Abstract
Objective:
Intraperitoneal administration of large doses of l-arginine is known to induce severe acute pancreatitis in rats. We therefore set out to determine whether metabolites of l-arginine (l-ornithine, l-citrulline, and nitric oxide) cause pancreatitis.
Design:
The authors conducted an in vivo animal study.
Setting:
This study was conducted at a university research laboratory.
Subjects:
Study subjects were male Wistar rats.
Interventions:
Dose–response and time course changes of laboratory and histologic parameters of pancreatitis were determined after l-arginine, l-ornithine, l-citrulline, or sodium nitroprusside (nitric oxide donor) injection.
Measurements and Main Results:
Intraperitoneal injection of 3 g/kg l-ornithine but not l-citrulline or nitroprusside caused severe acute pancreatitis; 4 to 6 g/kg l-ornithine killed the animals within hours. Serum and ascitic amylase activities were significantly increased, whereas pancreatic amylase activity was decreased after intraperitoneal injection of 3 g/kg l-ornithine. The increase in pancreatic trypsin activity (9–48 hrs) correlated with the degradation of IκB proteins and elevated interleukin-1β levels. Oxidative stress in the pancreas was evident from 6 hrs; HSP72 synthesis was increased from 4 hrs after l-ornithine administration. Morphologic examination of the pancreas showed massive interstitial edema, apoptosis, and necrosis of acinar cells and infiltration of neutrophil granulocytes and monocytes 18 to 36 hrs after 3 g/kg l-ornithine injection. One month after l-ornithine injection, the pancreas appeared almost normal; the destructed parenchyma was partly replaced by fat. Equimolar administration of l-arginine resulted in lower pancreatic weight/body weight ratio, pancreatic myeloperoxidase activity, and histologic damage compared with the l-ornithine-treated group. l-ornithine levels in the blood were increased 54-fold after intraperitoneal administration of l-arginine.
Conclusions:
We have developed a simple, noninvasive model of acute necrotizing pancreatitis in rats by intraperitoneal injection of 3 g/kg l-ornithine. Interestingly, we found that, compared with l-arginine, l-ornithine was even more effective at inducing pancreatitis. Large doses of l-arginine produce a toxic effect on the pancreas, at least in part, through l-ornithine. (Crit Care Med 2008; 36:2117–2127)
Keywords: l-ornithine, l-arginine, acute pancreatitis
Acute pancreatitis is a sudden inflammatory disorder of the pancreas (1). The disease can present in a mild or severe form. Although the overall mortality of patients with acute pancreatitis is approximately 5%, a great proportion of deaths is the result of the necrotizing form of the disease. To study the pathomechanism and to test possible treatment options of severe acute pancreatitis, a number of experimental models have been developed. However, most of these models are invasive (such as the retrograde ductal injection of bile acids and the closed duodenal loop method) (2, 3), or pancreatic damage is not that reproducible or homogeneous in distribution (like in choline-deficient ethionine-supplemented diet) (4). However, the noninvasive nature, reproducibility, and homogeneity (as found with the edematous cerulein pancreatitis model [5]) are preferential in an experimental setup. Severe acute pancreatitis induced by intraperitoneal (IP) injection of rats and mice with l-arginine fulfills these criteria and its use is getting more popular with researchers investigating this challenging disease (6–9). The mechanism by which l-arginine produces pancreatic injury is unknown. Free radicals, including nitric oxide (NO), inflammatory mediators (nuclear factor-κB, interleukins), endoplasmic reticulum stress, and polyamines, have all been implicated in this response (8, 10, 11).
The metabolism of l-arginine can occur through a number of different pathways. Two key enzymes that are involved in this process are NO synthase (NOS) and arginase. NOS (which has three isoforms: the constitutive endothelial and neuronal, and an inducible form [iNOS]) catalyze the conversion of l-arginine into NO and l-citrulline, whereas arginase hydrolyzes l-arginine to l-ornithine and urea (12). Therefore, we set out to determine whether equimolar doses of the l-arginine metabolites l-ornithine or l-citrulline and/or the NO donor sodium nitroprusside cause pancreatitis in rats. Intraperitoneally injected l-ornithine but not l-citrulline or nitroprusside induced severe acute necrotizing pancreatitis. The main aim of this study was to characterize the dose–response and time course changes of l-ornithine administration.
MATERIALS AND METHODS
Materials
Chemicals were from Sigma-Aldrich (Munich, Germany) unless stated otherwise.
Experimental Protocol
Animals.
Male Wistar rats weighing 200 to 240 g were used. The animals were kept at a constant room temperature of 24°C with a 12-hr light–dark cycle and were allowed free access to water and standard laboratory chow (Biofarm, Zagyvaszántó, Hungary). In each experimental group, four to ten rats were used. The experiments performed in this study were approved by the Animal Care Committee of the university.
Pilot study.
In a pilot study, rats (n = 3–5) were injected IP with equimolar (11.7 mL/kg 1.424 M/L) l-arginine (cat. A5131), l-citrulline (cat. 27510), and/or the NO donor sodium nitroprusside (cat. 71780), l-ornithine (cat. O8305), or d-ornithine (cat. 75480). The animals were killed by exsanguination through the abdominal aorta 24 hrs after the IP injection. To determine the serum concentrations of arginine, citrulline, and ornithine after injection of l-arginine, rats were killed at 2, 4, 6, and 12 hrs. The pancreas was quickly removed, cleaned from fat and lymph nodes, weighed, and frozen in liquid nitrogen and stored at −80°C until use. All blood samples were centrifuged at 2500 g for 20 mins and the serum was stored at −25°C.
Dose–response and Time Course Changes of l-ornithine Injection.
To study the dose–response (n = 6) of l-ornithine, rats were injected IP with 1 to 6 g/kg body weight of l-ornithine (dissolved in physiological saline at a concentration of 300 mg/mL, pH = 7.4) and were killed after 24 hrs as described previously. For the time course studies (n = 4–10), the rats were injected with 3 g/kg l-ornithine and were killed 2 to 72 hrs, 1 wk, or 1 month after the injection. The control animals received physiological saline IP and were killed 24 hrs after the injection.
Assays
Serum, Pancreatic and Ascites Amylase Activity, Serum Aspartate Aminotransferase Activity, and Concentrations of Glucose, Calcium, Triglyceride, Urea, Creatinine, Arginine, Ornithine, and Citrulline.
Laboratory parameters, excluding the amino acids, were determined by standard kits from Dialab (Vienna, Austria). The amino acids were assayed in dried serum specimens according to the method of Chace et al (13).
Pancreatic Weight/Body Weight Ratio.
This ratio was used to evaluate the degree of pancreatic edema.
Pancreatic Trypsin Activity.
Active trypsin in pancreatic tissue homogenates was measured as described previously (14).
Expression of Pancreatic HSP72, IκB-α, and IκB-β.
Western blot analysis of pancreatic HSP72, IκB-α, and IκB-β expression was performed from the cytosolic fraction of the pancreas homogenate as described previously (15).
Pancreatic Interleukin-1β Concentrations.
The proinflammatory interleukin-1β concentrations were measured in the pancreatic cytosolic fractions with an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions.
Pancreatic Nonprotein Sulfhydryl Group Content and the Activities of Glutathione Peroxidase and Superoxide Dismutase.
To determine nonprotein sulfhydryl group content and activities of glutathione peroxidase, Mn-, and Cu/Zn-superoxide dismutase (SOD), a part of the pancreas was homogenized, the homogenates centrifuged at 3000 g for 10 mins, and the supernatants were used for measurements as described previously (16).
Pancreatic Myeloperoxidase Activity.
Pancreatic myeloperoxidase activity, as a marker of tissue leukocyte infiltration, was assessed by the method of Kuebler et al (17).
Detection of Apoptosis in the Pancreas
Pancreatic Genomic DNA Analysis.
A biochemical hallmark of apoptosis is a characteristic form of DNA degradation in which the genome is cleaved at internucleosomal sites, generating a ladder-like pattern of DNA fragments (multiples of 180 bp) when analyzed by agarose gel electrophoresis. For a qualitative assessment of genomic DNA fragmentation/degradation, rat pancreata were ground to a powder with a mortar and pestle under liquid nitrogen and were then homogenized by five strokes in a glass Dounce homogenizer with 1.5- to four-fold excess of extraction buffer (50 mmol/L Tris-HCl [pH = 8.0], 50 mmol/L EDTA, .5% sodium dodecyl sulfate, and .2 mg/mL proteinase K). The homogenates were transferred to Eppendorf tubes and rotated at 55°C overnight. The DNA solution was extracted twice with TE-saturated phenol, once with 1:1 Tris-EDTA-saturated phenol: chloroform and once with chloroform. The DNA was precipitated by adding .1 vol 3 mol/L sodium acetate (pH = 5.5) and 2 vol 96% ethanol. DNA precipitate was collected by centrifugation at 13,000 g for 10 mins, rinsed with 70% ethanol, vacuum-dried, re-suspended in 100 to 200 μL Tris-EDTA buffer, and finally digested for 1 hr at 37°C with DNAse-free RNAse. Fifteen to 20 μg of DNA was electrophoretically fractionated on a 1.8% agarose gel with .5 μg/mL ethidium bromide.
TdT-mediated dUTP Nick End-labeling Technique.
Apoptotic cells were quantitated by TdT-mediated dUTP nick end-labeling (TUNEL) assay using an In Situ Cell Death Detection Kit from Roche Diagnostics (Mannheim, Germany) according to the manufacturer’s instructions. The number of apoptotic cells was counted in .5 mm2 of pancreatic tissue. Results are expressed as percentage of the number of cell nuclei in the same area in control tissue. Note that this method of calculation will underestimate the rates of apoptosis in edematic pancreata.
Microscopy.
Cells showing characteristic changes of apoptosis were also recognized by light and electron microscopic techniques (see subsequently).
Histologic Examination
Light Microscopy.
A portion of the pancreas, liver, kidney, and lungs was fixed in 6% neutral formaldehyde solution and subsequently embedded in paraffin. Sections were cut at 4 μm thickness and stained with hematoxylin and eosin. The slides were coded and read by two independent observers who were blind to the experimental protocol. Pancreatic tissue injury was evaluated as follows: semi-quantitative grading of interstitial edema (0–3), vascular congestion (0–1), leukocyte adhesion (0 –3) and infiltration (0 – 4), and apoptosis (0–3) and necrosis (0–4) of acinar cells was determined in each animal (described in more detail in Table 1). Signs indicative of regeneration: mitotic figures, ductuloacinar structures, and basophilia of acinar cells were recorded.
Table 1.
Histological scoring system for the evaluation of pancreatic injury in rats
| Scores | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Interstitial edema | 0 | Mild | Moderate | Severe | — |
| Vascular congestion | 0 | Present | — | — | — |
| Leukocyte adherence to vessel walls | 0 | Mild | Moderate | Extensive | — |
| Leukocyte infiltration | 0 | Focal | Diffuse/mild | Diffuse/moderate | Diffuse/severe |
| Vacuolization (% of total acinar cells) | 0 | Focal (<10) | 11–25 | 26–50 | 51–75 |
| Necrosis (% of total acinar cells) | 0 | Focal (<10) | 11–25 | 26–50 | 51–75 |
| Number of apoptotic bodies (% of total acinar cells) | 0 | <5 | 6–10 | 11–20 | — |
| Regeneration | 0 | Present | — | — | — |
The slides were coded and read for the traditional histological markers of pancreatic tissue injury by two independent observers who were blind to the experimental protocol.
Transmission Electron Microscopy.
Small pieces of pancreata were prepared for electron microscopy 0, 6, and 24 hrs and 1 wk after l-ornithine treatment as described previously (18).
Statistical Analysis
Results are expressed as means ± sem. Experiments were evaluated by using the analysis of variance followed by Dunnett’s multiple comparison post hoc test. Values of p < .05 were accepted as significant.
RESULTS
Pilot Study
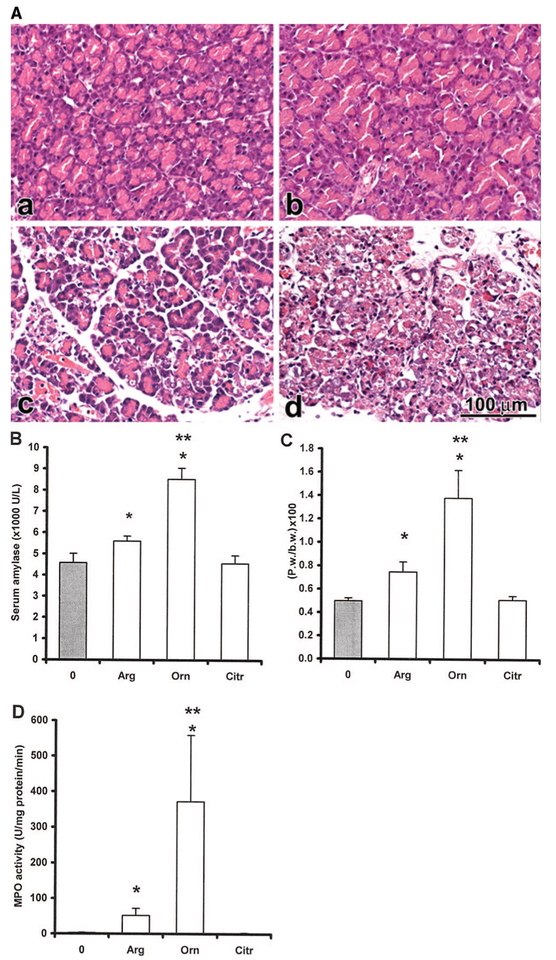
We first tested the effects of 3.5 g/kg l-arginine and its metabolites (administered at equimolar doses) on serum amylase activity, pancreatic weight/body weight ratio, pancreatic myeloperoxidase activity, and pancreatic histology 24 hrs after the injection (n = 3). Interestingly, the IP injection of 2.8 g/kg l-ornithine caused a more severe pancreatitis compared with the l-arginine group (Fig. 1; Table 2). Similar effect was seen with the 3 g/kg l-ornithine dose, so this was used throughout the rest of the study. In contrast, IP administration of 3 g/kg d-ornithine did not result in pancreatic injury (n = 5, results not shown). A dose of 2.9 g/kg l-citrulline did not cause an alteration in any of the measured parameters and the pancreas seemed normal in histology (Fig. 1; Table 2). The animals that received sodium nitroprusside (4.95 g/kg) alone or in combination with l-citrulline became lethargic soon after the injection and died by the next morning. This is in accord with the material safety data sheet provided with nitroprusside by Sigma-Aldrich, who report the IP LD50 for this compound at only 7 mg/kg. Autopsy did not show pancreatitis in these animals. Most likely they died as a result of vascular complications, that is, hypotension.
Figure 1.
Intraperitoneal (IP) administration of l-ornithine to rats resulted in necrotizing acute pancreatitis, which was more severe than that induced with l-arginine. Rats were injected intraperitoneally with physiologic saline (control, 0) or equimolar l-arginine (Arg, 3.5 g/kg), l-ornithine (Orn, 2.8 g/kg), l-citrulline (Citr, 2.9 g/kg), and/or the nitric oxide donor sodium nitroprusside (4.95 g/kg). The animals that received sodium nitroprusside alone or in combination with l-citrulline died by the next morning. The rest of the rats survived the treatment and were killed by exsanguination through the abdominal aorta 24 hrs after the IP injection. A, The diagrams show light micrographs (hematoxylin and eosin staining) of the pancreata of (a) control, (b) citrulline (Ctr.), (c) arginine (Arg), or (d) ornithine (Orn)-treated rats. The bar diagrams show the (B) serum amylase activity, (C) pancreatic weight (Pw) body weight (b.w.) ratio, and (D) pancreatic myeloperoxidase activity. Data are shown as means ± sem, n = 3–5. *Significant difference (p < .05) vs. the control group (0 hr). **Significant difference (p < .05) vs. the Arg group. MPO, myeloperoxidase.
Table 2.
Evaluation of pancreatic injury 24 hrs after the intraperitoneal injection of physiological saline (control, 0), equimolar l-citrulline (Citr, 2.9 g/kg), l-arginine (Arg, 3.5 g/kg) or l-ornithine (Orn, 2.8 g/kg)
| Control, 0 | Citr | Arg | Orn | |
|---|---|---|---|---|
| Interstitial edema | 0 | 0 | 1 | 3 |
| Vascular congestion | 0 | 0 | 1 | 1 |
| Leukocyte adherence | 0 | 0 | 1 | 3 |
| Leukocyte infiltration | 0 | 0 | 1 | 3 |
| Vacuolization | 0 | 0 | 1 | 1 |
| Apoptosis | 0 | 0 | 1 | 2 |
| Necrosis | 0 | 0 | 3 | 4 |
Scores are shown for an average of three animals.
Time Course Changes of Serum Arginine, Citrulline, and Ornithine Concentrations in Rats Injected Intraperitoneally with 3.5 g/kg l-arginine.
Serum arginine, citrulline, and ornithine concentrations (n = 5) were all significantly increased after the injection of l-arginine (Fig. 2). Importantly, there were much greater increases in serum ornithine versus citrulline levels after l-arginine injection.
Figure 2.
Time course changes of serum arginine, citrulline, and ornithine concentrations in rats injected intraperitoneally with 3.5 g/kg l-arginine. 2 hrs after the l-arginine injection; serum (A) arginine concentration was increased by approximately 25-fold, (B) citrulline concentration by approximately three-fold, and (C) ornithine concentration by approximately 54-fold. Data are shown as means ± sem, n = 5. *Significant difference (p < .05) vs. the control group (0 hrs).
Dose–response of Intraperitoneal Injection of 1 to 6 g/kg l-ornithine
The rats injected with 1 or 2 g/kg l-ornithine did not develop any pancreatic lesions (n = 6, results not shown). However, 3 g/kg of this basic amino acid caused a severe acute pancreatitis as described subsequently. A dose of 4 to 6 g/kg (n = 6) killed the animals within a couple of hours after the injection after developing lethargy, neurologic and neuromuscular symptoms (tremor, twitching, convulsion, and in some cases jumping all over the cage).
Time Course Studies After Intraperitoneal Injection of Rats with 3 g/kg l-ornithine
Macroscopic Observations.
The pancreas appeared edematous from 18 to 36 hrs, its peak being at 24 hrs. Ascites and adhesions of organs were seen from 4 to 6 hrs (peaking at 24 hrs). Occasionally, yellow–white foci indicative of chalky fat necrosis was detected in the mesentery of the bowels and retroperitoneum at 24 to 72 hrs. Dilated small and large bowels suggesting functional ileus was apparent at 72 hrs to 1 wk after l-ornithine injection.
Histologic Examination of the Pancreas (Table 3)
Table 3.
Evaluation of pancreatic injury by histological examination 2–168 hrs and 1 month after intraperitoneal injection of rats with 3 g/kg l-ornithine
| 2 hrs | 4 hrs | 6 hrs | 9 hrs | 12 hrs | 18 hrs | 24 hrs | 36 hrs | 48 hrs | 72 hrs | 168 hrs | 1 Month | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial edema | 0 | 0 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 0 | 0 | 0 |
| Vascular congestion | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Leukocyte adherence | 0 | 0 | 0 | 1 | 3 | 3 | 3 | 2 | 1 | 0 | 1 | 0 |
| Leukocyte infiltration | 0 | 0 | 0 | 1 | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 0 |
| Vacuolization | 0 | 1 | 2 | 3 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Apoptosis | 0 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 0 | 0 |
| Necrosis | 0 | 0 | 1 | 2 | 3 | 4 | 4 | 3 | 3 | 0 | 0 | 0 |
| Regeneration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
Scores are shown for an average of four animals.
0 to 6 Hrs.
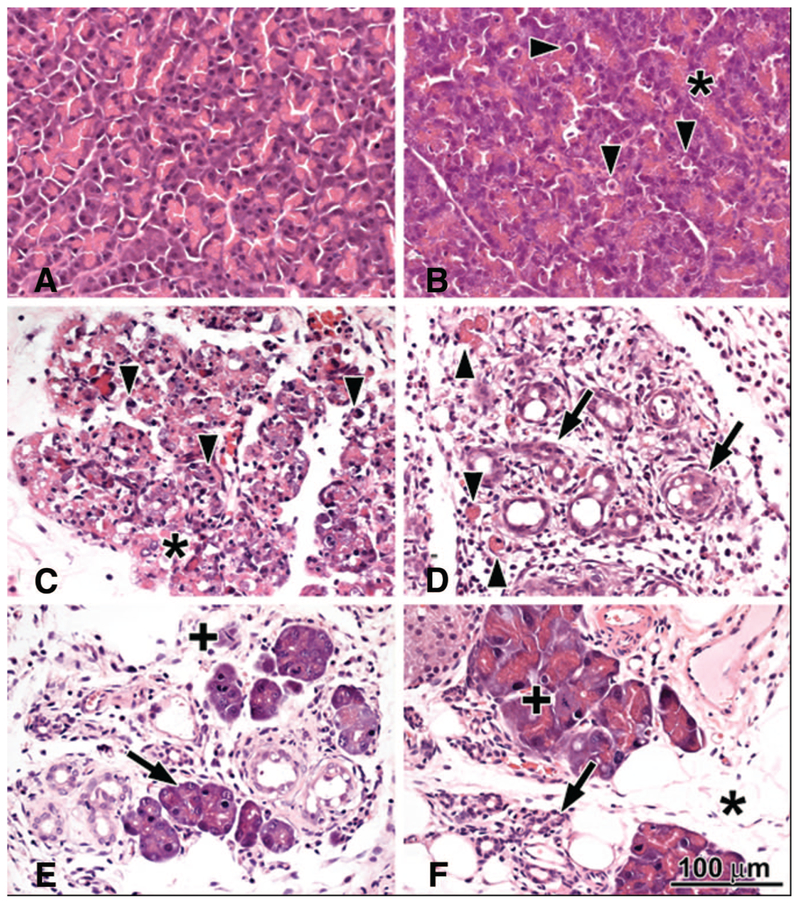
The pancreas appeared normal 2 hrs after l-ornithine (3 g/kg) injection. At 4 hrs, mild interstitial edema and foamy vacuolization of the acini and vascular congestion were observed. At 6 hrs, the number of apoptotic bodies was greatly increased and we could also observe focal necrosis (<10%) of acini (Fig. 3). Electron microscopy revealed the appearance of large autophagic vacuoles containing zymogen granules in varying stages of degradation, dense material, myelin figures, and mitochondrial remnants in acinar cells at 6 hrs (Fig. 4). The foamy vacuolization corresponded to accumulation of lipid droplets of medium density in the basal compartment of the cells. Overall, tight junctions, mitochondria, and endoplasmic reticulum were well preserved. In some acinar cells, condensation of the chromatin under the nuclear membrane and disappearance of the nucleoli with or without shrinkage of the cytoplasm (ongoing apoptosis) were detected. In these cells, the mitochondrial cristae and the rough endoplasmic reticulum were mildly dilated with disengagement of ribosomes from the latter.
Figure 3.
Histopathologic changes of the pancreas in response to intraperitoneal administration of 3 g/kg l-ornithine. A, Two hours: no major histopathologic changes. B, Six hours: foamy degeneration (asterisk) of acinar cells; arrowheads show apoptotic bodies. C, Twenty-four hours: widespread necrosis of acinar cells (asterisk) and lots of apoptotic bodies. D, Seventy-two hours: ductuloacinar structures (arrow) in place of disappeared acini; fibroblasts, macrophages, and some neutrophils in the interstitium. E, One week: some acini have regenerated (arrow) with mitotic figures (+). F, One month: acini show mitotic figures (+) and regenerative atypia; some ductuloacinar structure had undergone atrophy (arrow) with fatty ingrowth around them (asterisk).
Figure 4.
Electron micrographs of the rat pancreas 0 hrs (a–b), 6 hrs (c–d), 24 hrs (e–f), and 1 wk (g–h) after l-ornithine administration (3 g/kg intraperitoneally). A, Cell nucleus (arrowhead) and nucleolus (asterisk). B, Mitochondrion (arrowhead), rough endoplasmatic reticulum (asterisk), and zymogen granules (cross) (scale bar = 1 μm). C, Mitochondria seemed unaltered (inset); autophagic vacuoles (arrows) and lipid droplets (arrowhead) appeared. D, Autophagic vacuole with zymogen granules (arrow), myelin figures (asterisk), and mitochondrial remnants (arrowhead). E, Lysis of nuclei (arrows); mitochondria seemed unaltered (inset) and occasional condensation of chromatin with dense shrinkage of the cytoplasm (arrowhead) were observed (scale bar = 5 μm). F, Dilated rough endoplasmatic reticulum (asterisk); peripheral crescents (arrow) of compacted chromatin. G, Ductuloacinar structures (arrows) between capillaries (asterisk) and fibroblasts (scale bar = 10 μm). H, Ductuloacinar structure (arrow) differentiated into acinar cell (arrowhead) with few zymogen granules (scale bar = 5 μm). Scale bars = 2 μm, except indicated otherwise.
9 to 12 Hrs.
At 9 hrs, there was interstitial edema, neutrophilic and monocytic adherence, and focal infiltration. There were great numbers of large autophagic vacuoles and apoptotic bodies. The extent of acinar cell necrosis was 15% to 25%. At 12 hrs, there was diffuse moderate infiltrate of monocytes and neutrophils, and the necrosis of acinar cells was 26% to 50%.
18 to 24 Hrs.
Eighteen hours after the l-ornithine injection, the extent of pancreatic edema was greatly increased and there was a decrease in the number of autophagic vacuoles in the acinar cells. Necrosis of acinar cells was increased to 51% to 75%. The most severe interstitial edema was observed at 24 hrs. Electron microscopy of tissue at 24 hrs showed severe but zonal damage to the acini as a whole and several types of cell death (Fig.4). Damaged, necrotic cells predominantly localized to the center of the acini, and the less severely injured cells were observed in the basal part of the acini. There were large autophagic vacuoles containing zymogen granules in varying stages of degradation, myelin figures, and/or granular electron-dense material. There was a marked reduction or complete disappearance of zymogen granules. Two main types of nuclear damage were observed: the disappearance of the nuclear membrane with entire lysis of the nucleus or the condensation of the chromatin beneath the nuclear membrane (the extent of the latter was lower). Disappearance of the nucleoli with or without shrinkage of the cytoplasm was extensively seen. Lipid droplets of medium density persisted in the basal compartment of the acinar cells. The mitochondria seemed not primarily altered, but the rough endoplasmic reticulum was dilated with the separation of ribosomes. Large numbers of neutrophils and monocytes could be observed in the interstitial space.
36 to 48 Hrs.
At 36 hrs, pancreatic edema was still severe, but was decreased versus 24 hrs. There was also a decrease in monocytic and neutrophilic adherence, neutrophilic infiltration, and necrosis of acinar cells (26%–50%). Lobular architecture was distorted. Fibroblasts also appeared in the inflamed interstitium. Regeneration starting with undifferentiated, ductuloacinar structures with scanty cytoplasm and densely basophilic nuclei in the peripheral zone of the pancreas appeared. At 48 hrs, there was diffuse severe infiltrates of macrophages/monocytes, fibroblasts, and neutrophils.
At 72 hrs, there was no pancreatic edema, but there was a diffuse severe infiltrate of fibroblasts, macrophages/monocytes, eosinophils, and neutrophils (Fig. 3D). There was a mild degree of collagen deposition within the lobules and around ductuloacinar structures. Ductuloacinar structures were budding from tubular lumina. Mitotic figures and a decrease in the number of apoptotic bodies were evident.
At 1 wk, diffuse moderate infiltrates of fibroblasts and macrophages and relatively smaller number of eosinophils and neutrophils were observed (Fig. 3E). There was a mild deposition of collagen within the lobules and around ductuloacinar structures. Adipose tissue replaced some of the destructed lobules. Budding ductuloacinar structures started to form ductules and acini and a few newly formed acinar cells displayed zymogen granules. Dilation of small ducts and ductules were observed; in some cases, they contained eosinophilic material. Low-power electron micrographs revealed several ductuloacinar structures, an increased number of capillaries, mononuclear cells, and collagen in the interstitial space (Fig. 4). Some cells were enlarged and contained huge number of small electron-dense granules, indicating ductoendocrine proliferation.
One month after injection, the pancreas appeared normal, except that part of the parenchyma was replaced by fat (Fig. 3F). The acini showed numerous mitotic figures and regenerative atypia. Fibroblasts and macrophages were no longer present. One of four animals had focal periductal infiltrate composed of lymphocytes, macrophages, and eosinophils.
Overall, there were no major pathologic alterations of the pancreatic duct cells, islets of Langerhans, and the liver in the hematoxylin and eosin sections. However, in one animal, striking Langerhans islet hyperplasia with mitotic figures was found at 1 mo. At 9 to 36 hrs, some eosinophilic cylinders in the tubular lumina were noted in the kidneys with attenuation and dilation of the proximal tubules. Occasional detachment of microvilli and mild peritubular capillaritis also were seen. These latter changes are consistent with mild acute tubular necrosis. At the same time, alveolar thickening with predominant neutrophil infiltration and occasional hemorrhage were seen in the lungs indicative of mild respiratory distress syndrome.
Activities of Serum, Pancreatic, and Ascitic Amylase
The serum amylase activity significantly increased from 9 to 24 hrs, but thereafter (at 48 hrs) fell below control values (n = 4–10, Fig. 5A). Pancreatic amylase activity was significantly decreased from 24 hrs to 1 mo after l-ornithine injection and was just about detectable at 72 and 168 hrs (n = 4–8, Fig. 5B). The ascites recovered from rats 24 hrs after l-ornithine administration had a huge amylase activity (98,096 ± 25,590 U/L, n = 7).
Figure 5.
Time course of serum amylase and pancreatic amylase, trypsin and myeloperoxidase activities after intraperitoneal administration of 3 g/kg l-ornithine. A, Serum amylase and pancreatic; (B) amylase, (C) trypsin, and (D) myeloperoxidase (MPO) activities. The serum amylase activity significantly increased from 9 to 24 hrs, but thereafter (at 48 hrs) fell below control values. Pancreatic amylase activity was significantly decreased from 24 hrs to 1 mo after l-ornithine injection and was just about detectable at 72 and 168 hrs. Trypsin activity was gradually increased from 9 hrs and peaked at 24 hrs after l-ornithine administration. MPO activity gradually increased during the course of the disease and was highest in the 72- and 168-hr groups. Data are shown as means ± sem, n = 4–10. *Significant difference (p < .05) vs. the control group (0 hrs).
Pancreatic Trypsin Activity
Premature activation of trypsinogen is thought to play an important role in the development of acute pancreatitis. Pancreatic trypsin activity was significantly increased 9 to 48 hrs after IP injection of 3 g/kg l-ornithine (Fig. 5C).
Pancreatic Myeloperoxidase Activity
The extent of neutrophil infiltration was also judged by the measurement of myeloperoxidase activity (Fig. 5D). Interestingly, inflammatory infiltration had two phases; the first one coincided with the peak of amylase activity (9–36 hrs) and the second one occurred much later (at 72 hrs).
Induction of Pancreatic HSP72 Synthesis
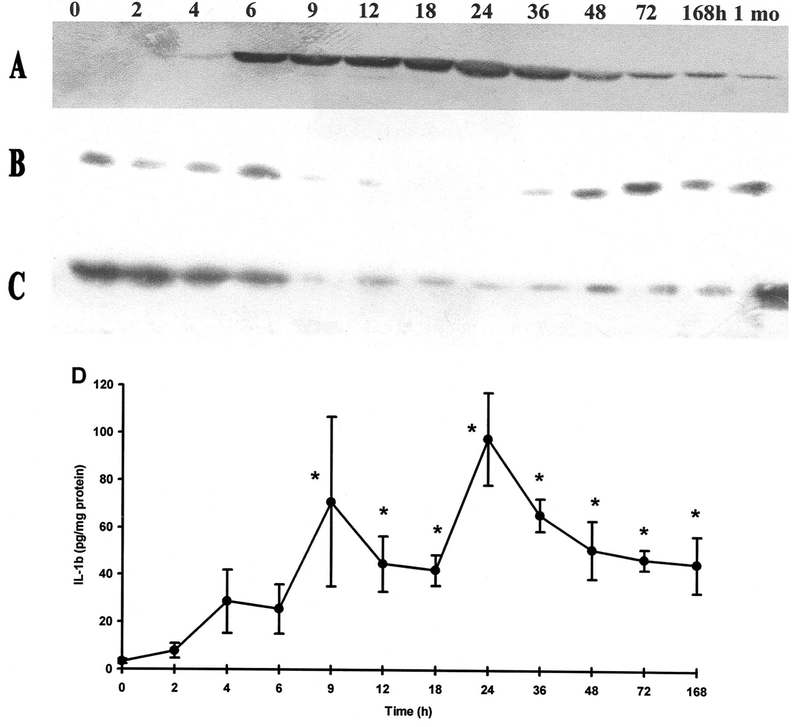
HSP72, the highly inducible form of the HSP70 family, could not be detected in the physiological saline-treated control group (Fig. 6A). However, by 4 hrs after the injection of 3 g/kg l-ornithine, the levels of HSP72 were significantly increased, peaked at 18 hrs, and remained elevated until 1 mo.
Figure 6.
Effects of l-ornithine administration (3 g/kg intraperitoneally) on pancreatic HSP72, IκB-α, IκB-β, and interleukin-1β expression as a function of time. A–C, Representative Western immunoblot analysis of protein lysates (50 μg/lane) from the pancreata of rats 0 to 168 hrs and 1 mo after injection, showing the levels of (A) HSP72, (B) IκB-α, and (C) IκB-β as a function of time after 3 g/kg l-ornithine injection. D, The graph shows the expression of the proinflammatory cytokine, interleukin-1β, as determined from the cytosolic fractions of pancreatic homogenates by enzyme-linked immunosorbent assay. Means ± sem for four to six animals are shown. *Significant difference (p < .05) vs. the control group (0 hrs).
Degradation of Pancreatic IκB-α and IκB-β and Induction of Interleukin-1β Synthesis
Pancreatic IκB levels in response to l-ornithine injection were significantly decreased from 9 hrs (Fig. 6B–C). IκB-α levels (Fig. 6B) returned to normal by 36 hrs; however, IκB-β level was significantly lower for up to 168 hrs after injection (Fig. 6C). Corresponding to IκB degradation, and consequently to activation of NF-κB, pancreatic interleukin-1β synthesis significantly increased from 9 hrs (Fig. 6D).
Confirmation of Pancreatic Apoptosis Observed on Histologic Examination
Genomic DNA Analysis.
Genomic DNA was extracted from pancreata 9 or 24 hrs after l-ornithine administration. Figure 7A shows that 9 hrs after l-ornithine injection, we could detect a ladder pattern on agarose gel electrophoresis. On the other hand, DNA showed unspecific degradation (smear) 24 hrs after the administration of l-ornithine indicating severe necrosis. This is in accordance with our histologic findings because at this latter time point, apoptosis was overcome by necrosis of the cells.
Figure 7.
Pancreatic apoptosis is greatly increased in rats treated with 3 g/kg l-ornithine intraperitoneally. A, Genomic DNA analysis of l-ornithine (3 g/kg) injected rats in agarose gel electrophoresis. Genomic DNA was isolated from the pancreata of rats 0, 9, and 24 hrs after l-ornithine injection. Fifteen to 20 μg of DNA was electrophoretically fractionated on a 1.8% agarose gel with .5 μg/mL ethidium bromide. Data presented are representative of three independent experiments. MW, molecular weight marker; bp, base pairs. B, The percentage of apoptotic cells in the pancreas was quantitated by using the TdT-mediated dUTP Nick end-labeling assay. Data are shown as means ± sem, n = 4. *Significant difference (p < .05) vs. the control group (0 hrs).
TdT-mediated dUTP Nick End-labeling Technique.
According to the histologic examination, the number of apoptotic cells was greatly elevated by 6 hrs after l-ornithine administration. To obtain quantitative data, the number of apoptotic cells was counted at selected time points 0 to 168 hrs after l-ornithine administration. As shown in Figure 7B, the percentage of apoptotic cells was greatly increased in response to l-ornithine administration. The peak of apoptosis was around 6 to 9 hrs after the injection (n = 4).
Pancreatic Nonprotein Sulfhydryl Group Content and the Activities of Glutathione Peroxidase and Superoxide Dismutase
Nonprotein sulfhydryl group content was significantly increased at 6 hrs and decreased thereafter in the ornithine-treated group compared with the control group (Fig. 8A). The activities of glutathione peroxidase (Fig. 8B) and Cu/Zn-SOD (Fig. 8C) significantly increased from 24 hrs. In contrast, Mn-SOD activity (Fig. 8D) was significantly decreased at 24 hrs and significantly increased at 48 hrs versus the control. Taken together these findings suggest the presence of oxidative stress in the pancreas of rats in response to l-ornithine treatment.
Figure 8.
l-ornithine injection (3 g/kg intraperitoneally) induces pancreatic oxidative stress. Changes in pancreatic (A) nonprotein sulfhydryl group (NSG) content, (B) glutathione–peroxidase (GSH-Px), (C) Cu/Zn, and (D) Mn superoxide dismutase (SOD) are depicted. Means ± sem for five to six animals are shown. *Significant difference (p < .05) vs. the control group (0 hrs).
Body Weight and Pancreatic Weight/Body Weight Ratio
The body weight of the rats was significantly decreased from 1 day to 1 mo after the administration of 3 g/kg l-ornithine versus the physiological saline-treated control (n = 5–10, Fig. 9A). Pancreatic weight/body weight ratio was significantly elevated at 18 to 48 hrs and significantly decreased at 168 hrs to 1 mo after l-ornithine injection (Fig. 9B).
Figure 9.
Time course of body weight (b.w.), pancreatic weight (p.w.)/b.w. serum aspartate aminotransferase (ASAT) activity and glucose concentration after intraperitoneal administration of 3 g/kg l-ornithine. A, B.w., (B) p.w./b.w., serum (C) ASAT activity, and (D) glucose concentration were determined as described in the Materials and Methods section. Data are shown as means ± sem, n = 4–10. *Significant difference (p < .05) vs. the control group (0 hrs).
Serum Aspartate Aminotransferase Activity and Concentrations of Glucose, Calcium, Triglyceride, Urea, and Creatinine
Serum ASAT activity was significantly increased by approximately five-fold at 24 hrs and three-fold at 48 hrs after l-ornithine injection (Fig. 9C). Serum concentrations of glucose were significantly decreased from 24 to 72 hrs and returned to normal by 1 wk (Fig. 9D). Serum levels of triglyceride were only significantly effected at 72 hrs (.40 ± .03 mM/L vs. .71 ± .18 mM/L in the control group). Calcium, urea, and creatinine concentrations were not significantly different versus the control (results not shown).
DISCUSSION
The present study characterizes a novel model of severe acute necrotizing pancreatitis induced by IP injection of 3 g/kg l-ornithine showing typical laboratory and morphologic signs of that observed in the human disease. The present pancreatitis model is noninvasive, more diffuse, and reproducible compared with those induced by retrograde ductal injection of bile acids, the closed duodenal loop method, or choline-deficient ethionine diet. l-ornithine-induced pancreatitis is superior to the l-arginine-induced model in that it produces a much severe disease with massive edema and without the confounding effects of possible excessive NO synthesis (at least in the initial phase of pancreatitis induction). IP administration of 4 to 6 g/kg l-ornithine killed the rats within hours (before pancreatitis could develop). The death of these animals may be the result of effects on the central nervous system. Our results also suggest that l-arginine-induced pancreatitis is due to metabolism of l-arginine to l-ornithine by arginase and is not caused by its metabolism to l-citrulline (and NO) by NOS. That is, l-arginine administration results in a greater increase in blood concentration of ornithine than citrulline, and administration of l-ornithine but not l-citrulline causes severe pancreatitis.
There has been longstanding debate on whether NO is involved in the pathogenesis of acute pancreatitis. Studies have shown a protective (19–21), no (22), or detrimental (23–25) effect of NO on experimental pancreatitis. One very important finding deduced from the present study is that NO is unlikely to be a primary factor in inducing l-ornithine (or l-arginine) pancreatitis. Serum citrulline levels were only increased by approximately three-fold after injection of rats with 3.5 g/kg l-arginine; l-ornithine levels were up by approximately 54-fold. In fact, this fits in well with our previous findings. which have shown that inhibition of NO formation by N-nitro-l-arginine methyl ester in l-arginine-induced pancreatitis only ameliorated laboratory parameters and did not influence pancreatic damage on histology(26). In contrast, others have found that aminoguanidine, an isoform-specific inhibitor of iNOS, ameliorated l-arginine induced pancreatic injury (24). Anyway, NO is unlikely to be involved in the early events (development) of pancreatitis given the time course of NOS induction. In the l-arginine-induced pancreatitis model, pancreatic cNOS activity was found to be depleted at 6 hrs followed by a gradually increasing level to a peak as observed at 24 hrs (26). The activity of iNOS was not increased until 24 hrs (peak of injury).
Morphologic examination of the pancreas showed the typical signs of severe acute necrotizing pancreatitis with massive interstitial edema, apoptosis, and necrosis of acinar cells and infiltration of neutrophil granulocytes (also confirmed by myeloperoxidase activity measurements) and monocytes. The necrotic process lacked morphologic signs indicative of lytic and ischemic damage to the cells and was evidently the result of toxic injury to the acini. The acini eventually regenerated. Acini that did not regenerate were replaced by fat tissue. Electron microscopy of pancreatitic tissue revealed large autophagic vacuoles containing zymogen granules, lipid droplets, severe nuclear damage, and dilated endoplasmic reticulum in acinar cells. Zymogen granules completely disappeared by 72 to 168 hrs, which are in accord with our pancreatic amylase activity measurements.
Interestingly, apoptosis of pancreatic acini (confirmed by light microscopic, electron microscopy, TUNEL, and DNA ladder analysis) was greatly increased within a few hours after l-ornithine administration. The rate of pancreatic apoptosis strongly increased at 16 to 24 hrs after the administration of 2.5 g/kg or 4 g/kg l-arginine (10, 27). In fact, Kubish et al. found that endoplasmic reticulum stress-regulated mechanisms are likely to be involved in the apoptosis observed in this model of the disease (10). Notably, in our study, apoptosis was already at is highest level at 6 to 9 hrs. A possible explanation for this is that in fact l-ornithine is mediating the apoptotic process (that is, l-arginine has been metabolized).
Several molecular features that have been observed in other models of pancreatitis were observed with administration of l-ornithine. These included intrapancreatic activation of the digestive enzyme trypsinogen (28), degradation of IκB proteins associated with activation of the transcription factor, NF-κB (29), increased interleukin-1β production, increased HSP72 content (30), and signs of oxidative stress (31). The time course changes of these laboratory parameters were similar to that observed in the l-arginine-induced pancreatitis model and were delayed compared with the cerulein-induced pancreatitis model (8). These point to possible differences in pathophysiology.
The mechanism underlying l-ornithine-induced pancreatitis is not clear. It is known that l-ornithine can serve as substrate for ornithine decarboxylase, the initial and rate-limiting enzyme in the polyamine biosynthetic pathway. Polyamines are essential for normal cell growth and development. A large dose of l-ornithine/l-arginine will inevitably influence their levels. Both increased and decreased levels of polyamines have been implicated in mediating apoptosis (32). Although one would probably expect to find an increase in polyamine levels, large doses of l-arginine have been shown to paradoxically reduce spermine and spermidine and increase putrescine levels by elevating polyamine catabolism (11). Spermine and spermidine depletion are also associated with human acute necrotizing pancreatitis (11). A decrease in pancreatic polyamine levels is likely to result in an inhibition of DNA and protein synthesis, which will result in the death of acini.
In conclusion, we have developed a simple, noninvasive, reproducible model of acute necrotizing pancreatitis by intraperitoneal injection of 3 g/kg l-ornithine and examined its time course in rats. Large doses of l-arginine (known to induce acute pancreatitis) may also produce a toxic effect on the pancreas, at least in part, through l-ornithine. Further studies are needed to investigate the mechanism of l-ornithine-induced pancreatitis.
Acknowledgments
Supported by the Hungarian Scientific Research Fund (K60406 to TT and PF63951 to ZR), the Hungarian Academy of Sciences (BO 00276/04 to PH and BO 00218/06 to ZR), and The Physiological Society, UK (Junior Fellowship to ZR).
The rabbit anti-HSP72 antibody was a generous gift from Dr. István Kurucz (IVAX Drug Research Institute, Budapest, Hungary) (33). We thank the Rosztóczy Foundation for helping to establish contact between the University of Szeged and UCLA through their generous scholarship awarded to ZR.
REFERENCES
- 1.Pandol SJ, Saluja AK, Imrie CW, et al. : Acute pancreatitis: Bench to the bedside. Gastroenterology 2007; 132:1127–1151 [DOI] [PubMed] [Google Scholar]
- 2.Nevalainen TJ, Seppä A: Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol 1975; 10:521–527 [PubMed] [Google Scholar]
- 3.Aho HJ, Koskensalo SM, Nevalainen TJ: Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol 1980; 15: 411–416 [DOI] [PubMed] [Google Scholar]
- 4.Lombardi B, Estes LW, Longnecker DS: Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol 1975; 79:465–480 [PMC free article] [PubMed] [Google Scholar]
- 5.Lampel M, Kern HF: Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 1977; 373:97–117 [DOI] [PubMed] [Google Scholar]
- 6.Mizunuma T, Kawamura S, Kishino Y: Effects of injecting excess arginine on rat pancreas. J Nutr 1984; 114:467–471 [DOI] [PubMed] [Google Scholar]
- 7.Tani S, Itoh H, Okabayashi Y, et al. : New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci 1990; 35:367–374 [DOI] [PubMed] [Google Scholar]
- 8.Hegyi P, Rakonczay Z Jr, Sári R, et al. : l-arginine-induced experimental pancreatitis. World J Gastroenterol 2004; 10:2003–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawra R, Sherif R, Phillips PA, et al. : Development of a new mouse model of acute pancreatitis induced by administration of l-arginine. Am J Physiol Gastrointest Liver Physiol 2007; 292:G1009–1018 [DOI] [PubMed] [Google Scholar]
- 10.Kubisch CH, Sans MD, Arumugam T, et al. : Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2006; 291:G238–245 [DOI] [PubMed] [Google Scholar]
- 11.Hyvönen MT, Herzig KH, Sinervirta R, et al. : Activated polyamine catabolism in acute pancreatitis: Alpha-methylated polyamine analogues prevent trypsinogen activation and pancreatitis-associated mortality. Am J Pathol 2006; 168:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris SM: Enzymes of arginine metabolism. J Nutr 2004; 134:2743S–2747S [DOI] [PubMed] [Google Scholar]
- 13.Chace DH, Millington DS, Terada N, et al. : Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem 1993; 39: 66–71 [PubMed] [Google Scholar]
- 14.Gukovsky I, Gukovskaya AS, Blinman TA, et al. : Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol 1998; 275:G1402–1414 [DOI] [PubMed] [Google Scholar]
- 15.Rakonczay Z Jr, Takács T, Boros I, et al. : NF-κB activation is detrimental in arginine-induced acute pancreatitis. Free Radic Biol Med 2003; 34:696–709 [DOI] [PubMed] [Google Scholar]
- 16.Czakó L, Takács T, Varga IS, et al. : Involvement of oxygen-derived free radicals in l-arginine-induced acute pancreatitis. Dig Dis Sci 1998; 43:1770–1777 [DOI] [PubMed] [Google Scholar]
- 17.Kuebler WM, Abels C, Schuerer L, et al. : Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int J Microcirc Clin Exp 1996; 16: 89–97 [DOI] [PubMed] [Google Scholar]
- 18.Rakonczay Z Jr, Takács T, Mándi Y, et al. : Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: Possible role of HSP72. Int J Hyperthermia 2001; 17:520–535 [DOI] [PubMed] [Google Scholar]
- 19.Molero X, Guarner F, Salas A, et al. : Nitric oxide modulates pancreatic basal secretion and response to cerulein in the rat: effects in acute pancreatitis. Gastroenterology 1995; 108:1855–1862 [DOI] [PubMed] [Google Scholar]
- 20.Werner J, Fernández-del Castillo C, Rivera JA, et al. : On the protective mechanisms of nitric oxide in acute pancreatitis. Gut 1998; 43:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama Y, Kato S, Mitsufuji S, et al. : Pathogenic role of endothelial nitric oxide synthase (eNOS/NOS-III) in cerulein-induced rat acute pancreatitis. Dig Dis Sci 2006; 51: 1396–1403 [DOI] [PubMed] [Google Scholar]
- 22.Weidenbach H, Lerch MM, Gress TM, et al. : Vasoactive mediators and the progression from edematous to necrotizing experimental acute pancreatitis. Gut 1995; 37:434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabrowski A, Gabryelewicz A: Nitric oxide contributes to multiorgan oxidative stress in acute experimental pancreatitis. Scand J Gastroenterol 1994; 29:943–948 [DOI] [PubMed] [Google Scholar]
- 24.Lomis TJ, Siffring CW, Chalasani S, et al. : Nitric oxide synthase inhibitors N-monomethylarginine and aminoguanidine prevent the progressive and severe hypotension associated with a rat model of pancreatitis. Am Surg 1995; 61:7–10 [PubMed] [Google Scholar]
- 25.Shields CJ, Delaney CP, Winter DC, et al. : Induction of nitric oxide synthase is a key determinant of progression to pulmonary injury in experimental pancreatitis. Surg Infect (Larchmt) 2006; 7:501–511 [DOI] [PubMed] [Google Scholar]
- 26.Takács T, Czakó L, Morschl É et al. : The role of nitric oxide in edema formation in L-arginine induced acute pancreatitis. Pancreas 2002; 25:31–38 [DOI] [PubMed] [Google Scholar]
- 27.Trulsson L, Sandström P, Sundqvist T, et al. : The influence of a load of l-arginine on serum amino acids and pancreatic apoptosis/proliferation and ATP levels in the rat. Pancreas 2004; 29:e113–120 [DOI] [PubMed] [Google Scholar]
- 28.Saluja AK, Donovan EA, Yamanaka K, et al. : Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 1997; 113:304–310 [DOI] [PubMed] [Google Scholar]
- 29.Rakonczay Z Jr, Hegyi P, Takács T, et al. : The role of NF-κB activation in the pathogenesis of acute pancreatitis. Gut 2008; 57:259–267 [DOI] [PubMed] [Google Scholar]
- 30.Rakonczay Z Jr, Takács T, Boros I, et al. : Heat shock proteins and the pancreas. J Cell Physiol 2003; 195:383–391 [DOI] [PubMed] [Google Scholar]
- 31.Schulz HU, Niederau C, Klonowski-Stumpe H, et al. : Oxidative stress in acute pancreatitis. Hepatogastroenterology 1999; 46: 2736–2750 [PubMed] [Google Scholar]
- 32.Schipper RG, Penning LC, Verhofstad AA: Involvement of polyamines in apoptosis. Facts and controversies: Effectors or protectors? Semin Cancer Biol 2000; 10:55–68 [DOI] [PubMed] [Google Scholar]
- 33.Kurucz I, Tombor B, Prechl J, et al. : Ultrastructural localization of HSP-72 examined with a new polyclonal antibody raised against the truncated variable domain of the heat shock protein. Cell Stress Chaperones 1999; 4:139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]