Abstract
Objective:
The goal of this study was to quantify short- and long-term outcomes of Clostridium difficile infection (CDI) in the elderly, including all-cause mortality, transfer to a facility, and hospitalizations.
Design:
Retrospective study using 2011 Medicare claims data, including all elderly persons coded for CDI and a sample of uninfected persons. Analysis of propensity score-matched pairs and the entire population stratified by the propensity score was used to determine the risk of all-cause mortality, new transfer to a long-term care facility (LTCF) and short-term skilled nursing facility (SNF), and subsequent hospitalizations within 30, 90, and 365 days.
Results:
174,903 persons coded for CDI were compared with 1,318,538 controls. CDI was associated with increased risk of death (OR 1.77, 95% CI: 1.74–1.81, attributable mortality 10.9%), new LTCF transfer (OR 1.74, 95% CI: 1.67–1.82) and new SNF transfer (OR 2.52, 95% CI: 2.46–2.58) within 30 days in matched pairs analyses. In stratified analysis CDI was associated with greatest risk of 30-day all-cause mortality in persons with lowest baseline probability of CDI (HR 3.04, 95% CI: 2.83–3.26); the risk progressively decreased as the baseline probability of CDI increased. CDI was also associated with increased risk of subsequent 30-day, 90-day, and one year hospitalization.
Conclusions:
CDI was associated with increased risk of short- and long-term adverse outcomes, including transfer to short- and long-term care facilities, hospitalization, and all-cause mortality. The magnitude of mortality risk varied depending on baseline probability of CDI, suggesting even lower-risk patients may benefit from interventions to prevent CDI.
summary:
Clostridium difficile is associated with acute and chronic morbidity in the elderly, including increased risk of skilled nursing and long-term care facility transfers, and additional hospitalizations. The risk of mortality was highest in patients with lowest baseline CDI probability.
Clostridium difficile is the most common microorganism associated with death in persons with gastroenteritis,1,2 and the single most common organism responsible for U.S. healthcare-associated infections.3 Although C. difficile infection (CDI) is clearly associated with morbidity and mortality, the incremental impact of CDI on mortality is not clear. In a 2015 review of CDI outcomes, all-cause mortality ranged from 11.8% to 38%, and attributable mortality ranged from 0–16.7%, depending on the time frame to assess mortality, statistical methods, and whether the investigations were conducted during endemic or epidemic periods of CDI.4 A prior review of European studies found similar heterogeneity in all-cause hospital mortality (4–37%) and CDI-attributable mortality (0–23%).5
Variation in all-cause and attributable CDI mortality is also likely due to differences in patient populations. Since CDI incidence and risk of complicated infection are much higher in older than younger persons,2,6,7 focus on outcomes in the elderly is important. Recently two studies reported CDI mortality in the elderly using Medicare data. Drozd found 1.9% 30-day attributable mortality after hospital-onset CDI, although their analysis included younger beneficiaries (i.e., end-stage renal disease, disabled).8 Shorr reported attributable CDI mortality of 14.9% at 60 days and 19.2% at one year in the elderly.9 Prior studies estimating the risk of mortality due to CDI have not considered the possibility of effect modification, in which the risk is not uniform but varies depending on other factors.10
The data on CDI-attributable morbidity are even more limited than that for mortality. Adults with CDI in a managed care plan were at higher risk of subsequent hospitalization, intensive care unit stay, and emergency department utilization than enrollees without CDI, particularly if they had recurrent CDI.11 Patients with CDI have been shown to be at increased risk of short- and longer-term hospital readmission,8,12–14 and discharge to a nursing care facility after hospitalization.15
We used Medicare data to better understand the impact of CDI on all-cause mortality, and short- and long-term morbidity in the elderly. We performed two different analyses to estimate differential risk of outcomes in CDI compared to uninfected persons, pooled across all persons and within strata of CDI risk. This was done to determine if heterogeneity in risk of poor outcomes exists, as this may impact how CDI prevention efforts should be targeted.
METHODS
The source of data for CDI patients was 2010–2012 Medicare claims data for all persons coded for CDI in 2011. For control patients we used the 2010–2012 5% random sample Medicare data, excluding persons coded for CDI in 2011.16 Long term care facility (LTCF) stays were identified using the 2010–2012 Minimum Data Set (MDS), which includes standardized assessments of patients in nursing facilities that accept federal payment.17
Eligible patients were those aged ≥ 66 years with complete Medicare fee-for-service enrollment during the 12 months prior to the CDI/control index date. Persons with no claims in 2010 and 2011 were excluded to ensure use of health benefits. Patients coded for CDI in the last quarter of 2010 were excluded to restrict the population to newly diagnosed individuals in 2011.
Index Date
Patients coded for CDI (ICD-9-CM diagnosis code 008.45) were identified from 1/1/2011–12/31/2011 in the Inpatient, Outpatient, Carrier (i.e., physician) or Skilled Nursing Facility (SNF) files. The CDI onset date was assigned as described previously,18 and an analogous index date was randomly selected for control patients, such that the distribution of index dates among control patients mirrored the distribution of CDI index dates.19 The first episode of CDI in 2011 was used for all analyses.
Outcomes
All-cause mortality within one year was identified using the death date in the Beneficiary Summary file. Secondary outcomes included new transfer to a LTCF, new transfer to a SNF, and one or more hospitalizations within 30 and 90 days, and one year after the index date. New transfer to a SNF was identified using the Medicare SNF file. LTCF residence was identified using method 2 as described by Goodwin,20 using the SNF file and MDS to distinguish long-term from short-term SNF encounters.17,20 For new transfers to SNF and LTCF, patients were excluded if they met the definition for these encounters in the year prior to the index date.
Acute care hospitalizations with admission date after the index date were identified using the Inpatient file. For individuals hospitalized on their index date, same-day transfers to another hospital were excluded, since they were not new hospitalizations. A subsequent hospitalization to treat CDI in patients diagnosed as an outpatient was considered a new hospitalization.
Covariates
Risk factors for CDI in the year prior to the index date were identified using ICD-9-CM diagnosis, Current Procedural Terminology, and Uniform Billing revenue codes. Risk factors included comorbidities, infections, and healthcare exposures, as defined previously,19 and acute noninfectious conditions and frailty indicators (Appendix Table 1). Acute noninfectious conditions included conditions expected to require hospitalization or outpatient treatment that may result in antibiotic exposure (Appendix Table 1). Frailty indicators were conditions associated with declining health (e.g., decubitus ulcer, difficulty walking, Appendix Table 1). Comorbidities were identified as recommended by Klabunde,21 while acute conditions required only a single code.
Statistical Analysis
Descriptive statistics were performed using Chi–square and Mann–Whitney U tests. To create the propensity score we used multivariable logistic regression with the dependent variable CDI and independent variables in Appendix Table 2. The independent variables were assessed in the year prior, in order to calculate the probability of CDI at the index date. The logit of the propensity score was used to match cases and controls 1:1 without replacement, using a caliper of 0.2 times the standard deviation of the logit of the propensity score.22,23 Standardized differences for all covariates were calculated before and after matching, with values > 0.1 indicating imbalance (Appendix Figure 1).24,25
The McNemar’s test was used to compare mortality, LTCF, and SNF transfer, with calculation of odds ratios for matched pairs. For the LTCF analysis the population was restricted prior to matching to exclude individuals previously residing in a LTCF, and individuals hospitalized at their index date who died during the hospitalization, since they would not have the opportunity for LTCF transfer. The population was restricted similarly for the SNF analysis. Attributable risk was calculated in the matched-pairs by subtraction of the percent of controls with outcome from the percent of CDI cases with outcome. For analysis of subsequent hospitalizations, Cox proportional hazards models were performed with a robust variance estimator, to account for the matching.26
For stratified analyses the probabilities were divided into 20 strata (i.e., ventiles) based on the propensity score in the CDI group, to obtain approximately equal numbers of CDI cases across strata for analysis of mortality (resulting in variable numbers of control patients/stratum). For secondary analyses, since individuals were excluded based on specific criteria, the number of CDI cases was no longer equal across strata. Analyses were performed using Cox proportional hazards models, with CDI the only independent variable, and stratification by the propensity score ventiles. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
The population of fee-for-service Medicare beneficiaries aged 66 and older included 1,510,046 persons. Of these, 16,605 were excluded due to CDI diagnosed in the last quarter of 2010, resulting in a final population of 1,493,441 persons for analysis: 174,903 coded for CDI and 1,318,538 control patients. Extrapolation to the entire 2011 fee-for-service Medicare population with at least one health care claim in 2010–2011 resulted in a comparison population of approximately 26.4 million, and an estimated CDI incidence of 663/100,000 elderly persons.
The mean age of the study population was 77.5 years, with 62.0% (n=925,316) female and 87.1% (n=1,301,397) white race (Table 1). Approximately 18% (n=271,128) of the population had dual eligibility in Medicare and Medicaid, indicative of low socioeconomic status. LTCF residence prior to the index date was identified in 95,775 (6.4%), while 404,227 (27.1%) had at least one hospitalization in the prior year.
Table 1.
Demographics and other Characteristics of the Medicare Elderly Population
| Characteristic | Total (%) N = 1,493,441 | CDI Cases N = 174,903 n (%) |
Controls N = 1,318,538 n (%) |
|---|---|---|---|
| Age (mean, SD) | 77.5 (7.9) | 80.5 (8.0) | 77.1 (7.7) |
| Female | 925,316 (62.0) | 112,251 (64.2) | 813,065 (61.7) |
| Race | |||
| White | 1,301,397 (87.1) | 153,857 (88.0) | 1,147,540 (87.0) |
| Black | 110,870 (7.4) | 13,801 (7.9) | 97,069 (7.4) |
| Other race | 81,174 (5.4) | 7,245 (4.1) | 73,929 (5.6) |
| Prior LTCF residence | 95,775 (6.4) | 40,143 (23.0) | 55,632 (4.2) |
| Dually eligible for Medicare and Medicaid | 271,128 (18.2) | 56,376 (32.2) | 214,752 (16.3) |
| Acute care hospitalization in past year | 404,227 (27.1) | 148,066 (84.7) | 256,161 (19.4) |
| Short-term skilled nursing facility encounter in past year | 183,539 (12.3) | 93,595 (53.5) | 89,944 (6.8) |
A total of 169,073 patients (11.3%) died within one year after CDI/control index date, with all-cause death in 40.9% of CDI cases and 7.4% of control patients. Of the persons eligible for new LTCF and SNF, 23,700/1,381,830 (1.72%) became resident in a LTCF (7.39% of CDI and 1.17% of control patients), and 119,780/1,429,750 (8.38%) entered a SNF (37.52% of CDI and 5.48% of control patients) within one year. Of the patients who newly entered a LTCF, 23.6% (n= 5,598) were also resident in a SNF before transitioning to the LTCF. Twenty-three percent of patients newly resident in a LTCF died within one year and 33.4% of patient who newly entered a SNF died within one year after the CDI/control onset date. The population eligible for subsequent hospitalization(s) included individuals whose CDI or control index date occurred as an outpatient, or for those hospitalized at their index date, individuals alive at hospital discharge (n = 1,474,999). At least one hospitalization within 30, 90, and 365 days after the index date occurred in 76,691 (23.21% of CDI and 3.03% of control patients), 162,022 (39.91% of CDI and 7.50% of control patients), and 369,931 (58.93% of CDI and 21.00% of control patients), respectively.
CDI was associated with 1.77-fold increased risk of 30-day all-cause mortality (95% CI: 1.74–1.81) in the propensity-score matched pairs analysis, and attributable mortality risk of 10.9% (Table 2 and Appendix Table 3). In secondary analyses CDI was associated with 1.74-fold increased risk of new LTCF transfer (95% CI: 1.67–1.82, attributable risk 2.7%), and 2.52-fold increased risk of new SNF entry (95% CI: 2.46–2.58, attributable risk 15.8%) within 30 days (Table 2). Matching was successful in 73.4% (128,406/174,903) of the CDI cases. The standardized differences and distribution of propensity scores are shown in Appendix Figures 1 and 2. Unmatched CDI cases were older and had higher frequencies of virtually all risk factors, consistent with the propensity score distribution for CDI cases (Appendix Table 2 and Figure 2).
Table 2.
Outcomes Attributable to CDI in Propensity Score-Matched Pairs Analyses in the Elderly Medicare Population
| Outcome | OR* (95% CI) | Risk Difference (%) |
|---|---|---|
| Mortality within one year | 1.77 (1.74–1.81) | 10.9% |
| New transfer to LTCF within one year | 1.74 (1.67–1.82) | 2.7% |
| New transfer to SNF within one year | 2.52 (2.46–2.58) | 15.8% |
| Acute care hospitalization | ||
| Within 30 days | 2.27 (CI:2.22–2.32) | 11.4% |
| Within 90 days | 1.95 (CI:1.92–1.98) | 15.7% |
| Within one year | 1.52 (CI:1.51–1.54) | 12.4% |
Hazard ratios presented for acute care hospitalizations
The results of stratified analysis for all-cause mortality are shown in Figure 1. The risk of mortality was highest in patients with lowest likelihood of CDI (ventile 1, HR 3.04, 95% CI: 2.83–3.26), and progressively decreased as the probability of CDI increased. In the highest-risk stratum the risk of mortality was much lower, but still statistically significant (ventile 20, HR 1.09, 95% CI: 1.01–1.17). As can also be seen in Figure 1, the percentage of CDI and control patients who died within one year progressively increased with increasing probability of CDI, from 8.8% of CDI cases and 3.0% of control patients in ventile 1, to 64.2% of CDI cases and 59.7% of control patients in ventile 20 with the highest probability of CDI.
Figure 1.
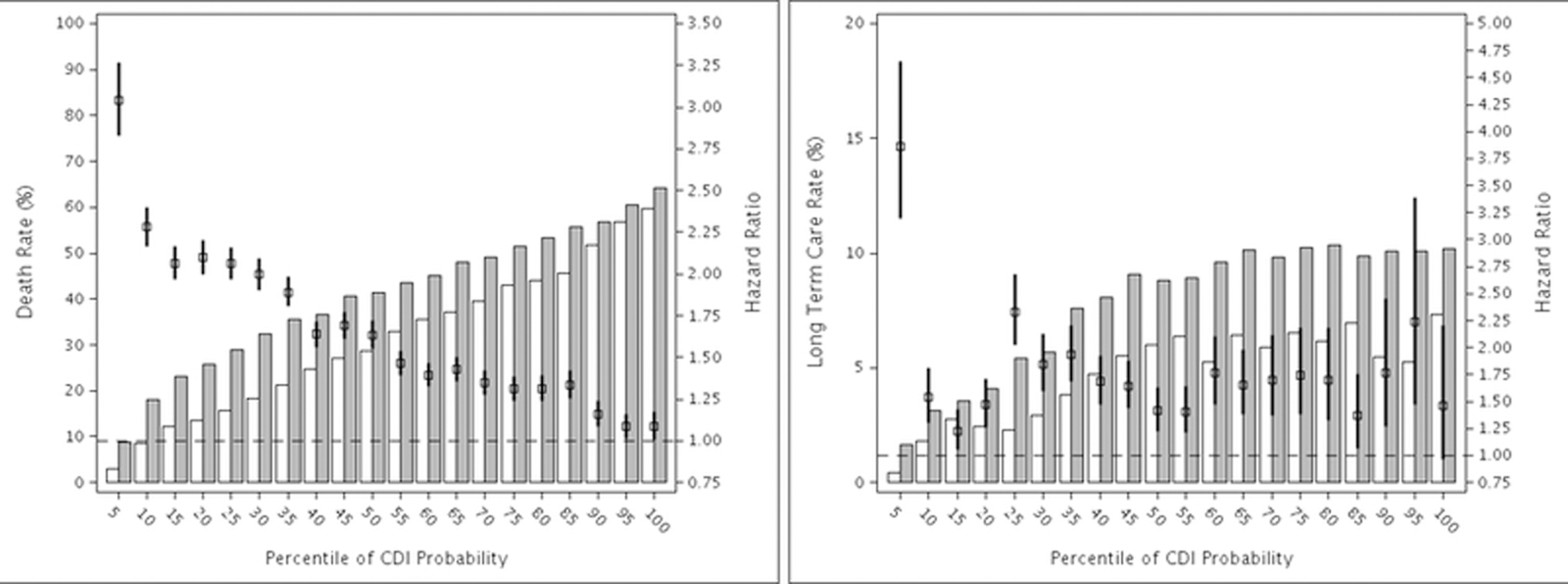
Stratified Hazard Ratios and Rates of Outcomes within One year after CDI or Control Index Date. Outcomes are: A. Mortality, B. New Entry into a Long-Term Care Facility. The bars represent the respective event rates controls (open bars) and CDI cases (grey bars).
 Hazard ratio and 95% confidence interval.
Hazard ratio and 95% confidence interval.
The same pattern was found for new LTCF (Figure 1) and SNF transfers (Appendix Figure 3). The highest risk of both outcomes occurred in the first stratum with lowest likelihood of CDI (HR 3.86, 95% CI: 3.20–4.65 for LTCF and HR 4.51, 95% CI: 4.17–4.89 for SNF). The risk of both outcomes decreased with increasing likelihood of CDI, albeit not as dramatically as mortality.
There were 121,830 matched pairs available for analysis of acute care hospitalizations. CDI was associated with increased risk of 30-day (HR 2.27, CI: 2.22–2.32), 90-day (HR 1.95, CI:1.92–1.98), and one year (HR 1.52, CI:1.51–1.54) hospitalization, with attributable risk ranging from 11.4%−15.7% (Table 2, Appendix Table 4). Similar results were found in the stratified analyses (Figure 2, Appendix Figure 4). The percentage of cases with at least one subsequent hospitalization was consistently higher for CDI cases across all strata compared to control patients. The risk of hospitalization was highest in individuals in the lowest risk stratum, and progressively decreased with increasing baseline probability of CDI. The magnitude of difference with increasing baseline CDI probability was greatest for 30-day hospitalization.
Figure 2.
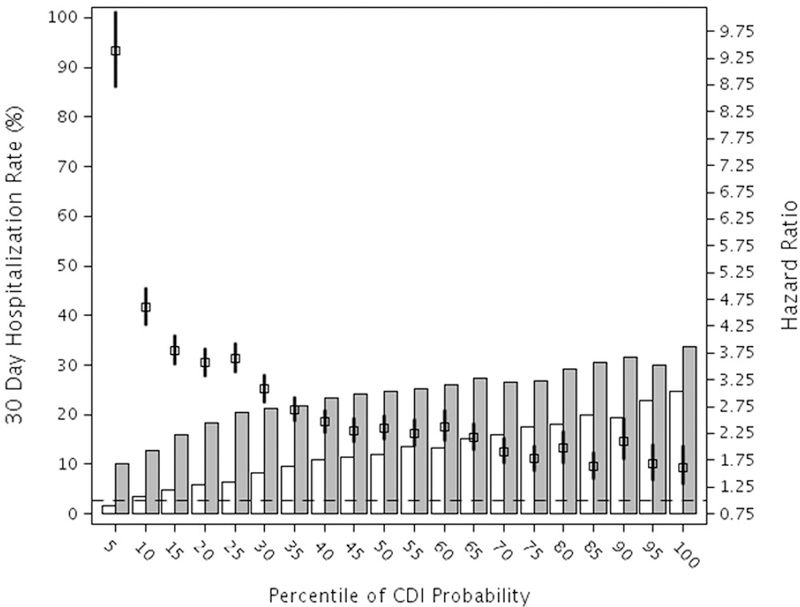
Stratified Hazard Ratios and Rates of Hospitalization within 30 days after CDI or Control Index Date. The number of total patient included in the hospitalization analyses was 1,475,045 (158,558 CDI case patients and 1,316,487 control patients), after excluding 18,396 persons who died during the index hospitalization. The bars represent the hospitalization rates in control patients (open bars) and CDI case patients (grey bars).
 Hazard ratio and 95% confidence interval. The null hazard ratio of 1.0 is indicated by the horizontal dashed line.
Hazard ratio and 95% confidence interval. The null hazard ratio of 1.0 is indicated by the horizontal dashed line.
DISCUSSION
Using 2011 Medicare data we found 10.9% excess 30-day mortality in CDI cases matched on the probability of CDI to control patients, with odds of mortality of 1.8. In stratified analysis the risk of mortality was highest in persons with lowest probability of CDI, with progressively decreased risk of mortality as the probability of CDI increased. The minimal contribution of CDI to risk of mortality in the strata with highest baseline CDI probability makes clinical sense, since persons at very high risk of CDI also have very high underlying severity of illness, with 60% one-year mortality in the control uninfected population in the highest stratum. In contrast, persons in the lowest baseline CDI stratum had 3-fold increased risk of one-year all-cause mortality if they developed CDI. The stratified analysis demonstrates that the increased risk of mortality associated with CDI is not uniform in all elderly persons.
In matched-pairs analysis the attributable risk of 30-day LTCF transfer due to CDI was 2.7%, while the attributable risk of 30-day SNF transfer due to CDI was much higher at 15.8%. In contrast to prior studies we developed algorithms to distinguish long-term care residence (i.e., nursing home) from short-term care stays, in order to determine the impact of CDI on these distinct outcomes. SNF stays (median 29 days) are reimbursed by Medicare for the purpose of rehabilitation following a hospitalization,27 reflective of acute events requiring additional medical care before patients can return home. In contrast, LTCF implies continual residence with no transition back to the community. The increased risk of 30-day SNF admission is suggestive of acute CDI-attributable morbidity, further supported by the increased risk of 30- and 90-day hospitalizations in CDI patients. The increased risk of LTCF transfer suggests that CDI also contributes to chronic morbidity from which patients are unable to fully recover. This increased risk of transition to a LTCF has additional implications in terms of quality of life and economics, since these costs are largely borne out-of-pocket or by the Medicaid program. The reduced impact of effect modification on these outcomes suggests that CDI-attributable morbidity impacts patients regardless of their underlying CDI risk.
The pooled CDI attributable mortality of 10.9% we calculated using propensity score matched pairs is similar to results reported by Nanwa in a Canadian population.28 In that study the attributable risk of mortality due to community-onset CDI was 13% at 1 year, although they did not report mortality specifically in the elderly. In contrast, Kuntz reported much lower attributable mortality risk of 4% due to non-recurrent CDI in adult Kaiser Health Plan members.11 One possible explanation for the lower CDI mortality risk in the Kuntz study was the requirement of a negative CDI test in control patients, thus selecting for control patients suspected of having CDI with likely higher underlying severity of illness than a non-tested group.
Our results also differ from those of Shorr in the Medicare elderly population, in which they reported higher attributable mortality due to CDI of 10% at 30 days and 19% at one year.9 Although Shorr also used propensity scores and matched pairs, they included fewer variables in their model and less stringent methods for matching than we did in this study. Since we were able to match only 73% of CDI cases to controls (vs. 99% by Shorr), our analysis consisted of patients likely more similar in baseline characteristics, resulting in lower attributable risk than that reported by Shorr.
Limitations of this study include use of administrative data, which lack clinical detail concerning some CDI risk factors (e.g., antibiotic utilization), CDI verification, and medications used to treat CDI in the hospital. Previously we found the CDI ICD-9-CM diagnosis code reported by hospitals to have sensitivity of 78% and specificity of 99.7% compared to C. difficile toxin assay results.29 Although identification of CDI using claims data is imperfect, the impact on our findings should be minimal, since the net effect of this misclassification will result in bias towards the null hypothesis. The use of older Medicare data is also a limitation, and our results should be confirmed with more recent data in which the incidence of CDI is lower. Despite the imperfection of claims data, the CDI incidence of 663/100,000 we calculated is almost identical to that reported by Lessa (628/100,000 elderly persons) using 2011 EIP surveillance data and laboratory tests to identify CDI.2 To mitigate the lack of clinical detail concerning prior antibiotic utilization, we included variables for a wide range of infections and classified them by expected type and duration of antibiotic therapy.19 We also included variables for numerous acute and chronic conditions that may result in antibiotic treatment and healthcare exposure. We calculated risk of outcomes based on exposures prior to the CDI/control index date, in order to quantify the probability of CDI. Although this does not take into account subsequent exposures, variation in CDI treatment, and other conditions after the index date, we used this method since our intent was to calculate differing risks of outcomes after balancing baseline exposures between the CDI and uninfected groups.
Strengths of this study include the very large population size, including all elderly beneficiaries coded for CDI in 2011, and generalizability of results to the Medicare fee-for-service population. We included a comprehensive set of variables into the propensity score model and achieved good balance in baseline characteristics, ensuring comparable case and control patients for the matched-pairs analyses. We performed stratified analyses of outcomes based on the propensity score, which demonstrated heterogeneity in the impact of CDI on mortality depending on baseline CDI risk.
We found that CDI was associated with increased risk of mortality, new LTCF, and short-term SNF transfer within 30 days and one year in elderly persons. The increased mortality risk associated with CDI was much greater in persons with low baseline CDI risk, and progressively decreased as the baseline risk of CDI increased. The increased risk of SNF and LTCF admissions, as well as 30-day and 1-year hospitalization demonstrates that CDI negatively impacts patients in both the short and long term. Our findings suggest that CDI prevention strategies should not be limited to just high-risk populations, since lower-risk elderly populations may have the greatest benefit. New strategies to prevent CDI focused on the elderly need to be developed to reduce mortality, morbidity, and decline resulting in loss of independence and institutionalization.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors thank Cherie Hill for support with the CMS data files.
Potential conflicts of interest. M.A.O. reports consulting and speaking fees from Pfizer. C.D. reports that she is an employee of Sanofi Pasteur. E.R.D. reports grant funding from Pfizer, Rebiotix, and Merck, and consulting fees from Sanofi Pasteur, Pfizer, Synthetic Biologics, Valneva, Abbott, Biofire, Rebiotix, and Merck.
FUNDING: E.R.D. received support for this study from Sanofi Pasteur. The sponsor participated in study design, interpretation of data, and final review of the manuscript. M.A.O and E.R.D had full access to all of the data in the study and final responsibility for the decision to submit for publication. Additional funding for access to data and services through the Washington University Center for Administrative Data Research was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR002345), and the Agency for Healthcare Research and Quality (R24 HS19455).
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE: The Washington University Human Research Protection Office gave approval to conduct this research with a waiver of informed consent.
Preliminary findings presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) conference, April 2016.
D.S. reports no conflicts of interest relevant to this manuscript.
References
- 1.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012;55(2):216–223. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372(9):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370(13):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 2015;29(1):123–134. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012;81(1):1–14. [DOI] [PubMed] [Google Scholar]
- 6.Abou Chakra CN, McGeer A, Labbe AC, et al. Factors Associated With Complications of Clostridium difficile Infection in a Multicenter Prospective Cohort. Clin Infect Dis 2015;61(12):1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS ONE 2014;9(6):e98400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drozd EM, Inocencio TJ, Braithwaite S, et al. Mortality, Hospital Costs, Payments, and Readmissions Associated With Clostridium difficile Infection Among Medicare Beneficiaries. Infect Dis Clin Pract (Baltim Md) 2015;23(6):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and Costs in Clostridium difficile Infection Among the Elderly in the United States. Infect Control Hosp Epidemiol 2016;37(11):1331–1336. [DOI] [PubMed] [Google Scholar]
- 10.Rothman KJ, S. G, T.L. L. Modern Epidemiology Third ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 11.Kuntz JL, Baker JM, Kipnis P, et al. Utilization of Health Services Among Adults With Recurrent Clostridium difficile Infection: A 12-Year Population-Based Study. Infect Control Hosp Epidemiol 2017;38(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanwa N, Kwong JC, Krahn M, et al. The Economic Burden of Hospital-Acquired Clostridium difficile Infection: A Population-Based Matched Cohort Study. Infect Control Hosp Epidemiol 2016;37(9):1068–1078. [DOI] [PubMed] [Google Scholar]
- 13.Magee G, Strauss ME, Thomas SM, Brown H, Baumer D, Broderick KC. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: A multicenter retrospective study of inpatients, 2009–2011. Am J Infect Control 2015;43(11):1148–1153. [DOI] [PubMed] [Google Scholar]
- 14.Barbut F, Bouee S, Longepierre L, Goldberg M, Bensoussan C, Levy-Bachelot L. Excess mortality between 2007 and 2014 among patients with Clostridium difficile infection: a French health insurance database analysis. J Hosp Infect 2018;98(1):21–28. [DOI] [PubMed] [Google Scholar]
- 15.Khanna S, Gupta A, Baddour LM, Pardi DS. Epidemiology, outcomes, and predictors of mortality in hospitalized adults with Clostridium difficile infection. Intern Emerg Med 2016;11(5):657–665. [DOI] [PubMed] [Google Scholar]
- 16.Center RDA. Chronic Conditions Data Warehouse 2017; https://www.ccwdata.org/web/guest/home. Accessed July 2, 2017.
- 17.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen MA, Young-Xu Y, Stwalley D, et al. The burden of clostridium difficile infection: estimates of the incidence of CDI from U.S. Administrative databases. BMC Infect Dis 2016;16(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubberke ER, Olsen MA, Stwalley D, et al. Identification of Medicare Recipients at Highest Risk for Clostridium difficile Infection in the US by Population Attributable Risk Analysis. PLoS One 2016;11(2):e0146822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res 2017;17(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca-Perraillon M. Local and global propensity score matching. SAS Global Forum 2007. http://www2.sas.com/proceedings/forum2007/185-2007.pdf. Accessed 6/8/2015. [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Dalton J. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012. http://www.lerner.ccf.org/qhs/software/lib/stddiff.pdf. Accessed 6/8/2015. [Google Scholar]
- 26.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33(7):1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowblis JR, Horowitz J, Brunt CS. Ownership Status and Length of Stay in Skilled Nursing Facilities: Does Endogeneity Matter? J Appl Gerontol 2016;35(3):303–320. [DOI] [PubMed] [Google Scholar]
- 28.Nanwa N, Sander B, Krahn M, et al. A population-based matched cohort study examining the mortality and costs of patients with community-onset Clostridium difficile infection identified using emergency department visits and hospital admissions. PLoS One 2017;12(3):e0172410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubberke ER, Butler AM, Yokoe DS, et al. Multicenter study of surveillance for hospital-onset Clostridium difficile infection by the use of ICD-9-CM diagnosis codes. Infect Control Hosp Epidemiol 2010;31(3):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


