Myelin basic protein (MBP) is one of the most abundant proteins in the human brain. Active immunization with MBP induces experimental autoimmune encephalomyelitis, and anti-MBP antibodies have been repeatedly described in MS.1 However, its role in MS pathogenesis or prediction of disease progression is still unclear.2,3 Previous studies utilized enzyme-linked immunosorbent assay or immunoblot assays with linear epitopes of MBP, thus potentially overlooking autoantibodies that bind to MBP's natural conformation. These initial studies also included antibodies against another myelin protein, myelin oligodendrocyte glycoprotein (MOG). As happened for MBP, conflicting results stimulated the discussion of whether MOG antibodies contribute to MS pathogenesis.2,3 More recent work demonstrated that there are presumably pathogenic MOG antibodies defining the new entity of MOG antibody-associated disease;4 however, they bind to conformational MOG only.
Here we report on a patient with MS with immunotherapy-responsive severe cognitive impairment having high-level immunoglobulin A (IgA) autoantibodies against conformational MBP, suggesting the possibility of myelin-directed humoral autoimmunity beyond MOG.
Case report
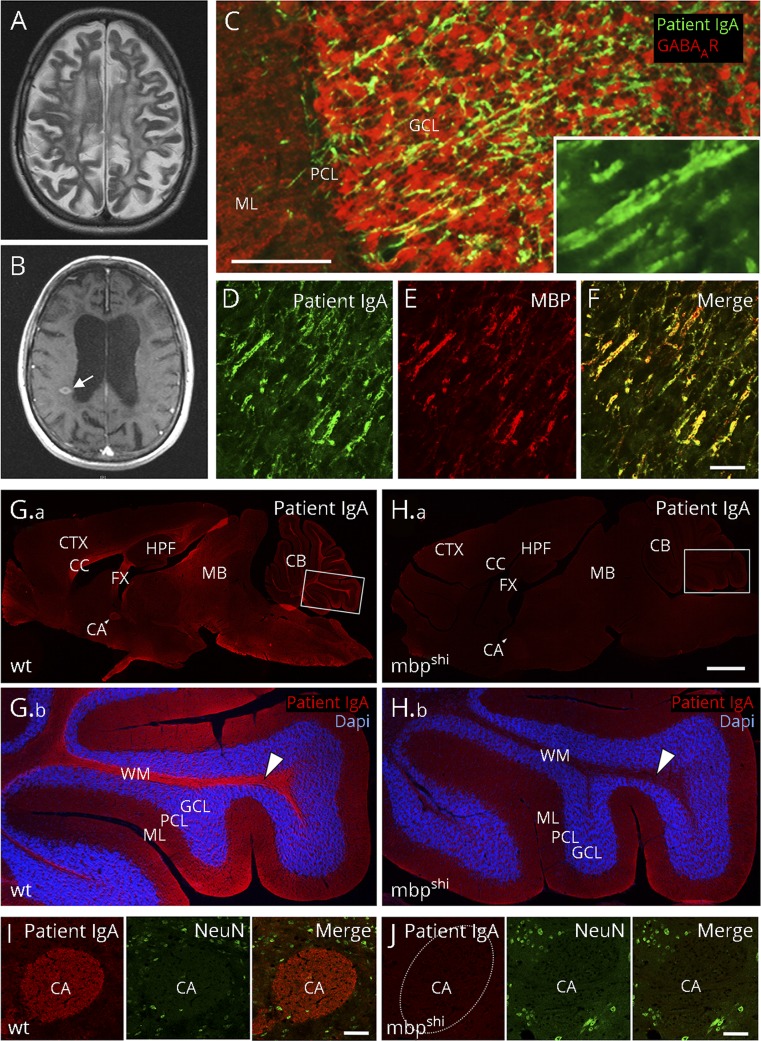
A 54-year-old woman with a 20-year history of relapsing–remitting MS (Expanded Disability Status Scale 3.5) was admitted for a suspected relapse with subacute-onset rapidly progressing cognitive decline, presenting with dementia and echolalia. Apart from unsteady gait, double vision, and lack of coordination, cerebellar and motor signs were relatively spared, and the MRI showed new lesions (figure, A and B). Previous treatments included mitoxantrone (19 cycles, cumulative dose 137 mg/m2) and beta-1a interferon (3 years of 44 μg 3 times per week). Given the unusual predominance of cognitive symptoms with rapid deterioration from 18 to 14/30 points in Mini-Mental State Examination, secondary autoimmune encephalitis was considered. Indirect immunofluorescence revealed high titers of brain-reactive IgA antibodies (serum 1:3,200, CSF 1:32, antibody index 6.1 indicating intrathecal synthesis; immunoglobulin M/G negative) labeling axonal fibers throughout the unfixed brain, particularly in cerebellum (figure, C), corpus callosum, and hippocampus. The fine parallel fiber staining suggested binding to myelin epitopes (figure, C, insert). MOG antibodies were excluded (Prof. Höftberger, Vienna, Austria). Immunotherapy, including plasma exchange (10 sessions every other day) and rituximab (1,000 mg every 6 months for 2 years), resulted in the disappearance of MBP antibodies after 6 months and improvement of cognitive symptoms (Mini-Mental State Examination 16/30), which remained stable for 3 years until the last follow-up, antibodies remained negative.
Figure. Myelin binding of high-level MBP IgA antibodies from a patient with MS.
(A) Cerebral MRI shows atrophy, widespread postinflammatory changes and (B) new contrast-enhancing lesions (arrow). (C) Using 20 μm unfixed rat brain sections, patient IgA (4.25 mg/mL, dilution 1:10) labels fine axonal fibers (green, goat anti-human IgA, Dianova, Hamburg, Germany, dilution 1:200) throughout the brain, in particular in the cerebellar cortex (colabeling with a GABAA receptor antibody [red; Santa Cruz Biotechnology, Dallas, TX, USA, dilution 1:200] for better anatomical visualization of the cerebellar cortex). (C, inset) Higher magnification shows parallel staining of fibers, indicative of myelin antigens. (D–F) Double-labeling of patient IgA (green) with a commercial anti-MBP antibody [red, Santa Cruz Biotechnology, Dallas, TX, USA, dilution 1:200] demonstrates complete overlap in rat cerebellar cortex (merged in [F]). The characteristic immunofluorescence with strong binding to axonal fiber tracts on a 20 µm paraformaldehyde-fixed mouse brain section (G, red) was completely absent in shiverer MBP knockout (mbpshi) littermate mice (H), exemplarily shown at higher magnification in the white matter of the cerebellum (arrowheads in G.b and H.b; double-labeling with DAPI for cell nuclei in blue) or the anterior commissure (I, J; double-labeling with the neuronal marker NeuN in green). Bars represent 50 μm in C–F, 1 mm in G–H and 50 μm in I, J. CA = anterior commissure; CB = cerebellum; CC = corpus callosum; CTX = cortex; FX = fornix; GCL = granule cell layer; HPF = hippocampal formation; MB = midbrain; ML = molecular layer; PCL = Purkinje cell layer; WM = white matter; and wt = wild-type.
To identify the antigen, immunoprecipitation and mass spectrometry were performed. One hundred micrograms of IgA purified from the plasma exchange eluate were incubated overnight with rat brain lysate and samples run on sodium dodecyl sulfate (SDS) gels. Bands were analyzed with mass spectrometry,5 and data were analyzed as described,6 matching MBP only. Double immunolabeling showed exact co-localization of patient antibody with a commercial anti-MBP antibody (figure, D–F). In contrast to the commercial antibody, the patient's IgA did not bind to rat brain lysate in denaturing Western blots (not shown), suggesting that they recognize the natural epitope conformation. Direct proof for the target antigen was obtained using MBP knockout mice in which the antibody binding was completely lost (figure, G–J).
Discussion
We report the case of a patient with MS with rapidly progressing cognitive decline having high-level autoantibodies against conformational MBP. Previous studies using denatured epitopes in enzyme-linked immunosorbent assay and Western blots could not establish a clear link between MBP antibodies and disease,2,3 potentially because they overlooked specific binding to the conformational epitope. This first report of MBP autoantibodies against native MBP revives the discussion of whether such antibodies might be related to a subgroup of MS patients, convey pathology, or serve as a biomarker for progression or cognitive symptoms.
Similar to MBP, the pathogenic role of MOG antibodies has been debated intensely. Only more recent studies focusing on antibody interactions with native MOG could convincingly demonstrate their pathogenic potential.7 For example, such MOG antibodies were present in a subgroup of patients with severe MS.
The clinical improvement with immunotherapy in our patient paralleled the disappearance of antibody titers, suggesting that the antibodies may have contributed to the disease. IgA antibody transfer into animals should be an important future experimental step to confirm pathogenicity.
It is unclear at present whether autoantibodies against native MBP are also detected in a subgroup of patients with MS. We did not find a similar immunofluorescence pattern using the serum and CSF of 352 consecutive patients with suspected encephalitis (including 46 patients with MS) and serum of 82 healthy controls, suggesting that—similar to MOG antibodies—the specific myelin staining observed in the present patient is rare. Prospective studies using established cohorts with MS and clinically isolated syndrome (CIS) should therefore be screened systematically to determine the frequency of native MBP-targeting autoantibodies, the association with clinical phenotypes, CIS conversion to MS, relapses, and disease progression.
Acknowledgment
The authors are grateful to Professor Brian Popko, Department of Neurology, University of Chicago, for providing MBP knockout mouse brains.
Author contributions
H. Schumacher: major role in the acquisition of data, analysis and interpretation of data, and drafting the manuscript. N.K. Wenke: acquisition of data. J. Kreye: acquisition of data. M. Höltje: acquisition of data. K. Marcus: acquisition of data. C. May: acquisition, analysis and interpretation of data, and critical revision of manuscript. H. Prüss: acquisition, analysis and interpretation of data, and critical revision of manuscript.
Study funding
This work was supported by the HUPO Brain Proteome Project (HBPP) and PURE, a project of North Rhine-Westfalia, a federal German state.
Disclosure
The authors report no disclosures. Disclosures available: Neurology.org/NN.
References
- 1.Olsson T, Baig S, Höjeberg B, Link H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann Neurol 1990;27:132–136. [DOI] [PubMed] [Google Scholar]
- 2.Berger T, Rubner P, Schautzer F, et al. . Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 2003;349:139–145. [DOI] [PubMed] [Google Scholar]
- 3.Kuhle J, Pohl C, Mehling M, et al. . Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med 2007;356:371–378. [DOI] [PubMed] [Google Scholar]
- 4.Pröbstel AK, Dornmair K, Bittner R, et al. . Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 2011;77:580–588. [DOI] [PubMed] [Google Scholar]
- 5.Plum S, Helling S, Theiss C, et al. . Combined enrichment of neuromelanin granules and synaptosomes from human substantia nigra pars compacta tissue for proteomic analysis. J Proteomics 2013;94:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina M, Steinbach S, Park YM, et al. . Enrichment of single neurons and defined brain regions from human brain tissue samples for subsequent proteome analysis. J Neural Transm (Vienna) 2015;122:993–1005. [DOI] [PubMed] [Google Scholar]
- 7.Reindl M, Rostasy K. MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm 2015;2:e60 doi: 10.1212/NXI.0000000000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]