INTRODUCTION
There have been marked improvements in outcomes among patients diagnosed with cancer. These improvements are related, in part, to the improved understanding of cancer biology leading to the development of more effective therapies. However, as the number of cancer survivors increases, there has been a renewed focus on the potential cardiotoxic effects of cancer therapies. Additionally, the types of therapies available for cancer treatment have expanded from traditional radiation and chemotherapy to targeted and immune therapies. There are over 15 million cancer survivors in the US, and this number is projected to increase(1). Both pre-existing risk factors for CV disease and possible cardiotoxic effects of cancer therapy contribute to enlarge the likelihood of CV disease development and exacerbation to these individuals (2, 3). Moreover, the number of older patients with multiple risk factors for CV disease who receive a diagnosis of a malignancy has considerably increased during the last three decades(4). The combination of the increased complexities of these cancer therapies, the increased number of cancer survivors, and the recognition of the increased risk of cardiovascular disease among cancer survivor’s has led to the development of a specialty called cardio-oncology or onco-cardiology (5).
Cardiovascular complications may occur after many types of cancer therapies, including traditional chemotherapy, radiotherapy, targeted and immune therapies regimens(6–8). The manifestations of cardiovascular disease among patients with cancer are broad and although cardiac injury is more frequently observed in the myocardium as left ventricular (LV) systolic dysfunction, it may also appear at other heart structures such as valves, coronary arteries, pericardium, great arteries and electrical system(9).
Despite advances, the effective detection and quantification of cancer therapy related cardiotoxicity is challenged by several factors. Firstly, in many cases cardiac dysfunction occurs only after many years of chemotherapy and in the absence of clinical symptoms, confounding its association with cancer therapy(10). Additionally, while the definition of cardiotoxicity has proven its clinical utility (a decrease in LVEF of ≥5% to < 55% in the presence of symptoms of HF or an asymptomatic decrease in LVEF by ≥10% to less than 55%) (11), this definition relies exclusively on longitudinal monitoring of LV systolic function and ejection fraction (EF), commonly performed by standard transthoracic echocardiogram which has several limitations such as poor reproducibility, lack to provide tissue characterization and to identify LV remodeling beyond systolic dysfunction. Finally, reductions in LV EF frequently take place within the normal range indicating that subtle myocardial injury may occur before LV dysfunction(12, 13).
Consistent data have established that cardiac magnetic resonance (CMR) is one of the most accurate imaging modalities for the assessment of cardiac toxicity and adverse LV remodeling. Its ability to assess cardiac morphology and function is highly accurate and reproducible(14). CMR also integrates different types of imaging sequences (e.g., cine imaging for morphology, T2-weighted imaging for myocardial edema, perfusion for ischemia assessment, late gadolinium enhancement (LGE) for scar and tagging for myocardial strain imaging), providing a very comprehensive evaluation of the LV remodeling associated with cancer therapy. More recently, different groups(15–17) have applied more novel sequences to charactize the effects of cancer therapy, such as the use of T1 and T2 mapping techniques; providing not only data regarding the early abnormalities in myocardial tissue composition, but also improving the current mechanistic understanding of cancer-related cardiotoxicity.
Mechanisms of the most common chemotherapy-induced cardiotoxicity -Anthracyclines
Anthracyclines are commonly implicated as a cause of CV toxicity among patients with cancer. The risk of HF with anthracyclines is related to cumulative dose, with the risk increasing with higher cumulative doses. The cardiotoxicity of anthracyclines is far higher than the rates of clinical heart failure. Specifically, rates of subclinical cardiotoxicity are higher, occurring even with lower cumulative doses, particularly when over 350 mg/m2 (18, 19). In a retrospective analysis Swain et al. identified an increased risk of cardiotoxicity even with doses previously considered safe (≤300 mg/m2)(20). Once HF is established, Mortality from HF secondary to anthracycline therapy ranges from 30 to 70%(21). There may be a long latency period between anthracycline and clinical heart failure especially among young patients; however, among adults toxicity generally appears early with 90% of cases occurring within the first year. (10). The cardiotoxicity of anthracyclines is likely a multifactorial process, related to oxidative stress, mitochondriopathy, changes in iron and calcium homeostasis and in respiratory chain components(22, 23). Zhang et al. proposed a unifying mechanism of cardiotoxicity, implicating topoisomerase-IIβ as an essential driver in this mechanism, because in its presence, doxorubicin activates the DNA response and apoptosis pathways and affects oxidative phosphorylation and mitochondrial biogenesis in cardiomyocytes(24).
Trastuzumab
Trastuzumab is a monoclonal antibody and inhibits the human epidermal growth factor by the receptor tyrosine-protein kinase erbB-2 pathway. ErbB-2 is also present in cardiomyocytes and downstream pathways that regulates apoptosis, mitosis, cell hypertrophy and elongation, cellular adhesion, angiogenesis and sensitivity to adrenergic signaling(25), being potentially deleterious to myocardial tissue. Trastuzumab has been associated with an increased incidence of cardiac dysfunction of 3% to 7%(11). The risk rises with concomitant use of paclitaxel(13%) and even more when concomitantly administered with anthracyclines and/or cyclophosphamide (27%). Trastuzumab-induced cardiotoxicity is not dose-dependent (as when determined by anthracyclines) but is often reversible, although it is difficult to predict which patients are at risk to develop toxicity(26).
Mitoxantrone
Mitoxantrone, an anthracenedione agent, is a DNA-topoisomerase II inhibitor with antineoplastic activity and potent anti-inflammatory and immunomodulating properties. It acts intercalating into DNA, reducing DNA repair and interfering with RNA synthesis(27). Mitoxantrone is associated with cumulative dose-related cardiotoxicity, and myocytes exposed to it experience similar alterations at electron microscopy to those seen with anthracycline(28). Mitoxantrone-induced cardiotoxicity manifests as systolic and diastolic dysfunction(29). It is recognized that around 2% of cancer patients treated with drug will develop cardiotoxicity. While this condition is asymptomatic in most cases it may transition to congestive HF related symptoms may transition to congestive cardiac failure increasing the risk of death (30).
Cyclophosphamide
Cyclophosphamide is a nitrogen mustard-alkylating agent with potent antineoplastic, immunosuppressive and immunomodulatory properties. Although the precise mechanism of cyclophosphamide-induced cardiac toxicity has not been entirely established, their metabolites can cause oxidative stress and endothelial damage. Cyclophosphamide cardiac toxicity is associated with cumulative doses and typically manifests as a fulminant myocarditis.
TKI’s
Sunitinib targets the vascular endothelial growth factor molecular pathway through tyrosine kinase inhibition. A recent review indicated that sunitinib was the preferred initial therapeutic option for metastatic renal cell carcinoma(31). However, sunitinib affects the AMP-activated protein kinase and platelet derived growth factor receptor, which are critical for cardiomyocyte function and survival(32, 33), leading to hypertension, left ventricular dysfunction and heart failure(34–36).
Radiation therapy: Heart failure, valve disease
Immune therapies: Myocarditis
Initial Management
Cardiac complication after cancer therapy may manifest in different ways, including cardiac systolic dysfunction, cardiac ischemia, arrhythmias, pericarditis and electrical repolarization abnormalities. A close interaction and cooperation between oncology and cardiology are required to improve care of many cancer patients at risk to develop cancer related cardiac complications. Patients considered for antineoplastic therapy should undergo initial CV evaluation to diagnose pre-existing cardiac diseases, including physical examination, electrocardiogram and analysis of ventricular function, identifying those with conditions that can be readily treated or might require more surveillance. Serial imaging assessment of cardiac function is suggested, even in asymptomatic patients, as discontinuation of cardiotoxic chemotherapy might allow reversible improvement in cardiac function when left ventricular systolic dysfunction is identified in this group(37).
Cardiac Magnetic Resonance Imaging
Morphological and Functional Parameters
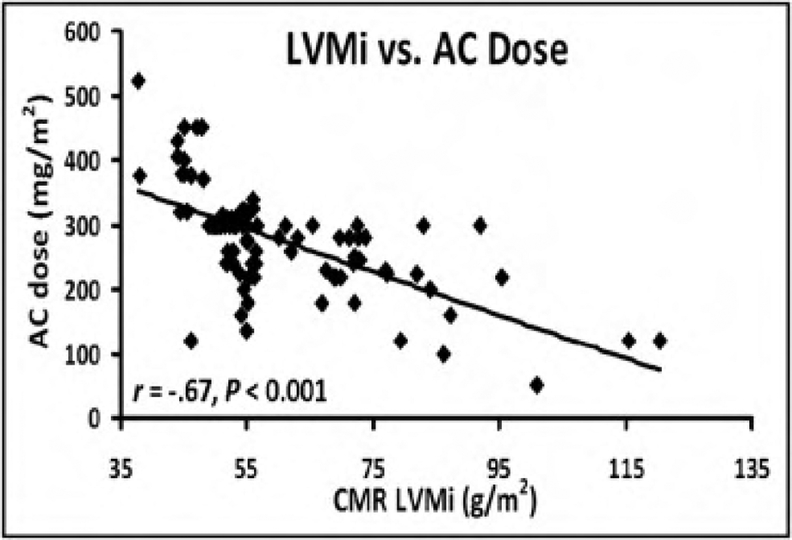
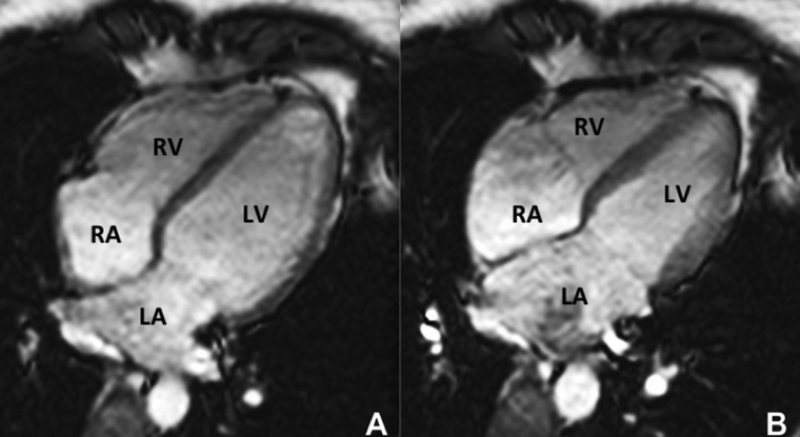
CMR is the method of choice for assessing changes in cardiac morphology and function caused by cancer treatment and cardiotoxicity (38–40). Its high contrast to noise ratio, superior accuracy and excellent reproducibility, allows detection of slight changes that may benefit from preventive therapies. Both LV and right ventricle (RV) volumes and mass can be precisely obtained using standard cine CMR images, independently of any assumption of ventricles shape and the degree of remodeling(41). Commonly used steady state free precession (SSFP) pulse sequences, which delivers high signal-to-noise and tissue-to-blood contrast (Figure-1 A and B), provides precise data on both global and regional wall motion, capturing even subtle functional changes(42). Drafts and coworkers demonstrated that CMR cine images were able to detect early and significant abnormalities in cardiac structure and function secondary to cardiotoxicity therapy, even after moderate to low doses of anthracycline chemotherapy. Performing a series of CMR examinations, before and up to 6 months after anthracycline therapy, the authors showed that reduction in LV EF occurred early as 1-month after chemotherapy initiation, identifying individuals at high risk to maintain significant later decline in LV function. Interestingly they also demonstrated that the decrease in LV function was associated with LV enlargement, highlighting the potential negative effect of anthracycline-based chemotherapy on systolic function. Using LGE imaging, new areas with infarcts or scar were not seen, suggesting that anthracycline induced cardiotoxicity appeared not to be caused by myocardial ischemia or myocardial infarction. A study comparing different imaging approaches for screening adult survivors of childhood cancer treated with anthracycline chemotherapy and/or radiation therapy(43), has shown that 2D echocardiogram, using the biplane method, may not achieve a clinically reasonable accuracy for detecting LV EF under 50% measured by CMR, demonstrating in this scenario limited sensitivity (25%) with high rates of false-positives (75%). While 3D echocardiogram may improve sensitivity (53%), this approach also fails to accomplish results comparable to CMR imaging(43). LV mass can also change during cancer therapy and especially after anthracycline-based chemotherapy(15). Nearby 50% of childhood cancer survivors have been shown to have LV mass > or = 2 standard deviations below the mean normative values(43). Neilan and colleagues, examining 91 individuals with decreased LV EF after anthracycline-based chemotherapy at a median follow up 88 months, found that indexed LV mass measured by CMR had a negative correlation with the cumulative given dose of anthracycline (Figure 2A). In the same study, LV mass was shown to be a strong predictor of major CV events(44) and patients with indexed LV mass < 57 g/m2 had significantly higher rates of CV deaths, appropriate implantable cardioverterdefibrillator therapies and admissions for decompensated heart failure compared to the ones with indexed LV mass >/= to 57 g/m2 (Figure 2B). A recent study, using novel CMR markers of myocardial tissue remodeling, indicates that left ventricular atrophy could be accounted not only for the expansion of the extra-cellular space, but also by a reduction in cardiomyocyte size(17). In addition, Jordan et al(45) suggested not only that the decrease in LV mass occurs early after anthracycline initiation, but may also take place regardless of factors that increase myocardial wall tension and stress. They also showed that a reduction in LV mass was associated with worsening HF symptoms.
Figure 1:
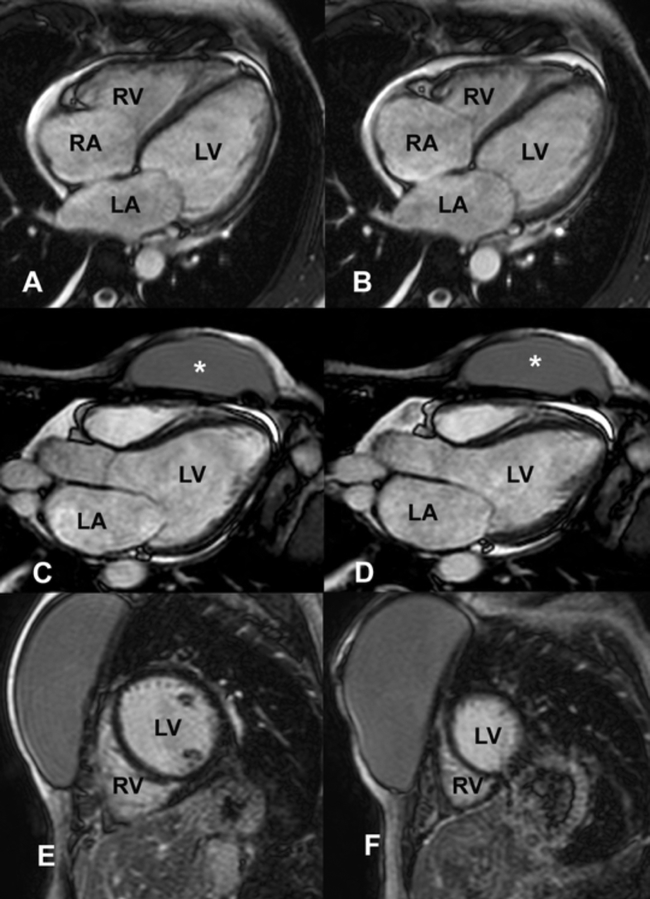
Four chamber steady-state free precession showing the high resolution and excellent soft tissue contrast of cardiac magnetic resonance imaging in end diastole (A) and end systole (B). RV: right ventricle, LV: left ventricle, RA: right atrium, LA: left atrium.
Figure 2:
A) Association of LV mass derived by CMR with anthracycline dose. B) Kaplan-Meier curves showing event-free probability according to LVM mass index by CMR (>/= 57 or < 57 g/m2). (From Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110(11):1679–86; with permission).
Although few observations have specifically focused on the effects of cancer therapy on RV, recent reports have demonstrated significant decline in RV systolic function after anthracycline alone or in combination with trastuzumab (46–48). RV failure has been associated with significant morbidity and mortality in HF patients with both reduced and preserved ejection fraction(49). Because RV is a thin and complex structure compare to LV, non-invasive imaging may be challenging. CMR has been show to be very accurate(50) and reproducible(51) to assess RV volumes and function(52, 53), having several advantages over 2D echocardiography, including outstanding spatial resolution, volumetric quantification and definition of complex structures and anatomy.
Tissue characterization
Myocardial Edema
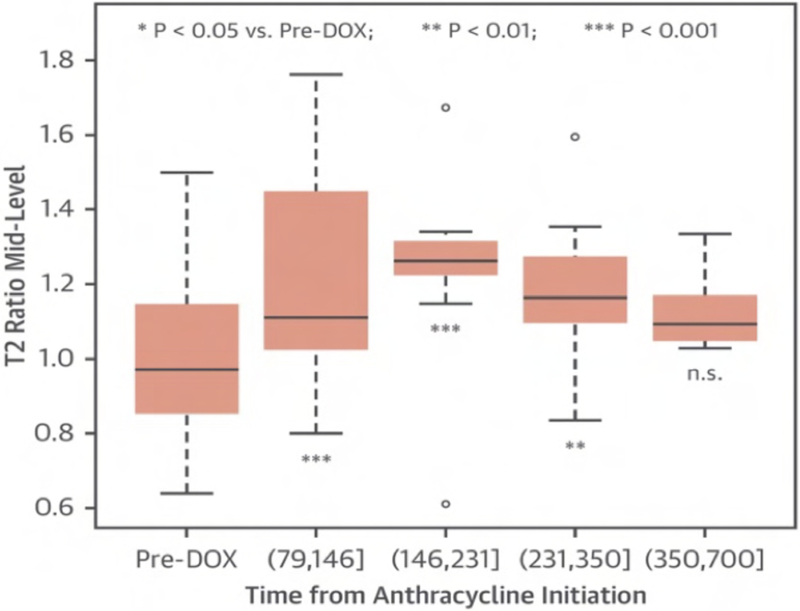
CMR has the advantage to offer information on myocardial tissue remodeling complementary to traditional morphological and functional measurements. Compelling evidence indicates that myocardial edema, inflammation, abnormal strain and expansion of interstitial fibrosis occur before cardiac dysfunction develops. Indeed, experimental(54) and clinical observation(15, 17, 55) have shown that a multiple parametric CMR protocol, incorporating recently developed T1 and T2 mapping techniques, may detect very early signs of myocardial tissue remodeling, improving the current understanding of chemotherapy-induced cardiotoxicity, facilitating future development and implementation of potential preventive treatments. Detection of myocardial edema and inflation, which are mostly based on increased ratio of T2-weighted signal intensity of myocardium normalized to skeletal muscle, has been successfully applied to ischemic(56) and non-ischemic cardiomyopathy(57–59). In a well-designed animal study, comprising baseline and post doxorubicin multiple-parametric CMR examination, Farhad et al. demonstrated that both myocardial edema and expansion of interstitial fibrosis occurred prematurely, having also a significant association with animal mortality rates. Using widely available black-blood T2-weighted sequences Ferreira de Souza and colleagues(17) observed a significant rise in myocardial T2-weighted signal intensity ratio to skeletal early after a moderate cumulative dose of doxorubicin (total dose of 240 mg/m2) (Figure 3 and 4). Also, data suggests that myocardial edema, assessed by myocardial T2-weighted signal intensity normalized to skeletal muscle, has been shown to be significantly associated with decreased RV systolic function, 12 months after anthracycline and/or trastuzumab treatment(47). Various groups are current investigating the role of novel T2 mapping sequences to serially detect myocardial edema after cancer therapy, but up to now T2-weighted imaging has not been broadly studied to establish the usefulness of edema quantification to monitor patients after cancer therapy.
Figure 3:
The T2-weighted signal intensity ratio (myocardium/skeletal muscle) significantly increased early after anthracycline therapy, maintaining significantly higher until the third follow-up quartile compared with baseline (all p < 0.05) (From Ferreira de Souza T, Quinaglia A C Silva T, Osorio Costa F, et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc Imaging. 2018 Aug;11(8):1045–1055; with permission).
Figure 4:
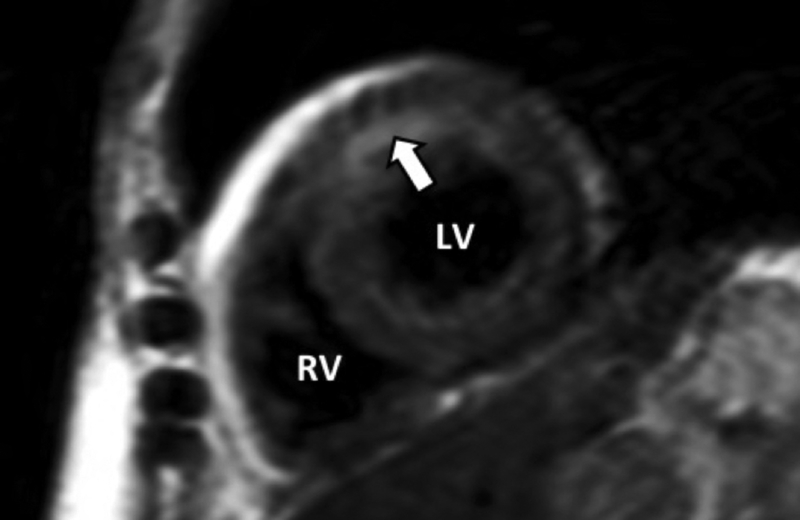
Short-axis T2-weighted imaging exhibiting myocardial edema in anterior wall (white arrow) in patient breast cancer patient treated with anthracycline-based chemotherapy.
Myocardial Fibrosis
While LGE imaging has been show to precisely recognize both myocardial scar and replacement fibrosis(60, 61), this technique may only accomplish partial assessment of the myocardial fibrosis burden(62). Because LGE imaging relies on relative signal intensity differences after gadolinium-based contrast administration, it can fail to identify interstitial myocardial fibrosis. In numerous reports, including retrospective and prospective studies, LGE was not uniformly detected after anthracycline-based chemotherapy(15, 63, 64). Actually a negative LGE imaging study, frequently seem in anthracycline-induce cardiomyopathy (Figure 5), may not represent true absence of fibrosis, highlighting the limitation of LGE imaging in this setting. Although Fallah-Rad et al. showed evidence of subepicardial LGE in all patients who developed trastuzumab-induced cardiac dysfunction(65), several subsequent studies reveled conflicting results(66), with a report from Lawley et al. showing LGE in only 8% of the 25 women treated with trastuzumab(67). In addiction, Neilan and colleagues also observed that LGE is an infrequent finding in patients treated with anthracyclinecardiomyopathy, occurring only in 6% of cases(44).
Figure 5:
46-year-old women with breast cancer with anthracycline-induce cardiomyopathy. 4 and 3 chamber views steady-state free precession in diastole (A, C) and systole (B and D) showing severe LV dysfunction and an absence of scar on LGE images (E, F). RV: right ventricle, LV: left ventricle, RA: right atrium, LA: left atrium, *: breast prosthesis.
Myocardial T1 mapping
Recently developed CMR techniques, based on T1 measurements done before and after administration of usual dose of gadolinium-based contrast agents, allow accurate quantification of the extra cellular volume fraction (ECV), a marker of myocardial interstitial fibrosis. Even before the advent of T1 mapping approaches, Wassmuth et al. (63) using T1-weighted sequences, were able to demonstrate abnormal myocardial accumulation of gadolinium within the myocardium of cancer survivors. Although this approach has several important limitations, this study confirmed that T1 measurements after gadolinium administration might detect subclinical cardiotoxic effects of chemotherapy. Most proposed T1 mapping techniques measure the longitudinal relaxation(68) before and after gadolinium administration, which induces more prominent changes in the areas of the myocardium with increased interstitial fibrosis. Using pre and post contrast T1 measurements, signal intensity versus time curves for the myocardium and the blood pool were used to determine partition coefficient for gadolinium. ECV can then be obtained correcting blood pool measurements for patient’s hematocrit. Both animal(69–71) and clinical (72–76) studies, using slight different approaches and pulse sequences to measure T1 in myocardium and blood pool, have shown excellent agreement between the ECV derived from CMR with fibrosis quantification measured by histology. Numerous studies have examined the usefulness of CMR T1 mapping to investigate myocardial tissue remodeling after cancer therapy. In a pediatric cohort of cancer survivals treated with anthracycline, Tham el al. (77) showed a positive association of ECV measured by CMR with the chemotherapy dose. Interestingly, while the entire cohort has preserved LV EF, the CMR measurements of interstitial fibrosis were also associated with impaired physical capacity measured by cardiopulmonary exercise testing. These unique findings indicate that ECV is directly related to the total anthracycline dose, being also an early marker of cardiotoxicity, which also predict physical impairment(63). Data from another cohort study(16), revealed that patients previously exposed to anthracyclines had significantly higher ECV compared to age-matched controls, which also correlated with LV volume and diastolic function. In an interesting study, Jordan and colleagues(78) investigated a relatively large cohort of cancer patients, including patients not yet treated (n=37) and treated with anthracycline (n=27) or nonanthracycline (n=17) chemotherapy. Native T1 was significantly higher in pre-(1058±7 ms) and post-treatment (1040±7 ms) cancer patients compared to cancer free individuals (965±3 ms; P<0.0001). Authors also demonstrated that ECV was elevated in anthracycline-treated cancer patients (30.4±0.7%) compared to pre-treatment cancer (27.8±0.7%; P<0.01) or controls (26.9±0.2%; P<0.0001). In the same study, using multivariable models, they could confirm that both native T1 and ECV remained elevated in cancer survivors after adjusting to previous risk factors for CV disease. Finally changes in ECV occurred in parallel to reduction in LV systolic function.
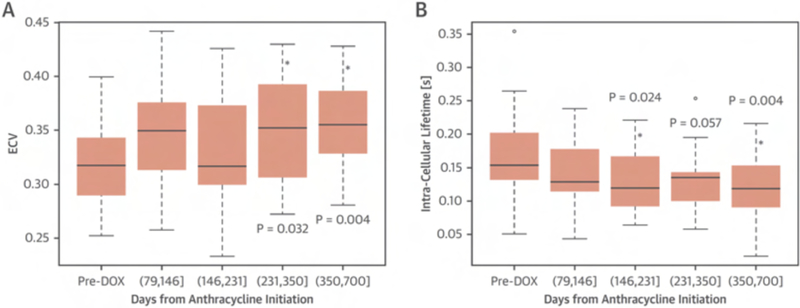
Our group has shown that quantification of the intra-cellular lifetime of water (τic) in the myocardium provides an innovative approach to detect changes in cardiomyocyte size, thereby expanding the capability for myocardial tissue profiling by CMR.(79) Both intra-cellular lifetime of water (τic) and extra-cellular volume (ECV) can be simultaneously measured by CMR T1 mapping over a range of contrast concentrations. Ferreira de Souza et al. (17), using this novel CMR approach, revealed that anthracycline therapy was associated with cardiomyocyte atrophy and early signs of heart disease. In this study, 27 breast cancer patients were studied before and serially after anthracycline (240 mg/m2), including CMR imaging and biomarkers. At [351 to 700] days after anthracycline LV EF decline by 12% to 58±6% (p < 0.001) and LV mass index by 19 g/m2 to 36±6 g/m2 (p < 0.001) and ECV increased by 0.037 to 0.36 ±0.04, p < 0.004), while intra-cellular lifetime of water (τic) decreased by 62 ms to 119± 54 ms (p< 0.004) (Figure 6). One of the main findings of this study is that anthracycline-induced remodeling is associated with a decline in cardiomyocyte size, suggesting that not only interstitial fibrosis and/or myocardial interstitial edema (19) can increase ECV. These findings support further investigations of the role for this novel CMR-based tissue characterization in patients treated with anthracycline-based chemotherapy.
Figure 6:
Extracellular volume (ECV) increased (A) and the intracellular lifetime of water (τic) declined (B) after anthracycline. The p values in A and B are for the fixed effect of follow-up time (in quartiles) versus baseline in linear mixed-effects models for ECV and τic respectively. (From Ferreira de Souza T, Quinaglia A C Silva T, Osorio Costa F, et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc Imaging. 2018 Aug;11(8):1045–1055; with permission).
Left ventricular strain
Strain and strain rate are sensitive and reproducible measurements of systolic changes, with proved prognostic significance. CMR strain methods perform better then echocardiography for applications focused on segmental function(80) with the advantage to also demonstrate tissue abnormalities, such as myocardial fibrosis or edema. Jolly et al. (81) used an automated method in 72 patients undergoing chemotherapy to measure 3-month serial changes in left ventricle mean mid-wall circumferential strain through the analysis of cine white blood images. The results indicated that circumferential strain worsened from −18.8±2.89 at baseline to −17.6±3.08 at 3-month visit, had strong correlation with subclinical declines in LV EF, and do not require extra image acquisition. CMR strain techniques are not widely clinically available, and future studies are needed to assess the prognostic value of CMR strain in cardio-oncology patients. More recently developed CMR strain methods using feature tracking assessing global longitudinal, radial and circumferential strain have been proposed and its feasibility has also been proved in small studies. Additional well-conducted long-term follow-up trials are necessary to further investigate the role of CMR strain imaging in cancer survivals. Since this method can detect early signs of myocardial dysfunction, occurring before LV dysfunction, it may be suitable to identify those at higher risk to develop cardiotoxicity, allowing earlier intervention that can potentially improve clinical outcomes.
Vascular remodeling
Cancer therapy may also impact vasculature and contribute to CV events. Anthracycline can be deleterious to CV system, with direct cardiotoxic effect by suppression of endothelin-1 production, leading to apoptosis(82). While the nature of vascular remodeling remains not entirely understood, some reports have shown that the pulse wave velocity in the aortic arch determined by CMR, which is a measure of aortic stiffness, increased four months after anthracycline therapy(83). In the same study, patients with elevated pulse wave velocity had three times more chance to experience a CV event.
Recommendation in Cardiovascular Imaging
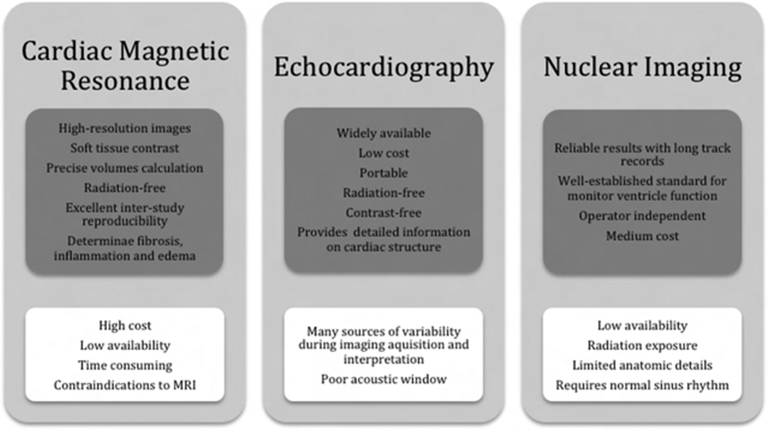
Cardiotoxicity screening and detection includes cardiac imaging and biomarkers, and the choice of modality should consider reproducibility, likelihood to provide additional clinical information, availability throughout the treatment pathway, and a high-quality radiation-free imaging(39). A comparison between the most common used imaging modalities is shown in Figure 7. The ideal imaging modality should identify those at high-risk for future CV events, guide the initiation of protective therapies, and be economic viable. To assist referring physicians make the most appropriate imaging decision and enhance quality of care, a joint report of the American College of Radiology and the American College of Cardiology wrote an evidence-based document determining the appropriate use of CV imaging in patients with heart failure(84), where echocardiography, radionuclide ventriculography and CMR are appropriate in evaluation of patients with malignancy on current or planned cardiotoxic therapy and no prior imaging evaluation.
Figure 7:
Comparison between imaging modalities with advantages in upper gray boxes and disadvantages in bottom white boxes.
A statement from the American Heart Association(85) suggested that echocardiography is likely to be the mainstay of monitoring given its widespread availability, however CMR offers several advantages over it. Evaluation of ventricular mass and function are highly reproducible, even in challenging cases for other modalities, when low image quality and assumptions of cardiac geometry are important limitations. The use of CMR tissue characterization capability can identify edema, inflammation, scarring, perfusion abnormalities, and even diffuse fibrosis, quantification of extracellular volume fraction and cardiomyocyte size, as previously discussed. It also evaluates pericardium, great vessels and characterizes cardiac masses.
Conclusion
Several strengths of CMR imaging, including its ability to accurately assess cardiac morphology, function, scar as well as myocardial tissue remodeling, have been supporting its use to investigate myocardial injury in cancer survivals. Because CMR incorporates several different types of imaging strategies it provides a very comprehensive evaluation of the CV system, which is very helpful in cancer patients. Novel CMR techniques, incorporating T1 and T2 mapping, have the potential not only to improve the current knowledge of cardiotoxicity, but also has the promising capability to detect early markers of LV remodeling which may facilitate developing of prevention and therapeutic interventions. Future clinical studies, investigating both widely availed and novel CMR methods in a broad cancer population are required to further explore its clinical role.
Key Points.
Several strengths of CMR imaging, including its ability to accurately assess cardiac morphology, function, scar as well as myocardial tissue remodeling, have been supporting its use to investigate myocardial injury in cancer survivals.
Because CMR incorporates several different types of imaging strategies it provides a very comprehensive evaluation of the CV system, which is very helpful in cancer patients.
Novel CMR techniques, incorporating T1 and T2 mapping, have the potential not only to improve the current knowledge of cardiotoxicity, but also has the promising capability to detect early markers of LV remodeling which may facilitate developing of prevention and therapeutic interventions.
SYNOPSIS.
Chemotherapy is associated with cardiovascular injury, including the development of a cardiomyopathy and vascular remodeling. Each agent has a mechanistic pathway to cardiovascular injury yielding different strategies to recognize, prevent or minimize cardiotoxicity. Cardiovascular injury may manifest as systolic dysfunction, cardiac ischemia, vascular dysfunction, arrhythmias, pericarditis and electrical repolarization abnormalities. Cardiac magnetic resonance (CMR) is sensitive to detect not only established morphological and functional abnormalities but also early, potentially reversible, signs of myocardial injury. It robustly detects and quantifies myocardial edema, inflammation and focal fibrosis, as well as, interstitial fibrosis, and vascular remodeling. These capabilities support the role of CMR as an excellent tool for evaluating cardiotoxicity. Beyond improving knowledge of mechanisms of cardiotoxicity, novel CMR markers may even enhance patient management by facilitating the early detection of reversible myocardial tissue remodeling before classical morphological and functional changes appear.
Sources of Funding
Dr Coelho-Filho is supported by National Council for Scientific and Technological Development (CNPq) Productivity in Research award grant (303366/2015–0) and travel award grant (453960/2016–2). Dr. Coelho-Filho is also supported by a Young Investigators Grant from The São Paulo Research Foundation (2015/15402–2). Dr. Neilan has the following support: The Kohlberg Foundation, National Institutes of Health/ National Heart, Lung, and Blood Institute (1R01HL130539–01A1; 1R01HL137562 – 01A1) and National Institutes of Health/ Harvard Center for AIDS Research (P30 AI060354).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
REFERENCES
- 1.https://http://www.cancer.gov/about-Cancer/understanding/statistics). U.S. Department of Health and Human Services; 2018. [cited 2018 November, 4]. [DOI] [PubMed]
- 2.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. Journal of the National Cancer Institute. 2007;99(5):365–75. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. Journal of the National Cancer Institute. 2007;99(3):206–14. [DOI] [PubMed] [Google Scholar]
- 4.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. The oncologist. 2007;12(1):20–37. [DOI] [PubMed] [Google Scholar]
- 5.Hong RA, Iimura T, Sumida KN, Eager RM. Cardio-oncology/onco-cardiology. Clin Cardiol. 2010;33(12):733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89(5 Pt 1):942–9. [PubMed] [Google Scholar]
- 7.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. Journal of the American College of Cardiology. 2018;71(16):1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts RG, George M, Johnson WH Jr. Pretreatment and routine echocardiogram monitoring during chemotherapy for anthracycline-induced cardiotoxicity rarely identifies significant cardiac dysfunction or alters treatment decisions: a 5-year review at a single pediatric oncology center. Cancer. 2012;118(7):1919–24. [DOI] [PubMed] [Google Scholar]
- 10.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. Jama. 1991;266(12):1672–7. [PubMed] [Google Scholar]
- 11.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–21. [DOI] [PubMed] [Google Scholar]
- 12.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1201–3. [DOI] [PubMed] [Google Scholar]
- 13.Eidem BW. Identification of anthracycline cardiotoxicity: left ventricular ejection fraction is not enough. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2008;21(12):1290–2. [DOI] [PubMed] [Google Scholar]
- 14.Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovascular imaging. 2008;1(4):413–23. [DOI] [PubMed] [Google Scholar]
- 15.Drafts BC, Twomley KM, D’Agostino R Jr., Lawrence J, Avis N, Ellis LR, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovascular imaging. 2013;6(8):877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira de Souza T, Quinaglia ACST, Osorio Costa F, Shah R, Neilan TG, Velloso L, et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovascular imaging. 2018;11(8):1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander J, Dainiak N, Berger HJ, Goldman L, Johnstone D, Reduto L, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. The New England journal of medicine. 1979;300(6):278–83. [DOI] [PubMed] [Google Scholar]
- 19.Buzdar AU, Marcus C, Smith TL, Blumenschein GR. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55(12):2761–5. [DOI] [PubMed] [Google Scholar]
- 20.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–79. [DOI] [PubMed] [Google Scholar]
- 21.Aiken MJ, Suhag V, Garcia CA, Acio E, Moreau S, Priebat DA, et al. Doxorubicin-induced cardiac toxicity and cardiac rest gated blood pool imaging. Clin Nucl Med. 2009;34(11):762–7. [DOI] [PubMed] [Google Scholar]
- 22.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. [DOI] [PubMed] [Google Scholar]
- 23.Menna P, Paz OG, Chello M, Covino E, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity. Expert Opin Drug Saf. 2012;11 Suppl 1:S21–36. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. [DOI] [PubMed] [Google Scholar]
- 25.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106(1):35–46. [DOI] [PubMed] [Google Scholar]
- 26.Rushton M, Johnson C, Dent S. Trastuzumab-induced cardiotoxicity: testing a clinical risk score in a real-world cardio-oncology population. Curr Oncol. 2017;24(3):176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray TJ. The cardiac effects of mitoxantrone: do the benefits in multiple sclerosis outweigh the risks? Expert Opin Drug Saf. 2006;5(2):265–74. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin RS, Chawla SP, Ewer MS, Carrasco CH, Mackay B, Holmes F. Evaluation of mitoxantrone cardiac toxicity by nuclear angiography and endomyocardial biopsy: an update. Invest New Drugs. 1985;3(2):117–21. [DOI] [PubMed] [Google Scholar]
- 29.Shaikh AY, Suryadevara S, Tripathi A, Ahmed M, Kane JL, Escobar J, et al. Mitoxantrone-Induced Cardiotoxicity in Acute Myeloid Leukemia-A Velocity Vector Imaging Analysis. Echocardiography. 2016;33(8):1166–77. [DOI] [PubMed] [Google Scholar]
- 30.Ghalie RG, Edan G, Laurent M, Mauch E, Eisenman S, Hartung HP, et al. Cardiac adverse effects associated with mitoxantrone (Novantrone) therapy in patients with MS. Neurology. 2002;59(6):909–13. [DOI] [PubMed] [Google Scholar]
- 31.Jonasch E, Signorovitch JE, Lin PL, Liu Z, Culver K, Pal SK, et al. Treatment patterns in metastatic renal cell carcinoma: a retrospective review of medical records from US community oncology practices. Curr Med Res Opin. 2014;30(10):2041–50. [DOI] [PubMed] [Google Scholar]
- 32.Rainer PP, Doleschal B, Kirk JA, Sivakumaran V, Saad Z, Groschner K, et al. Sunitinib causes dose-dependent negative functional effects on myocardium and cardiomyocytes. BJU Int. 2012;110(10):1455–62. [DOI] [PubMed] [Google Scholar]
- 33.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44. [DOI] [PubMed] [Google Scholar]
- 34.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(32):5204–12. [DOI] [PubMed] [Google Scholar]
- 35.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76(3):348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarneri V, Lenihan DJ, Valero V, Durand JB, Broglio K, Hess KR, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(25):4107–15. [DOI] [PubMed] [Google Scholar]
- 38.Goenka AH, Flamm SD. Cardiac magnetic resonance imaging for the investigation of cardiovascular disorders. Part 1: current applications. Tex Heart Inst J. 2014;41(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801. [DOI] [PubMed] [Google Scholar]
- 40.Armenian SH, Lacchetti C, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. Journal of oncology practice. 2017;13(4):270–5. [DOI] [PubMed] [Google Scholar]
- 41.Walsh TF, Hundley WG. Assessment of ventricular function with cardiovascular magnetic resonance. Cardiology clinics. 2007;25(1):15–33, v. [DOI] [PubMed] [Google Scholar]
- 42.Sarwar A, Shapiro MD, Abbara S, Cury RC. Cardiac magnetic resonance imaging for the evaluation of ventricular function. Seminars in roentgenology. 2008;43(3):183–92. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110(11):1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan JH, Castellino SM, Melendez GC, Klepin HD, Ellis LR, Lamar Z, et al. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circulation Heart failure. 2018;11(7):e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottin Y, Touzery C, Coudert B, Richebourg S, Cohen M, Toubeau M, et al. Diastolic or systolic left and right ventricular impairment at moderate doses of anthracycline? A 1-year follow-up study of women. European journal of nuclear medicine. 1996;23(5):511–6. [DOI] [PubMed] [Google Scholar]
- 47.Grover S, Leong DP, Chakrabarty A, Joerg L, Kotasek D, Cheong K, et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. 2013;168(6):5465–7. [DOI] [PubMed] [Google Scholar]
- 48.Calleja A, Poulin F, Khorolsky C, Shariat M, Bedard PL, Amir E, et al. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J Oncol. 2015;2015:609194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19(7):873–9. [DOI] [PubMed] [Google Scholar]
- 50.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. American heart journal. 2004;147(2):218–23. [DOI] [PubMed] [Google Scholar]
- 51.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. Journal of magnetic resonance imaging : JMRI. 2008;28(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, et al. Right Ventricular Volumes and Systolic Function by Cardiac Magnetic Resonance and the Impact of Sex, Age, and Obesity in a Longitudinally Followed Cohort Free of Pulmonary and Cardiovascular Disease: The Framingham Heart Study. Circulation Cardiovascular imaging. 2016;9(3):e003810. [DOI] [PubMed] [Google Scholar]
- 53.Crean AM, Maredia N, Ballard G, Menezes R, Wharton G, Forster J, et al. 3D Echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated With Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circulation Cardiovascular imaging. 2016;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melendez GC, Jordan JH, D’Agostino RB Jr., Vasu S, Hamilton CA, Hundley WG. Progressive 3-Month Increase in LV Myocardial ECV After Anthracycline-Based Chemotherapy. JACC Cardiovascular imaging. 2017;10(6):708–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109(20):2411–6. [DOI] [PubMed] [Google Scholar]
- 57.Torreao JA, Ianni BM, Mady C, Naia E, Rassi CH, Nomura C, et al. Myocardial tissue characterization in Chagas’ heart disease by cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2015;17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zagrosek A, Abdel-Aty H, Boye P, Wassmuth R, Messroghli D, Utz W, et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovascular imaging. 2009;2(2):131–8. [DOI] [PubMed] [Google Scholar]
- 59.Carbone I, Childs H, Aljizeeri A, Merchant N, Friedrich MG. Importance of Reference Muscle Selection in Quantitative Signal Intensity Analysis of T2-Weighted Images of Myocardial Edema Using a T2 Ratio Method. BioMed research international. 2015;2015:232649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. [DOI] [PubMed] [Google Scholar]
- 61.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48(10):1977–85. [DOI] [PubMed] [Google Scholar]
- 62.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2011;57(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141(6):1007–13. [DOI] [PubMed] [Google Scholar]
- 64.Ylanen K, Poutanen T, Savikurki-Heikkila P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. Journal of the American College of Cardiology. 2013;61(14):1539–47. [DOI] [PubMed] [Google Scholar]
- 65.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology. 2011;57(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 66.Wassmuth R, Schulz-Menger J. Late gadolinium enhancement in left ventricular dysfunction after trastuzumab. Journal of the American College of Cardiology. 2011;58(25):2697–8; author reply 9–700. [DOI] [PubMed] [Google Scholar]
- 67.Lawley C, Wainwright C, Segelov E, Lynch J, Beith J, McCrohon J. Pilot study evaluating the role of cardiac magnetic resonance imaging in monitoring adjuvant trastuzumab therapy for breast cancer. Asia Pac J Clin Oncol. 2012;8(1):95–100. [DOI] [PubMed] [Google Scholar]
- 68.Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coelho-Filho OR, Mongeon FP, Mitchell R, Moreno H Jr., Nadruz W Jr., Kwong R, et al. Role of transcytolemmal water-exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circulation Cardiovascular imaging. 2013;6(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coelho-Filho OR, Shah RV, Neilan TG, Mitchell R, Moreno H Jr., Kwong R, et al. Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. Journal of the American Heart Association. 2014;3(3):e000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, et al. Assessment of diffuse myocardial fibrosis in rats using small-animal Look-Locker inversion recovery T1 mapping. Circulation Cardiovascular imaging. 2011;4(6):636–40. [DOI] [PubMed] [Google Scholar]
- 72.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. American journal of physiology Heart and circulatory physiology. 2008;295(3):H1234–H42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138–44. [DOI] [PubMed] [Google Scholar]
- 75.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. Journal of the American College of Cardiology. 2008;52(19):1574–80. [DOI] [PubMed] [Google Scholar]
- 76.Vita T, Grani C, Abbasi SA, Neilan TG, Rowin E, Kaneko K, et al. Comparing CMR Mapping Methods and Myocardial Patterns Toward Heart Failure Outcomes in Nonischemic Dilated Cardiomyopathy. JACC Cardiovascular imaging. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jordan JH, Vasu S, Morgan TM, D’Agostino RB Jr., Melendez GC, Hamilton CA, et al. Anthracycline-Associated T1 Mapping Characteristics Are Elevated Independent of the Presence of Cardiovascular Comorbidities in Cancer Survivors. Circulation Cardiovascular imaging. 2016;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H Jr., Simonson B, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation. 2013;128(11):1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amzulescu MS, Langet H, Saloux E, Manrique A, Boileau L, Slimani A, et al. Head-to-Head Comparison of Global and Regional Two-Dimensional Speckle Tracking Strain Versus Cardiac Magnetic Resonance Tagging in a Multicenter Validation Study. Circulation Cardiovascular imaging. 2017;10(11). [DOI] [PubMed] [Google Scholar]
- 81.Jolly MP, Jordan JH, Melendez GC, McNeal GR, D’Agostino RB Jr., Hundley WG. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2017;19(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keltai K, Cervenak L, Mako V, Doleschall Z, Zsary A, Karadi I. Doxorubicin selectively suppresses mRNA expression and production of endothelin-1 in endothelial cells. Vascul Pharmacol. 2010;53(5–6):209–14. [DOI] [PubMed] [Google Scholar]
- 83.Chaosuwannakit N, D’Agostino R Jr., Hamilton CA, Lane KS, Ntim WO, Lawrence J, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(1):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel MR, White RD, Abbara S, Bluemke DA, Herfkens RJ, Picard M, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. Journal of the American College of Cardiology. 2013;61(21):2207–31. [DOI] [PubMed] [Google Scholar]
- 85.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–95. [DOI] [PubMed] [Google Scholar]