Abstract
Background
Stepped care is a rational resource allocation approach to reduce population obesity. Evidence is lacking to guide decisions on use of low cost treatment components such as mobile health (mHealth) tools without compromising weight loss of those needing more expensive traditional treatment components (e.g., coaching, meal replacement). A sequential multiple assignment randomization trial (SMART) will be conducted to inform the development of an empirically based stepped care intervention that incorporates mHealth and traditional treatment components.
Objective
The primary aim tests the non-inferiority of app alone, compared to app plus coaching, as first line obesity treatment, measured by weight change from baseline to 6 months. Secondary aims are to identify the best tactic to address early treatment non-response and the optimal treatment sequence for resource efficient weight loss.
Study Design
Four hundred participants, 18–60 years old with Body Mass Index between 27–45 kg/m2 will be randomized to receive a weight loss smartphone app (APP) or the app plus weekly coaching (APP + C) for a 12 week period. Those achieving less than 0.5 lb weight loss on average per week, assessed by wireless scale at 2, 4, and 8 weeks, will be classified as non-responders and re-randomized once to step-up modestly (adding another mHealth component) or vigorously (adding mHealth and traditional treatment components) for the remaining treatment period. Weight will be assessed in person at baseline, 3, 6, and 12 months.
Significance
Results will inform construction of an obesity treatment algorithm that balances weight loss outcomes with resource consumption.
Keywords: Weight loss, Optimization, mHealth, Stepped Care, Obesity, Resource Allocation
1. Introduction
Obesity’s high prevalence and associated costs are a significant public health concern [1, 2]. Efficacious weight loss interventions are costly and burdensome because they provide expensive traditional treatment components (e.g., coaching, meal replacement) over multiple sessions [3–8]. Mobile health (mHealth) tools (e.g., smartphone applications, text messaging) have shown efficacy in weight loss programs at lower cost and scalability to reach a broader population [9–11]. However, considerable heterogeneity exists in response to mHealth treatments [11–15]. While a nontrivial proportion of the population achieves clinically meaningful improvement in response to minimal behavioral treatments, many need more than technology support [16–18]. Since traditional obesity treatment components produce greater weight loss than technology tools, [4, 6] but at higher cost [8], a rational approach to manage population level obesity might combine technology and traditional tools [19].
Rather than dispensing the same fixed package of treatment components to all patients throughout treatment, adaptively allocating treatment resources depending on patient need has the potential to improve weight loss outcomes for a greater proportion of the population [20–22]. Stepped-care is an adaptive approach that conserves resources by initiating treatment with minimal support (i.e., offering relatively low cost or low burden treatments first), and stepping-up (i.e., offering more costly or burdensome treatments) only to those who show signs of suboptimal response [23]. The decision rule for stepping up treatments is common to all participants and only the path participants take through that rule is adapted based on their progress (i.e., response status). This adaptive approach mirrors the way in which clinicians typically make decisions; i.e., sequentially manner, after considering response to prior treatment administrations. Stepped care has become best practice policy for several health conditions [24–26], with inexpensive web or mobile phone treatments are often an initial step on the care pathway [27]. For obesity, however, stepped care has neither equaled the weight loss outcomes attained with fixed treatment nor maximized resource efficiency by incorporating mHealth tools [28, 29]. Previous stepped care obesity trials evaluated weight loss over an observation period lasting between 6 weeks and 3 months before determining whether to augment treatment. This long evaluation period may allow those experiencing little weight loss from initially insufficient treatment to become discouraged and non-adherent. Given evidence that a 2 week observation period predicts 6 month weight loss, the current study shortened the augmentation decision interval to 2 weeks [30].
An assumption underlying stepped care is that greater support (i.e., more intensive intervention) uniformly leads to superior clinical outcomes and would be prescribed for all if resource limitations did not exist. Conversely, self-determination theory posits that provision of more intensive external support may come at a cost of reduced internal control, lowered autonomous motivation, and increased dependency [16, 31, 32]. Per self-determination theory, treatment components should offer the least possible support from external sources so as to not undermine autonomous motivation [33, 34]. Hence, an app alone could be the best first line treatment if it provides unobtrusive, low level support and feedback that reinforce autonomous learning and self-competence. Findings show that not everyone needs significant external support to attain weight loss [35]. Indeed, coaching, [36], could introduce interpersonal dependency that undermines autonomy if implemented in a manner perceived as controlling.
Abbreviations
SMART
APP
APP + C
Hence, the study’s primary aim is to identify the optimal first line obesity treatment, testing the hypothesis that app alone will be non-inferior to app plus coaching, the secondary aim tests whether the most resource efficient tactic to address early treatment non-response is to modestly or vigorously augment treatment. Exploratory aims examine degree of self-monitoring as a moderator and change in autonomous motivation as a mediator of weight loss, and maintenance of weight loss to 12 months.
2. Materials and Methods
The SMART study protocol and consent procedures will be approved by the Northwestern University Institution Review Board before enrolling participants. The trial will be registered on clinicaltrials.gov and the full study protocol and results will be published on clinicaltrials.gov upon completion of the trial.
2.1. Research Design
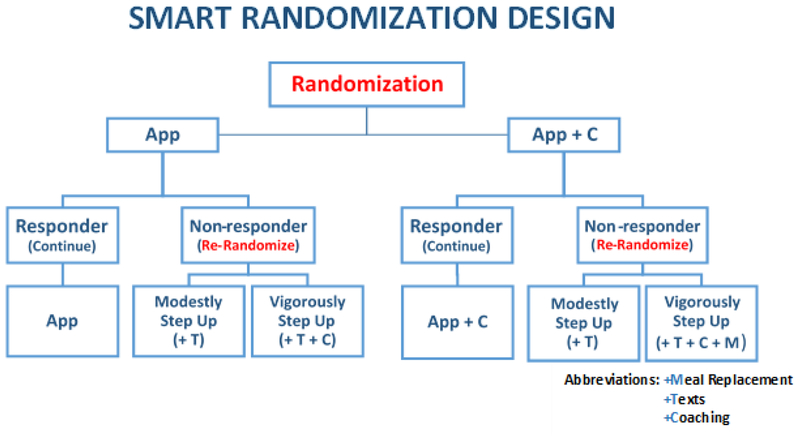
The research design is a Sequential Multiple Assignment Randomized Trial (SMART), an efficient experimental approach developed explicitly to construct efficacious adaptive interventions [20]. In the current SMART study, participants are randomized at baseline to one of two first line treatments. Those who show early signs of non-response (i.e., non-responders) are then re-randomized to one of two treatment augmentation tactics as soon as non-response becomes evident (at predetermined assessment time-points). Specifically, as diagrammed in Figure 1, participants will be randomized with equal probability to one of two first-line treatments, either (1) an mHealth component in the form of a mobile app that supports self-monitoring of weight, dietary intake and physical activity (APP), or (2) the app plus a more traditional treatment component in the form of weekly telephone-based health coaching sessions with a trained coach (APP + C).
Figure 1.
Smart Weight Loss Management Study design. Follow up assessments will occur at months 3, 6, and 12.
Prior data from our group [37] and others [38] indicated that weight loss of less than 1 pound by week 2 is highly predictive of ultimate non-response, namely losing less than 5% of baseline weight by 6 months of a weight loss program. Evidence suggests that weight loss at one and two months is also predictive of long term weight loss [39, 40]. Hence, response status, operationalized in terms of whether or not the participant lost an average of at least 0.5 lbs per week, will be assessed at weeks 2, 4, and 8.
At the first time point when a participant is classified as a non-responder (i.e., the participant did not lose at least an average of 0.5 lbs per week, as measured by home wi-fi scale), the individual will be re-randomized with equal probability to one of two treatment augmentation tactics, involving either: (1) Modest Step-Up: adding another mHealth component in the form of supportive messaging, or (2) Vigorous Step-Up: adding both supportive messaging plus a more traditional treatment component (either coaching or meal replacement) that was not offered to the participant initially. Individuals will be re-randomized as soon as they are classified as non-responders (at week 2, 4, or 8) and will continue with their new treatment assignment through 12 weeks. Re-randomization following nonresponse will occur only once per participant. All participants who prove responsive to their initial treatment (losing at least 0.5 lbs. on average per week) at the 2, 4, and 8 week assessments will continue receiving their first line treatment assignment through 12 weeks. At 12 weeks, all participants will revert back to intervention involving App only.
2.2. Aims
Since the best way to sequence the provision of mHealth tools and traditional treatment components in a stepped program of obesity treatments is unknown, we designed the SMART Weight Loss Management study to address two main aims. The primary aim is to determine whether the optimal first line weight loss treatment for a population of adults with overweight and obesity is an mHealth treatment component alone (APP) or an mHealth component plus coaching (APP + C). We hypothesize that at 6 months, the weight loss of those who receive the App alone as a first line treatment will be non-inferior to the weight loss of those initially assigned to APP + C. This hypothesis assumes that App alone will anchor the participant in framing weight regulation as an autonomously guided behavior and will thus provide as much (or more) benefit as the more expensive option, APP + C.
The secondary aim of the SMART study is to determine the optimal treatment augmentation tactic for early non-responders. We will compare the effects on weight loss at 6 months of augmenting the first-line treatment with an mHealth component alone: messaging (Modest Step Up) versus augmenting with an mHealth component (messaging) plus a traditional obesity treatment component: coaching for those who haven’t yet received it, or meal-replacement for those who have already received coaching (Vigorous Step Up).
The SMART study also has three exploratory aims. The first is to determine the optimal sequence of treatment tactics by comparing effects on 6 month weight loss and cost-effectiveness (cost/pound lost) of the four treatment sequences embedded in the SMART design. The second is to identify the best treatment for whom, when, and why by identifying baseline and time varying moderators (socioeconomic status, self-efficacy, extent of self-monitoring) that influence 6 month weight loss, and by determining whether increased autonomous motivation mediates greater weight loss. The third is to explore maintenance by examining how well the weight loss produced by the different treatment sequences is maintained through 12 months of follow-up.
2. 3. Core Intervention
All participants will be given the goal of 5% weight loss via calorie reduction from usual intake and increased physical activity. Participants will receive a calorie goal based on their initial weight, as well as a fat gram goal (based on 25% of their total daily calories coming from fat). Individuals weighing ≤174 lb. at baseline will be instructed to follow a 1200-kcal/d diet (33 grams fat/d); participants weighing between 175 and 219 lb. will be asked to follow a 1500-kcal/d diet (42 grams fat/d); those between 220 to 249 lb. will be asked to follow an 1800-kcal/d diet (50 grams fat/d); and any participants weighing ≥250 lb. will be instructed to follow a 2000-kcal/d diet (55 grams fat/d). Participants are also encouraged to engage in moderate-vigorous physical activity in order to meet a study prescribed physical activity goal. The goal will progress from 60 min/week to a maximum prescription of 300 min/week at a rate that averages a participant’s past 2 weeks of activity plus an increase of 20%, lending to a feasible increase in activity. Participants will be asked to login to the SMART study website to read online lessons on evidence based weight loss and behavioral strategies (e.g., portion size estimation, becoming active, stress management).
2.4. Selection of Treatment Components
The two categories of treatment components to be examined are interactive mHealth technologies and traditional weight loss treatment components. The former will be evaluated as potential early steps in obesity treatment; the latter will be evaluated as both potential initial and later steps in treatment.
2.4.1. mHealth Components
Two mHealth treatment components will be studied: an app and text messaging. These tools show evidence of weight loss efficacy and can be disseminated broadly at lower cost than traditional obesity treatment components, albeit they produce less weight loss [17, 41].
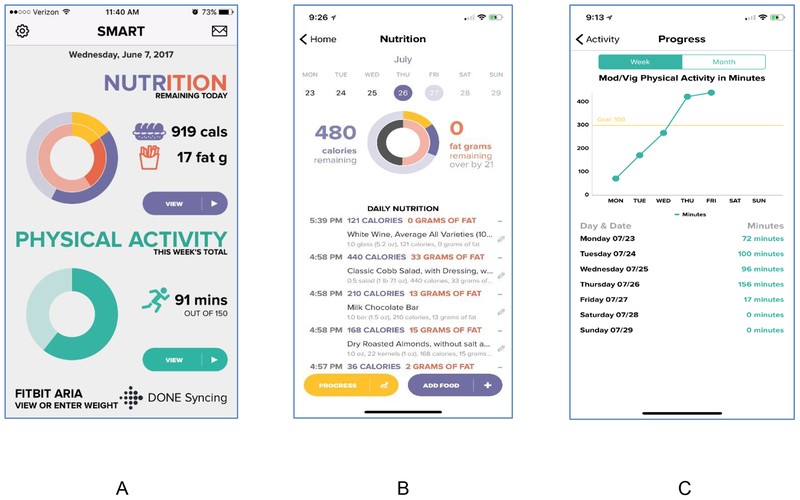
SMART App. The smartphone app for this trial is a custom-built, native application that is operable on either Android or iOS devices. Figure 1 shows display screens from the SMART App. Participants use the app to record dietary intake and to monitor graphical feedback that displays their dietary intake, physical activity, and weight relative to daily and long-term goals. Physical activity data are transmitted to the app by a study-provided Fitbit zip, and weight data are transmitted from a study-provided Fitbit Aria Scale. Upon study entry, participants are trained on how to estimate portion sizes, as well as how to locate and enter foods into the app which uses the Calorie King food and nutrient database. They are also trained on how to sync, verify, and correct weight and physical activity data from their Fitbit devices. All participants receive the app as either a part or all of their first line obesity treatment.
Push Notifications. All early non-responders (i.e., those achieving less than 0.5 lb weight loss on average per week when assessed at 2, 4, and 8 weeks) to first line treatment receive one-way messaging as either part or all of the components added to step up to their second line treatment. Messages are delivered as push notifications (delivered within the app) so that they are free of charge. Up to 3 push notifications are delivered daily during three pre-specified intervals (morning, afternoon, evening). Messages do not repeat, are tailored to the individual’s adherence and progress in weight loss and are designed to reinforce self-monitoring, mastery, and self-efficacy, and support autonomous motivation.
2.5. Traditional Obesity Treatment Components.
Coaching [18, 36, 42, 43] and meal replacement [44, 45], two traditional obesity treatment components, both have well established efficacy for producing weight loss in prior obesity intervention trials. Needed ongoing expenditures for coaching personnel and purchase costs for meal replacement foods make these traditional components more expensive than mHealth intervention components that entail lower delivery cost, once programmed. Coaching is offered as a step up intervention to those who failed to respond to initial treatment with app alone. Due to the additional cost of food provision, meal replacement is offered only as an additional step up to those who failed to respond to app plus coaching.
Coaching. Diet and activity coaching are provided together with the app to half of the participants as first line treatment (APP + C). Coaching also is provided for the first time as part of the Vigorous Step up second line treatment given to those who initially received App alone (APP), but showed early signs of non-response. For those assigned to receive it, health coaching occurs weekly until week 12 of the trial, at which point coaching ceases, to enable individuals to continue independently. Calls last approximately 10–15 minutes and include feedback and problem solving delivered within a motivational interviewing (MI) framework. Bachelors level coaches are trained, audio-recorded, monitored for treatment fidelity, and supervised weekly by a licensed clinical health psychologist. Coaches view all participant data via a custom web application, enabling them to monitor progress and tailor counseling.
Meal Replacements. Participants who are non-responsive to the initial combination of App and Coaching (APP + C) and are re-randomized to Vigorous Step Up will receive meal replacements. They will be given powdered meal replacement mixes to consume twice per day until week 12. This component is reserved for the most intensive level of intervention because of its high cost and high level of external control. By supplying portion-controlled, low calorie meal substitutes, meal replacement simplifies the dieter’s food environment, reducing the need to maintain a high level of self-control over food intake choices.
3. Participants and Procedures
3.1. Eligibility
The study will enroll 400 participants between the ages of 18 and 60 years old with a body mass index (BMI) between 27–45 kg/m2. Eligibility criteria require participants to be interested in losing weight, weight stable (no loss or gain >25 lbs. for the past 6 months), and not enrolled in a concurrent formal weight loss program. They must own a smartphone, be willing to install the study app, discontinue use of other weight loss apps, and provide informed consent. Candidates will not be eligible if they have had a cerebrovascular accident or myocardial infarction in the past six months, have diabetes treated with insulin, are pregnant, intending to become pregnant, or lactating, have active suicidal ideation, anorexia, bulimia, or substance abuse or dependence (other than nicotine), require an assistive device for mobility, or do not plan to remain in the Chicago area for the next 6 months.
3.2. Recruitment
Participants will be recruited through multiple channels including flyers, research registries, and advertisements online, in local newspapers, and on local public transportation. All recruitment materials will refer volunteers to the study website which displays information about the study and includes an invitation to link to a REDCap survey to determine initial eligibility. Prior studies using similar recruitment strategies in our urban location have yielded a diverse sample including many minority participants [46, 47].
3.3. Initial Screening, Orientation, and Run-in Procedures
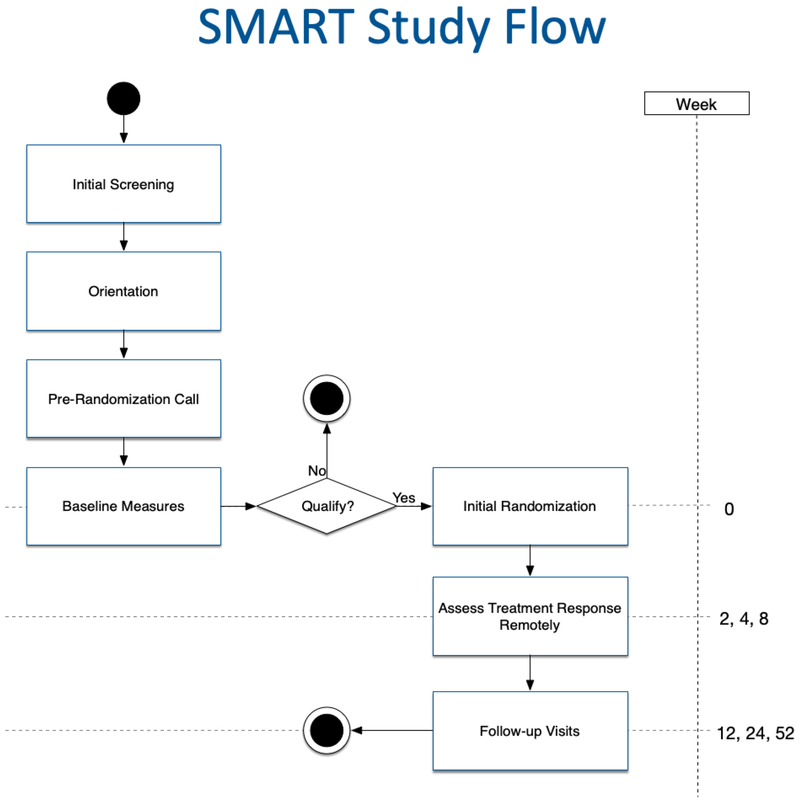
The SMART study flow and timeline are depicted in Figure 3. Candidates who initially screen in as eligible based upon their responses to a brief web survey will be contacted by telephone for further screening. The short telephone screening interview will confirm information from the web survey such as contact information, demographics, and weight, and will gather a more detailed medical history. Eligible volunteers will be invited to a group orientation session and equipoise induction, wherein potential participants discuss the pros and cons of being randomly assigned to alternative treatment conditions and learn about the impact of drop-out and missing data on the ability to validly interpret study findings. The goal of the orientation is to engage participants collaboratively in the research process, so as to minimize drop-out after randomization and foster protocol adherence [48]. Volunteers who remain interested will undergo an informed consent process and be scheduled for an in-person visit that involves a pre-randomization behavioral interview, baseline assessment and randomization. Prior to that appointment, all participants must complete a run-in period of 7 days, during which they self-monitor dietary intake and physical activity through the completion of a food and physical activity diary. The diary may be completed electronically or in a pencil and paper format. Those who demonstrate commitment to the program by logging 7 consecutive days of food and activity data in the diary, in addition to completing an online baseline questionnaire, will remain eligible for the study if during a telephone call they also demonstrate comprehension of the research requirements that were reviewed during their orientation session.
Figure. 3.
Smart Weight Loss Management Study Protocol sequence
3.4. Baseline Assessment and Randomization
Potential participants will complete baseline questionnaires, the run-in period, and medical approval forms prior to attending the assessment. Trained, blinded staff will collect anthropometric data (height, weight, and waist circumference). They will also conduct brief interviews using the PRIME-MD and a modified version of the Mini-International Neuropsychiatric Interview to identify and exclude individuals with bulimia, current substance abuse, major depressive disorder, or active suicidal ideation [49, 50].
Eligible participants will then be randomized with equal probability to one of two first-line treatments: APP or APP + C. Randomization will be stratified by gender and baseline BMI. Staff will discuss the randomized assignment with the participant, train the individual to use the smartphone application, understand the calculated weight, dietary, and physical activity goals, train the participant on connecting the scale to wifi at home, and anticipate the assessment time points. Participants will be provided with a FitBit zip and instructed to wear it every day, using the clip or wristband. A wireless Fitbit Aria body weight scale will also be provided; participants will be trained to weigh themselves on a hard surface daily upon awakening, after urinating, without clothes, with their phone nearby to ensure that their weight is received by the app. Finally, participants will be reminded that they will be remotely assessed at the end of weeks 2, 4, and 8 at which point they could be re-randomized to receive a new treatment strategy.
3.5. Re-randomization of Early Non-Responders
Participants will be loaned a wireless scale that uses wifi or Bluetooth technology to send data to the SMART app. The app will calculate weekly weight loss by subtracting the wireless scale measured weight closest to the start of the intervention (an at-home weight) from the most current weight on Sunday night. If the average weekly weight loss is found to be less than 0.5 pounds at the 2, 4, or 8 week time point, the participant will be classified as a non-responder. Once a participant is classified as a non-responder the app will re-randomize him/her to either a Modest Step Up or a Vigorous Step Up. Once a participant has been re-randomized, the new assigned treatment will continue through the end of week 12, and that participant will not be re-randomized again. Re-randomizations of early non-responders will be stratified by whether the non-responder did or did not lose any weight by the time of re-randomization, relative to baseline. Based on pilot data and prior studies, we anticipate that 50% of study participants will be classified as non-responders during the trial [12, 51].
3.6. Treatment Fidelity
To evaluate fidelity for the delivery of traditional weight loss treatment components, telephone coaching sessions will be audio recorded. On a quarterly basis, 15% of all calls will be sampled and rated using the fidelity checklist shown in Supplementary Materials. Fidelity items are scored positively when prescribed session content is delivered as intended (e.g., discussion of core values, goal attainment, problem solving with a participant assigned to receive coaching), zero when proscribed content is omitted (e.g., no discussion of meal replacements with a participant assigned to receive them), and negatively if proscribed content is discussed. If fidelity falls below 90%, coaches will be retrained.
To ensure fidelity of mHealth treatment component delivery through the smartphone app, 15% of participants’ smartphone data will be checked through the custom dashboard every quarter. Fidelity of the app treatment component will be coded by checking the integrity of the re-randomization algorithm’s functioning. This will be done by evaluating each participant’s weight change data over time in tandem with their re-randomization assignment to ensure that there were no failures of logic in the system in either timing or re-randomization. Finally, fidelity of the messaging component will be monitored by evaluating whether messages were sent by the app.
Treatment receipt will be measured by examining the login and time spent on the SMART lesson website, the log data of the smartphone app, the recorded receipt of meal replacements and reported use in the app, the recorded number and minutes spent on coaching calls, and interaction with messages (i.e. dismissing or opening). Self-monitoring, a mediator that is part of the exploratory analyses, is indicative of treatment enactment. Adherence to the study goals over time will also be examined as an indicator of treatment enactment.
3.7. Outcome Assessment
Participant anthropometrics will be assessed in person at 3, 6, and 12 months by trained, blinded staff. Participants will be paid $20 for completing each follow up assessment. See Figure 3 below for complete protocol sequence.
3.8. Weight, Height, and BMI
Height, weight, and waist circumference will be measured while the participant dons a standard hospital gown and wears no shoes. Height will be measured using a wall-mounted stadiometer to the nearest 0.25 in. Body weight will be measured to the nearest 0.25 lb using a calibrated balance beam scale. BMI will be calculated using the Quetelet Index as weight in pounds / (height in inches)2 × 704.5 [52, 53]. Waist circumference is measured twice during expiration using a Gulick tape placed midway between the palpated iliac crest and the palpated lowest rib margin in the mid-axillary lines, per the CARDIA protocol [54].
3.9. Measurement of Moderators and Mediators
Age, gender, and ethnicity will be assessed by a demographic questionnaire. SES will be measured using the Hollingshead Four-Factor Index of Socioeconomic Status [55]. This measure is designed to assess the social status of an individual based on four domains: marital status, retired/employed status, educational attainment, and occupational prestige. Self-monitoring adherence is collected by the smartphone application and is measured in terms of the quantity, frequency, and consistency of a participant entering diet and exercise data.
Self-Efficacy will be measured for both eating and exercise. Eating self-efficacy will be assessed from the scenario-based Dieting Self-Efficacy Scale (DIET-SE) [56]. The DIET-SE includes 11 items that are broken down into three subscales to reflect self-efficacy for managing different kinds of challenges to eating self-control: 1) high caloric food (HCF, 4 items), 2) social and internal factors (SIF, 4 items), and 3) negative emotional events (NEE, 3 items). The internal consistency of the DIET-SE is satisfactory (α = .77 for HCF; α = .79 for SIF; α = .79 for NEE; α = .87 for total score). Test–retest correlations for a 2- to 3-week interval were r = .83 for the DIETSE scale (r = .75 for HCF, r = .77 for SIF, and r = .80 for NEE), indicating good test–retest reliability. The Physical Activity Self-Efficacy [35] scale was modified to include 18 items. Participants use a 5-point Likert scale (1 = not at all confident; 5 = completely confident) to rate how confident they are that they will be able to exercise when “other things get in the way” such as being depressed, anxious, busy, or fatigued. A total score is calculated and a higher score indicates higher self-efficacy for physical activity.
Autonomous Motivation will be measured by an adapted and abbreviated version of the Treatment Self-Regulation Questionnaire [48, 57]. Four statements measuring autonomous motivation will be rated on a 5-point scale ranging from 1 (not at all true) to 5 (very true). Each item begins with the same stem: “The reason I want to achieve a healthier weight is…,” followed by, for example, “…because I personally believe it is the best thing for my health.” The abbreviated scale shows satisfactory internal consistency (α =.77) [56].
To examine cost-effectiveness we will focus only on cost of delivery, rather than costs to the patient. We will create a detailed accounting system to capture all costs associated with implementation of treatments [58]. Salary and fringe benefit information obtained from the U.S. Department of Labor, Bureau of Labor Statistics will be used to calculate total expenses associated with coaching personnel. We will also measure health-related quality of life at baseline and 6 months via the 5-item EQ-5D [55, 59, 60]; scores will be converted to quality-adjusted life years (QALYs) [56].
3.10. Statistical Analysis
The primary outcome measure for the SMART Weight Loss Management trial is change in weight (kilograms) from baseline to 6 months. For exploratory analyses, we also are interested in change in weight from baseline to 12 months. Each participant, once randomized, will be included in the intent-to-treat sample. Thus, every effort will be made to collect all primary and secondary outcomes even if a participant does not engage in the assigned treatment.
3.10.1. Primary Aim Analysis
To determine whether first line treatment with app alone is noninferior to first line treatment with app plus coaching, we will compare the effects of initial APP versus initial APP + C (regardless of subsequent treatments) in terms of weight change from baseline to 6 months. A Covariance Pattern Models (CPM [61]) using SAS PROC MIXED will be used to analyze the longitudinal weight data. CPMs use all available outcome data allowing for unequal number of observations and, under full-likelihood estimation, accommodate missingness when observations are missing at random (MAR) [62]. Thus, all available data from weight at baseline, 3 months, and 6 months will be used. Pattern-mixture models will be used to perform sensitivity analyses to investigate how robust our inferences are to departures from the MAR assumption [61, 63]. The CMP will include fixed effects for time (treated as a nominal variable with baseline as the reference cell), group, and group by time interaction. The group indicator will be defined as APP (Cells A+B+C in Figure 2) versus APP + C (Cells D+E+F in Figure 2). All fixed effects will be tested using Wald statistics. The CPM will include an unstructured residual variance-covariance matrix, to allow for differing variances and covariances across time, and will adjust for gender and baseline BMI.
Figure 2.
Main display interface, daily calorie view, and weekly physical activity view for the custom-built SMART App. Meters allow participants to view cumulative daily calorie and fat gram intakes, amount of physical activity completed, and weight on a single screen (A) or to see daily calorie and fat grams contributed by specific foods (B), or to see physical activity (C), diet, or weight graphed over days or weeks.
Model diagnostics will be used to determine the suitability of more parsimonious correlation structures and nonlinear effects for time. From the fitted CPMs, the primary hypothesis will be examined by attending to the coefficient of the group-by-month 6 interaction term (the difference in weight loss between APP and APP + C). Non-inferiority will be established if the lower limit of a (1–2α) × 100% confidence interval for the mean difference in weight loss between APP and APP + C is above –δ, where α is the Type I error, and δ is the non-inferiority margin [64]. Jones et al. [65] recommend a non-inferiority margin to be 50% of the difference between a treatment and control condition. In a recent weight loss trial, Ross and Wing (2016), found a difference of 11.24 pounds between similar treatment groups over a 6 month time period. Thus, we define the non-inferiority margin to be 50% of the difference found in the Ross and Wing study, specifically, 5.62 pounds [66].
3.10.2. Secondary Aim Analysis
To determine the optimal treatment augmentation tactic for early non-responders, we will compare: modest versus vigorous step-up among non-responders, in terms of change in weight from baseline to 6-months. This analysis will only include non-responders to the first line treatment, and group will be defined as Modest Step-Up (Cells B+E in Figure 2) versus Vigorous Step-Up (Cells A+D in Figure 2).
3.10.3. Exploratory Aims Analyses
The first exploratory aim is to determine the optimal sequence of treatment tactics by comparing effects on 6 month weight loss and cost-effectiveness (cost/pound lost) of the 4 treatment sequences embedded in the SMART design (Table 2). To address this aim, we will first compare the 4 embedded treatment sequences in terms of change in weight from baseline to 6-months. The weight and replicate method [22, 67, 68] will be used to perform this comparison. Secondly, we will compare each treatment sequence in terms of cost-effectiveness. For the purpose of this analysis, intervention costs include personnel and materials cost associated with developing and delivering each sequence. The principal measure of cost-effectiveness will be incremental cost per pound lost, calculated by taking the difference in average costs per patient involved in any two of the treatment sequences divided by the difference in weight loss between the two groups. Additionally, health-related quality of life will be evaluated and converted to quality-adjusted life years (QALYs) [69].
Table 2.
The four treatment sequences (TS’s) embedded in the SMART study design (Figure 1)
| Embedded Treatment sequences (TS) | Initial treatment | Subsequent tactic (treatment) for non-responders | Subsequent tactic for responders | Subgroups in Figure 1 |
|---|---|---|---|---|
| 1 | APP | Modest Step-Up (APP+T) | Continue first-line intervention | B+C |
| 2 | APP | Vigorous Step-Up (APP+T+C) | A+C | |
| 3 | APP + C | Modest Step-Up (APP + C+T) | E+F | |
| 4 | APP + C | Vigorous Step-Up (APP + C+T+MR) | D+F |
The second exploratory aim is to identify the optimal treatment for whom, when, and why by identifying baseline and time varying moderators (socioeconomic status, self-efficacy, extent of self-monitoring) that influence 6 month weight loss, and by determining whether increased autonomous motivation mediates greater weight loss for groups receiving APP alone (vs. APP + C) as their first line treatment. Investigating baseline and time-varying moderators to inform further individualization of treatment options and tactics will be done with Q-learning [70–72] which is a generalization of moderated regression analysis to sequence of treatments. Mediation will be tested using CPM and marginal structural modeling (MSM [72]).
The third exploratory aim is to explore maintenance by examining how well the weight loss produced by the different treatment sequences is maintained through 12 months of follow-up. Since we are interested in sustaining behavior change, we will compare treatment sequences in terms of weight change through 12 months. We will again use the weight and replicate method to compare the four treatment sequences embedded in the SMART study (Table 2) in terms of change in weight from baseline to 12-months.
3.10.4. Sample Size and Power
Sample size calculations were based on the primary outcome contrast: change in weight from baseline to 6-months in App versus App plus coaching. Recall that our goal is to test whether App alone is non-inferior to App plus coaching as first-line treatment. As discussed earlier, we define the non-inferiority margin to be 5.62 pounds. Based on preliminary data, we assume an overall SD of 29.6 with a correlation of 0.80 between weight at baseline and weight at month 6. Therefore, to obtain 90% statistical power with a 1-sided alpha equal to 0.05, a total of 344 participants will be required before attrition to establish the non-inferiority of App compared to App plus coaching. Assuming up to 14% attrition rate by month 6 (based on our previous studies; [35, 73]), recruiting 400 participants will provide adequate power for the primary contrast.
Enrolling 400 participants would result in 172 participants in each initial arm after attrition. By assuming a non-response rate of 50%, we estimate that 86 participants will be randomized to each of the two augmentation arms. Thus we will also have 82% power to detect at least a small to moderate effect size (Cohen’s d= 0.30) between augmentation tactics (Table 2).
3. Discussion
More than 69% of adults in the US are individuals with overweight or obesity [66], and existing practice standards for the treatment of obesity endorse a fixed and one-size-fits-all approach. Current guidelines recommending intensive lifestyle intervention for all obesity treatment result in significant burden and cost, impeding scalability and widespread uptake. The SMART study takes a significant step toward providing evidence for two important aspects of conserving resources in the allocation of treatment. First, it will test whether a low cost, app only intervention is a sufficient first line treatment when compared to a more costly option of including coaching. Secondly, it will provide evidence for an adaptive approach to weight loss intervention that also seeks to minimize resource use unless necessary. By progressively allocating more costly treatment components only when non-responsivity to prior treatment demonstrates need, the resulting treatment sequences conserve resources and support an individual’s autonomous motivation. The goal of this study is to evaluate intervention sequences that have potential for scalability at a population level and sustainability at an individual level.
The evidence based practice process is often misunderstood as advising clinicians to deliver a single fixed best treatment package continuously over time without regard to how the patient responds. However, an important part of what expert clinicians do is to assess the adequacy of an individual’s response to intervention and adjust treatment as warranted [74]. For weight loss, as for other health conditions, clinicians adapt treatment dynamically by making changes that respond to patients’ progress. For example, when a self-help intervention is not producing benefit for an individual, the clinician may add expert coaching in order to prevent demotivation. In clinical practice, clinicians often adjust the treatment in a way that is implicit (i.e., not well-specified) and based on intuition, rather than on evidence based practices that were subject to research validation. The aim of designs like SMART is to explicate and objectively test which parametric decision rules (e.g., about the timing, magnitude, or nature of treatment adaptation) serve to optimize the targeted clinical outcome.
It is often assumed that, from the perspective of the individual care recipient, “more is better,” such that, if resources were infinite, the “best” i.e., maximally intensive, costly care should be given to all. Indeed, the well-replicated association between treatment intensity (number of provided treatment sessions [75, 76]) and magnitude of weight loss has led health policy entities to restrict coverage for behavioral obesity treatment to care that incorporates multiple counseling sessions[77–79]. On the other hand, a nontrivial proportion of adults with obesity achieves clinically meaningful weight loss in response to minimalist behavioral intervention [77, 78]. Currently we cannot pre-identify who will respond to minimal intensity intervention (e.g., self-help) and who needs more provision of external support and as such, the SMART study includes exploratory aims to investigate such moderating effects as sociodemographic variables. The apparent heterogeneity in response to obesity intervention leads us to question the conventional wisdom that more treatment can always be assumed to be better. Instead, we suggest that offering more support than is minimally necessary for behavioral accomplishment can undermine feelings of competency and autonomy, thereby hindering longer term motivation and positive behavior change [31, 32, 34]. In weight loss, specifically, it has been shown that gaining experience overcoming short-term difficulties with weight gain fosters subsequent success at weight loss and maintenance [80]. Consequently, we are at equipoise in terms of being able to predict whether the best stepped care policy for allocating resources across a population with obesity should involve starting with minimal intensity self-help treatment (i.e., an app alone) and stepping up minimally to add another mHealth component (i.e., texts) in the event of nonresponse, or, alternatively, whether investing in more traditional external treatment resources initially (e.g., coaching) and at subsequent step-ups is sounder policy to prevent discouragement and sustained weight loss failure. Thus, the SMART study will explore such variables as self-monitoring, self-efficacy, and autonomy to shed light on heterogeneity of response and provide evidence to inform further treatment optimization to produce greater and sustained weight loss over time.
For these reasons, the SMART Weight Loss Management study tests a non-inferiority hypothesis about optimal obesity treatment strategy. The study will provide evidence regarding what first line treatment components are necessary to maximize weight loss while conserving resources. As such, if app alone is found to be non-inferior to APP+C, our conclusion with respect to the primary aim would be to suggest that app is the “better” first line treatment for the entire population because it is less expensive to implement and the policy of starting intervention with app alone produces weight loss results that are non-inferior to app plus coaching. Hence, stepped care policy that starts with the less expensive care option would make sense to scale up to a population level because of its lower resource utilization. If starting treatment with App alone is found to be non-inferior, we have also posited that this treatment strategy would be the best choice because it supports autonomous motivation, which we will test as an exploratory mediator. A different policy question is whether the rescue strategies implemented to respond to unsuccessful weight loss efforts should be vigorous, i.e., externally introducing more expensive traditional treatment supports such as meal replacement, or whether augmentation should be more modestly incremental, adding low-cost automated text messaging to the components initially provided. Since our secondary aim will compare modest and vigorous augmentation tactics, SMART will provide evidence as to whether tactics that use fewer resources and introduce less external control are able to initiate weight loss among early non responders.
With the help of technology, we can now remotely monitor engagement, adherence, and progress towards health goals in real time. Investigating engagement with the mHealth intervention can inform when and how to and make reasonable adaptations to treatments [81]. Adaptive treatments present an opportunity to provide appropriate treatment to those who need it, when they need it and, ultimately, to improve long-term outcomes for greater numbers of people. Additionally, the best treatment for whom, when, and why will be tested in the SMART study by investigating moderators (socioeconomic status, self-efficacy, self-monitoring) and a mediator (autonomous motivation) consistent with our conceptual model. Such systematic investigation will add to the evidence base about treatment sequences that can create population level obesity treatment. Developing evidence-based treatment sequence protocols suggesting how to proceed, particularly when a first line treatment proves inadequate, can help advance treatment guidelines beyond the current fixed and one size fits all approach.
There are limitations to the current study that warrant discussion. First, although the SMART study does assess treatment response at multiple time points, it will not address whether additional step ups later in treatment could further optimize weight loss outcome. Second, the current study only focuses on developing an adaptive treatment strategy for weight loss initiation. Other important questions about obesity treatment policy remain to be answered, such as those regarding optimal treatment discontinuation policy to achieve maintenance of weight loss. Despite these limitations, the current study will provide foundational evidence on which to build a cost effective adaptive treatment strategy for population level obesity intervention.
Obesity is a significant public health threat whose high prevalence warrants an effective population based strategy to reverse the trend. Research efforts to date have primarily aimed to identify a best treatment for the average person, rather than tailored solutions that are adapted to different individuals. Consequently, the most efficacious behavioral obesity treatments identified to date and now covered by third party payers, include requisite intensive and expensive elements, limiting their scalability and reach. Yet, it is unknown what proportion of the population actually requires intensive behavioral intervention to lose weight, versus what proportion could succeed and benefit motivationally from less expensive self-help approaches. We have posited non-inferiority between obesity treatments that begin with a self-help app alone, versus those that begin with externally introduced expert coaching. We believe that heterogeneity in treatment needs is baked into the population, and we currently lack precision indicators that could accurately predict who needs how much care. Lacking such markers, we suggest that treatment policy that steps and adapts treatment intensity based on monitoring treatment response represents sounder husbanding of health care resources than does providing very intensive, costly treatment to all comers, including those who need less care. By leveraging modern research designs, such as the SMART design, we can develop pre-specified treatment policies that are comprised of adaptive sequences of treatments that optimize weight loss and manage resources equitably and judiciously. Both efficacious treatment and sound management of scarce resources are necessary to move the needle on obesity prevalence across a diverse population.
Supplementary Material
Table 1:
SMART inclusion and exclusion criteria
| Inclusion Criteria |
|---|
| BMI between 27–45 kg/m2 |
| 18–60 years of age |
| <350 lbs |
| Weight stable over the past 6 months |
| Interested in losing weight and not enrolled in a formal weight loss program or taking medications or supplements that may cause weight change |
| Own a Smartphone and be willing to install the SMART App |
| Reside in the Chicago area for the duration of their participation (12 months) |
| Exclusion Criteria |
| Unstable medical conditions (uncontrolled hypertension, diabetes - uncontrolled or treated with insulin, uncontrolled hypothyroidism, unstable angina pectoris, transient ischemic attack, cancer undergoing active treatment, cerebrovascular accident or myocardial infarction within the past six months, or Crohn’s disease) |
| Pregnancy, lactation, or intended pregnancy |
| Active suicidal ideation, anorexia, bulimia, binge eating disorder, current substance abuse or dependence (besides nicotine dependence) |
| Require assistive device for mobility or current condition that may limit or prevent participation in moderate activity |
| Use of pacemaker or other electrical implanted device |
| History of bariatric (or LapBand) surgery, or considering or currently on a wait-list for bariatric or |
| LapBand surgery |
| May not live with a current or past SMART study participant |
Acknowledgements
Funding: This work was supported in part by National Institutes of Health grants R01DK108678, R01DK097364, R01DA039901, UL1TR001422, and P30CA60553.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hales CM, et al. , Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA, 2018. 319(16): p. 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, et al. , Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA, 2016. 315(21): p. 2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeBlanc ES, et al. , Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA, 2018. 320(11): p. 1172–1191. [DOI] [PubMed] [Google Scholar]
- 4.Look ARG and Wing RR, Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med, 2010. 170(17): p. 1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, et al. , Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama, 2012. 307(5): p. 491–497. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, et al. , Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med, 2002. 346(6): p. 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor EA, et al. , Screening for Obesity and Intervention for Weight Management in Children and Adolescents Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA, 2017. 317(23): p. 2421–2444. [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Prevention Program Research Group. The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention. Diabetes Care, 2012. 35(4): p. 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellegrini CA, et al. , Smartphone applications to support weight loss: current perspectives. Adv Health Care Technol, 2015. 1: p. 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spring B, D. JM, Janke EA, Kozak AT, McFadden HG, DeMott A, Pictor A, Epstein LH, Siddique J, Pellegrini CA, Buscemi J, Hedeker D, Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Internal Medicine, 2013. 173(2): p. 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke LE, Ma J, Azar KMJ, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, & Quinn CC, Current science on consumer use of mobile health for cardiovascular disease prevention. A scientific statement from the American Heart Association. Circulation, 2015. 132(12): p. 1157–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spring B, et al. , Effects of an abbreviated obesity intervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity (Silver Spring), 2017. 25(7): p. 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coons MJ, et al. , Technology Interventions to Curb Obesity: A Systematic Review of the Current Literature. Curr Cardiovasc Risk Rep, 2012. 6(2): p. 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR, et al. , A self-regulation program for maintenance of weight loss. N Engl J Med, 2006. 355(15): p. 1563–71. [DOI] [PubMed] [Google Scholar]
- 15.Burke LE, et al. , Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med, 2012. 43(1): p. 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzlicht M, Schmeichel BJ, and Macrae CN, Why self-control seems (but may not be) limited. Trends Cogn Sci, 2014. 18(3): p. 127–33. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. , A Systematic Review of Application and Effectiveness of mHealth Interventions for Obesity and Diabetes Treatment and Self-Management. Adv Nutr, 2017. 8(3): p. 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spring B, Pellegrini C, Pfammatter AF, McFadden HG, Driver S, Hedeker D, Siddique J . Advanced Mobile Technology to Evaluate and Intervene on Eating Behaviors to Produce Weight Loss in The Obesity Society’s Annual Scientific Meeting 2014. Boston, MA. [Google Scholar]
- 19.Bhardwaj NN, et al. , Can mHealth Revolutionize the Way We Manage Adult Obesity? Perspectives in health information management, 2017. 14(Spring): p. 1a–1a. [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy SA, An experimental design for the development of adaptive treatment strategies. Stat Med, 2005. 24(10): p. 1455–81. [DOI] [PubMed] [Google Scholar]
- 21.Collins LM, Murphy SA, and Strecher V, The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med, 2007. 32(5 Suppl): p. S112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahum-Shani I, et al. , Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods, 2012. 17(4): p. 457–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower P and Gilbody S, Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry, 2005. 186: p. 11–7. [DOI] [PubMed] [Google Scholar]
- 24.Li M, et al. , Management of Depression in Patients With Cancer: A Clinical Practice Guideline. J Oncol Pract, 2016. 12(8): p. 747–56. [DOI] [PubMed] [Google Scholar]
- 25.Sveum R BJ, Brottman G, Hanson M, Heiman M, Johns K, Malkiewicz J, Manney S, Moyer L, Myers C, Myers N, O’Brien M, Rethwill M, Schaefer K, Uden D, Diagnosis and management of asthma, in Institute for Clinical Systems Improvement (ICSI). 2012: Bloomington, MN,. p. 1–86. [Google Scholar]
- 26.Haug T, et al. , Stepped care versus face-to-face cognitive behavior therapy for panic disorder and social anxiety disorder: Predictors and moderators of outcome. Behav Res Ther, 2015. 71: p. 76–89. [DOI] [PubMed] [Google Scholar]
- 27.Guide N, NICE Guideline. Commissioning stepped care for people with common mental health disorders. 2014: London, UK. [Google Scholar]
- 28.Carels RA, et al. , The failure of therapist assistance and stepped-care to improve weight loss outcomes. Obesity (Silver Spring), 2008. 16(6): p. 1460–2. [DOI] [PubMed] [Google Scholar]
- 29.Jakicic JM, et al. , Effect of a stepped-care intervention approach on weight loss in adults: a randomized clinical trial. JAMA, 2012. 307(24): p. 2617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waring ME, et al. , Early-treatment weight loss predicts 6-month weight loss in women with obesity and depression: implications for stepped care. J Psychosom Res, 2014. 76(5): p. 394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorin AA, et al. , Autonomy support, self-regulation, and weight loss. Health Psychol, 2014. 33(4): p. 332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teixeira PJ, et al. , Self-regulation, motivation, and psychosocial factors in weight management. J Obes, 2012. 2012: p. 582348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan RM and Deci EL, Self-regulation and the problem of human autonomy: does psychology need choice, self-determination, and will? J Pers, 2006. 74(6): p. 1557–85. [DOI] [PubMed] [Google Scholar]
- 34.Deci EL and Ryan RM, The “What” and “Why” of Goal Pursuits: Human Needs and the Self-Determination of Behavior. Psychological Inquiry, 2000. 11(4): p. 227–268. [Google Scholar]
- 35.Hartmann-Boyce J, et al. , Self-Help for Weight Loss in Overweight and Obese Adults: Systematic Review and Meta-Analysis. American Journal of Public Health, 2015. 105(3): p. e43–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appel LJ, et al. , Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med, 2011. 365(21): p. 1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spring B, Pellegrini CA, Nahum-Shani I How long does it take to identify non-responders to weight loss treatment in The Obesity Society’s Annual Scientific Meeting 2014. Boston, MA. [Google Scholar]
- 38.Waring ME, et al. , Early-treatment weight loss predicts 6-month weight loss in women with obesity and depression: Implications for stepped care. Journal of Psychosomatic Research, 2014. 76(5): p. 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unick JL, et al. , Examination of the Consistency in Affective Response to Acute Exercise in Overweight and Obese Women. J Sport Exerc Psychol, 2015. 37(5): p. 534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unick JL HP, Neiberg R, Cheskin L, Dutton G, Evans-Hudnall G, Jeffery R, Kitabchi A, Nelson J, Pi-Sunyer X, West D, Wing RR for the Look AHEAD Research Group. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring), 2014. 22(7): p. 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin JB, et al. , My Quest, an Intervention Using Text Messaging to Improve Dietary and Physical Activity Behaviors and Promote Weight Loss in Low-Income Women. J Nutr Educ Behav, 2018. 50(1): p. 11–18 e1. [DOI] [PubMed] [Google Scholar]
- 42.Hammond GC, et al. , Comparative effectiveness of cognitive therapies delivered face-to-face or over the telephone: an observational study using propensity methods. PLoS One, 2012. 7(9): p. e42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherwood NE, et al. , Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes (Lond), 2006. 30(10): p. 1565–73. [DOI] [PubMed] [Google Scholar]
- 44.Ard JD, et al. , Impact on weight and physical function of intensive medical weight loss in older adults with stage II and III obesity. Obesity (Silver Spring), 2016. 24(9): p. 1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moldovan CP, et al. , Effects of a meal replacement system alone or in combination with phentermine on weight loss and food cravings. Obesity (Silver Spring), 2016. 24(11): p. 2344–2350. [DOI] [PubMed] [Google Scholar]
- 46.Spring B, et al. , Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med, 2012. 172(10): p. 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spring B, et al. , Multicomponent mHealth Intervention for Large, Sustained Change in Multiple Diet and Activity Risk Behaviors: The Make Better Choices 2 Randomized Controlled Trial. J Med Internet Res, 2018. 20(6): p. e10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg JH and Kiernan M, Innovative techniques to address retention in a behavioral weight-loss trial. Health Educ Res, 2005. 20(4): p. 439–47. [DOI] [PubMed] [Google Scholar]
- 49.Spitzer RL, Kroenke K, and Williams JB, Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA, 1999. 282(18): p. 1737–44. [DOI] [PubMed] [Google Scholar]
- 50.Hergueta T, Baker R, and Dunbar GC, The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IVand ICD-10. J clin psychiatry, 1998. 59(Suppl 20): p. 2233. [PubMed] [Google Scholar]
- 51.Pellegrini CA, et al. , A smartphone-supported weight loss program: design of the ENGAGED randomized controlled trial. BMC Public Health, 2012. 12: p. 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McInnis KJ, Franklin BA, and Rippe JM, Counseling for physical activity in overweight and obese patients. Am Fam Physician, 2003. 67(6): p. 1249–56. [PubMed] [Google Scholar]
- 53.Esherick JS, Slater ED, and David JA, Chapter 4: Appendices, in CURRENT Practice Guidelines in Primary Care 2018. 2017, McGraw-Hill Education: New York, NY. [Google Scholar]
- 54.Smith D, The CARDia trial protocol. Heart, 2003. 89(10): p. 1125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holingshead AB, Four Factor Index of Social Status. Yale Journal of Sociology, 2011. 8: p. 21–52. [Google Scholar]
- 56.Stich C, Knauper B, and Tint A, A scenario-based dieting self-efficacy scale: the DIET-SE. Assessment, 2009. 16(1): p. 16–30. [DOI] [PubMed] [Google Scholar]
- 57.Thomas S, Reading J, and Shephard RJ, Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci, 1992. 17(4): p. 338–45. [PubMed] [Google Scholar]
- 58.Feldman PJ and Steptoe A, Psychosocial and socioeconomic factors associated with glycated hemoglobin in nondiabetic middle-aged men and women. Health Psychol, 2003. 22(4): p. 398–405. [DOI] [PubMed] [Google Scholar]
- 59.Carels RA, et al. , Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol, 2007. 26(3): p. 369–74. [DOI] [PubMed] [Google Scholar]
- 60.EuroQol G, EuroQol--a new facility for the measurement of health-related quality of life. Health Policy, 1990. 16(3): p. 199–208. [DOI] [PubMed] [Google Scholar]
- 61.Hedeker D, Longitudinal data analysis. 2006.
- 62.Molenberghs G, et al. , Analyzing incomplete longitudinal clinical trial data. Biostatistics, 2004. 5(3): p. 445–64. [DOI] [PubMed] [Google Scholar]
- 63.Mj D, Missing data in longitudinal studies: Strategies for Bayesian modeling and sensitivity analysis. 2008.
- 64.Walker E and Nowacki AS, Understanding equivalence and noninferiority testing. J Gen Intern Med, 2011. 26(2): p. 192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones B, et al. , Trials to assess equivalence: the importance of rigorous methods. BMJ, 1996. 313(7048): p. 36–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ross KM and Wing RR, Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: A randomized pilot study. Obesity (Silver Spring), 2016. 24(8): p. 1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orellana L, Rotnitzky A, and Robins JM, Dynamic regime marginal structural mean models for estimation of optimal dynamic treatment regimes, Part II: proofs of results. Int J Biostat, 2010. 6(2): p. Article 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robins J, Orellana L, and Rotnitzky A, Estimation and extrapolation of optimal treatment and testing strategies. Stat Med, 2008. 27(23): p. 4678–721. [DOI] [PubMed] [Google Scholar]
- 69.Hagberg LA, et al. , Cost-utility analysis of a randomized controlled weight loss trial among lactating overweight/obese women. BMC Public Health, 2014. 14: p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nahum-Shani I, et al. , Q-learning: a data analysis method for constructing adaptive interventions. Psychol Methods, 2012. 17(4): p. 478–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy SA, Collins LM, and Rush AJ, Customizing treatment to the patient: adaptive treatment strategies. Drug Alcohol Depend, 2007. 88 Suppl 2: p. S1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robins JM, Hernan MA, and Brumback B, Marginal structural models and causal inference in epidemiology. Epidemiology, 2000. 11(5): p. 550–60. [DOI] [PubMed] [Google Scholar]
- 73.Azar KM, et al. , Mobile applications for weight management: theory-based content analysis. Am J Prev Med, 2013. 45(5): p. 583–9. [DOI] [PubMed] [Google Scholar]
- 74.Straus S, et al. , Evidence-Based Medicine. 2018, London: Elsevier. [Google Scholar]
- 75.Cramer JS, et al. , An adaptation of the diabetes prevention program for use with high-risk, minority patients with type 2 diabetes. Diabetes Educ, 2007. 33(3): p. 503–8. [DOI] [PubMed] [Google Scholar]
- 76.ter Bogt NC, et al. , Preventing weight gain by lifestyle intervention in a general practice setting: three-year results of a randomized controlled trial. Arch Intern Med, 2011. 171(4): p. 306–13. [DOI] [PubMed] [Google Scholar]
- 77.Glasgow RE, et al. , Minimal intervention needed for change: definition, use, and value for improving health and health research. Transl Behav Med, 2014. 4(1): p. 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wieland LS, et al. , Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev, 2012(8): p. CD007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Force USPST, et al. , Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA, 2017. 317(23): p. 2417–2426. [DOI] [PubMed] [Google Scholar]
- 80.Kiernan M, et al. , Promoting healthy weight with “stability skills first”: a randomized trial. J Consult Clin Psychol, 2013. 81(2): p. 336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alshurafa N, et al. , Is More Always Better?: Discovering Incentivized mHealth Intervention Engagement Related to Health Behavior Trends. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol, 2018. 2(4): p. 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.