Abstract
Background: Vascular occlusion during the injection of facial fillers is uncommon, but can result in serious adverse events, including necrosis, blindness, and stroke. Objectives: We explored factors that influence the frequency and severity of vascular complications during filler injections. Methods: This was a meta-analysis that included case reports and case series published during the years 2004 to 2016 describing patients who experienced any type of vascular complication after an aesthetic procedure. In addition to the descriptive analysis of the variables retrieved, a logistic regression for predicting the outcome of the vascular event was performed. Results: The analysis included 93 cases described in 30 articles. Blindness was the main consequence of the vascular complications (n=57; 61%). The reported outcome was partial or total recovery in 24 cases (28%) and no improvement in 61 cases (72%). Hyaluronic acid (HA) and autologous fat were the two fillers most frequently involved in vascular occlusions, with autologous fat showing a stronger trend toward no improvement than HA. Involvement of the ophthalmic and retinal arteries was most frequently associated with no improvement. Conclusion: Injury to ophthalmic and retinal arteries during the injection of facial fillers can result in irreversible serious adverse events. Physicians performing facial filler injections should have a proficient knowledge of anatomy.
Keywords: Dermal fillers, vascular complications, hyaluronic acid, autologous fat, collagen, adverse events
Nonsurgical cosmetic procedures are a growing trend worldwide. Included among these minimally invasive techniques are botulinum toxin and soft-tissue augmentation with fillers, which are used restore tissue loss and correct aging-related rhytides and folds. In 2011, dermal fillers were used in nearly 1.6 million aesthetic procedures, increasing to 2.3 million in 2013 and 5.5 million in 2014.1–3
Hyaluronic acid (HA) fillers are the most commonly used injectable fillers, followed by autologous fat. According to the American Society for Aesthetic Plastic Surgery, nearly 900,000 soft-tissue augmentation procedures were performed with HA in 2004.4,5 Other commonly used filler materials include bovine and human collagen (active for 1–3 months before degradation); poly-L-lactic acid, which stimulates endogenous collagen production for up to 15 months; and calcium hydroxylapatite, which offers up to 2 years of activity.3 These fillers can all be used for volume replacement and enhancement, such as cheek and chin augmentation, tear trough valley correction, nose reshaping (rhinoplasty), midface volumization, and lip enhancement.1,5–7 Although these procedures are generally considered safe, some local adverse events, aside from the relatively common site-injection reactions (e.g., swelling, tenderness, pain, bruising), have been observed.8–10 These include edema, erythema, scarring, granuloma formation, hyper- and hypopigmentation, infection, abscess formation, herpetic outbreaks, nodular masses, and paresthesia (if a nerve has been pinched during the procedure).While these adverse reactions are usually transient, the common use of three-dimensional facial volume restoration techniques, where the filler material can be injected at any depth, has brought about infrequent but serious and often irreversible vascular complications caused by symptomatic arterial occlusion.6,11–13 These vascular complications can result in persistent skin necrosis, ophthalmoplegia, permanent unilateral or bilateral vision loss, and stroke.11–13 Ocular and cerebral embolism occurs when the injected material travels from the distal to proximal retinal and ophthalmic arteries, causing sudden, excruciating pain, persistent blindness, and further tissue necrosis.11–13 In addition to fillers accessing the vessel lumen, vascular occlusion can occur by external compression of the stiff gel bolus deposited in direct contact with the vessel wall.14–16
Based on the available literature, some authors have suggested that the injection technique, site, and substance can have significant influence on the level of risk for an adverse vascular event.2,5,7,11,12,17 However, most of these reviews were not systematic, and the potential influence of other variables on the incidence of adverse events has not been addressed. Therefore, we reviewed the literature regarding vascular complications and performed a meta-analysis of the variables that potentially affect the frequency and severity of adverse events.
METHODS
Literature search and article selection. This meta-analysis included data from case reports and case series of patients experiencing any type of vascular complication after an aesthetic procedure published during the years 2004 to 2016. The main source for article retrieval was the PubMed. Additional sources included Google Scholar, where the search was restricted to the article title, and a case series by Park et al,18 which provided details from 19 cases previously published as case reports. The database search, performed on December 2016, combined the term filler with the following terms: injection (or injected), blindness, visual loss, ophthalmoplegia, artery occlusion, embolism, ischemia (or ischemic), necrosis, and complication. Only full-text articles written in English were considered for eligibility. To be included in the analysis, cases had to report a vascular event occurring after an aesthetic procedure on the human face.
Data extraction and management. Data for the meta-analysis were extracted from each case and transferred to a predefined form containing the following variables: case reference, age, sex, injected product, aesthetic procedure, needle diameter, injected volume, person who injected the product, injection site, blood vessel affected, main consequence(s) of the vascular event, concomitant symptoms, time to symptom onset, intervention performed to treat the vascular complication, and outcome. Additionally, diagnostic tests performed to confirm the occurrence of vascular complications were recorded to address the quality of the articles included in our review. The main consequences of a vascular complication were blindness, visual loss, necrosis, and other. Blindness was only considered when explicitly stated in the text, whereas visual loss included a reduction in visual acuity, the perception of light only, and the perception of hand movement only. Time-to-onset values were grouped into three categories: less than one hour postprocedure, 1 to 24 hours postprocedure, and more than 24 hours postprocedure. The final outcome was categorized as no change, partial recovery, or full recovery based on the progress of the main consequence of the vascular complication.
Statistical analysis. Categorical variables were described as frequency and percentage, whereas quantitative variables were described as means and standard deviations (SDs). To assess the factors possibly influencing the outcome of vascular complications, the percentages of cases with no improvement and those showing partial or full recovery were compared using the chi-squared test. For variables showing statistically significant differences, a post-hoc analysis was performed by computing the chi-squared values of the adjusted residuals and applying the Bonferroni correction, as described by Beasley et al.19 A prediction model (multivariate analysis) for the vascular event outcomes was built using logistic regression. The multivariate analysis included all variables regarding events occurring prior to any vascular complication, which showed significant differences when comparing patients without improvement and those with partial or total recovery. The significant threshold was set at α=0.05 and all analyses were performed using the Statistical Package for the Social Sciences version 22.0 for Windows software program (IBM Corp., Armonk, New York).
RESULTS
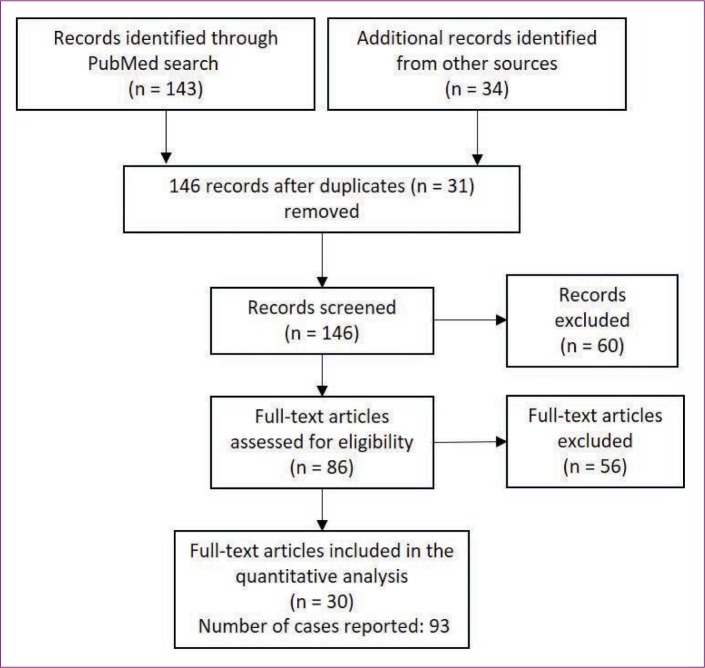
Study selection. The initial search (including articles retrieved from additional sources) yielded 143 articles, published during the years 2004 to 2016, on vascular events potentially associated with the use of injected fillers (Figure 1). After removing duplicates and excluding non-English articles and those without full-text availability, 86 were considered eligible. Of these, 56 either reported results at injection sites other than the face or did not report any vascular complication, and thus were discarded. The final selection included 30 full-text articles reporting 93 cases: 22 case reports (i.e., articles containing a full description of one or more cases),2,8,13,20–39 seven case series (i.e., articles containing a tabulated description of various cases with vascular complications),18,40–44 and one observational trial (i.e., an article retrieving data from a cohort of patients, including at least one patient experiencing a vascular complication).45 Most cases (n=62; 66.7%) were reported in Korea, while 14 (15.1%) were reported in China, 10 (10.8%) were reported in the United States, three (3.2%) were reported in Germany, three (3.2%) were reported in Taiwan, and one (1.1%) was reported in Japan.
FIGURE 1.
Flow diagram of study inclusion.
All cases had information regarding the injection site and main consequences of vascular complications. Other key variables, such as injected substance, outcome, and affected blood vessel were reported in 92 (98.9%), 85 (91.4%), and 82 (88.2%) cases, respectively. In 80 cases (86.0%), the vascular complication and identity of the affected blood vessel were confirmed by at least one of the following imaging techniques: optical coherence tomography, magnetic resonance imaging, ultrasonography, or fundoscopy. In six cases, the affected vessel was deduced from the signs (e.g., necrosis affecting a skin area clearly irrigated by the facial artery) or the treatment outcome (e.g., prostaglandins injected into a vein, leading to the improvement of signs and symptoms). Conversely, in four cases, the physician failed to identify the affected vessel despite performing imaging diagnostic tests. Needle diameter, injected volume, and the professional who performed the injection were only reported in 11, 17, and 13 cases, respectively; due to their low representation in the study sample, these variables were excluded from analysis.
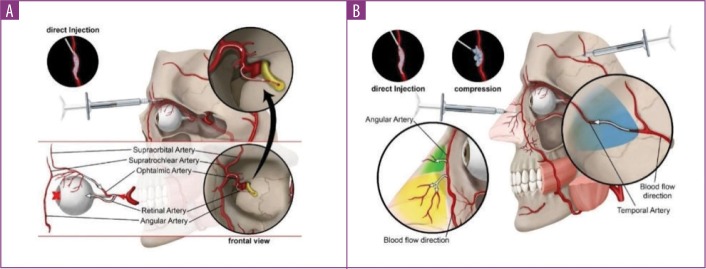
Case characteristics. Table 1 summarizes the main characteristics of the cases described in the selected articles. In most cases (n=57; 61.3%), blindness was the main consequence of vascular complication. In five cases (5.4%), the patients experienced blindness and skin necrosis simultaneously. Whereas blindness was typically assumed to be a consequence of a vascular embolization of the filler material, necrosis was sometimes attributed to compression (Figure 2).2,39,45 However, none of these cases reported evidence regarding the etiology of skin necrosis, and compression was suggested based on the time-to-onset or necrosis progression. Nine patients (9.7%) experienced neither necrosis nor visual loss or blindness despite a diagnosis of vascular occlusion. Eight patients (8.6%) reported mild consequences (e.g., pain, erythema), all of which resolved completely. One patient that was injected with autologous fat in the glabella experienced occlusion of the retinal artery with concomitant brain infarction, which resulted in hemiplegia and death.43
TABLE 1.
Characteristics of cases included in the meta-analysis
| CHARACTERISTIC | n | %* | |
|---|---|---|---|
| Sex (n=93) | Female | 84 | 90.3 |
| Male | 9 | 9.7 | |
| Injected substance (n=92) | Hyaluronic acid | 40 | 43.5 |
| Autologous fat | 38 | 41.3 | |
| Collagen | 7 | 7.6 | |
| Calcium hydroxylapatite | 4 | 4.3 | |
| Poly-(L)-lactic acid | 3 | 3.3 | |
| Injection site** (n=90) | Glabella | 44 | 48.9 |
| Nose | 41 | 45.6 | |
| Periocular | 9 | 9.7 | |
| Frontal/temple area | 11 | 12.2 | |
| Affected blood vessel (n=82) | Ophthalmic artery | 36 | 43.9 |
| Central retinal artery | 29 | 35.4 | |
| Nasociliary artery | 8 | 9.8 | |
| Other | 9 | 11.0 | |
| Main consequence** (n=93) | Blindness | 57 | 61.3 |
| Visual loss | 21 | 22.6 | |
| Skin necrosis | 11 | 11.8 | |
| Concomitant symptoms** (n=68) | Pain | 32 | 47.1 |
| Erythema | 3 | 4.4 | |
| Ptosis | 31 | 45.6 | |
| Edema | 11 | 16.2 | |
| Imaging diagnostic tests** (n=80) | Angiography | 31 | 38.8 |
| OCT | 4 | 5.0 | |
| MRI | 52 | 65.0 | |
| Fundus imaging | 15 | 18.8 | |
| Ultrasonography | 1 | 1.3 | |
| Time to symptoms onset (n=73) | < 1 hour | 13 | 17.8 |
| 1–24 hours | 47 | 64.4 | |
| > 24 hours | 12 | 16.4 | |
| Outcome (n=85) | Total or partial recovery | 24 | 28.2 |
| No improvement | 61 | 71.8 |
Percentages are shown based on available cases
More than one category can apply to each case
FIGURE 2.
Etiological details of blindness caused by A) direct injection of the filler into the vessel lumen and B) skin necrosis caused by either direct injection or vascular compression
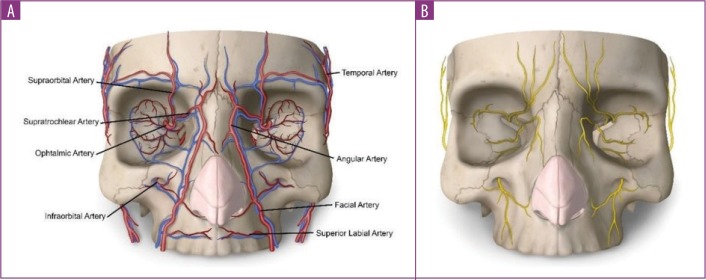
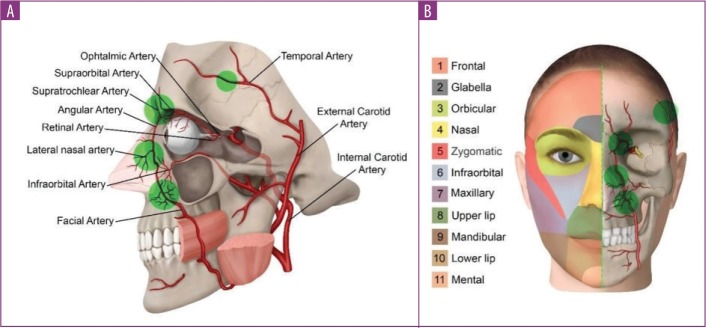
Theoretically, multiple blood vessels and nerves can be reached by the needle during filler injection (Figure 3). However, the paths of facial, nasal, temporal, and ophthalmic arteries define anatomical areas with increased risk of injury during filler injection (Figure 4). In the case of the ophthalmic artery, the increased risk included occlusion of one of its most important branches: the retinal artery. In our analysis, the ophthalmic retinal arteries accounted for 79.3 percent of the cases in which the affected blood vessel was reported. In addition to the nasociliary artery, other blood vessels affected by the aesthetic procedure were the choroid vessels, the internal carotid artery, the middle cerebral artery, and the facial vein and artery. The occlusion of the ophthalmic artery was mostly due to injections in the nose (n=18, 42.9% of all cases affecting the ophthalmic artery). Conversely, the occlusion of the retinal artery was mostly due to injections in the glabella (n=18, 55% of all cases affecting the retinal artery).
FIGURE 3.
A) main vascular and B) nerve structures of the face
FIGURE 4.
A) depiction of facial arteries illustrating the primary areas of risk and B) their associated anatomical structure
In 12 cases (12.9%), vascular occlusion progressed to brain infarction, identified by magnetic resonance imaging. Two of these were associated with ophthalmic artery occlusion, whereas eight were associated with retinal artery occlusion. With the exception of two cases—one leading to the patient’s death and another resulting in neurological sequelae—blindness was the main consequence for all patients affected by brain infarction.
Full recovery was reported in seven cases (8.2%): one case of blindness, one of visual loss, and five cases of vascular occlusion with minor consequences. Temporary blindness was caused by an HA injection in the eyebrow. The patient reported foggy and hazy vision immediately after the filler injection; 10 days later, the filler was successfully removed by irrigation and aspiration after creating a temporal limbal incision in the affected eye. Eight days after removal, visual acuity was restored.27
Hyaluronidase was used only in 10 of 40 cases in which HA was the cause of vascular occlusion. The time between symptom onset and hyaluronidase injection exceeded three hours in all cases. The dose of hyaluronidase injected, reported only in five cases, ranged from 1,000 to 9,000 units. In five of these cases, blindness was the main consequence of the vascular event; only one patient experienced partial recovery,25 whereas the rest remained blind despite attempts to remove the HA obstruction by injecting hyaluronidase.
Factors influencing outcome. To explore possible baseline factors influencing the outcome, cases with either visual loss or blindness as the main consequence were grouped into two categories based on the outcome: total or partial recovery and no improvement (Table 2). A chi-squared test revealed significant differences in the injected substance, the affected blood vessel, and the time to symptom onset. The post-hoc analysis of the injected substance showed that both HA and autologous fat were significantly associated with no improvement (p=0.003 and p<0.001 for the chi-squared adjusted residuals of HA and autologous fat, respectively; the significance threshold after Bonferroni correction was set at α=0.005). Regarding the affected vessel, only the ophthalmic artery was significantly associated with no improvement (p=0.001 for the chi-squared adjusted residuals; the significant threshold after the Bonferroni correction was set at α =0.006). A post-hoc analysis of time-to-onset did not reveal significant differences in any of the three categories.
TABLE 2.
Distribution of cases in variables potentially influencing the outcome
| VARIABLE | TOTAL OR PARTIAL RECOVERY, n=24 n (%) | NO IMPROVEMENT, n=61 n (%) | p- VALUE | |
|---|---|---|---|---|
| Sex | Female | 21 (27.6) | 55 (72.4) | 0.719 |
| Male | 3 (33.3) | 6 (66.7) | ||
| Injected substance | Hyaluronic acid | 16 (45.7) | 19 (54.3) | |
| Autologous fat | 1 (2.7) | 36 (97.3) | ||
| Collagen | 4 (66.7) | 2 (33.3) | <0.001 | |
| Calcium hydroxylapatite | 3 (75.0) | 1 (25.0) | ||
| Poly-(L)-lactic acid | 0 | 3 (100.0) | ||
| Injection site* | Nose | 14 (35.9) | 25 (64.1) | 0.132 |
| Glabella | 8 (19.0) | 34 (81.0) | 0.063 | |
| Periocular | 1 (16.7) | 5 (83.3) | 0.519 | |
| Frontal/temple area | 3 (30.0) | 7 (70.0) | 0.883 | |
| Affected blood vessel | Ophtalmic artery | 2 (5.6) | 34 (94.4) | |
| Retinal artery | 6 (26.1) | 17 (73.9) | 0.004 | |
| Nasociliary artery | 4 (50.0) | 4 (50.0) | ||
| Other | 4 (50.0) | 4 (50.0) | ||
| Time to onset | <1 hour | 5 (41.7) | 7 (58.3) | |
| 1–24 hours | 10 (22.2) | 35 (77.8) | 0.024 | |
| >24 hours | 6 (66.7) | 3 (33.3) |
Patients could have more than one injection site
The injected substance, the affected blood vessel, and the time to symptoms onset were included in a logistic regression analysis. The resulting model explained 22 percent of the outcome’s variance, categorized as “no improvement” and “total or partial recovery” (R2=0.219; p=0.027). However, only the affected blood vessel significantly contributed to the overall model (Table 3).
TABLE 3.
Individual contribution of variables in the logistic regression to predict the outcome of the vascular complication
| VARIABLE | OR (95% CI) | P -VALUE |
|---|---|---|
| Injected substance | 1.3 (0.7–2.5) | 0.391 |
| Affected blood vessel | 0.4 (0.2–0.8) | 0.007 |
| Time to symptom onset | 0.8 (0.2–2.6) | 0.708 |
CI: confidence interval; OR: odds ratio
DISCUSSION
In this systematic review and meta-analysis of patients with vascular complications occurring after aesthetic procedures, we found that unilateral blindness was the most frequent vascular adverse event associated with cosmetic fillers for facial tissue augmentation. Of these, autologous fat tended to cause more cases of permanent vascular damage. Among all blood vessels affected, the ophthalmic artery was significantly associated with irreversible blindness.
The risk of vascular complications associated with facial aesthetic procedures has been addressed previously in case reports, case series, and literature reviews. In an attempt to further understand the factors influencing the risks and outcomes of vascular complications, we extracted data from individual cases to provide a quantitative approach. Moreover, considering that the number of products available for soft-tissue augmentation has been progressively and continuously increasing for the last 10 years, our review aimed to present an updated picture of vascular complications associated with these fillers. All analyses based on case reports are constrained by the amount and accuracy of the information published. Eighty-six percent of cases reported using imaging diagnostic techniques to verify the diagnosis of vascular occlusion, and most of them provided details regarding key variables such as the injected substance, the blood vessel affected, the outcome of the vascular complication, and the time to symptom onset.
In terms of clinical correlation, one of the most relevant variables was the filler injected. In our study selection, the absolute number of cases with vascular complications after the use of HA and autologous fat was similar. However, considering that HA is, by far, the most used filler in the world for aesthetic procedures,4,5 this observation suggests that autologous fat is more often associated with vascular complications than HA. Regarding the recovery rate of vascular complications, both HA and autologous fat were significantly associated with a lower frequency of improvement, but the latter showed a stronger trend towards more severe outcomes. This result is consistent with that of previous reviews, which concluded that autologous fat is the filler material that most frequently causes permanent blindness.12,17,46 In a previous review by Beleznay et al,12 autologous fat was responsible for 47.9 percent of cases of unilateral permanent blindness, followed by HA (23.5%), collagen (8.2%), poly-L-lactic acid (3.1%), and calcium hydroxylapatite (2%).The increased risk of major vascular complications associated with autologous fat injections could be explained by its large particle size, enabling it to occlude relatively large vessels, such as the ophthalmic artery.12,17
Regarding safety, one of the advantages of HA is the availability of an effective rescue procedure (i.e., hyaluronidase injection into or around the occluded blood vessel).45,47,48 This is one of the reasons why HA has been claimed as the safest substance indicated for tissue augmentation.48,49 However, in our review, the number of cases in which hyaluronidase was administered accounted for only a quarter of all cases in which HA was used (10 vs. 40). Furthermore, although the reduced number of cases limited our statistical analysis, it is worth mentioning that only half of these cases resulted in the total recovery of the main outcome related to vascular occlusion. The low recovery rate despite the use of hyaluronidase could be partially explained by the excessive time gap between symptoms onset and hyaluronidase injection, ranging from 3 to 24 hours, with five over seven cases exceeding the four-hour threshold, below which significant differences are seen.45 These observations suggest that the safer profile of HA compared with autologous fat might be better explained by the properties of the filler material rather than the availability of a rescue procedure. Due to the different physical properties of each substance, the injector’s ability to inject the filler using the right pressure might become an overriding factor influencing the risk of vascular complications.12,50 Rapid injections not only result in greater amounts of filler but also limit the capacity of the injector to identify and amend any vascular occlusion. Furthermore, various authors have proposed that, when exerting too much pressure on the plunger, even during the injection of small amounts of filler, arterial pressure can easily be overcome, with the filler reaching deeper arteries.6,12 Of course, injection pressure and rate cannot be monitored unless the professional performing the injection uses a motorized injector to deliver the filler; hence, this information could not be included in our analysis. Motorized injectors have been proposed as a means to reduce injection risks, as they provide a comfortable flow rate and allow physicians to keep their attention on the patient.50,51
Considering that shorter onset times are more likely to prompt early interventions, we expected time to symptom onset to influence the outcome. However, no significant differences were found between the times before and after one hour. The importance of the time gap between the vascular complication and the intervention was investigated in animal models by Kim et al38 and Cavallini et al,47 who found that rescue procedures performed less than four hours after a filler injection significantly reduced the area of necrotic ear skin. However, these studies were based on hyaluronidase injections as rescue procedures, which were barely used in our case collection. Notwithstanding the lack of correlation with other studies, two important drawbacks limited our analysis of the potential influence of the time-to-onset on symptom recovery. First, our dataset did not include time frames more accurate than a 24-hour interval. Second, most of these cases were reported by ophthalmologists with patients showing sudden blindness concurrent with filler injections; therefore, the time from the aesthetic intervention to the onset of vision loss or blindness was assessed retrospectively.
Our results also showed that the affected blood vessel significantly influenced the outcome of the vascular complication. Based on the statistical analysis, ophthalmic artery occlusion was more frequently associated with no improvement than that of other blood vessels, particularly the nasociliary artery. However, individual case examinations revealed that the most dangerous adverse events (i.e., cerebral infarctions) occurred as an ultimate consequence of retinal artery occlusion. Since the retinal artery is a final branch of the ophthalmic artery, it could be assumed that an occlusion of the retinal artery is not likely to have consequences at more central areas. However, as previously discussed, when the tip of the needle penetrates the artery and pressure is applied to the plunger, the filler can reverse the flow in it, moving as a column proximal to the origin of the retinal artery. If the injector exerts more pressure on the plunger for a longer time, the column can reach the origin of the ophthalmic artery, and part of the filler embolus can access the internal carotid artery and subsequently reach cerebral circulation (Figure 2).6,12 The use of motorized devices, which enable accurate pressure control, has been proposed to minimize this risk.50,51 We found no differences in the outcome when the occlusion occurred in other blood vessels, particularly the nasociliary and facial arteries. Although this observation is consistent with the larger diameter of these vessels, due to the limited number of cases in which there was occlusion in blood vessels other than the ophthalmic and retinal arteries, no firm conclusions could be reached.
Finally, we addressed the influence of the injection site on the outcome of the vascular event. Previous studies reported the glabella and the forehead as areas more frequently associated with blindness and visual loss than the nose.5,7,11 However, in our analysis, injections in the nose accounted for nearly half of the cases of vascular complications and had a similar frequency to that of injections in the glabella. These observations indicated that the nose might not be a safer injection site than the glabella. Lazzeri et al11 suggested that the dorsal nasal artery (i.e., the second terminal branch of the ophthalmic artery) might be responsible for the transmission of emboli following injections in either the glabella or the area proximal to the nasal root.Other injection sites did not yield significant results upon comparing the outcomes of the vascular procedures. However, it is worth mentioning that our analysis was compromised by the fact that a single patient could be injected at various sites, which precludes the identification of the precise injection responsible for the vascular complication.
Limitations. The fact that our meta-analysis was based mostly on case reports implies some limitations that should not be dismissed. Case reports do not always provide all details of the procedures performed. This was particularly notable for some variables identified as risk factors for vascular complications, such as injection technique, injected volume, pressure applied, and needle diameter, which were omitted in most cases. Some of these factors were investigated by Glogau et al,5 who concluded that low injection pressures (i.e., flow rates of less than 0.3mL/minute) and small volume injections (i.e., less than 0.5mL) might prevent retrograde embolization of the filler; the authors also recommended avoiding the fan-like technique, which was identified as the main cause of iatrogenic vascular occlusion.Other variables that could not be analyzed because of data omission, despite their potential interest, include the specialty of the person performing the injection, the characteristics of the device (e.g., needle, cannula), and the concentration of hyaluronidase used in the rescue procedure. In addition to a few poor-quality case reports, some of the cases analyzed were not reported by the physician injecting the filler but rather by the ophthalmologist who treated the complication, thus omitting details of the initial aesthetic procedure. The variables most affected by this lack of data were time-to-onset, initial rescue treatment, and concomitant symptoms, which, in most cases, were retrospectively reported by the physician treating the complication. Another potential source of inaccuracy was the ad-hoc data transformation. The heterogeneity in the way the various case reports reported the data prevented the use of pure, raw data for the analysis, which would have been a factor adding simplicity and clarity to our conclusion.
In addition to presenting an updated and quantitative perspective of vascular complications associated with filler injections, the results obtained in our analysis might serve to support a few recommendations to help clinicians who perform filler augmentation procedures avoid vascular adverse events or minimize their consequences. Table 4 provides a list of key recommendations for preventing and minimizing vascular adverse events when performing filler injections. However, as mentioned before, our analysis has important limitations associated with the accuracy and diversity of data presentation in the source articles. Hence, the recommendations we present in Table 4 should not be interpreted as being strictly supported by the results of our meta-analysis; our recommendations are also based on our own insights gained from our extensive experience as plastic surgeons.
TABLE 4.
Recommendations for preventing and managing vascular complications associated with filler injections
| PREVENTIVE STRATEGIES | |
| Practitioner | |
Deep knowledge of the vascular anatomy is key for preventing vascular complications. In addition to good anatomical background knowledge, practitioners should consider the following aspects:
|
|
| Filler choice | |
| Use reabsorbable products appropriate for the type of correction and therefore for the implant level. Hyaluronic acid fillers are typically noninflammatory products and have a purely mechanical effect, unlike collagen and autologous fat, which seem to activate the “clotting mechanism.” | |
| Injection technique | |
|
|
| MANAGEMENT OF COMPLICATIONS | |
| Immediate pain and/or bleaching of the area (typically a few seconds after injection) | |
| Immediately stop injecting; vigorously massage the area. | |
| Possible livedoreticularis or reactive hyperemia (it may occur up to 10 minutes after injection) | |
| Treat immediately to restore the vascular flow. | |
| Possible arterial insufficiency (slow capillary reloading with acupressure) | |
| Apply warm gauzes, topical paste or patch of nitro-derivatives; inject hyaluronidase (independently from the type of filler injected) and apply a local massage. | |
| Dark-blue discoloration of the area (it may occur from ten minutes to hours) | |
| Contact your plastic surgeon and consider using systemic antibiotics, steroids, aspirin, low molecular weight heparin, prostaglandin. | |
| Blisters and boils after a few days | |
| Gently disinfect by swabbing the area; pierce the boils and gently favor the spillage of the serum; leave a gras gauze dressing with antibiotic on the skin for no more than three days, then remove it (with clamp and scissors), gently disinfect with 3% boric acid and medicate with a gras gauze dressing and antibiotic ointment until complete repitelization of the area. | |
| Necrosis (can appear after days or weeks) | |
| Apply antibiotic ointments until eschar demarcation; after removal of the necrotic tissue, apply products intended to improve tissue regeneration such as hydrocolloids gel, plates or collagen tablets on the loss of residual substance. | |
| Ocular complications | |
| Contact an eye surgeon immediately. In the meantime, try to reduce eye pressure through ocular massage, timolol drops, acetazolamide/manitol, steroids, haemodilution, oxygen therapy, antiplatelet/anticoagulant, thrombolysis, decompression of the eye anterior chamber. |
CONCLUSION
This meta-analysis provides an up-to-date overview of vascular complications associated with the injection of facial fillers. Our results support the hypothesis that autologous fat is more likely to cause serious vascular events than HA, irrespective of the use of hyaluronidase to treat the vascular occlusion. In light of the information published in the literature, it seems that accidental injection in the terminal branches of the facial artery, particularly the retinal artery, almost invariably leads to unilateral, and occasionally bilateral, blindness. The incidental occlusion of the retinal artery most frequently occurs when treating the nose, but this artery can also be reached from the glabella. Thus, to prevent vascular adverse effects, it is essential that the physician performing the filler injections has a proficient knowledge of anatomy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the time and effort of Dr. Patrice Delobel, Dr. Kévin Legent, and Dr. Luana Consolini, who provided scientific advice and helped with laying the groundwork of this research. The authors also thank the i2e3 Biomedical Research Institute team for providing statistical and medical writing assistance.
REFERENCES
- 1.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6(1):295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunebaum LD, Allemann IB, Dayan S et al. The risk of alar necrosis associated with dermal filler injection. Dermatologic Surg. 2009;35(Suppl 2):1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31(1):110–121. doi: 10.1177/1090820X10391083. [DOI] [PubMed] [Google Scholar]
- 4.Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane) Plast Reconstr Surg. 2006;117(Suppl 3):3S–34S. doi: 10.1097/01.prs.0000204759.76865.39. discussion 35S–43S. [DOI] [PubMed] [Google Scholar]
- 5.Glogau RG, Kane MAC. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatologic Surg. 2008;34(Suppl 1):S105–S109. doi: 10.1111/j.1524-4725.2008.34251.x. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: scientific and technical considerations. Plast Reconstr Surg. 2007;120(6):33S–40S. doi: 10.1097/01.prs.0000248808.75700.5f. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009;25(5):324–328. doi: 10.1055/s-0029-1243081. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann F, Erdmann R, Hartmann V et al. The spectrum of adverse reactions after treatment with injectable fillers in the glabellar region: results from the injectable filler safety study. Dermatologic Surg. 2009;35(Suppl 2):1629–1634. doi: 10.1111/j.1524-4725.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 9.McCracken MS, Khan JA, Wulc AE et al. Hyaluronic acid gel (Restylane) filler for facial rhytids: lessons learned from American Society of Ophthalmic Plastic and Reconstructive Surgery member treatment of 286 patients. Ophthal Plast Reconstr Surg. 2006;22(3):188–191. doi: 10.1097/01.iop.0000217562.64529.ff. [DOI] [PubMed] [Google Scholar]
- 10.Zielke H, Wölber L, Wiest L, Rzany B. Risk profiles of different injectable fillers: results from the Injectable Filler Safety Study (IFS Study) Dermatologic Surg. 2008;34(3):326–335. doi: 10.1111/j.1524-4725.2007.34066.x. [DOI] [PubMed] [Google Scholar]
- 11.Lazzeri D, Agostini T, Figus M et al. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129(4):995–1012. doi: 10.1097/PRS.0b013e3182442363. [DOI] [PubMed] [Google Scholar]
- 12.Beleznay K, Carruthers JDA, Humphrey S, Jones D. Avoiding and treating blindness from fillers: a review of the world literature. Dermatologic Surg. 2015;41(10):1097–1117. doi: 10.1097/DSS.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 13.Chen YY, Wang WY, Li JP et al. Fundus artery occlusion caused by cosmetic facial injections. Chin Med J (Engl). 2014;127(8):1434–1437. [PubMed] [Google Scholar]
- 14.Loh KTD, Chua JJ, Lee HM et al. Prevention and management of vision loss relating to facial filler injections. Singapore Med J. 2016;57(8):438–443. doi: 10.11622/smedj.2016134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker L, King M. This month’s guideline: visual loss secondary to cosmetic filler injection. J Clin Aesthet Dermatol. 2018;11(5):E53–E55. [PMC free article] [PubMed] [Google Scholar]
- 16.Urdiales-Gálvez F, Delgado NE, Figueiredo V, Lajo-Plaza JV et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510. doi: 10.1007/s00266-017-1063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinendegen DL, Baumgartner RW, Vuadens P et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163–167. doi: 10.1007/s002669900185. [DOI] [PubMed] [Google Scholar]
- 18.Park KH, Kim YK, Woo SJ et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132(6):714–723. doi: 10.1001/jamaophthalmol.2013.8204. [DOI] [PubMed] [Google Scholar]
- 19.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 1995;64(1):79–93. [Google Scholar]
- 20.Kim SK, Kim JH, Hwang K. Skin necrosis of the nose after injection of ribose cross-linked porcine atelocollagen. J Craniofac Surg. 2015;26(7):2211–2212. doi: 10.1097/SCS.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 21.Chou CC, Chen HH, Tsai YY et al. Choroid vascular occlusion and ischemic optic neuropathy after facial calcium hydroxyapatite injection—a case report. BMC Surg. 2015;15(1):21. doi: 10.1186/s12893-015-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin YC, Chen WC, Liao WC, Hsia TC. Central retinal artery occlusion and brain infarctions after nasal filler injection. QJM. 2015;108(9):731–732. doi: 10.1093/qjmed/hcu235. [DOI] [PubMed] [Google Scholar]
- 23.McGuire LK, Hale EK, Godwin LS. Post-filler vascular occlusion: a cautionary tale and emphasis for early intervention. J Drugs Dermatol. 2013;12(10):1181–1183. [PubMed] [Google Scholar]
- 24.Kim EG, Eom TK, Kang SJ. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel. J Craniofac Surg. 2014;25(2):684–686. doi: 10.1097/SCS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 25.Hu XZ, Hu JY, Wu PS et al. Posterior ciliary artery occlusion caused by hyaluronic acid injections into the forehead: a case report. Medicine (Baltimore). 2016;95(11):e3124. doi: 10.1097/MD.0000000000003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung MS, Kim HG, Woo KI, Kim YD. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthal Plast Reconstr Surg. 2010;26(4):289–291. doi: 10.1097/IOP.0b013e3181bd4341. [DOI] [PubMed] [Google Scholar]
- 27.Kim DY, Eom JS, Kim JY. Temporary blindness after an anterior chamber cosmetic filler injection. Aesthetic Plast Surg. 2015;39(3):428–430. doi: 10.1007/s00266-015-0477-9. [DOI] [PubMed] [Google Scholar]
- 28.Roberts SAI, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)- lactic acid (PLLA) injection. Ophthal Plast Reconstr Surg. 2012;28(3):e68–e70. doi: 10.1097/IOP.0b013e3182288e4d. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Choi KS. Bilateral blindness after filler injection. Plast Reconstr Surg. 2013;131:298e–299e. doi: 10.1097/PRS.0b013e318278d6e1. [DOI] [PubMed] [Google Scholar]
- 30.Kwon SG, Hong JW, Roh TS et al. Ischemic oculomotor nerve palsy and skin necrosis caused by vascular embolization after hyaluronic acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333–334. doi: 10.1097/SAP.0b013e31824f21da. [DOI] [PubMed] [Google Scholar]
- 31.Kim SN, Byun DS, Park JH et al. Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci. 2014;21(4):678–680. doi: 10.1016/j.jocn.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10(3):224–231. doi: 10.1111/j.1473-2165.2011.00562.x. [DOI] [PubMed] [Google Scholar]
- 33.Carle M V, Roe R, Novack R, Boyer DS. Cosmetic facial fillers and severe vision loss. JAMA Ophthalmol. 2014;132(5):637–639. doi: 10.1001/jamaophthalmol.2014.498. [DOI] [PubMed] [Google Scholar]
- 34.Kim SG, Lee CJ, Kim YJ, Lee S Il. Salvage of nasal skin in a case of venous compromise after hyaluronic acid filler injection using prostaglandin E. Dermatol Surg. 2011;37(12):1817–1819. doi: 10.1111/j.1524-4725.2011.02188.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee CM, Hong IH, Park SP. Ophthalmic artery obstruction and cerebral infarction following periocular injection of autologous fat. Korean J Ophthalmol. 2011;25(5):358. doi: 10.3341/kjo.2011.25.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the glabellar area. Clin Ophthalmol. 2008;2(3):679–683. [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon DY, Park MH, Koh SB et al. Multiple arterial embolism after illicit intranasal injection of collagenous material. Dermatologic Surg. 2010;36(7):1196–1199. doi: 10.1111/j.1524-4725.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim YJ, Kim SS, Song WK et al. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27(6):E152–E155. doi: 10.1097/IOP.0b013e3182082f37. [DOI] [PubMed] [Google Scholar]
- 39.Tracy L, Ridgway J, Nelson JS et al. Calcium hydroxylapatite associated soft tissue necrosis: a case report and treatment guideline. J Plast Reconstr Aesthetic Surg. 2014;67(4):564–568. doi: 10.1016/j.bjps.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Park SW, Woo SJ, Park KH et al. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154(4):653–662. doi: 10.1016/j.ajo.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with Hyaluronic acid-filler complications. J Plast Reconstr Aesthetic Surg. 2011;64(7):892–897. doi: 10.1016/j.bjps.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Kim YK, Jung C, Woo SJ, Park KH. Cerebral angiographic findings of cosmetic facial filler-related ophthalmic and retinal artery occlusion. J Korean Med Sci. 2015;30(12):1847–1855. doi: 10.3346/jkms.2015.30.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong JH, Ahn SJ, Woo SJ et al. Central retinal artery occlusion with concomitant ipsilateral cerebral infarction after cosmetic facial injections. J Neurol Sci. 2014;346:310–314. doi: 10.1016/j.jns.2014.08.030. (1-2) [DOI] [PubMed] [Google Scholar]
- 44.Lee SK, Kim SM, Cho SH et al. Adverse reactions to injectable soft tissue fillers: memorable cases and their clinicopathological overview. J Cosmet Laser Ther. 2015;17(2):102–108. doi: 10.3109/14764172.2014.968584. [DOI] [PubMed] [Google Scholar]
- 45.Kim DW, Yoon ES, Ji YH et al. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthetic Surg. 2011;64(12):1590–1595. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Rzany B, DeLorenzi C. Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136((5S)):196S–203S. doi: 10.1097/PRS.0000000000001760. [DOI] [PubMed] [Google Scholar]
- 47.Cavallini M, Gazzola R, Metalla M, Vaienti L. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J. 2013;33(8):1167–1174. doi: 10.1177/1090820X13511970. [DOI] [PubMed] [Google Scholar]
- 48.Buhren BA, Schrumpf H, Hoff NP et al. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res. 2016;21(1):5. doi: 10.1186/s40001-016-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vartanian AJ, Frankel AS, Rubin MG. Injected hyaluronidase reduces restylane-mediated cutaneous augmentation. Arch Facial Plast Surg. 2005;7(4):231–237. doi: 10.1001/archfaci.7.4.231. [DOI] [PubMed] [Google Scholar]
- 50.Carey W, Weinkle S. Retraction of the plunger on a syringe of hyaluronic acid before injection: are we safe? Dermatologic Surg. 2015;41(c):S340–S346. doi: 10.1097/DSS.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 51.Cassuto D, Papagni M. Anteis injection system: positive results of a preliminary clinical evaluation. Updat Plast Surg. 2010;3:5–8. [Google Scholar]