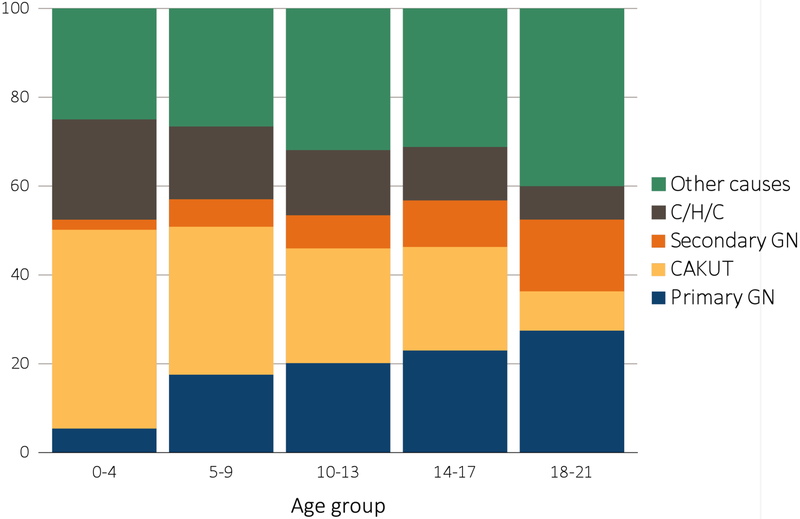INTRODUCTION AND OVERVIEW
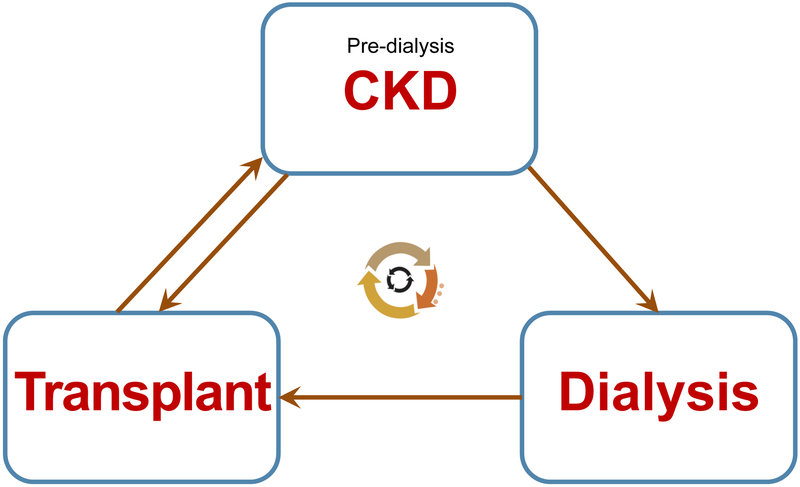
Chronic kidney disease (CKD) can be defined as a sustained damage of renal parenchyma leading to chronic deterioration of renal function that may gradually progress to end-stage renal disease (ESRD). ESRD remains uniformly fatal without renal replacement therapy (dialysis or kidney transplantation). The term CKD acknowledges that this condition exists on a continuum with differing degrees of renal impairment rather than a discrete state of renal insult (acute kidney injury). The term CKD has replaced previously used terms “chronic renal insufficiency” and “chronic renal failure”. While kidney transplantation revolutionized ESRD care, most children with transplanted kidneys presently have various degrees of allograft injury or dysfunction (CKD of a transplanted kidney). Throughout their lifetime, individuals with pediatric-onset CKD may repeatedly transition between pre-dialysis CKD, various dialysis modalities, and kidney transplants (Fig. 1). Kidney transplantation leads to better survival and quality of life than dialysis. However, complications and requirement for a rigorous supportive care still significantly limit the quality of life of pediatric kidney transplant recipients.
Figure 1.
Life cycle of children with CKD
Etiology
According to the 2017 United States Renal Data System (USRDS) annual data report, the leading causes of ESRD in children during 2011-2015, similar to earlier years, were congenital anomalies of the kidney and urinary tract (CAKUT, 22%), primary glomerular diseases (21.8%), cystic/hereditary/congenital disorders (12.5%), and secondary glomerular diseases/vasculitidies (10.7%) (Fig. 2). The most common individual diagnoses associated with pediatric ESRD included focal segmental glomerulosclerosis (11.6%), renal hypoplasia/dysplasia (10%), congenital obstructive uropathies (9.7%), and systemic lupus erythematosus (6.3%)1. This distribution is very different from CKD in adults, which in developed countries is most typically associated with diabetes mellitus or hypertension.2,3
Figure 2.
Distribution of reported incident pediatric end stage renal disease (ESRD) patients by primary cause of ESRD, 2011-2015
(From the United States Renal Data System, USRDS 2017 Annual Data Report. Available at: https://www.usrds.org/2017/view/Default.aspx).
CAKUT, congenital anomalies of the kidney and urinary tract;
C/H/C, cystic/hereditary/congenital diseases;
ESRD, end-stage renal disease;
GN, glomerulonephritis.
Kidney development and CKD
Prenatal factors play an important role in the development of CKD in many children. While genetic background contributes to the development of CAKUT, epigenetic and maternal influences may play a role in nephron endowment and account for differences in the final nephron number. Each human kidney has on average one million nephrons, but there is a substantial variability (from 200000 to 1800000 nephrons).4 The majority of nephrons are formed during the third trimester of pregnancy,5 and no newly forming nephrons can be found in fetal kidneys beyond the 36 week of gestation.6 It appears that premature babies born before 36-week gestation are able to form new nephrons postnataly, but this ability may be limited. Prematurity, low birth weight and other prenatal factors diminishing nephron endowment were shown in retrospective studies to increase the risk of CKD later in life, even without associated overt renal anomalies7 – thus supporting a concept of fetal programming of late-onset pediatric and adult CKD.8
However, more than half of the individuals who develop CKD in childhood are born with structurally and functionally abnormal kidneys and urinary tract. Regardless of the individual anatomic abnormality, prognosis largely depends on the number of functioning nephrons. Tubular dysfunction may predominate compared with glomerular impairment in the first years of life, manifesting as defective reabsorption of the filtered solutes, causing renal salt wasting. Survival of children with severe bilateral obstructive anomalies depends on their lung development, which requires factors present in amniotic fluid. While fetal oliguria or anuria due to urinary tract obstruction, usually bilateral, does not cause fetal uremia (as placenta provides the necessary clearance), it leads to oligohydramnios and pulmonary hypoplasia (Potter sequence). 9 Release of obstruction after birth may cause postobstructive diuresis and severe electrolyte abnormalities.10
Glomerular diseases tend to present later in childhood and to progress more rapidly11,12. In both early- and late-onset pediatric CKD, kidneys eventually become affected by tubulointerstitial fibrosis and exhibit nephron loss. This impairs the ability of the kidney to remove metabolic “waste”, including end-products of protein metabolism. Impaired renal clearance leads to accumulation of “uremic toxins” – the solutes that would normally be excreted by the kidneys but accumulate in CKD and have adverse impact on biologic functions.13 The European Uremic Toxin Work Group compiled a database that currently consists of more than 150 identified substances that were found at higher concentrations in the blood of patients with CKD as compared with normal individuals (http://www.uremic-toxins.org/). Uremic toxins are also produced by gut microbiota – particularly indoxyl sulfate and p-Cresyl sulfate.14 Alterations in the gut microbiota in patients with CKD may increase production of these uremic toxins.15
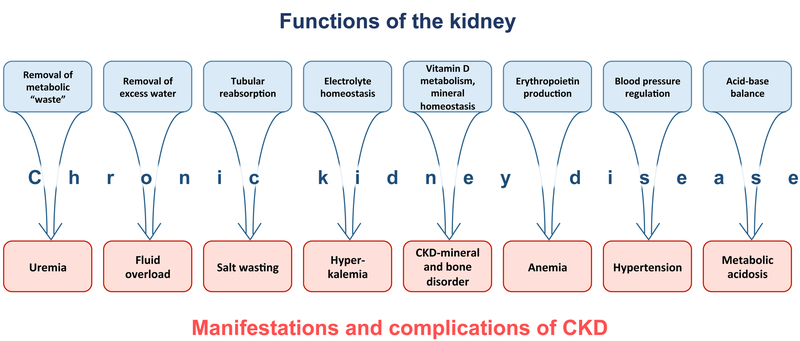
Serum urea and creatinine are routinely used as markers of renal dysfunction, while they do not appear to be intrinsically toxic in CKD. Furthermore, accumulation of uremic toxins only partially correlates with GFR in pediatric patients with CKD.16 The term “azotemia” is used to denote the biochemical presence of “uremic toxins”, while the term “uremia” usually refers to the clinical manifestations of azotemia. Systemic manifestations of CKD are related not only to uremia, but also to the impaired homeostatic functions of the kidney (Fig. 3).
Figure 3.
Impairment of different kidney functions in chronic kidney disease (CKD) leads to distinct manifestations and complications of CKD.
Clinical evaluation of renal function and CKD stages
Glomerular filtration rate (GFR) is a measure of kidney function. GFR is defined as the amount of plasma filtered by the kidneys during a certain period of time (usually one minute). Given differences in circulating blood volume, GFR in children is normalized by body surface area (units of measurement: mL/min/1.73m2). Healthy children younger than 2 years of age have physiologically lower GFR even when corrected for body surface area.17 GFR is equal to the sum of the filtration rates in all of the functioning nephrons, and therefore it correlates with the number of functioning nephrons. Healthy kidneys have substantial reserve to maintain adequate GFR under various physiologic conditions. While providing a tremendous biologic benefit, functional nephron “excess” complicates clinical evaluation of the early CKD stages. This is because unaffected nephrons are able to provide compensation to the damaged nephrons.18 Hypertrophy of remaining nephrons may occur resulting in partial restoration of GFR. Therefore, there is no linear correlation between nephron loss and the loss of GFR.
GFR can be measured indirectly using clearance of substances that are freely filtered in the glomeruli but not secreted or reabsorbed by the tubules, such as inulin. Inulin clearance require intravenous inulin infusion and repeated blood sampling and urine collection, which makes it impractical in clinical settings. Creatinine clearance can also be used to assess GFR, however tubules are able to secrete creatinine, which is one of the limitations of creatinine clearance as measure of GFR. Tubular creatinine secretion increases with decreased GFR. Therefore, creatinine clearance can overestimate GFR in children with CKD. Other limitations of serum creatinine as a marker of kidney function include its dependence on muscle mass and physical activity. Many physiologic substances and drugs interfere with creatinine assays, which can lead to falsely high or low serum creatinine values.19,20 Serum cystatin C is one of the most widely used alternative biomarkers to estimate GFR. Cystatin C, unlike creatinine, is produced by all nucleated cells, and is not dependent on muscle mass. Various formulas have been developed to estimate GFR based on serum markers and other variables (Box 1).21
Box 1. Formulas for estimation of glomerular filtration rate (eGFR, in mL/min/1.73m2) in children. Cystatin C based formulas are especially useful in children with reduced muscle mass.
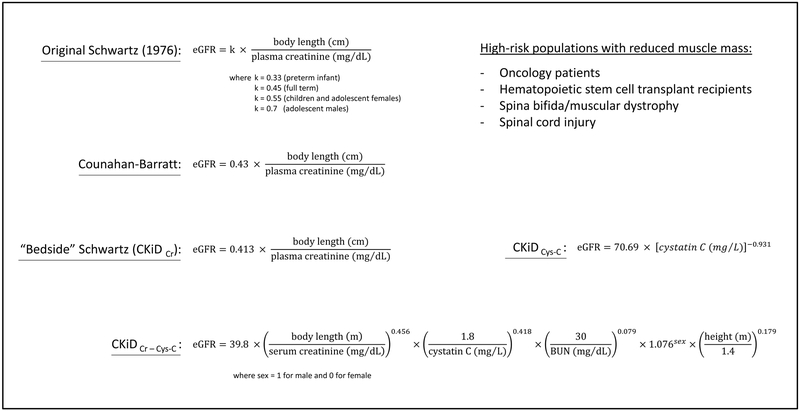
From Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Advances in chronic kidney disease. 2017;24(6):348-356; with permission.
The Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for Evaluation and Management of Chronic Kidney Disease recommends stratifying CKD in children older than 2 years based on the GFR into 5 stages (Table 1). For GFR > 60 ml/min/1.73 m2, the evidence of irreversible kidney damage is required to make a diagnosis of CKD, e.g., fixed proteinuria or characteristic renal imaging or histology. KDIGO criteria require the duration of renal insufficiency at least 3 months (except in babies younger than 3 months) to make a diagnosis of CKD.
Table 1.
Chronic kidney disease (CKD) staging based on the glomerular filtration rate (GFR).
| CKD stage / GFR category |
GFR (ml/min/1.73 m2) | Terms |
|---|---|---|
| G1 | ≥90 | Normal or high |
| G2 | 60-89 | Mildly decreased |
| G3a | 45-59 | Mildly to moderately decreased |
| G3b | 30-44 | Moderately to severely decreased |
| G4 | 15-29 | Severely decreased |
| G5 | <15 | Kidney failure |
If no evidence of kidney damage, neither GFR category G1 nor G2 fulfill the criteria for CKD.
From Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013;3(1):1-150; with permission.
Presentation and screening
The majority of children with CKD have CAKUT, which can be frequently detected on the prenatal ultrasound. Lack of prenatal care may delay diagnosis in these children. If not diagnosed in early childhood, children with non-glomerular CKD may present later with polyuria, growth impairment, or an accidental finding of elevated serum creatinine, blood urea nitrogen (BUN) or abnormal imaging (e.g., multiple renal cysts noted during abdominal ultrasound performed for a non-renal indication). Importantly, many children remain asymptomatic for years, sometime even until they reach ESRD. It is currently rare in the developed countries for children to have symptoms of chronic uremia at presentation (anorexia, vomiting, fatigue, cognitive dysfunction, and confusion). Classic findings of uremic pericarditis or uremic pruritus are currently also rare in children presenting with CKD. Children with glomerular CKD may present with more specific “renal” signs and symptoms, such as edema, elevated blood pressure, or may have an accidental finding of proteinuria.
Subtle and insidious onset of CKD prompted a consideration of CKD screening. The American Academy of Pediatrics currently does not recommend universal CKD screening with urinalyses in healthy children, due to concerns of its cost-ineffectiveness.22 However, targeted screening is appropriate in selected high-risk populations (Box 2),23,24 and should include quantification of urine protein based on a first morning void urine sample with or without urinalysis, and accurate blood pressure measurement.
Box 2. Conditions associated with increased risk of chronic kidney disease (CKD) in children*.
Low birth weight / prematurity
History of acute kidney injury
Obesity
Diabetes
Hypertension
History of glomerulopathies, including SLE, HSP, HUS
Sickle cell disease
CAKUT, congenital or acquired single kidney
Recurrent UTIs
Congenital heart disease
Family history of CKD
* CKD screening is required in these categories of children (see text for details).
SLE, systemic lupus erythematosus.
HSP, Henoch-Schonlein purpura.
HUS, hemolytic uremic syndrome.
CAKUT, congenital anomalies of kidney and urinary tract.
UTI, urinary tract infections.
MANIFESTATIONS AND COMPLICATIONS OF CKD, THEIR DIETARY AND PHARMACOLOGIC MANAGEMENT
CKD-mineral and bone disorder (CKD-MBD)
CKD is frequently associated with disorders of mineral and bone metabolism, which can manifest as one or all of the following interrelated abnormalities:
Disturbances of calcium, phosphorus, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and vitamin D metabolism.
Alterations of bone turnover, mineralization, elongation and strength.
Extraskeletal calcification.
The kidney is the only site of conversion of 25(OH)-vitamin D to the highly active 1,25(OH)2 form (Fig. 3). Recent data reveal a much more complex role of the kidney in mineral and bone metabolism than previously appreciated. The pathogenesis of CKD-MBD is still far from being completely understood. However, it is well established that subclinical changes in bone and mineral biomarkers can be detected very early in the course of CKD (at stage 2), before the drop in calcitriol levels.25,26 One of these early events is elevation of FGF23, a phosphatonin produced by osteocytes, which enhances renal phosphate excretion. The role of elevated FGF23 in early stages of CKD may be protective, as it likely helps to postpone the development of hyperphosphatemia. However, gradual decline in the number of functioning nephrons and deficiency of Klotho, an FGF23-co-receptor, eventually overwhelms the phosphaturic effect of FGF23. Moreover, FGF-23 worsens 1,25(OH)2-vitamin D deficiency, by directly inhibiting 1α-hydroxylase gene expression via activation of the ERK1/2 signaling pathway.27 Thus it contributes to the early pathogenesis of secondary hyperparathyroidism, before the development of serum mineral abnormalities.28 Hyperphosphatemia usually develops at stage 4 CKD, and stimulates parathyroid gland function, thus contributing to secondary hyperparathyroidism. The net effect of these systemic changes, coupled with current therapies, may paradoxically result either in excessive bone resorption or bone formation. However, in cases of apparent increase in bone mass, bone strength still suffers because the deposited bone collagen fibers are immature.29
The current goals of CKD-MBD management are:
Correction of hyperphosphatemia (phosphate binders, low phosphorus diet).
Avoiding positive calcium balance beyond what is needed by the growing child (limiting the dose of calcium-based phosphate binders, timely introduction of noncalcium-based phosphate binders) to prevent vascular calcifications.
Correction of secondary hyperparathyroidism (calcitriol and vitamin D analogues), as necessary to achieve the previous two goals. Importantly, the optimal PTH levels in patients with CKD currently remain unknown. The latest KDIGO guidelines suggest that calcitriol and vitamin D analogs not be routinely used in adults with CKD not on dialysis; while they may be considered in children with CKD to maintain serum calcium level in the age-appropriate normal range.30
Improvement of bone quality and prevention of vascular calcifications would be the ultimate goals; however, no therapies are currently available to directly target renal osteodystrophy or vascular quality in children. Bisphosphonates and other osteoporosis medications can be considered in adult CKD patients with osteoporosis and/or high risk of fracture.30
Dietary management of CKD-MBD include dietary phosphorus restriction in cases of hyperphosphatemia or hyperparathyroidism. Conventional hemodialysis does not provide adequate phosphate removal, and therefore dietary phosphorus control seem to be especially important in dialysis patients. KDOQI guidelines recommend limiting phosphorus intake to 100% of the DRI for age when PTH is elevated, and to 80% of the DRI for age when both PTH and serum phosphorus are elevated31
Age-specific normative values should be used for the assessment of hyperphosphatemia, because serum phosphorus is physiologically higher in younger children and babies.32 Breast milk is naturally low in phosphorus and is the preferred nutrition in babies with CKD. If breast milk is not available, a low-phosphorus “renal” formula should be used. Both breast milk and formulas can be additionally pretreated with sevelamer, a phosphate binder.33 It is well accepted that foods and beverages that are naturally high in phosphorus (Box 3), and those containing phosphorus additives should be avoided. Phosphorus additives contain non-organic phosphorus which is absorbed more avidly than organic phosphorus. Phosphorus in animal foods is absorbed more easily than that in plant foods. Low-phosphorus healthy choices for children with CKD include fruits and vegetables, corn, and rice.
Box 3. High phosphorus food items that should be limited or avoided in CKD.
Dark colas / sodas, chocolate drinks, cocoa
Dairy products (cheese, milk, ice cream, yogurt)
Organ meats, liver
Oysters, sardines
Dried beans and peas
Nuts and nut butters
Chocolate
Bran cereals and oatmeal, whole grain products
Egg yolks
Dietary phosphorus restriction in patients with CKD has many practical challenges, and its effects are surprisingly not clear. The association between hyperphosphatemia and adverse outcomes is well established in adults with CKD, and dietary phosphorus restriction led to improvement of renal function in animal models.34 However, no well-designed trials have been conducted to demonstrate the effect of lowering serum phosphorus on clinically meaningful outcomes in patients with CKD. Furthermore, the direct effects of dietary phosphorus restriction on serum phosphorus in clinical settings are still not definitively established.30 The conventional concept that correction of hyperphosphatemia and/or hyperparathyroidism in children with CKD should improve their growth is based on very small series.35 Because dietary protein is the major source of dietary phosphorus, there is a concern that strict adherence to low-phosphorus diet may contribute to protein-energy wasting. Therefore, a balanced consideration of the currently available evidence about clinical merits of phosphorus restricted diet coupled with the acknowledgement of its impact on patient-centered outcomes seems to be a reasonable approach to dietary counseling regarding phosphorus intake in CKD.
Metabolic acidosis of CKD
The kidneys play a major role in the maintenance of acid-base balance by means of hydrogen ions excretion, reabsorption of the filtered bicarbonate and generation of new bicarbonate ions and other buffers. The kidneys excrete hydrogen ion through titratable acid and urinary ammonium. Renal ammoniagenesis is the primary mechanism responsible for the regulation of hydrogen ion excretion. CKD can affect all components involved in renal regulation of acid-base balance leading to metabolic acidosis. Metabolic acidosis is especially common and develops earlier in the course of CKD in children with tubular dysfunctions, e.g., those with renal dysplasia. For example, 18% of children with GFR≥50 mL/min/1.73 m2 enrolled in the Chronic Kidney Disease in Children (CKiD) study had metabolic acidosis and were treated with alkaline therapy.36 Children typically have normal anion gap (hyperchloremic) metabolic acidosis in early CKD, but may develop high anion gap metabolic acidosis in advanced CKD, once the ability of the kidney to excrete organic acids becomes limited. When circulating buffers are depleted, bone begins to buffer excess hydrogen ions, which leads to release of calcium from bone. If left untreated, this may contribute to osteopenia and growth impairment, and increase the risk of secondary hyperparathyroidism and CKD progression.37 Advanced acidosis may contribute to hyperkalemia. Metabolic acidosis in CKD also adversely affects protein and muscle metabolism, stimulates inflammation, and enhances insulin resistance.38 Correction of acidosis appears to attenuate CKD progression39 and improve secondary hyperparathyroidism,40 as well as nutritional status, muscle strength,41 and quality of life in adults with CKD.42
Current guidelines by KDOQI and KDIGO recommend to maintain serum bicarbonate level in patients with CKD at or above 22 mEq/L (20 mEq/L for infants younger than 2 years of age).31,43 Dietary management of metabolic acidosis in CKD consists of dietary reduction of H+ intake by removing or limiting H+-producing dietary components, and adding base-producing dietary components. A typical “western” diet is acid-producing, particularly due to a substantial amount of animal protein, while fruits and vegetables are able to alkalinize the diet. However, in patients with advanced CKD dietary content of fruits and vegetables and the kinds of fruits and vegetables consumed have to be carefully monitored to prevent hyperkalemia. While dietary measures may be sufficient to prevent metabolic acidosis in early stages of CKD,44,45 many patients will also require additional alkali therapy, e.g. citric acid / sodium citrate or sodium bicarbonate. Alkali should be administered at least 2-3 times a day. Gastrointestinal side effects are relatively common, and are related in part to the release of CO2 when NaHCO3 gets in contact with gastric HCl. Close sodium intake monitoring is required to avoid volume expansion and hypertension. A novel sodium-free hydrochloric acid binding agent TRC101 showed promising efficacy and safety in adults with CKD.46 Caution is necessary when using alkali in patients with hypocalcemia because rapidly raising the pH in these patients may precipitate tetany. There is also a concern that long-term alkali therapy may promote vascular calcification.47 Blood collection for metabolic evaluation in children with CKD should be done prior to the first daily alkali dose.48
Potassium balance in CKD
Advanced CKD frequently leads to inadequate renal potassium excretion due to loss of nephron mass, and thus reduction in the number of collecting ducts to secrete K+. Hyperkalemia is a well-recognized risk factor for arrhythmias and cardiac arrest,49 and it predictably leads to higher mortality.50,51 Other factors that may contribute to hyperkalemia in patients with CKD include high dietary potassium intake, catabolic state with increased tissue breakdown, and inorganic metabolic acidosis. Hyperkalemia develops earlier in patients with diabetes, in part due to decreased mineralocorticoid activity / hyporeninemic hypoaldosteronism frequently seen in these patients.52 Hyperkalemia is a side effect of some medications commonly used in CKD, including ACE-inhibitors and angiotensin receptor blockers, potassium-sparing diuretics, calcineurin inhibitors, and prostaglandin inhibitors (e.g., NSAIDs). There is an adaptive increase in colonic potassium secretion in patients with advanced CKD and ESRD.53 Because the amount of stool potassium correlates with wet stool weight, constipation is a concern in patients with CKD and hyperkalemia.52 Traumatic blood collection and RBC hemolysis is the most common reason for laboratory reports of elevated potassium in children. Interpretation of serum potassium in children should be based on the age-specific normative ranges; younger children and infants have physiologically higher serum potassium.
Dietary management.
A typical diet of a healthy adult American contains about 3500 to 4500 mg of potassium a day. A low potassium diet provides about 2000 mg of potassium a day. In children, KDOQI recommends restriction of potassium intake to 40-120 mg/kg/day for infants and younger children and 30-40 mg/kg/day for older children31. Breast milk is naturally low in potassium and is the best nutrition for babies with CKD. If breast milk is not available, “renal” low-potassium formulas should be used. If necessary, formulas may be pre-treated with a potassium binder to further lower potassium content. Table 2 provides examples of high- and low-potassium foods. In addition, families should be educated about cooking techniques that can lower potassium content in meals, such as peeling, dicing, and presoaking potassium-rich vegetables, and discarding the broth.54 Similar to phosphorus, it is currently not required to list potassium on the Nutrition Facts Label Panel in the U.S.55
Table 2.
Examples of high- and low-potassium food items.
| High-potassium | Relatively low-potassium alternatives |
|
|---|---|---|
| Fruits | Orange and orange juice Banana Cantaloupe, honeydew Mango, papaya Nectarine Raisins and other dried fruits |
Apple, apple juice, applesauce Blueberries, cranberries Raspberries, strawberries Grapes and grape juice Mandarin oranges Pineapple and pineapple juice |
| Vegetables | Tomatoes and tomato products Potato (white and sweet) Pumpkin, butternut squash |
Eggplant Yellow squash and zucchini Onions Cucumber |
| Other foods | Chocolate Salt substitutes Nuts and seeds |
Rice Pasta Bread (not whole grains) |
Sodium polystyrene sulfonate is a cation-exchange resin (potassium binder) that can be used orally or rectally to treat hyperkalemia. Approximately 100 mg (4 mEq) of sodium is released per 1 gram of medication in exchange of potassium, which can lead to volume expansion. A rare but serious side effect of polystyrene sulfonate is colonic necrosis,56 which limits its use in neonates, especially those already at risk for necrotizing enterocolitis. Patiromer (RLY5016) and zirconium cyclosilicate (ZS-9) are novel emergent oral agents for the treatment of chronic hyperkalemia.57 Patiromer was approved by the US Food and Drug Administration for the management of nonemergent hyperkalemia in adults; no information is currently available on its use in children.
Sodium balance, intravascular volume, hypertension, and dyslipidemia in children with CKD
Children with CKD may experience a spectrum of dysnatremias, ranging from deficit to excess. Children with obstructive uropathies and/or renal dysplasia may experience excessive urine sodium loss, requiring sodium supplementation. Some peritoneal dialysis patients have excessive sodium loss with ultrafiltration, which may also need to be replaced. It has been suggested that without adequate repletion, chronic total body sodium deficit may contribute to growth impairment.58 Children with nephrotic syndrome, glomerulonephritidies, and severely decreased GFR frequently retain sodium, leading to expansion of the extracellular volume and / or hypertension. Elevated office blood pressure was associated with faster GFR decline in children with CKD in the CKiD study.11 The ESCAPE trial demonstrated that aggressive blood pressure control slows the progression of CKD.59 Restriction of sodium intake was beneficial in a few studies in adult patients with CKD,60,61 although more studies are needed to definitively determine the optimal sodium intake in children and adults with CKD.62 Excessive salt restriction can bring its own risks, such as stimulation of reninaldosterone axis, catecholamine production, and dyslipidemia.63 Recent studies suggested a U-shaped relationship between sodium intake and outcomes, including mortality, in the general population.64,65 Thus, dietary sodium intake should be closely monitored and individually optimized in patients with CKD. Urine sodium (ideally based on a 24hr collection) provides a good measure of dietary sodium intake and should complement dietary recall. KDOQI guidelines recommend 1500-2400 mg/day sodium intake for children who require sodium restriction31. At the same time, based on the Food Frequency Questionnaire, median daily sodium intake was 3089 mg in children with CKD from the CKiD study cohort, indicating that further efforts are needed for improvement of nutritional counseling66.
High prevalence of other factors that has been associated with cardiovascular disease in adults have been documented in children with CKD, including dyslipidemia.36,67 In the CKiD study cohort, 45% of children with moderate CKD had dyslipidemia; many of those children had nephrotic-range proteinuria.68 However, it remains unknown if dyslipidemia in children with CKD contributes to cardiovascular morbidity and mortality. KDIGO recommends dietary measures and weight control as the first line of dyslipidemia treatment in children. Due to very limited available data, the guidelines do not recommend the use of statins in children with CKD younger than 10 years. Boys older than 10 years and postmenarchal girls with severely elevated LDL-C “who place a higher value on the potential for preventing cardiovascular events and are less concerned about adverse events from statin use might be candidates for statin use – especially those with multiple additional risk factors such as family history of premature coronary disease, diabetes, hypertension, smoking, and ESRD”.69
Anemia and iron metabolism in children with CKD
Anemia is a well-recognized complication of CKD, and a major determinant of quality of life and morbidity in patients with CKD70,71. Anemia complicates CKD in about 50% of children before they reach ESRD72. Erythropoietin, a hormone essential for erythropoiesis, is produced in the kidney, and erythropoietin axis is predictably disrupted in CKD (Fig. 3). Introduction of erythropoiesis stimulating agents (ESA) revolutionized care for patients with CKD and ESRD, and resulted in significant reduction of blood transfusions in the CKD population. However, wide ranges of ESA doses are required to improve anemia in different patients with CKD, and high doses of ESAs, unfortunately, are associated with increased mortality in adult patients with CKD. Thus, hemoglobin targets in adults with CKD receiving ESAs are currently set significantly lower than in the general population73. It remains controversial whether hemoglobin targets in children with CKD should be higher than those in adults with CKD, in part due to concern that higher hemoglobin is needed to promote growth and cognitive development in children74.
Endogenous erythropoietin and ESAs are effective only when iron is readily available for erythropoiesis. Iron metabolism is disrupted in patients with CKD particularly due to elevated hepcidin75, a hepatic hormone that prevents iron egress from cells by binding to iron exporter, ferroportin. Hepcidin excess suppresses iron absorption and leads to iron sequestration in macrophages and hepatocytes, with leads to functional iron deficiency in CKD76. Therapeutic iron supplementation becomes necessary, but it further stimulates hepcidin77, which not only worsens anemia, but may contribute to growth impairment in juvenile CKD78. Hepcidin excess probably precedes erythropoietin dysfunction in the course of CKD, which is indirectly reflected by a much higher prevalence of iron therapy in children with mild to moderate CKD than ESA therapy79. There is a growing concern about iron overload and toxicity in CKD80,81. Indeed, increased iron content has been found in the liver and spleen of children with ESRD 82.
It remains unclear whether iron status has an impact on growth of children with CKD independently of anemia. Iron-deficiency anemia can impair growth in children without CKD and iron supplementation improves growth of these children 83-85. Iron excess, however, can be as devastating as iron deficiency, mainly because free iron has high potential for biologic toxicity. Specifically, iron excess negatively affects growth in children with transfusion-dependent thalassemia and hereditary iron overload disorders 86. Furthermore, even mild iron excess during childhood may negatively affect growth and neurocognitive development of otherwise healthy children 87-90. Therefore, while enteral or IV iron supplementation is currently essential in children with CKD, especially those requiring ESA therapy, it should be used judiciously and with a close (at least every 3 months)91 monitoring of iron status. Inflammation is a potent stimulation of hepcidin production, and reduction of inflammation in children with CKD has potential for the improvement of their iron status and anemia.92
Protein-energy metabolism and nutritional status in children with CKD
Nutritional status in children with CKD may be affected by several factors, including loss of appetite (anorexia), altered gastrointestinal motility, malabsorption, intestinal dysbiois, and uremic abnormalities of energy, protein, lipid, and carbohydrate metabolism. Assessment of nutritional status in children with CKD is complex (Box 4)31,93, and should be ideally conducted jointly be a pediatric nephrologist and a pediatric dietitian experienced in the nutritional management of patients with CKD.94 The term “malnutrition” refers to an inadequately low dietary intake, typically with adaptive increase in appetite and decrease in metabolic rate;95 therefore this term is usually not applicable to the alterations of nutritional status currently seen in children with CKD, especially in developed countries. Indeed, in the CKiD study, children with CKD consumed more protein and calories than recommended66. The majority of children with CKD in the U.S. have normal BMI when they reach ESRD, about 40% are overweight or obese, and only 10-15% are underweight, according to the USRDS1. Despite normal or elevated BMI in most children with CKD in the U.S., there is a concern that some of these children may have a disproportion between lean and fat body mass, and relative loss of muscle mass, including the possibility of sarcopenic obesity. The International Society of Renal Nutrition has developed diagnostic criteria for protein-energy wasting (PEW)96. Adaptation of these criteria to pediatric population has been proposed based on the validation in the CKiD study cohort97.
Box 4. Nutritional assessment in children with CKD.
- Dietary assessment
- Dietary recall
- Food Frequency Questionnaire
- Anthropometric assessment
- Body weight, length, BMI, head circumference
- Waist circumference, skinfold thickness, somatogram and frame size
- Advanced assessment of body composition
- Dual X-ray absorptiometry (DEXA)
- Bioelectrical impedance analysis (BIA)
- MRI (proton density contrast weighting, Dixon-related fat–water- separation)
- Biochemical assessment
- Serum albumin and prealbumin
- Normalized protein equivalent of nitrogen appearance (NPNA)
- Creatinine index (in dialysis patients)
The importance of protein metabolism in CKD has been recognized more than a century ago, particularly because the classical markers of uremia – BUN and creatinine – are both products of protein metabolism. In the pre-dialysis era, dietary protein restriction has been a mainstay of CKD management, making it possible to reduce the values of BUN and serum creatinine. However, the Modification of Diet in Renal Disease (MDRD) Study, the largest to date randomized clinical trial to test the effect of protein restriction on CKD progression in adults was inconclusive;98 Currently, protein restriction is usually not recommended in children with CKD. KDOQI recommends 100%-140% of the DRI protein intake for ideal body weight in children with stage 3 CKD, and 100%-120% in children with stages 4-5 CKD.31 Dialysis is associated with additional protein losses, which need to be taken into account when calculating protein requirements for children on dialysis, as well as urine protein losses in children with nephrotic syndrome. Certain vitamins may accumulate in advanced CKD due to reduced excretion and should not be taken in excess.99 Specific “renal” multivitamin preparation for patients with CKD are commercially available.
Statural growth in children with CKD
Growth impairment is a common problem in children with all stages of CKD. In the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) report, more than 35% of children had impaired growth at the time of enrollment100. In the era preceding the introduction of recombinant human growth hormone (rhGH) therapy, almost one-half of patients with childhood-onset ESRD had final adult heights below the 3rd percentile.101 Growth impairment is one of the major factors affecting the quality of life in children with CKD.102 Short children with CKD miss school more frequently and spend more time admitted in the hospital.103 Furthermore, growth impairment is a poor predictor of survival in children with ESRD.104 The risk of death increased by 14% for each unit of decrease of height z-score at the time of dialysis initiation in children from the USRDS.105
CKD appears to distinctly affect all phases of body growth.58 As with any chronic disease, growth velocity is most affected during the periods of rapid growth. Both prematurity and low birth weight / intrauterine growth restriction are common in children who subsequently develop CKD, and are risk factors for poor growth outcomes, independent of renal function106 (fetal phase). During infantile phase, adverse effects of CKD on growth may be most intense and are thought to be largely mediated by inadequate nutrition. Growth hormone (GH) axis becomes critical for growth in the childhood phase, while gonadotropin-sexual hormones axis is frequently affected by CKD in the pubertal phase.
Disturbance of (GH)/IGF-1 axis plays an important role in most children at some point in the course of CKD.107 In children with CKD, fasting serum GH levels are normal or elevated, suggesting GH insensitivity.107 Indeed, GH receptor expression is reduced in the liver tissue of juvenile mice108 and rats109 with experimental CKD, leading to inadequate production of IGF-1. Furthermore, the levels of circulating IGF-binding proteins are elevated in CKD,110 reducing the free, bioactive IGF-1.111 Other factors that may contribute to growth impairment in children with CKD include alterations in nutritional status, metabolic acidosis, fluid and electrolyte abnormalities, CKD-MBD, and anemia. While optimization of nutrition is important, sufficient or even excessive caloric and nutrient intake does not always prevent growth impairment in children with CKD.112,113 Gastrostomy feeding appears to be superior to oral or nasogastric feeding in infants on peritoneal dialysis.114 The relationship between anemia and growth in children with CKD remains incompletely understood.58 Growth impairment is a hallmark of untreated chronic anemias of non-renal origin. However, correction of anemia with ESA in several multicenter clinical trials did not result in a catch-up growth.115,116 The effect of iron therapy on growth has not been investigated in CKD, however preclinical data suggest that hepcidin blockade can be advantageous.108
rhGH has been FDA-approved for the treatment of short stature in children with CKD. Consensus guidelines for rhGH therapy in children with CKD are available.117 However, utilization of rhGH therapy in the U.S. seems to be low,118 and many families are non-compliant with rhGH treatment regimen.119 Recent survey of pediatric nephrologists have identified family refusal as a most common reason for rhGH underutilization, usually due to fear of injections.94 Logistical challenges, including those related to insurance approval were also reported to influence practice patterns of rhGH therapy in children with CKD in North America.94 Evaluation of bone age is required in short children older than 12 years with CKD prior to rhGH therapy initiation. Importantly, fusion of the growth plates can be significantly delayed in adolescents with CKD, in part due to delayed pubertal development.58 Thus, in many cases rhGH may be offered even in older adolescents with CKD.120 While not sufficiently studied in children with CKD, rhGH therapy may potentially have additional benefits, beyond the improvement in linear growth, such as mobilization of adipose tissue, increase in lean body mass, improvement in physical function117, stimulation of erythropoiesis and lowering circulating hepcidin.121
SUMMARY
CKD is a sustained loss of kidney function involving many organ systems and metabolic pathways, as well as impairing growth, physical and neurocognitive development of affected children. Pediatric CKD is associated with poor quality of life, high morbidity and mortality. Our understanding of the interventions that may slow disease progression and lead to improvement of patient-centered outcomes, including quality of life, has advanced in recent years. Aggressive control of hypertension; correction of metabolic acidosis, water and electrolyte abnormalities; and preventing episodes of acute on chronic kidney injury appear to constitute the most promising strategies. Nutritional management is a critically important component of care for children with CKD that can improve key outcomes. Effective dietary support requires coordinated team efforts of a pediatric nephrologist, pediatric renal dietitian, social worker, primary care provider, and the family. Timely introduction of transplant and / or dialysis services improves outcomes in most children with advanced CKD and ESRD. Further collaborative research is needed to resolve many outstanding controversies in the pathophysiology, diagnostic approaches, and management of pediatric CKD.
KEY POINTS.
Chronic Kidney Disease (CKD) is an irreversible deterioration of renal function that may progress to end-stage renal disease (ESRD).
The goals of CKD management in pediatric patients include slowing disease progression, prevention and treatment of complications, and optimizing growth, development, and quality of life.
Nutritional management is critically important for the prevention of acute and chronic complications and optimization of physical and neurocognitive development in children with CKD.
Control of blood pressure, proteinuria, and metabolic acidosis in CKD with dietary and pharmacologic measures may slow disease progression and postpone dialysis or kidney transplantation.
Further research is required to clarify the role of dietary protein intake and control of dyslipidemia in the progression of pediatric CKD.
SYNOPSIS.
Chronic Kidney Disease (CKD) is an ongoing deterioration of renal function that in many patients progresses to end-stage renal disease. The goals of CKD management in children include slowing disease progression, prevention and treatment of complications, and optimizing growth, development, and quality of life. Nutritional management is critically important to achieve all of these goals. Control of blood pressure, proteinuria, and metabolic acidosis with dietary and pharmacologic measures may slow progression of CKD. While significant progress in the management of CKD in children has been made in recent decades, further research is required to resolve many outstanding controversies. Here we review recent developments in the field of pediatric CKD, with a focus on dietary measures to improve outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Grant/Research support: Rohr Family Clinical Scholar Award (Weill Cornell Medicine)
References
- 1.USRDS. Annual data report. Chapter 7: ESRD among Children, Adolescents, and Young Adults. 2017.
- 2.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. The Lancet. 2013;382(9888):260–272. [DOI] [PubMed] [Google Scholar]
- 3.Hu J-R, Coresh J. The public health dimension of chronic kidney disease: what we have learnt over the past decade. Nephrology Dialysis Transplantation. 2017;32(suppl_2):ii113–ii120. [DOI] [PubMed] [Google Scholar]
- 4.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney International. 2003;63:S31–S37. [DOI] [PubMed] [Google Scholar]
- 5.Hinchliffe S, Sargent P, Howard C, Chan Y. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Laboratory investigation; a journal of technical methods and pathology. 1991;64(6):777–784. [PubMed] [Google Scholar]
- 6.Osathanondh V, Potter E. Development of human kidney as shown by microdissection. III. Formation and interrelationship of collecting tubules and nephrons. Archives of pathology. 1963;76:290–302. [PubMed] [Google Scholar]
- 7.Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki birth cohort study, 1924-1944. American Journal of Kidney Diseases. 2018;71(1):20–26. [DOI] [PubMed] [Google Scholar]
- 8.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney International. 2005;68:S68–S77. [DOI] [PubMed] [Google Scholar]
- 9.Pøtter EL. Bilateral renal agenesis. The Journal of pediatrics. 1946;29(1):68–76. [DOI] [PubMed] [Google Scholar]
- 10.Bülchmann G, Schuster T, Heger A, Kuhnle U, Joppich I, Schmidt H. Transient pseudohypoaldosteronism secondary to posterior urethral valves-a case report and review of the literature. European Journal of Pediatric Surgery. 2001;11(04):277–279. [DOI] [PubMed] [Google Scholar]
- 11.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. American Journal of Kidney Diseases. 2015;65(6):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furth SL, Pierce C, Hui WF, et al. Estimating Time to ESRD in Children With CKD. American Journal of Kidney Diseases. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duranton F, Cohen G, De Smet R, et al. Normal and pathologic concentrations of uremic toxins. Journal of the American Society of Nephrology. 2012:ASN. 2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yacoub R, Wyatt CM. Manipulating the gut microbiome to decrease uremic toxins. Kidney international. 2017;91(3):521–523. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney international. 2013;83(2):308–315. [DOI] [PubMed] [Google Scholar]
- 16.Snauwaert E, Van Biesen W, Raes A, et al. Accumulation of uraemic toxins is reflected only partially by estimated GFR in paediatric patients with chronic kidney disease. Pediatric Nephrology. 2017:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatric clinics of North America. 1987;34(3):571–590. [DOI] [PubMed] [Google Scholar]
- 18.Schnaper HW. Remnant nephron physiology and the progression of chronic kidney disease. Pediatric Nephrology. 2014;29(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg N, Roberts WL, Bachmann LM, et al. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and Jaffe method principles. Clinical chemistry. 2012;58(2):391–401. [DOI] [PubMed] [Google Scholar]
- 20.Weber J, Van Zanten A. Interferences in current methods for measurements of creatinine. Clinical chemistry. 1991;37(5):695–700. [PubMed] [Google Scholar]
- 21.Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Advances in chronic kidney disease. 2017;24(6):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekhar DL, Wang L, Hollenbeak CS, Widome MD, Paul IM. A cost-effectiveness analysis of screening urine dipsticks in well-child care. Pediatrics. 2010;125(4):660–663. [DOI] [PubMed] [Google Scholar]
- 23.Massengill SF, Ferris M. Chronic kidney disease in children and adolescents. Pediatrics in review. 2014;35(1):16–29. [DOI] [PubMed] [Google Scholar]
- 24.Seo-Mayer PW. Focus on Subspecialties: Children at risk for chronic kidney disease benefit from targeted screening AAP News. 2015. [Google Scholar]
- 25.Sabbagh Y, Graciolli FG, O'Brien S, et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. Journal of Bone and Mineral Research. 2012;27(8):1757–1772. [DOI] [PubMed] [Google Scholar]
- 26.Graciolli FG, Neves KR, Barreto F, et al. The complexity of chronic kidney disease– mineral and bone disorder across stages of chronic kidney disease. Kidney international. 2017;91(6): 1436–1446. [DOI] [PubMed] [Google Scholar]
- 27.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. American Journal of Physiology-Renal Physiology. 2007;293(5):F1577–F1583. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. Journal of the American Society of Nephrology. 2005;16(7):2205–2215. [DOI] [PubMed] [Google Scholar]
- 29.Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nature Reviews Nephrology. 2013;9(11):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler DC, Winkelmayer WC. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Foreword. Kidney International Supplements. 2017;7(1):1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53(3 Suppl 2):S11–104. [DOI] [PubMed] [Google Scholar]
- 32.Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. Amer Assn for Clinical Chemistry; 1994. [Google Scholar]
- 33.Raaijmakers R, Houkes LM, Schröder CH, Willems JL, Monnens LA. Pre-treatment of dairy and breast milk with sevelamer hydrochloride and sevelamer carbonate to reduce phosphate. Peritoneal Dialysis International. 2013;33(5):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alfrey A Effect of dietary phosphate restriction on renal function and deterioration. The American journal of clinical nutrition. 1988;47(1): 153–156. [DOI] [PubMed] [Google Scholar]
- 35.Jureidini KF, Hogg RJ, van Renen MJ, et al. Evaluation of long-term aggressive dietary management of chronic renal failure in children. Pediatric Nephrology. 1990;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 36.Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clinical Journal of the American Society of Nephrology. 2011. ;6(9):2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harambat J, Kunzmann K, Azukaitis K, et al. Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease. Kidney international. 2017;92(6):1507–1514. [DOI] [PubMed] [Google Scholar]
- 38.Kraut JA, Madias NE. Adverse Effects of the Metabolic Acidosis of Chronic Kidney Disease. Advances in chronic kidney disease. 2017;24(5):289–297. [DOI] [PubMed] [Google Scholar]
- 39.Kraut JA, Madias NE. Retarding progression of chronic kidney disease: use of modalities that counter acid retention. Current opinion in nephrology and hypertension. 2017. [DOI] [PubMed] [Google Scholar]
- 40.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Renal failure. 2006;28(1):1–5. [DOI] [PubMed] [Google Scholar]
- 41.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clinical Journal of the American Society of Nephrology. 2013:CJN. 08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. Journal of the American Society of Nephrology. 2009;20(9):2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013;3(1):1–150. [Google Scholar]
- 44.Goraya N, Simoni J, Jo C-H, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney international. 2014;86(5):1031–1038. [DOI] [PubMed] [Google Scholar]
- 45.Goraya N, Simoni J, Jo C-H, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical Journal of the American Society of Nephrology. 2013;8(3):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bushinsky DA, Hostetter T, Klaerner G, et al. Randomized, Controlled Trial of TRC101 to Increase Serum Bicarbonate in Patients with CKD. Clinical Journal of the American Society of Nephrology. 2017:CJN. 07300717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goraya N, Wesson DE. Management of the Metabolic Acidosis of Chronic Kidney Disease. Advances in chronic kidney disease. 2017;24(5):298–304. [DOI] [PubMed] [Google Scholar]
- 48.Kraut JA, Madias NE. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatric nephrology. 2011;26(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abuelo JG. Treatment of Severe Hyperkalemia: Confronting 4 Fallacies. Kidney international reports. 2018;3(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrology Dialysis Transplantation. 2017. [DOI] [PubMed] [Google Scholar]
- 51.Einhorn LM, Zhan M, Walker LD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Archives of internal medicine. 2009;169(12):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer BF, Clegg DJ. Hyperkalemia across the Continuum of Kidney Function. Clinical Journal of the American Society of Nephrology. 2018;13(1):155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayes C Jr, McLeod M, Robinson R. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Transactions of the Association of American Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- 54.Nguyen L, Levitt R, Mak RH. Practical Nutrition Management of Children with Chronic Kidney Disease. Clinical Medicine Insights: Urology. 2016;9:CMU. S13180. [Google Scholar]
- 55.Hill LJ, Herald AJ. Kidney-Friendly Label Reading for Chronic Kidney Disease Shoppers. Journal of Renal Nutrition. 2018;28(1):e1–e4. [DOI] [PubMed] [Google Scholar]
- 56.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. The American journal of medicine. 2013;126(3):264. e269–264. e224. [DOI] [PubMed] [Google Scholar]
- 57.Fried L, Kovesdy CP, Palmer BF. New options for the management of chronic hyperkalemia. Kidney International Supplements. 2017;7(3):x164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haffner D, Rees L. Growth and Puberty in Chronic Kidney Disease In: Geary DF, Schaefer F, eds. Pediatric Kidney Disease. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016:1425–1454. [Google Scholar]
- 59.Group ET. Strict blood-pressure control and progression of renal failure in children. New England Journal of Medicine. 2009;361(17):1639–1650. [DOI] [PubMed] [Google Scholar]
- 60.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. Journal of the American Society of Nephrology. 2012;23(1):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. Journal of the American Society of Nephrology. 2013;24(12):2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomura K, Asayama K, Jacobs L, Thijs L, Staessen JA. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney international. 2017;92(1):67–78. [DOI] [PubMed] [Google Scholar]
- 63.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). American journal of hypertension. 2012;25(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 64.Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low-and excessive-sodium diets are associated with increased mortality: a meta-analysis. American journal of hypertension. 2014;27(9):1129–1137. [DOI] [PubMed] [Google Scholar]
- 65.Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk—measurement matters. The New Englandjournal of medicine. 2016;375(6):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui WF, Betoko A, Savant JD, et al. Assessment of dietary intake of children with chronic kidney disease. Pediatr Nephrol. 2017;32(3):485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaefer F, Doyon A, Azukaitis K, et al. Cardiovascular phenotypes in children with CKD: the 4C study. Clinical Journal of the American Society of Nephrology. 2017;12(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saland JM, Pierce CB, Mitsnefes MM, et al. Dyslipidemia in children with chronic kidney disease. Kidney international. 2010;78(11): 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Group KK. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 70.Gerson A, Hwang W, Fiorenza J, et al. Anemia and health-related quality of life in adolescents with chronic kidney disease. American journal of kidney diseases. 2004;44(6):1017–1023. [DOI] [PubMed] [Google Scholar]
- 71.Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. American journal of kidney diseases. 2005;45(4):658–666. [DOI] [PubMed] [Google Scholar]
- 72.Fadrowski JJ, Pierce CB, Cole SR, Moxey-Mims M, Warady BA, Furth SL. Hemoglobin decline in children with chronic kidney disease: baseline results from the chronic kidney disease in children prospective cohort study. Clinical Journal of the American Society of Nephrology. 2008;3(2):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Locatelli F, Mazzaferro S, Yee J. Iron therapy challenges for the treatment of nondialysis CKD patients. Clinical Journal of the American Society of Nephrology. 2016:CJN.00080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hattori M Hemoglobin target in children with chronic kidney disease: valuable new information. Kidney international. 2017;91(1): 16–18. [DOI] [PubMed] [Google Scholar]
- 75.Atkinson MA, Kim JY, Roy CN, Warady BA, White CT, Furth SL. Hepcidin and risk of anemia in CKD: a cross-sectional and longitudinal analysis in the CKiD cohort. Pediatr Nephrol. 2015;30(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganz T, Nemeth E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Seminars in nephrology. 2016;36(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chand S, Ward DG, Ng Z-YV, et al. Serum hepcidin-25 and response to intravenous iron in patients with non-dialysis chronic kidney disease. Journal of nephrology. 2015;28(1):81–88. [DOI] [PubMed] [Google Scholar]
- 78.Akchurin O, Sureshbabu A, Doty SB, et al. Lack of Hepcidin Ameliorates Anemia and Improves Growth in an Adenine-induced Mouse Model of Chronic Kidney Disease. American journal of physiology Renal physiology. 2016;311(5):F877–F889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akchurin OM, Schneider MF, Mulqueen L, et al. Medication adherence and growth in children with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(9): 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney international. 2004;65(6):2279–2289. [DOI] [PubMed] [Google Scholar]
- 81.Lukaszyk E, Lukaszyk M, Koc-Zorawska E, Tobolczyk J, Bodzenta-Lukaszyk A, Malyszko J. Iron Status and Inflammation in Early Stages of Chronic Kidney Disease. Kidney & blood pressure research. 2015;40(4):366–373. [DOI] [PubMed] [Google Scholar]
- 82.Querfeld U, Dietrich R, Taira R, Kangarloo H, Salusky I, Fine R. Magnetic resonance imaging of iron overload in children treated with peritoneal dialysis. Nephron. 1988;50(3):220–224. [DOI] [PubMed] [Google Scholar]
- 83.Soliman AT, Al Dabbagh MM, Habboub AH, Adel A, Humaidy NA, Abushahin A. Linear growth in children with iron deficiency anemia before and after treatment. Journal of tropical pediatrics. 2009;55(5):324–327. [DOI] [PubMed] [Google Scholar]
- 84.Bandhu R, Shankar N, Tandon OP. Effect of iron on growth in iron deficient anemic school going children. Indian journal of physiology and pharmacology. 2003;47(1):59–66. [PubMed] [Google Scholar]
- 85.Soliman AT, De Sanctis V, Yassin M, Adel A. Growth and Growth hormone - Insulin Like Growth Factor -I (GH-IGF-I) Axis in Chronic Anemias. Acta bio-medica : Atenei Parmensis. 2017;88(1):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skordis N, Kyriakou A. The multifactorial origin of growth failure in thalassaemia. Pediatric endocrinology reviews : PER. 2011;8 Suppl 2:271–277. [PubMed] [Google Scholar]
- 87.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public health nutrition. 2006;9(7):904–920. [DOI] [PubMed] [Google Scholar]
- 88.Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. The Journal of nutrition. 2002;132(11):3249–3255. [DOI] [PubMed] [Google Scholar]
- 89.Perng W, Mora-Plazas M, Marin C, Villamor E. Iron status and linear growth: a prospective study in school-age children. European journal of clinical nutrition. 2013;67(6):646–651. [DOI] [PubMed] [Google Scholar]
- 90.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Archives of pediatrics & adolescent medicine. 2012;166(3):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.KDIGO. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney International Supplements. 2012;2:279–335. [Google Scholar]
- 92.Akchurin M, Kaskel F. Update on inflammation in chronic kidney disease. Blood purification. 2015;39(1-3):84–92. [DOI] [PubMed] [Google Scholar]
- 93.Mastrangelo A, Paglialonga F, Edefonti A. Assessment of nutritional status in children with chronic kidney disease and on dialysis. Pediatr Nephrol. 2014;29(8):1349–1358. [DOI] [PubMed] [Google Scholar]
- 94.Akchurin M, Kogon AJ, Kumar J, et al. Approach to growth hormone therapy in children with chronic kidney disease varies across North America: the Midwest Pediatric Nephrology Consortium report. BMC nephrology. 2017;18(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mak RH, Cheung WW, Zhan JY, Shen Q, Foster BJ. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2012;27(2):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. [DOI] [PubMed] [Google Scholar]
- 97.Abraham AG, Mak RH, Mitsnefes M, et al. Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2014;29(7):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. New England Journal of Medicine. 1994;330(13):877–884. [DOI] [PubMed] [Google Scholar]
- 99.Steiber AL, Kopple JD. Vitamin status and needs for people with stages 3-5 chronic kidney disease. Journal of Renal Nutrition. 2011;21(5):355–368. [DOI] [PubMed] [Google Scholar]
- 100.Seikaly MG, Salhab N, Gipson D, Yiu V, Stablein D. Stature in children with chronic kidney disease: analysis of NAPRTCS database. Pediatr Nephrol. 2006;21(6):793–799. [DOI] [PubMed] [Google Scholar]
- 101.André J-L, Bourquard R, Guillemin F, Krier M-J, Briançon S. Final height in children with chronic renal failure who have not received growth hormone. Pediatric Nephrology. 2003;18(7):685–691. [DOI] [PubMed] [Google Scholar]
- 102.Al-Uzri A, Matheson M, Gipson DS, et al. The impact of short stature on health-related quality of life in children with chronic kidney disease. The Journal of pediatrics. 2013;163(3):736–741 e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA. Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2002;109(5):909–913. [DOI] [PubMed] [Google Scholar]
- 104.Weaver DJ, Somers MJ, Martz K, Mitsnefes MM. Clinical outcomes and survival in pediatric patients initiating chronic dialysis: a report of the NAPRTCS registry. Pediatric Nephrology. 2017;32(12):2319–2330. [DOI] [PubMed] [Google Scholar]
- 105.Wong CS, Gipson DS, Gillen DL, et al. Anthropometric measures and risk of death in children with end-stage renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36(4):811–819. [DOI] [PubMed] [Google Scholar]
- 106.Greenbaum LA, Muñoz A, Schneider MF, et al. The association between abnormal birth history and growth in children with CKD. Clinical Journal of the American Society of Nephrology. 2010:CJN. 08481109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tönshoff B Pathogenesis evaluation and diagnosis of growth impairment in children with chronic kidney disease In: Niaudet P, ed. UpToDate. Waltham, MA: UpToDate Inc;2018. [Google Scholar]
- 108.Akchurin O, Sureshbabu A, Doty SB, et al. Lack of hepcidin ameliorates anemia and improves growth in an adenine-induced mouse model of chronic kidney disease. American Journal of Physiology-Renal Physiology. 2016;311(5):F877–F889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tönshoff B, Powell DR, Zhao D, et al. Decreased hepatic insulin-like growth factor (IGF)-I and increased IGF binding protein-1 and-2 gene expression in experimental uremia. Endocrinology. 1997;138(3):938–946. [DOI] [PubMed] [Google Scholar]
- 110.Tönshoff B, Blum WF, Wingen A-M, Mehls O. Serum insulin-like growth factors (IGFs) and IGF binding proteins 1, 2, and 3 in children with chronic renal failure: relationship to height and glomerular filtration rate. The European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. The Journal of Clinical Endocrinology & Metabolism. 1995;80(9):2684–2691. [DOI] [PubMed] [Google Scholar]
- 111.Frystyk J, Ivarsen P, Skærbæk C, Flyvbjerg A, Pedersen EB, Ørskov H. Serum-free insulin-like growth factor I correlates with clearance in patients with chronic renal failure. Kidney international. 1999;56(6):2076–2084. [DOI] [PubMed] [Google Scholar]
- 112.Abithol CL, Zilleruelo G, Montane B, Strauss J. Growth of uremic infants on forced feeding regimens. Pediatric Nephrology. 1993;7(2):173–177. [DOI] [PubMed] [Google Scholar]
- 113.Rees L, Jones H. Nutritional management and growth in children with chronic kidney disease. Pediatric Nephrology. 2013;28(4):527–536. [DOI] [PubMed] [Google Scholar]
- 114.Rees L, Azocar M, Borzych D, et al. Growth in very young children undergoing chronic peritoneal dialysis. Journal of the American Society of Nephrology. 2011:ASN.2010020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morris K, Sharp J, Watson S, Coulthard M. Non-cardiac benefits of human recombinant erythropoietin in end stage renal failure and anaemia. Archives of disease in childhood. 1993;69(5):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jabs K The effects of recombinant human erythropoietin on growth and nutritional status. Pediatric Nephrology. 1996;10(3):324–327. [DOI] [PubMed] [Google Scholar]
- 117.Mahan JD, Warady BA, Committee C. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatric Nephrology. 2006;21(7):917–930. [DOI] [PubMed] [Google Scholar]
- 118.Greenbaum LA, Hidalgo G, Chand D, et al. Obstacles to the prescribing of growth hormone in children with chronic kidney disease. Pediatric Nephrology. 2008;23(9):1531–1535. [DOI] [PubMed] [Google Scholar]
- 119.Akchurin M, Schneider MF, Mulqueen L, et al. Medication adherence and growth in children with CKD. Clinical Journal of the American Society of Nephrology. 2014;9(9):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gil S, Aziz M, Adragna M, Monteverde M, Belgorosky A. Near-adult height in male kidney transplant recipients started on growth hormone treatment in late puberty. Pediatric Nephrology. 2018;33(1):175–180. [DOI] [PubMed] [Google Scholar]
- 121.Troutt JS, Rudling M, Persson L, et al. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clinical chemistry. 2012;58(8):1225–1232. [DOI] [PubMed] [Google Scholar]