Abstract
Objective
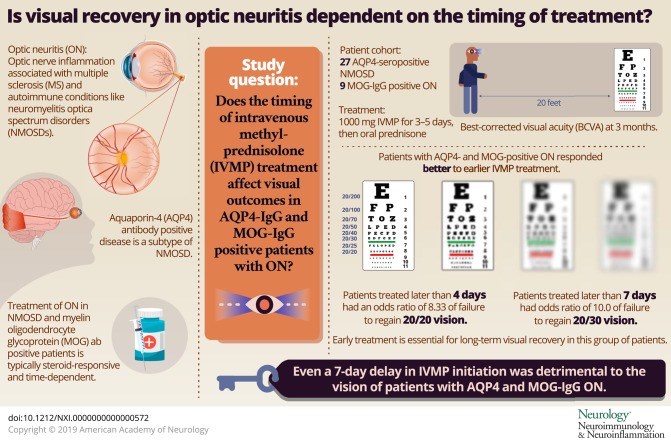
To investigate whether visual disability which is known to accumulate by poor recovery from optic neuritis (ON) attacks can be lessened by early treatment, we investigated whether the time from symptom onset to high-dose IV methylprednisolone (IVMP) affected visual recovery.
Methods
A retrospective study was performed in a consecutive cohort of patients following their first aquaporin-4 (AQP4)-IgG or myelin oligodendrocyte glycoprotein (MOG)-IgG-ON. Best-corrected visual acuity (BCVA) in ON eyes at 3 months (BCVA3mo) was correlated with time to IVMP (days). In cases of bilateral ON, 1 eye was randomly selected.
Results
A total of 29 of 37 patients had ON (27 AQP4-seropositive neuromyelitis optica spectrum disorder [NMOSD] and 9 MOG-IgG-ON), 2 of whom refused treatment. Of the 27 patients included, 10 presented later than 7 days from onset. The median BCVA3mo of patients treated >7 days was 20/100 (interquartile range 20/100–20/200). Patients treated >7 days had an OR of 5.50 (95% CI 0.88–34.46, p = 0.051) of failure to regain 0.0 logMAR vision (20/20) and an OR of 10.0 (95% CI 1.39–71.9) of failure to regain 0.2 logMAR vision (20/30) (p = 0.01) compared with patients treated within 7 days. ROC analysis revealed that the optimal criterion of delay in IVMP initiation was ≤4 days, with a sensitivity and specificity of 71.4% and 76.9%, respectively.
Conclusions
In this retrospective study of ON with AQP4 and MOG-IgG, even a 7-day delay in IVMP initiation was detrimental to vision. These results highlight the importance of early treatment for the long-term visual recovery in this group of patients. A prospective, multicenter study of the effects of timing of IVMP is currently underway.
Classification of evidence
This study provides Class IV evidence that hyperacute treatment of AQP4 and MOG-ON with IVMP increases the chance for good visual recovery (20/20 vision) and that even a greater than 7-day delay in treatment is associated with a higher risk for poor visual recovery.
Optic neuritis (ON) is a common inflammation of the optic nerve associated with numerous autoimmune conditions, including MS, neuromyelitis optica spectrum disorders (NMOSDs), chronic relapsing inflammatory optic neuritis (CRION), and autoimmune optic neuritis (AON).1–5 NMOSD is further subdivided into aquaporin-4 (AQP4) antibody–positive disease and a seronegative form.6 A subset of patients with ON have serum IgG autoantibodies to myelin oligodendrocyte glycoprotein (MOG).7–11 The protein and cellular targets of these 2 antibodies are distinct in that AQP4 is expressed on astrocytes and retinal Müller cells, whereas MOG is expressed by oligodendrocytes.12,13 Despite these pathogenic differences, ON attacks in both conditions are treated similarly with high-dose corticosteroids and/or plasma exchange (PE). Although some patients with MOG ab disease meet the 2015 criteria for NMOSD, there is an ongoing debate as to whether MOG ab-positive patients should receive a diagnosis of NMOSD.14 Although a significant number of MOG ab-positive patients have a relapsing course leading to accumulative disability, others do not relapse; thus, their inclusion together with other AQP4-seronegative patients with NMOSD could compromise the study of therapeutic candidates in NMOSD.14
Acute treatment of ON in MS was shaped by the North American Optic Neuritis Treatment Trial (ONTT), which showed that IV methylprednisolone (IVMP) accelerates recovery but does not affect the final visual outcome.15,16
However, the clinical course of ON in NMOSD and in MOG ab-positive patients differs from MS and is typically steroid responsive or dependent. Disability from both AQP4 and MOG-ON is accumulated by poor recovery from attacks.17 The recommended acute treatment options in antibody-mediated ON are high-dose IVMP, PE, and immunoadsorption.18,19
Historically, NMOSD-ON has been associated with a poor visual outcome.20 Studies have correlated the visual outcome of AQP4-ON attacks with the severity of visual loss at presentation, type of antibody, and with the use of additional PE.21,22 Visual disability has been shown to be accrued with each attack, resulting in poor quality of life.13 Three previous studies focused on the effect of timing of IVMP on visual outcome.23–25 These studies included several subtypes of ON, with only a few patients with NMOSD and no MOG-positive patients.
In this study, we tested the hypothesis that timing of IVMP affects visual outcome in a cohort of AQP4-IgG and MOG-IgG–positive patients with ON by analyzing the effect of the number of days until treatment commenced with the best-corrected visual acuity (BCVA) at 3 months.
Methods
Patients
We conducted a retrospective case review of a cohort of all consecutive patients presenting to a tertiary referral neuro-ophthalmology and neuroimmunology center at Rabin Medical Center, Israel, with a first event of AQP4 or MOG-ON between January 2005 and June 2018.
Standard protocol approvals, registrations, and patient consents
The study was performed following IRB approval in accordance with the World Medical Association Declaration of Helsinki. The neuro-ophthalmology unit database was searched for the diagnoses of NMOSD, AQP4, and MOG-associated ON.
Inclusion and exclusion criteria
ON was diagnosed based on a combination of clinical history, objective findings as determined by clinical examination of a neuro-ophthalmologist, and paraclinical tests. These included patients presenting with subacute onset vision loss, pain with eye movement, visual field defects consistent with an optic nerve injury, color defects, MRI evidence of optic nerve inflammation (increased T2 signal, gadolinium enhancement, and optic nerve swelling),26 and neurophysiologic abnormalities (delayed visual evoked potential latencies).27 Exclusion criteria were other ocular causes of poor visual acuity (VA) and treatment refusal. This retrospective cohort study focused on VA as a functional outcome and did not examine other functional parameters such as visual field or structural-anatomic outcome measures such as optical coherence tomography (OCT) outcomes because for some patients, these were either missing (3 patients) or performed by different machines (2 patients).
Patients had to have a diagnosis of NMOSD, AQP4, or MOG-associated ON based on established diagnostic criteria.6,28 AQP4 antibodies were tested using a commercial cell-based kit (EUROIMMUN, Lübeck, Germany). In addition, AQP4 IgG antibodies were tested at the Center for Autoimmune Neurology in Barcelona, Spain, using tissue immunohistochemistry and cell-based assays.28,29 MOG-IgG antibodies were tested by cell-based assays at the Center for Autoimmune Neurology in Barcelona, Spain.30
Treatment
The treatment received was IVMP at a daily dose of 1,000 mg for 3–5 days, followed by oral prednisone (starting at 1 mg/kg/d). At the time of presentation, antibody status was not known for the majority of patients, but oral prednisone treatment was prolonged in patients with relapse of visual loss following steroid cessation or in patients presenting with clinical or paraclinical findings suggestive of AQP4 or MOG antibody disease. Patients who refused acute treatment with IVMP for ON were excluded from this study (figure e-1, flowchart, links.lww.com/NXI/A116).
Clinical assessment and medical notes
Medical notes had to include a detailed report of the timing of patient-reported onset of visual loss, timing of IVMP treatment, and documentation of high-contrast BCVA examination in each eye at 3 months following the attack.
Main outcome measures
The main outcome measure of this study was 3-month BCVA. Secondary outcomes were failure to regain 0.0 logMAR (20/20) and 0.2 logMAR vision (20/30) vision at the 3-month follow-up visit.
Level of evidence
This is a level IV retrospective cohort study comparing the BCVA at 3 months of patients with AQP4 and MOG-ON presenting early for IVMP treatment vs those patients presenting late.
Statistical analysis
Descriptive statistics were calculated using SAS software (v9.4). Median logMAR BCVA at 3 months (“BCVA3mo”) and interquartile ranges were documented at 3 months. Patients were grouped according to BCVA3mo into those achieving 0.0 logMAR (20/20) vision and those whose BCVA3mo was worse than 0.0 logMAR. Outcome was correlated with time (in days) from symptom onset to IVMP (“time to IVMP”). For patients with bilateral ON, 1 eye was randomly included in the analysis. A receiver operator curve (ROC) was used to analyze the best sensitivity and specificity using the Youden index31 for the best cutoff time to IVMP to achieve the best BCVA3mo. The relative risk, OR, and confidence intervals for BCVA3mo worse than 0.0 logMAR (20/20) and 0.2 logMAR vision (20/30) were analyzed for patients treated early (time to IVMP ≤ 6 days) compared with patients treated after day 7. Two-tailed tests were used, and p < 0.05 was accepted as statistically significant.
Data availability
Data have been uploaded and will be made readily available upon publication at the following Mendeley data repository: dx.doi.org/10.17632/ht5s9cc845.1.
Results
Thirty-seven patients were enrolled. Twenty-eight patients fulfilled the 2015 diagnostic criteria6 for NMOSD (27 were AQP4 positive, and 1 patient was seronegative), and another 9 had MOG-IgG positive ON. Included in this study were 27 AQP4-positive and 9 MOG-positive patients with ON. Figure e-1 (links.lww.com/NXI/A116) depicts the flowchart of the patient files reviewed (n = 37) and those included in final analysis (n = 27). The mean age at presentation was 36.6 ± 13.7 (range 8.3–68.1) years, and 85.2% (n = 23) were female. The mean age at presentation for patients with MOG-ON was 41.8 ± 11.1 (range 26.2–55.6) years, and 78% (n = 7) were female. BCVA at nadir revealed no trend toward worst BCVA nadir in the delayed treatment group (4 days as cutoff), with a mean of 1.55 ± 0.74 for those treated >4 days and 0.99 ± 0.85 for those treated ≤4 days, p = 0.085. It is interesting to note that this trend leveled off to no difference in BCVA nadir when comparing those treated <7 days and those treated ≥7 days (BCVA nadir 1.17 ± 0.83 in those treated <7 days and 1.47 ± 0.84 in those treated later, p = 0.41). Patients were treated with IVMP on the same day they presented with ON. The median time to IVMP was 4 days for the whole cohort (range 1–65 days). Of those treated ≥7 days, the median time to IVMP was 21 days (range 9–65 days). The median time to IVMP for those treated earlier than <7 days was 3 days and 2 days for those treated within 4 days. Baseline demographic and clinical factors were similar in both the early treatment group (<4 day treatment group) and those treated >4 days (percentage of MOG positive p = 0.59, male p = 0.94, age p = 0.48, additional use of plasmapheresis p = 1).
Three-month VA
There was a significant inverse correlation between BCVA3mo (logMAR) and age (r = −0.41, p = 0.04) and days to IVMP treatment (r = 0.43, p = 0.03), with a nearly significant correlation between BCVA3mo and logMAR VA at nadir (r = 0.38, p = 0.06). The distribution of BCVA3mo is depicted in figure 1.
Figure 1. Distribution of BCVA3mo for patients treated with IVMP for AQP4 and MOG-ON.
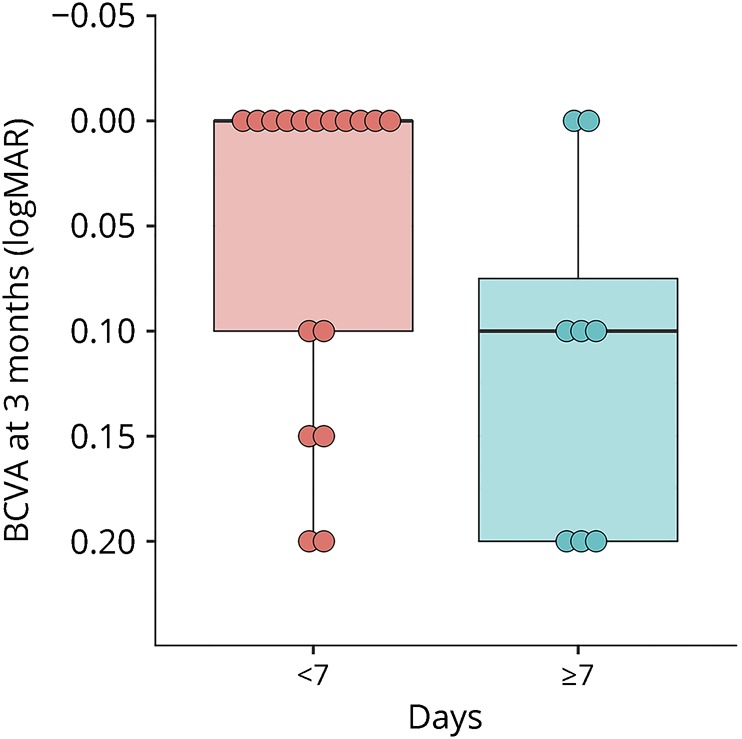
Note inverted logMAR scale: better acuity at top. Left boxplot: eyes of patients treated <7 days. Right boxplot: Eyes of patients treated ≥7 days. BCVA3mo = best-corrected visual acuity at 3 months after IVMP treatment for AQP4 and MOG-IgG-ON. Box plot details: thick horizontal bar: median; box: interquartile range (25%–75%). Dots: outliers. AQP = aquaporin; BCVA = best-corrected visual acuity; IVMP = IV methylprednisolone; MOG = myelin oligodendrocyte glycoprotein; ON = optic neuritis.
The BCVA3mo was similar between men and women (0.33 ± 0.52 vs 0.17 ± 0.47, p = 0.61) and similar between AQP4-positive and MOG-positive patients (0.11 ± 0.09 vs 0.22 ± 0.56, p = 0.38). Using multivariate analysis, with type of antibody (AQP4 vs MOG), age, days to IVMP treatment, logMAR VA at nadir, and plasmapheresis treatment as the independent variables, the 2 factors that remained significant in predicting BCVA3mo were days to IVMP treatment (r2 = 15.5%, p = 0.03) and age (r2 = 16.5%, p = 0.04).
Failure to regain 0.0 logMAR (20/20) vision
An ROC analysis was performed with days to IVMP treatment as the predictor and failure to regain 0.0 logMAR (20/20) vision as the dependent variable. An area under the curve (AUC) of 0.71 was achieved (figure 2), and with a Youden optimal criterion of days to treatment >4 days, a sensitivity and specificity of 71.4% and 76.9%, respectively, were achieved. Patients who were treated later than 4 days had an OR of 8.33 (95% CI 1.47–47.22) of failure to regain 0.0 logMAR vision (p = 0.01). The individual AUCs of age and nadir BCVA as individual predictors of failure to regain 0.0 logMAR vision at 3 months were lower (0.56 and 0.60, respectively), and the addition of these 2 predictors to days to IVMP treatment led to a minute improvement in the AUC (0.74) compared with days to treatment alone (0.71).
Figure 2. A receiver operating characteristic curve of days to IVMP as a predictor of failure to regain 0.0 logMAR (20/20) vision (AUC 0.71, p < 0.001).
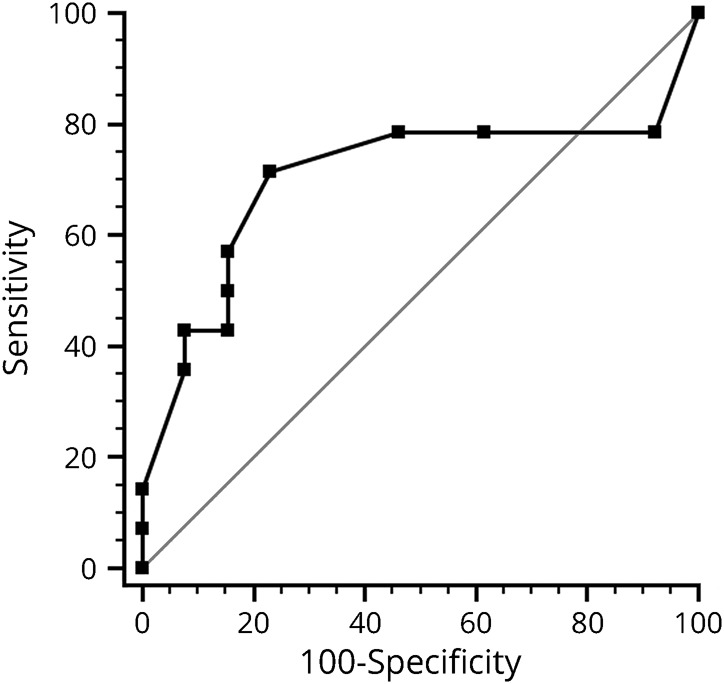
AUC = area under the curve; IVMP = IV methylprednisolone.
Failure to regain 0.2 logMAR vision (∼Snellen 20/30)
A similar analysis with failure to regain 0.2 logMAR as the dependent variable revealed a Youden optimal criterion of days to treatment >7 days with an AUC of 0.84 (figure 3), sensitivity of 71.4%, and specificity of 80.0%. Patients treated later than 7 days had an OR of 10.0 (95% CI 1.39–71.86) of failure to regain 20/30 vision (p = 0.01). The individual AUCs of age and nadir BCVA as individual predictors of failure to regain 0.2 logMAR vision at 3 months were lower (0.59 and 0.63, respectively), and the addition of these 2 predictors to days to IVMP treatment led to a reduced AUC (0.80) compared with days to treatment alone (0.84).
Figure 3. A receiver operating characteristic curve of days to IVMP as a predictor of failure to regain 0.2 logMAR (20/30) vision (AUC 0.84, p < 0.001).
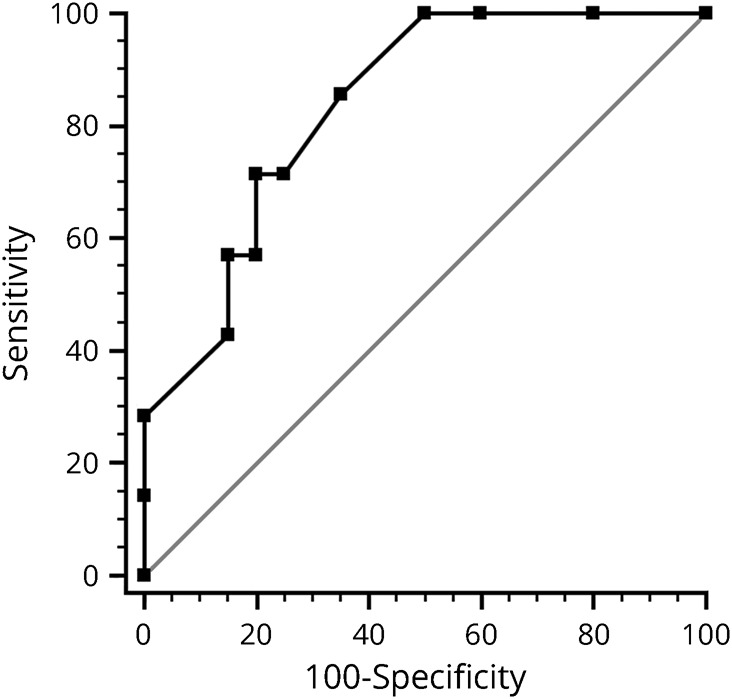
AUC = area under the curve; IVMP = IV methylprednisolone.
Discussion
In our cohort, patients with AQP4- and MOG-positive ON responded better to earlier IVMP. Of 27 patients with AQP4 or MOG-ON (18 AQP4-IgG+ and 9 MOG-IgG+), and those treated later than 4 days had an OR of 8.33 of failure to regain 20/20 0.0 logMAR vision (p = 0.01). Patients treated later than 7 days had an OR of 10.0 of failure to regain 20/30 0.2 logMAR vision (p = 0.01). This finding corroborates a study in patients with acute ON, demonstrating that retinal ganglion cell (RGC) layer loss starts within a few days of ON and may be a predictor of visual loss.32
ON in patients with AQP4-IgG and MOG-IgG antibodies is frequently steroid responsive or dependent, thus differing from MS-ON, in which IVMP does not affect visual outcome.1,33 We tested our hypothesis that timing of acute treatment affects visual outcome in AMDD-ON. Despite the small number of patients enrolled and investigated, we were able to construct ROC curves to identify cut points that optimize the balance between sensitivity and specificity in regard to the optimal time window for the administration of IVMP that would also translate into greater improvement of the BCVA at 3 months. Administration of IVMP treatment at day 4 or earlier was the identified cut point (71.4% sensitive; 76.9% specific). Two additional variables affecting visual outcome to a lesser degree were age and VA at nadir.
Offering a better visual outcome for AQP4-seropositive and MOG-seropositive patients with ON implies the need for action in all forms of ON because at presentation, the etiology is often unclear. In MS-related ON, the ONTT suggested that final visual outcome is not affected by acute treatment with IVMP,15,16 leading to a sense of nonurgency in the acute phase of ON. A change of treatment paradigm, especially in acceleration of IVMP timing in acute ON treatment, may be needed. For a significant number of patients who harbor AQP4 ab or MOG ab at presentation, it may be crucial to start IVMP treatment for ON as soon as possible.
Perhaps most salient about this submission was the recognition that as little as a 7-day delay in treatment inception (for NMOSD and anti–MOG-associated optic neuritides) was found to be detrimental in terms of the OR for improving BCVA at 3 months after symptom onset. “Time is Tissue” is a core principal that is evolving in the field of neuroimmunology,34 making it imperative to potentially view an antibody-mediated ON with a comparable sense of urgency in terms of diagnosis and treatment akin to that of heart attack and stroke. Our findings are in good alignment with the findings by Soelberg et al.,32 who reported that in ON, the majority of which were not antibody mediated, progressive ganglion cell layer loss at a rate of 0.2 μm/d can be observed as early as 8 days after onset.
Corroborating the contention of Time is Tissue has been the recognition of inflammation as a fundamental antecedent of the cardinal hallmark of irreversible disability in those with inflammatory syndromes of the CNS; that being axonal transection, the evolution of dying back and Wallerian degeneration. Among the most striking observations of this proposed model of sequential steps in the pathobiology of postinflammatory neurodegeneration has been the degeneration of RGCs within a time epoch as short as 2 days of the onset of clinical symptoms ultimately designated as a derivative of such inflammation.
The results of our study strengthen 3 previous reports in other forms of ON23–25 demonstrating a beneficial effect of hyperacute IVMP. These studies23–25 did not focus on AQP4 and MOG-ON; Osinga et al.23 described a cohort of 19 patients with recurrent ON, 9 of whom with relapsing isolated ON, 4 with MS-ON, 4 with chronic relapsing inflammatory optic neuropathy, and 2 with NMOSD-ON. These 19 patients were analyzed for the effects of treatment within 2 days (hyperacute treatment). The importance of hyperacute steroids in ON treatment has experimental logic in animal models. In mice with experimental autoimmune encephalomyelitis, the inflammatory process precedes axonal degeneration by 2 days.35 A goal of treatment within 2 days of symptom onset is difficult to achieve in clinical reality. Another study by Zhu et al.36 showed that irreversible axonal damage starts between days 5 and 7, supporting our clinical finding that optimal treatment is by day 4, but that treatment before day 7 still offers an opportunity for very good visual outcome.
Previous MRI and OCT studies have demonstrated that the bulk of axonal loss and neuronal damage is sustained early in the disease course for patients with MS.37,38 Although the rates of ganglion cell–inner plexiform layer atrophy may be influenced by disease-modifying therapies in patients with MS, further studies, using the detailed structural OCT tools currently at our disposal, should re-examine the effect of timing of IVMP on visual outcome in other forms of ON.
Few clinical studies on outcome of NMOSD-ON include details of accurate timing from symptom onset to acute treatment, and there is much need for this detail to be analyzed in larger cohorts. The results of this study show a trend indicating that even a 7-day delay in IVMP can be detrimental to vision in AQP4 and MOG-IgG ON. Several limitations should be taken into consideration when considering these results, including the study's retrospective design, the small sample size resulting in very large confidence intervals, the short follow-up duration, and the lack of paraclinical data to confirm the functional results with structural indices such as loss of retinal nerve fiber and ganglion cell layers on OCT.
A prospective study in a larger cohort of patients with NMOSD examining the effects of timing of IVMP on additional visual parameters such as OCT, visual fields BCVA, and on subsequent ON attacks seems warranted.
Glossary
- AON
autoimmune optic neuritis
- AQP
aquaporin
- AUC
area under the curve
- BCVA
best-corrected visual acuity
- IA
immunoadsorption
- IVMP
IV methylprednisolone
- MOG
myelin oligodendrocyte glycoprotein
- OCT
optical coherence tomography
- ON
optic neuritis
- ONTT
Optic Neuritis Treatment Trial
- PE
plasma exchange
- RGC
retinal ganglion cell
- VA
visual acuity
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
No targeted funding reported.
Disclosure
H. Kalish received research support from the Maratier Foundation, Tel Aviv University, and Israeli Car Accident Prevention Association. M.A. Hellmann and M. Mimouni report no disclosures. F. Paul served on the scientific advisory boards of Novartis and MedImmune; received speaker honoraria and travel funding from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; serves as academic editor of PLoS ONE and associate editor of Neurology: Neuroimmunology & Neuroinflammation; consulted for Sanofi-Genzyme, Biogen, MedImmune, Shire, and Alexion; and received research support from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Guthy Jackson Charitable Foundation, and NMSS. O. Bialer and M. Bach report no disclosures. L. Lotan received travel funding from Teva, Merck Serono, Biogen, and Sanofi-Genzyme. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med 2006;354:1273–1280. [DOI] [PubMed] [Google Scholar]
- 2.Galetta SL, Villoslada P, Levin N, et al. . Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015;2:e135 doi:10.1212/NXI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soelberg K, Jarius S, Skejoe HPB, et al. . A population-based prospective study of optic neuritis. Mult Scler J 2017;23:1893–1901. [DOI] [PubMed] [Google Scholar]
- 4.Petzold A, Wattjes MP, Costello F, et al. . The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 2014;10:447–458. [DOI] [PubMed] [Google Scholar]
- 5.Atkins EJ. Optic neuritis. Encycl Neurol Sci 2014;13:681–686. [Google Scholar]
- 6.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarius S, Kleiter I, Ruprecht K, et al. . MOG-IgG in NMO and related disorders: a multicenter study. Part 3: mOG-IgG-associated brainstem encephalitis. Mult Scler 2016; Conference:410-411. Available at: cochranelibrary.com/central/doi/10.1002/central/CN-01212258/full. [Google Scholar]
- 8.Biotti D, Bonneville F, Tournaire E, et al. . Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J Neurol 2017;264:2173–2175. [DOI] [PubMed] [Google Scholar]
- 9.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016;15:307–324. [DOI] [PubMed] [Google Scholar]
- 10.Akaishi T, Sato DK, Takahashi T, Nakashima I. Clinical spectrum of inflammatory central nervous system demyelinating disorders associated with antibodies against myelin oligodendrocyte glycoprotein. Neurochem Int Epub 2018 Oct 23. [DOI] [PubMed]
- 11.Chalmoukou K, Alexopoulos H, Akrivou S, Stathopoulos P, Reindl M, Dalakas MC. Clinical/scientific notes. Neurol Neuroimmunol Neuroinflamm 2015;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol 2018;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt F, Zimmermann H, Mikolajczak J, et al. . Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2017;11:45–50. [DOI] [PubMed] [Google Scholar]
- 14.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015;2:e62 doi:10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW. The optic neuritis treatment trial: three-year follow-up results. Arch Ophthalmol 1995;113:136–137. [DOI] [PubMed] [Google Scholar]
- 16.Kupersmith MJ, Anderson S, Kardon R. Predictive value of 1 month retinal nerve fiber layer thinning for deficits at 6 months after acute optic neuritis. Mult Scler J 2013;19:1743–1748. [DOI] [PubMed] [Google Scholar]
- 17.Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci 2016;1366:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato D, Callegaro D, Lana-Peixoto MA, Fujihara K; Brazilian Committee for Treatment and Research in Multiple Sclerosis. Treatment of neuromyelitis optica: an evidence based review. Arq Neuropsiquiatr 2012;70:59–66. [DOI] [PubMed] [Google Scholar]
- 19.Kleiter I, Gahlen A, Borisow N, et al. . Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions criteria for rating therapeutic and diagnostic studies. Neurol Neuroimmunol Neuroinflamm 2018;5:e504 doi:10.1212/NXI.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107. [DOI] [PubMed] [Google Scholar]
- 21.Bonnan M, Valentino R, Debeugny S, et al. . Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 2018;89:346–351. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Kurimoto T, Ueda K, Nakamura M. Short-term effect of additional apheresis on visual acuity changes in patients with steroid-resistant optic neuritis in neuromyelitis optica spectrum disorders. Jpn J Ophthalmol 2018;62:525–530. [DOI] [PubMed] [Google Scholar]
- 23.Osinga E, van Oosten B, de Vries-Knoppert W, Petzold A. Time is vision in recurrent optic neuritis. Brain Res 2017;1673:95–101. [DOI] [PubMed] [Google Scholar]
- 24.Plant GT, Sibtain NA, Thomas D. Hyperacute corticosteroid treatment of optic neuritis at the onset of pain may prevent visual loss: a case series. Mult Scler Int 2011;2011:815068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura M, Nakazawa T, Doi H, et al. . Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefe's Arch Clin Exp Ophthalmol 2010;248:1777–1785. [DOI] [PubMed] [Google Scholar]
- 26.Filippi M, Rocca MA, Ciccarelli O, et al. . MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balcer LJ, Miller DH, Reingold SC, Cohen JA. Vision and vision-related outcome measures in multiple sclerosis. Brain 2015;138(pt 1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarius S, Paul F, Aktas O, et al. . MOG encephalomyelitis: international recommendations on diagnosis and antibody testing [in German]. Nervenarzt 2018;89:1388–1399. [DOI] [PubMed] [Google Scholar]
- 29.Höftberger R, Sabater L, Marignier R, et al. . An optimized immunohistochemistry technique improves NMO-IgG detection: study comparison with cell-based assays. PLoS One 2013;8:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höftberger R, Sepulveda M, Armangue T, et al. . Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 2015;21:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 32.Soelberg K, Specovius S, Zimmermann HG, et al. . Optical coherence tomography in acute optic neuritis: a population-based study. Acta Neurol Scand 2018;138:566–573. [DOI] [PubMed] [Google Scholar]
- 33.Group RWB and the optic neuritis study; Beck RW, Cleary PA, Anderson MM Jr, et al. . A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992;326:581–588. [DOI] [PubMed] [Google Scholar]
- 34.Eshaghi A, Prados F, Brownlee WJ, et al. . Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 2018;83:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res 2008;87:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu B, Moore GRW, Zwimpfer TJ, et al. . Axonal cytoskeleton changes in experimental optic neuritis. Brain Res 1999;824:204–217. [DOI] [PubMed] [Google Scholar]
- 37.Balk LJ, Cruz-Herranz A, Albrecht P, et al. . Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 2016;263:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granberg T, Fan Q, Treaba CA, et al. . In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 2017;140:2912–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data have been uploaded and will be made readily available upon publication at the following Mendeley data repository: dx.doi.org/10.17632/ht5s9cc845.1.


