5375–80. [PubMed] [Google Scholar]
- [67].VONS-H SA, Schmeel LC, Schmeel FC, et al. Targeting the Wnt/beta-catenin pathway in renal cell carcinoma. Anticancer Res 2014; 34(8): 4101–8. [PubMed] [Google Scholar]
- [68].Yu Y, Kovacevic Z, Richardson D. Tuning cell cycle regulation with an iron key. Cell Cycle 2007; 6(16): 1982–94. [DOI] [PubMed] [Google Scholar]
- [69].Zhou H, Shen T, Shang C, et al. Ciclopirox induces autophagy through reactive oxygen species-mediated activation of JNK signaling pathway. Oncotarget 2014; 5(20): 10140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sen S, Hassane DC, Corbett C, et al. Novel mTOR inhibitory activity of ciclopirox enhances parthenolide antileukemia activity. Exp Hematol 2013; 41(9): 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149(2): 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhou H, Huang S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr Protein Pept Sci 2011; 12(1): 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev 2005; 57(4): 547–83. [DOI] [PubMed] [Google Scholar]
- [74].Hauber J Revisiting an old acquaintance: role for eIF5A in diabetes. J Clin Invest 2010; 120(6): 1806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ashcroft F, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell 2012; 148(6): 1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Maier B, Ogihara T, Trace A, et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest 2010; 120(6): 2156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117(5): 1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Martin A, Komada M, Sane D. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003; 23(2): 117–45. [DOI] [PubMed] [Google Scholar]
- [79].Ferrara N VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002; 2(10): 795–803. [DOI] [PubMed] [Google Scholar]
- [80].Lerman O, Galiano R, Armour M, et al. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 2003; 162(1): 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 2002; 105(3): 373–9. [DOI] [PubMed] [Google Scholar]
- [82].Ikeda E, Achen MG, Breier G, et al. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem 1995; 270(34): 19761–6. [DOI] [PubMed] [Google Scholar]
- [83].Finkenzeller G, Technau A, Marme D. Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun 1995; 208(1): 432–9. [DOI] [PubMed] [Google Scholar]
- [84].Thomas M, MacIsaac R, Tsalamandris C, et al. Elevated iron indices in patients with diabetes. Diabet Med 2004; 21(7): 798–802. [DOI] [PubMed] [Google Scholar]
- [85].Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009; 106(32): 13505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Killian M, Levy J. HIV/AIDS: 30 years of progress and future challenges. Eur J Immunol 2011; 41(12): 3401–11. [DOI] [PubMed] [Google Scholar]
- [87].Dybul M, Fauci A, Bartlett J, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep 2002; 51(RR-7): 1–55. [PubMed] [Google Scholar]
- [88].Hauber I, Bevec D, Heukeshoven J, et al. Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy. J Clin Invest 2005; 115(1): 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Andrus L, Szabo P, Grady R, et al. Antiretroviral effects of deoxyhypusyl hydroxylase inhibitors: a hypusine-dependent host cell mechanism for replication of human immunodeficiency virus type 1 (HIV-1). Biochem Pharmacol 1998; 55(11): 1807–18. [DOI] [PubMed] [Google Scholar]
- [90].Berkhout B, Arts K, Abbink TEM. Ribosomal scanning on the 5’-untranslated region of the human immunodeficiency virus RNA genome. Nucleic Acids Res 2011; 39(12): 5232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lakatta E Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 2002; 7(1): 29–49. [DOI] [PubMed] [Google Scholar]
- [92].Carlson-Banning KM, Chou A, Liu Z, et al. Toward repurposing ciclopirox as an antibiotic against drug-resistant Acinetobacter baumannii, Escherichia coli, and Klebsiella pneumoniae. PLoS One 2013; 8(7): e69646. [DOI] [PMC free article] [PubMed] [Google Scholar]