SUMMARY
OBJECTIVE:
To determine the proportion of recurrent tuberculosis (TB) due to relapse with the patient’s initial strain or reinfection with a new strain of Mycobacterium tuberculosis 1–2 years after anti-tuberculosis treatment in Uganda, a sub-Saharan TB-endemic country.
DESIGN:
Records of patients with culture-confirmed TB who completed treatment at an urban Ugandan clinic were reviewed. Restriction fragment length polymorphism (RFLP) patterns were used to determine relapse or reinfection. Associations between human immunodeficiency virus (HIV) positivity and type of TB recurrence were determined.
RESULS:
Of 1701 patients cured of their initial TB episode with a median follow-up of 1.24 years, 171 (10%) had TB recurrence (8.4 per 100 person-years). Rate and risk factors for recurrence were similar to other studies from sub-Saharan Africa. Insertion sequence (IS) 6110-based RFLP of paired isolates from 98 recurrences identified 80 relapses and 18 reinfections. Relapses among HIV-positive and -negative patients were respectively 79% and 85% of recurrences.
CONCLUSIONS:
Relapse was more common and presented earlier than reinfection in both HIV-positive and -negative TB patients 1–2 years after completing treatment. These findings impact both the choice of retreatment drug regimen, as relapsing patients are at higher risk for acquired drug resistance, and clinical trials of new TB regimens with relapse as clinical endpoint.
Keywords: RFLP, HIV, retreatment
RÉSUMÉ
OBJECTIF :
Déterminer en Ouganda, un pays subsaharien où la tuberculose (TB) est endémique, la proportion de reprises de TB dues à une rechute due à la souche initiale du patient ou à une réinfection par une nouvelle souche de Mycobacterium tuberculosis 1 à 2 ans après le traitement TB.
SCHÉMA :
On a révisé les dossiers de patients atteints d’une TB confirmée par la culture qui ont achevé leur traitement dans un dispensaire urbain d’Ouganda. On a utilisé les types de polymorphisme de longueur des fragments de restriction (RFLP) pour déterminer s’il s’agissait de rechute ou de réinfection. On a déterminé les associations entre le type de séropositivité au virus de l’immunodéficience humaine (VIH) et le type de reprise de la TB.
RÉSULTATS :
Sur 1701 patients guéris de leur épisode initial de TB, après un suivi médian de 1,24 année, on a observé une reprise de la TB chez 171 sujets (10%; 8,4 pour 100 années/personne). Les taux et facteurs de risque ont été similaires à ceux d’autres études provenant d’Afrique sub-saharienne. La RFLP basée sur la séquence d’insertion (IS) 6110 a été pratiquée sur des isolats appariés provenant de 98 cas de reprises et a identifié 80 rechutes et 18 réinfections. Dans les reprises de traitement, il s’agit de 79% de rechutes chez les sujets séropositifs pour le VIH et 85% chez les sujets séronégatifs.
CONCLUSIONS :
La rechute est plus courante et survient plus précocement que la réinfection chez les patients TB tant séropositifs que séronégatifs pour le VIH 1 à 2 ans après achèvement du traitement. Ces observations ont un impact à la fois sur le choix du régime médica-menteux de retraitement, puisque les patients en rechute encourent un risque plus élevé de résistance acquise à l’égard des médicaments, et sur les essais cliniques de nouveaux régimes TB ayant la rechute comme donnée clinique terminale.
RESUMEN
OBJETIVO:
Determinar la proporción de casos de recidiva de tuberculosis (TB) causada por la cepa inicial del paciente o por una reinfección externa con una nueva cepa de Mycobacterium tuberculosis, 1 a 2 años después del tratamiento antituberculoso en Uganda, un país subsahariano donde la TB es endémica.
MÉTODO:
Se analizaron las historias clínicas de los pacientes con TB confirmada por cultivo que completaron el tratamiento en un consultorio urbano en Uganda. Se diferenció la recaída de la reinfección mediante el patrón de las longitudes de los fragmentos de restricción (RFLP). Se determinó la asociación entre la seropositividad frente al virus de la inmunodeficiencia humana (VIH) y tipo de recidiva de la TB.
RESULTADOS:
De los 1701 pacientes curados de su episodio inicial de TB con una mediana de seguimiento de 1,24 años, 171 presentaron una recaída (10%; 8,4 por 100 años persona). La tasa de recidiva y los factores de riesgo asociados con la misma fueron análogos a los comunicados por otros estudios en África subsahariana. El análisis de las parejas de aislados en 98 casos de recidiva por RFLP con la secuencia de inserción IS6110 detectó 80 recaídas y 18 reinfecciones externas. Las recaídas en los casos de pacientes positivos frente al VIH fueron 79% y en los casos de pacientes seronegativos fueron 85%.
CONCLUSIÓN:
La recaída entre 1 a 2 años después de completar el tratamiento antituberculoso fue más frecuente y se presentó de manera más precoz que la reinfección externa en los pacientes TB coinfectados por el VIH y también en los pacientes sin coinfección. Estos resultados tienen repercusiones sobre la elección del régimen de retratamiento, pues los pacientes en recaída presentan un mayor riesgo de presentar farmacorresistencia adquirida, e influyen además sobre el diseño de los ensayos clínicos de nuevos regímenes antituberculosos en los cuales la recaída sea un criterio de evaluación.
RECURRENT TUBERCULOSIS (TB) occurs in patients who have recovered from a prior episode of TB. Recurrent TB is due either to reactivation of the original Mycobacterium tuberculosis strain or to reinfection with a different M. tuberculosis strain.1,2 The proportion of recurrent TB due to reinfection appears to increase with increasing incidence of TB and length of follow-up period.1–8 Three studies conducted in sub-Saharan Africa have shown that recurrence due to exogenous reinfection was more likely among human immunodeficiency virus (HIV) positive patients.6,9,10 Understanding the relative contributions of endogenous reactivation (relapse) and exogenous reinfection to recurrence is important, not only for guiding TB prevention and control efforts, but also for clinical trials of new TB drug combinations with relapse as critical endpoint.
Uganda is a sub-Saharan country with both a high incidence of TB (281 cases per 100 000 population per year)11 and a high prevalence of HIV infection (6.5% among adults).12,13 The extent of exogenous reinfection among recurrent TB patients has not been fully described for this area.14–16
We examined the incidence of recurrent TB among patients treated with short-course rifampin (RMP) containing chemotherapy in 13 cohort studies and clinical trials conducted in Kampala, Uganda, at a single large research unit serving a crowded urban population with a high incidence of TB and HIV co-infection (>400/100 000).12 Restriction fragment length polymorphism (RFLP) analysis was used to determine the cause of the recurrent TB; we examined risk factors associated with TB recurrence in this cohort during the first 1–2 years of follow-up after completion of anti-tuberculosis treatment.
METHODS
Study design
This was a retrospective review of culture-positive recurrent pulmonary TB cases. Patients with recurrent TB were identified from existing databases and their records were reviewed.
Patients
Consenting adults without a history of TB treatment and with a first episode of culture-confirmed pulmonary TB were enrolled in 13 research protocols at the Uganda–Case Western Reserve University Research Collaboration TB Clinic at Mulago Hospital in Kampala, Uganda. The results of these clinical trials and epidemiologic studies have been published.17–22 The clinic utilizes a general screening process, whereby individuals presenting with symptoms suspicious for TB, such as cough and fever, undergo a diagnostic work-up for TB disease, including chest X-ray and sputum culture. This clinic treated and followed over 1800 HIV-positive and -negative TB patients from 1993 to 2006. All consecutive eligible subjects enrolled in studies during this period were included in the analysis.
The majority of the patients (94%) were treated with short-course chemotherapy, consisting of 2 months of ethambutol (EMB), isoniazid (INH), RMP and pyrazinamide (PZA), followed by 4–6 months of RMP and INH (n = 1598) or 6 months of EMB and INH (n = 103). HIV status and drug susceptibility testing (DST) patterns at baseline were recorded. Patients were followed monthly until completion of treatment and then every 3 months until the end of the study. At every visit, patients underwent history and physical examination, sputum examination and an end of treatment chest X-ray. Antiretroviral therapy (ART) was introduced in Uganda in 2004, but was not widely available at Mulago Hospital until 2008.
Patients were identified retrospectively for this study by searching study databases for clinically and bacteriologically cured patients who returned with culture-positive recurrent TB. The criteria for identification of patients with recurrent TB were uniform across studies.
Processing of sputum samples
Sputum specimens for initial and recurrent TB episodes were decontaminated and processed using a standard sodium hydroxide (NaOH) N-acetyl L-cysteine/sodium citrate method with a 1.5% final NaOH concentration. Buffered sediments were cultured on Middlebrook 7H10 agar medium and in liquid media using BD BACTEC™ 460 or MGIT™ 960 systems (BD, Sparks, MD, USA).23 Positive cultures were confirmed as M. tuberculosis complex with the p-nitro-α-acetylamino-β-hydroxy propiophenone (NAP) differentiation method23 or insertion sequence (IS) 6110 polymerase chain reaction (PCR).24 Initial and recurrent isolates were analyzed using standard IS6110 RFLP methodology.25,26 Initial fingerprint patterns were entered into Bionumerics 3.0 software (Applied Maths, St Martens Latem, Belgium) and the dendrogram was calculated (Pearson’s correlation). Recurrent fingerprint patterns were visually compared with initial patterns. Matching fingerprint patterns were defined as those with identical size and number of bands. No patterns had fewer than six bands.
Definitions
Recurrent TB was defined as culture of M. tuberculosis on more than one occasion from a patient who was declared cured. A cured patient was defined as a patient whose sputum became culture-negative, who became symptom free or was unable to produce sputum at completion of treatment.
Endogenous reactivation (hereafter referred to as relapse) was defined as the occurrence of matching RFLP patterns for initial and recurrence isolates. Exogenous reinfection was defined as the occurrence of non-matching RFLP patterns for initial and recurrent isolates differing by more than one band. Extent of disease was described as ‘far advanced’ or ‘moderate or minimal’, according to Falk et al.27
Study oversight
The parent studies were approved by the ethics committees of the Uganda National Council of Science and Technology and the University Hospitals Case Medical Center Institutional Review Board.
Statistical analysis
Incidence of TB recurrence was expressed as rate per 100 person-years (py) of observation (PYO). Calculation of py began at treatment completion and ended at date of recurrence, date last seen at clinic, date of death or end of study. Univariate analysis was performed to identify potential risk factors for TB recurrence. Cox proportional hazards modeling was used for multivariate analysis to calculate adjusted hazard ratios (aHRs) for HIV status and other risk factors. When testing hypotheses a 5% level of significance was used; for relative hazards, 95% confidence intervals (CIs) are presented. The Kaplan-Meier method tested the equality of survival distribution at 2 years, stratified by HIV status. SAS version 9.2 software (SAS Institute Inc, Cary, NC, USA) was used for analysis.
RESULTS
Patient enrollment
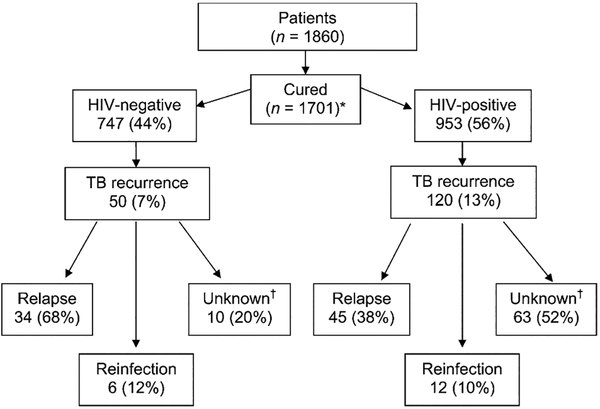
Of the 1701 patients who completed anti-tuberculosis treatment and were declared cured, 953 (56%) were HIV-positive (Figure 1). Of 142 deaths, 139 occurred in HIV-positive TB patients. Baseline DST patterns were available for 932 patients. Seventy-three patients (8%) had isolates resistant to one or more drugs; 69 were monoresistant to INH (n = 48), EMB (n = 12), RMP (n = 5) or PZA (n = 4). Four were resistant to both INH and RMP, and treated with second-line drugs. Median follow-up was 1.24 years (14.9 months).
Figure 1.
Patient study profile and outcomes. *HIV status was missing for one patient. †RFLP patterns not completed for these patients. HIV = human immunodeficiency virus; TB = tuberculosis; RFLP = restriction fragment length polymorphism.
Recurrent tuberculosis
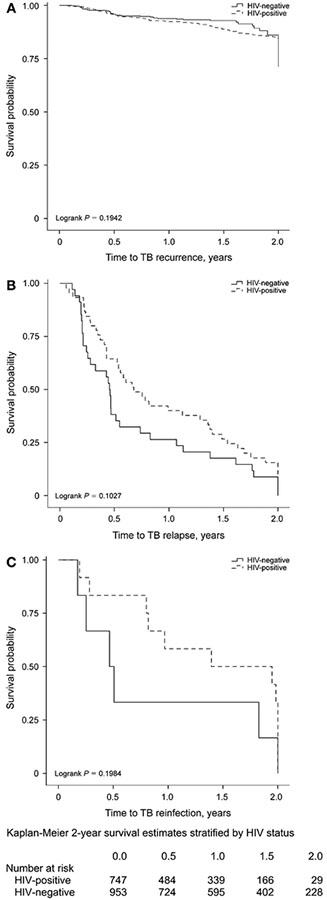
A total of 171 patients (10% of all cured patients) developed recurrent TB. Of these, 120 (70%) were HIV-positive. The incidence rate for TB recurrence was 8.4 per 100 py (Table 1). The incidence for HIV-positive patients was 9.4/100 py compared to 6.7/100 py for HIV-negative patients. HIV-positive patients were at increased risk for recurrence during the first 2 years after completing anti-tuberculosis treatment (Figure 2A).
Table 1.
Incidence rates and HRs for patients with TB recurrence (univariate and multivariate analysis)
| n | PYO | Patients with TB recurrence | Rate per 100 PYO (95%CI) | Crude HR (95%CI) | aHR (95%CI) | |
|---|---|---|---|---|---|---|
| Overall | 1701 | 2027.27 | 171 | 8.4 (7.3–9.8) | ||
| Sex | ||||||
| Male | 939 | 1088.66 | 96 | 8.8 (7.2–10.8) | 1.1 (0.8–1.4) | |
| Female | 762 | 938.61 | 75 | 8.0 (6.4–10.1) | Reference | |
| Age group, years* | ||||||
| ⩾30 | 735 | 895.0 | 86 | 9.6 (7.8–11.9) | 1.2 (0.9–1.7) | 1.2 (0.8–1.6) |
| <30 | 966 | 1129.73 | 85 | 7.5 (6.1–9.3) | Reference | Reference |
| HIV status | ||||||
| Positive | 953 | 1283.36 | 120 | 9.4 (7.8–11.2) | 1.3 (0.9–1.8) | 1.1 (0.7–1.6) |
| Negative | 747 | 740.81 | 50 | 6.7 (5.1–8.9) | Reference | Reference |
| CD4 count, cells/mm3† | ||||||
| <350 | 139 | 223.47 | 23 | 10.3 (6.8–15.5) | 2.8 (1.3–5.9) | |
| ⩾350 | 206 | 284.52 | 10 | 3.5 (1.9–6.5) | Reference | |
| Hemoglobin concentration, g/dl* | ||||||
| <12 | 960 | 1129.85 | 121 | 10.7 (9.0–12.8) | 1.8 (1.3–2.5) | 1.5 (1.1–2.3) |
| ⩾12 | 741 | 897.42 | 50 | 5.6 (4.2–7.4) | Reference | Reference |
| BMI at baseline* | ||||||
| <18.9 | 872 | 1031.11 | 99 | 9.6 (7.9–11.7) | 1.3 (0.9–1.7) | |
| ⩾18.9 | 829 | 996.16 | 72 | 7.2 (5.7–9.1) | Reference | |
| Weight gain at 2 months, kg* | ||||||
| <3 | 918 | 1018.35 | 105 | 10.3 (8.5–12.5) | 1.8 (1.3–2.4) | 1.9 (1.3–2.6) |
| ⩾3 | 783 | 1008.91 | 66 | 6.5 (5.1–8.3) | Reference | Reference |
| Continuation phase regimen‡ | ||||||
| 6H3E3 | 103 | 160.81 | 24 | 14.9 (10.0–22.3) | 1.9 (1.2–2.9) | 1.6 (0.9–2.7) |
| 6RH | 244 | 363.75 | 20 | 5.5 (3.5–8.5) | 0.7 (0.4–1.1) | 0.8 (0.5–1.4) |
| 4RH | 1354 | 1502.21 | 127 | 8.5 (7.1–10.1) | Reference | Reference |
| Drug resistance§ | ||||||
| Resistant to H and/or R | 73 | 96.06 | 9 | 9.4 (4.9–18.0) | 1.1 (0.6–2.2) | |
| Susceptible | 859 | 999.00 | 83 | 8.3 (6.7–10.3) | Reference | |
| Extent of disease (end of treatment) | ||||||
| Far advanced | 156 | 171.26 | 25 | 14.6 (9.9–21.6) | 2.0 (1.3–3.1) | 1.3 (0.8–2.1) |
| Moderate or minimal | 1278 | 1551.52 | 121 | 7.8 (6.5–9.3) | Reference | Reference |
| Cavity (end of treatment) | ||||||
| Yes | 467 | 541.22 | 63 | 11.6 (9.1–14.9) | 1.6 (1.2–2.3) | 1.4 (1.0–1.9) |
| No | 965 | 1176.48 | 83 | 7.1 (5.7–8.7) | Reference | Reference |
| Fibrosis (end of treatment) | ||||||
| Yes | 385 | 481.68 | 54 | 11.2 (8.6–14.6) | 1.4 (1.0–2.0) | 1.2 (0.8–1.7) |
| No | 1050 | 1240.53 | 92 | 7.4 (6.0–9.1) | Reference | Reference |
Variable dichotomized based on median value.
CD4 count available for 345 patients.
Numbers before the letters indicate the duration in months of the phase of treatment; numbers in subscript indicate the number of times the drug is taken each week.
Drug susceptibility testing results available for 932 patients.
HR = hazard ratio; TB = tuberculosis; PYO = person-years of observation; CI = confidence interval; aHR = adjusted HR; HIV = human immunodeficiency virus; H = isoniazid; E = ethambutol; R = rifampicin.
Figure 2.
A. Survival estimates for TB recurrence at 2 years post treatment. B. Time to relapse at 2 years post treatment. C. Time to reinfection at 2 years post treatment. TB = tuberculosis; HIV = human immunodeficiency virus.
The median time to recurrence was 6.5 months. Among HIV-positive patients, the median time to recurrence was 9.8 months (interquartile range [IQR] 4.9–21.0) vs. 5.6 months (IQR 2.6–13.2) among HIV-negative patients (P = 0.02). Fifty-six per cent of recurrent TB occurred in males, with a median age of 30 years (range 18–53). Of 53 patients with INH or RMP monoresistance, seven (all INH) had TB recurrence. No multidrug-resistant patients recurred. There was no association between drug resistance and TB recurrence.
Risk factors for recurrence
Risk factors associated with recurrent TB in univariate analysis were age group ⩾30 years, CD4+ T-cell count <350 cells/mm3, baseline hemoglobin concentration <12 g/dl, weight gain <3 kg after 2 months of treatment and a continuation phase regimen consisting of 6 months of intermittent (thrice weekly) INH and EMB. Radiological features associated with recurrent TB were far advanced disease, residual cavity and fibrosis at the end of treatment (Table 1). HIV-positive status was associated with recurrence; however, this result did not reach statistical significance (P = 0.20).
Multivariate analysis revealed that lower hemoglobin concentration (aHR = 1.5, 95%CI 1.1–2.3), weight gain of <3 kg after 2 months (aHR = 1.9, 95%CI 1.3–2.6) and cavitation (aHR = 1.4, 95%CI 1.0–1.9) remained significantly associated with TB recurrence. CD4+ count available for only 345 TB patients was not included in the model.
Relapse vs. reinfection
Ninety-eight patients with recurrent TB had paired initial and recurrent culturable M. tuberculosis isolates for comparison by RFLP. To determine if the 63 HIV-positive patients with unknown RFLP contributed to underestimating reinfection, we conducted a sub-analysis comparing HIV-positive patients with unknown RFLP to those with RFLP results. There were no significant differences between the two groups for all risk factors examined. Median follow-up during the 2 years post treatment was 9.8 for those with vs. 15.0 months for those without RFLP (P = 0.33).
The range of IS6110 numbers was 7–18, median 14. No clusters were observed. Eighty patients (82%) had identical isolates at baseline and recurrence (relapse), and 18 (18%) had different isolates for the two episodes (reinfection). When stratified by HIV status, recurrence was more likely due to relapse than to reinfection for both HIV-positive (79% relapse) and HIV-negative (85% relapse) patients (P = 0.45) (Table 2). Among 57 HIV-positive patients with recurrent TB, 19 had both CD4+ T-cell counts and outcome data. Of 13 with CD4+ count < 350 cells/mm3, 9 relapsed and 4 were re-infected; of 6 with CD4+ count ⩾ 350, 5 relapsed and 1 was re-infected. Low hemoglobin increased the risk of relapse and fibrosis at the end of treatment increased the risk of reinfection.
Table 2.
Multivariate analysis of factors associated with relapse and reinfection among 98 patients with confirmed RFLP results
| Relapse | Reinfection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Events | Rate per 100 PYO | HR (95%CI) | aHR (95%CI) | Events | Rate per 100 PYO | HR (95%CI) | aHR (95%CI) | |
| Overall | 98 | 80 | 1.0 | 18 | 0.7 | ||||
| HIV status* | |||||||||
| Positive | 57 | 45 | 1.0 | 0.8 (0.5–1.2) | 0.4 (0.2–0.8) | 12 | 0.6 | 0.8 (0.3–2.5) | 0.6 (0.1–3.6) |
| Negative | 40 | 34 | 1.1 | Reference | 6 | 1.0 | Reference | ||
| Hemoglobin, g/dl† | |||||||||
| <12 | 69 | 57 | 1.2 | 1.4 (0.9–2.4) | 2.2 (1.1–4.7) | 12 | 0.7 | 0.8 (0.3–2.3) | 0.8 (0.2–3.8) |
| ⩾12 | 29 | 23 | 0.8 | Reference | 6 | 0.7 | Reference | ||
| Weight gain at 2 months, kg† | |||||||||
| <3 | 62 | 54 | 1.1 | 1.1 (0.7–1.7) | 1.2 (0.6–2.4) | 8 | 0.7 | 1.0 (0.4–3.1) | 1.5 (0.4–6.3) |
| ⩾3 | 36 | 26 | 1.0 | Reference | 10 | 0.8 | Reference | ||
| Continuation phase regimen‡ | |||||||||
| 6H3E3 | 9 | 7 | 1.4 | 1.2 (0.5–2.6) | 1.4 (0.6 3.5) | 2 | 0.6 | 1.0 (0.2–4.6) | 1.6 (0.2–11.2) |
| 6RH | 7 | 7 | 0.7 | 0.7 (0.3–1.5) | 0.8 (0.3–2.3) | 0 | — | — | — |
| 4RH | 82 | 66 | 1.1 | Reference | 16 | 0.7 | Reference | — | |
| Fibrosis (end of treatment)§ | |||||||||
| Yes | 31 | 26 | 1.1 | 1.0 (0.6–1.6) | 0.9 (0.5–1.7) | 5 | 1.3 | 3.0 (0.9–10.0) | 3.9 (1.0–15.8) |
| No | 52 | 41 | 1.0 | Reference | 11 | 0.6 | Reference | ||
HIV status missing for 1 patient (relapse).
Variable dichotomized based on median value.
Numbers before the letters indicate the duration in months of the phase of treatment; numbers in subscript indicate the number of times the drug is taken each week.
Variable missing for 15 patients (13 relapse; 2 reinfection).
RFLP = restriction fragment length polymorphism; PYO = person-years of observation; HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; HIV = human immunodeficiency virus; H = isoniazid; E = ethambutol; R = rifampicin.
The overall median time to relapse during the first 2 years post treatment was 6.5 months; it was 14.2 months to reinfection (P = 0.08; Table 3). The median time to relapse was shorter than the time to reinfection (8.1 vs. 20.1 months, P = 0.07) for HIV-positives and HIV-negatives (5.5 vs. 5.9 months, P = 0.60). When time to recurrence was evaluated separately for those with ⩽1 year vs. those with >1 year of follow-up, similar trends were observed.
Table 3.
Associations between median time to recurrence during the first 2 years of follow-up and HIV serostatus among 98 patients with confirmed RFLP results*
| Overall | ⩽1 year of follow-up (n = 61) | 1–2 years of follow-up (n = 37) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relapse | Reinfection | Relapse | Reinfection | Relapse | Reinfection | ||||||||||||
| n | Median months | n | Median months | n | Median months | P value | n | Median months | n | Median months | P value | n | Median months | n | Median months | P value | |
| All | 98 | 7.1 | 80 | 6.5 | 18 | 14.2 | 0.08 | 52 | 4.7 | 9 | 5.6 | 0.44 | 28 | 21.1 | 9 | 23.9 | 0.09 |
| HIV-positive | 57 | 9.8 | 45 | 8.1 | 12 | 20.1 | 0.07 | 27 | 5.1 | 5 | 9.6 | 0.28 | 18 | 20.6 | 7 | 23.9 | 0.21 |
| HIV-negative | 40 | 5.6 | 34 | 5.5 | 6 | 5.9 | 0.60 | 25 | 3.4 | 4 | 4.3 | 0.88 | 9 | 21.2 | 2 | 22.9 | 0.35 |
HIV status was missing for 1 patient (relapse).
HIV = human immunodeficiency virus; RFLP = restriction fragment length polymorphism.
DISCUSSION
This is the largest study to date of recurrent TB. It includes 98 paired isolates with RFLP fingerprints for both initial and recurrent isolates. Patients were screened and followed up under similar clinical and laboratory conditions. The observed recurrence rate of 8.4/100 PYO is similar to other studies reported from sub-Saharan Africa.9,10 HIV co-infection, low CD4+ count and radiological severity of disease were associated with recurrent TB.6,28 Most recurrences in this population were due to relapse rather than reinfection for both HIV-positive and -negative patients. Reinfection with a TB strain with the same RFLP is unlikely in Kampala, where RFLP and SNP (single nucleotide polymorphism) analyses reveal significant strain variation.29
Our results contrast with earlier reports from high TB and HIV prevalence areas that suggested reinfection as a major cause of recurrent TB. In studies by Verver et al. and Charalambous et al., exogenous reinfection occurred in respectively 24/31 (77%) and 11/16 (68%) patients.7,9 Among HIV-positive South African miners reinfection was reported in respectively 13/21 (62%) and 10/14 (71%) patients, and in rural Malawi in 11/25 (44%) recurrences.6,9,10 A study from Kenya found reinfection among 1/5 (20%) recurrences.30
A number of factors contribute to the contrasting results of our study. First, our study evaluated recurrences during the first 1–2 years of follow-up. This duration of follow-up is used for Phase 3 clinical trials, which is when most recurrences occur.31 Studies by Sonnenberg et al. and Crampin et al. had longer follow-up periods, although increased reinfection rates were detected in HIV-positives after 12–24 months of follow-up.6,10 A separate study in South African miners found high reinfection rates in HIV-positives, with a median follow-up period of 1.0 years.9 Second, our studies were conducted in crowded urban Kampala, with a high annual risk of M. tuberculosis infection that may differ from rural Malawi and South African mines in risk of reinfection.12 Circulating lineages of M. tuberculosis likely also differ among these settings, and could contribute to differential reinfection risk. Third, selection of TB-HIV patients in our research studies may have been biased towards better performance and immune status compared to those enrolled in large cohort studies. Performance status may impact risk of reinfection, as antiretrovirals did not become widely available in Uganda until 2008. This may have impacted the long-term survival of TB-HIV patients and reduced opportunities for reinfection.
The issue of missing RFLP patterns was addressed not only by comparing clinical and demographic characteristics of those with and without RFLP results, but also by sensitivity analysis for the effect of missing values. For HIV-negatives, if all 10 patients with unknown RFLP patterns were due to reinfection, the maximum reinfection rate would be 32%. For HIV-positives, if all 63 recurrences with unknown RFLP patterns were due to reinfection, the rate would be 62%. Conversely, if the unknown RFLP patterns were all due to relapse, the reinfection rates would remain the same between HIV-positives and -negatives. As baseline predictors of TB recurrence did not differ between HIV-positives with and without RFLP results, we infer that the distribution of relapse or reinfection would not be altered by the missing information.
The absence of treatment adherence data is an-other limitation. Non-adherence to anti-tuberculosis treatment is associated with recurrence.5,32 Our study included patients from clinical trials and community studies. Adherence to anti-tuberculosis treatment may be lower in community studies. Most patients were treated on an ambulatory unsupervised out-patient basis, with routine patient education, defaulter tracing, monthly follow-up visits and, in many studies, urine INH metabolite testing in clinic. DST patterns of recurrent isolates were available for 57/98 patients with RFLP results and did not reveal emergence of resistance. The presence of multiple M. tuberculosis strains at baseline occurs in HIV-positive patients in Uganda, and could result in an overestimation of reinfection.33
Concerns about combining different studies for this study were addressed by performing sub-analysis on TB cases in our longstanding household contact study that had two phases (1995–1999 and 2002–006), conducted by the same clinical team with consistent enrollment criteria, treatment and follow-up. Among 37 recurrences with RFLP results, relapse occurred in 30 and reinfection in 7 in this study.
Strengths of our study include 1) large sample size, 2) availability of 98 paired DNA fingerprints, 3) patients recruited from the same clinic and catchment area, and 4) use of the same TB laboratory.
The median duration to TB recurrence (9.9 months) is in agreement with earlier African studies where 90% of clinical relapses occurred within 12 months after completing treatment.34,35 Time to reinfection was longer than to relapse (20.1 vs. 8.1 months) for HIV-positive patients. This criterion was not useful for HIV-negative patients, where 15% of recurrences were reinfections. The implications of our results are that in urban Uganda relapse is more likely than reinfection in the first 1–2 years after completing anti-tuberculosis treatment for both HIV-positive and -negative persons. This impacts choice of retreatment regimen, as patients who relapse are at greater risk of acquired drug resistance. The data also support recommendations for wider use of culture and DST against first-line drugs in patients who require retreatment for recurrent TB. Our study also suggests that biomarkers other than clinical parameters will be needed to identify those requiring longer treatment to prevent relapse.
Acknowledgements
The authors thank the patients who participated in the research studies and the staff at the project clinic in Kampala. They acknowledge the following study medical officers, laboratory and data staff: F Byekwaso, M Kamya, E Ssekasanvu, B Sewali, A Wajja, R S Wallis, P Kyambadde, A Namale, F Mubiru, P Nsubuga, J G Nakibali, S Nyole, M Millard, M Pohle, L Nshuti, S Zalwango, M Walusimbi, P Gitta, M Nsereko, R Mugerwa, K Chervenak, S Kayes, N Fomukong, P Orikiriza, K Morgan, P Peters, A Etwom, F Ashaba, L Malone, D Dobbs, M Breda, B Thiel, A Chiunda, L Horter and L Kucharski. The authors also thank C Stein for her review of this manuscript.
This project was funded by the Tuberculosis Research Unit, Case Western Reserve University, through the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (Contracts N01-AI-95383 and HHSN266200700022C/N01-AI-70022). This project was also supported by the AIDS International Training and Research Program at Case Western Reserve University (Grant #D43-TW000011), funded by the Fogarty International Center of the NIH.
Footnotes
Conflict of interest: none declared.
References
- 1.Dobler CC, Marks GB, Simpson SE, Hamish Crawford AB. Recurrence of tuberculosis at a Sydney chest clinic between 1994 and 2006: reactivation or reinfection? Med J Aust 2008; 188: 153–155. [DOI] [PubMed] [Google Scholar]
- 2.Van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 1999; 341: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 3.Jasmer RM, Bozeman L, Schwartzman K, et al. Recurrent tuberculosis in the United States and Canada: relapse or reinfection? Am J Respir Crit Care Med 2004; 170: 1360–1366. [DOI] [PubMed] [Google Scholar]
- 4.Caminero JA, Pena MJ, Campos-Herrero MI, et al. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am J Respir Crit Care Med 2001; 163(3 Pt 1): 717–720. [DOI] [PubMed] [Google Scholar]
- 5.Garcia de Viedma D, Marin M, Hernangomez S, et al. Tuberculosis recurrences: reinfection plays a role in a population whose clinical/epidemiological characteristics do not favor reinfection. Arch Intern Med 2002; 162: 1873–1879. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 7.Verver S, Warren R, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005; 171: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 8.Wang JY, Lee L, Lai H, et al. Prediction of the tuberculosis reinfection proportion from the local incidence. J Infect Dis 2007; 196: 281–288. [DOI] [PubMed] [Google Scholar]
- 9.Charalambous S, Grant AD, Moloi V, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis 2008; 12: 942–948. [PubMed] [Google Scholar]
- 10.Crampin AC, Mwaungulu JM, Mwaungulu FD, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS 2010; 24: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Global tuberculosis control 2010. WHO/HTM/TB/2010.7 Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 12.Guwatudde D, Zalwango S, Kamya MR, et al. Burden of tuberculosis in Kampala, Uganda. Bull World Health Organ 2003; 81: 799–805. [PMC free article] [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS 2010 Uganda HIV and AIDS estimates 2009. Geneva, Switzerland: UNAID, 2010. [Google Scholar]
- 14.Johnson JL, Okwera A, Nsubuga P, et al. Efficacy of an unsupervised 8-month rifampicin-containing regimen for the treatment of pulmonary tuberculosis in HIV-infected adults. Int J Tuberc Lung Dis 2000; 4: 1032–1040. [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick LK, Okwera A, Mugerwa R, Ridzon R, Ellner J, Onorato I. An investigation of suspected exogenous reinfection in tuberculosis patients in Kampala, Uganda. Int J Tuberc Lung Dis 2002; 6: 550–552. [DOI] [PubMed] [Google Scholar]
- 16.Okwera A, Johnson JL, Luzze H, et al. Comparison of intermittent ethambutol with rifampicin-based regimens in HIV-infected adults with PTB, Kampala. Int J Tuberc Lung Dis 2006; 10: 39–44. [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JL, Kamya RM, Okwera A, et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected Ugandan adults with newly diagnosed pulmonary tuberculosis. J Infect Dis 2000; 181: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JL, Ssekasanvu E, Okwera A, et al. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med 2003; 168: 185–191. [DOI] [PubMed] [Google Scholar]
- 19.Mayanja-Kizza H, Jones-Lopez E, Okwera A, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis 2005; 191: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whalen CC, Chiunda A, Zalwango S, Nshuti L, Jones-Lopez E. Immune correlates of acute Mycobacterium tuberculosis infection in household contacts in Kampala, Uganda. Am J Trop Med Hyg 2006; 75: 55–61. [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JL, Hadad DJ, Dietze R, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med 2009; 180: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanteza MW, Mayanja-Kizza H, Charlebois E, et al. A randomized trial of punctuated antiretroviral therapy in Ugandan HIV-seropositive adults with pulmonary tuberculosis and CD4+ T-cell counts of ⩾350 cells/μl. J Infect Dis 2011; 204: 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BD. BBL MGIT products for the detection of mycobacteria. Cockeysville, MD, USA: BD Microbiology Systems, 1994. [Google Scholar]
- 24.Otal I, Samper S, Asensio MP, et al. Use of a PCR method based on IS6110 polymorphism for typing Mycobacterium tuberculosis strains from BACTEC cultures. J Clin Micro 1997; 35: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behr MA, Small PM. Molecular fingerprinting of Mycobacterium tuberculosis: how can it help the clinician? Clin Infect Dis 1997; 25: 806–810. [DOI] [PubMed] [Google Scholar]
- 27.Falk A, O’Connor JB, Pratt PC, Webb WR, Wier JA, Wolinsky E. Chapter 6. Classification of pulmonary tuberculosis In: Diagnostic standards and classification of tuberculosis. 12th ed. New York, NY, USA: National Tuberculosis and Respiratory Disease Association, 1969: pp 68–76. [Google Scholar]
- 28.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis 2007; 11: 828–837. [PubMed] [Google Scholar]
- 29.Joloba ML, Whalen CC, Cave DM, et al. Determination of drug susceptibility and DNA fingerprint patterns of clinical isolates of Mycobacterium tuberculosis from Kampala, Uganda. East Afr Med J 2000; 77: 111–115. [PubMed] [Google Scholar]
- 30.Godfrey-Faussett P, Githui W, Barchelor B, et al. Recurrence of HIV-related tuberculosis in an endemic area may be due to relapse or reinfection. Tubercle Lung Dis 1994; 75: 199–202. [DOI] [PubMed] [Google Scholar]
- 31.Nunn AJ, Phillips PPJ, Mitchison DA. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int J Tuberc Lung Dis 2010; 14: 241–242. [PubMed] [Google Scholar]
- 32.Ormerod LP, Prescott RJ. Inter-relations between relapses, drug regimens and compliance with treatment in tuberculosis. Respir Med 1991; 85: 239–242. [DOI] [PubMed] [Google Scholar]
- 33.Dickman KR, Nabyonga L, Kateet DD, et al. Detection of multiple strains of Mycobacterium tuberculosis using MIRU-VNTR in patients with pulmonary tuberculosis in Kampala, Uganda. BMC Infect Dis 2010; 10: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulido F, Pena J, Rubio R, et al. Relapse of tuberculosis after treatment in human immunodeficiency virus-infected patients. Arch Intern Med 1997; 157: 227–232. [PubMed] [Google Scholar]
- 35.Chang KC, Leung CC, Yew WW, Ho SC, Tam CM. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med 2004; 170: 1124–1130. [DOI] [PubMed] [Google Scholar]