Abstract
Background and Aim Congenital segmental intestinal dilatation (CSID) is a neonatal condition with unclear etiology and pathogenesis. Typically, the newborn with CSID presents with a limited (circumscribed) bowel dilatation, an abrupt transition between normal and dilated segments, neither intrinsic nor extrinsic perilesional obstruction, and no aganglionosis or neuronal intestinal dysplasia. We aimed to review this disease and the long-term follow-up at the Children's Hospital of the Medical University of Innsbruck, Tyrol, Austria.
Study Design Retrospective 25-year review of medical charts, electronic files, and histopathology of neonates with CSID.
Results We identified four infants (three girls and one boy) with CSID. The affected areas included duodenum, ileum, ascending colon, and sigmoid colon. Noteworthy, all patients presented with a cardiovascular defect, of which two required multiple cardiac surgical interventions. Three out of the four patients recovered completely. To date, the three infants are alive.
Conclusion This is the first report of patients with CSID and cardiovascular defects. The clinical and surgical intervention for CSID also requires a thorough cardiologic evaluation in these patients. CSID remains an enigmatic entity pointing to the need for joint forces in identifying common loci for genetic investigations.
Keywords: dilatation, intestine, obstruction, aganglionosis, Hirschsprung's disease, heart defect
Congenital segmental intestinal dilatation (CSID) is a neonatal condition with unclear etiology and pathogenesis. Typically, the newborn with CSID presents with a limited (confined) bowel dilatation with three- to fourfold increase, an abrupt transition between normal and dilated segments, neither intrinsic nor extrinsic perilesional obstruction, and presence of normal ganglionic cells of the gastrointestinal autonomous plexus. 1 There is no evidence of Hirschsprung's disease (aganglionosis) or neuronal intestinal dysplasia using the most updated laboratory diagnostic procedures. 2 3 4 5 In some cases, a segmental absence of intestinal musculature may cause spontaneous bowel perforation, intestinal obstruction, or intussusception. 6 The resection of the affected segment usually leads to complete recovery, but the clinical polymorphism and the lack of specificity of diagnostic imaging may point to the difficulty to have a complete preoperative picture of the disease. 7 8
We aimed to review this disease and the long-term follow-up at the Children's Hospital of the Medical University of Innsbruck, Tyrol, Austria.
Materials and Methods
We reviewed the medical charts, electronic medical records, and histopathology at our institution (Children's Hospital, Innsbruck, Austria) of the Medical University of Innsbruck, Austria.
Results
We found four infants with CSID who underwent surgery at the Innsbruck Department of Pediatric Surgery. The clinical findings of the four infants (three girls and one boy) are shown in Table 1 , while the surgical sites and the histopathology findings are illustrated in Figs. 1 and 2 , respectively. Briefly, in the first patient, the affected area was the ascending colon. It was reduced by tapering and later used to close the colonic defect caused by atresia of the distal descending colon. The second patient had a dilated duodenum. The infant underwent to open exploratory surgery, and the duodenum was then tapered. The third infant had dilatation of the lower sigmoid colon, which was primarily resected, the rectum was closed, and an artificial anus was created in the sigmoid colon (sigmoidostomy). Six weeks later, a colostomy reversal was performed, and continuity was re-established by an end-to-end anastomosis between the descending colon and rectum. The fourth infant had a dilated segment of the ileum which was resected, and an end-to-end ileoileostomy performed. In our patients, all histological findings showed data like the findings mentioned earlier. We found regular configured bowel wall segments with thin external muscle layers ( Fig. 2a–c ). The pylorus tumor of patient 2 showed glandular structures lined by cuboidal to columnar epithelium surrounded by hypertrophic smooth muscle bundles, which corresponds to a gastric adenomyoma of the pylorus ( Fig. 2d ). Ganglia cells were found in all specimens, although occasionally some ganglia cells were reported as immature.
Table 1. Data of patients with CSID, associated anomalies, surgical interventions, and outcome.
| Case | Sex | Year | Birth | BW | CSID | MCA | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 1994 | Term, VD | 2,410 | Ascending colon | High anal atresia and atresia of the descending colon, VUR III, ASD | (1) Tapering of ascending colon, double-barreled transverse loop colostomy (2) Resection of the residual descending colon, ascending colon pull-through operation (Soave) with the construction of a neoanus |
Alive, uneventful, apart from pubertas praecox |
| 2 | M | 1996 | Term, VD | 2360 | Duodenum | Pyloric “tumor,” aortic arch hypoplasia (isthmus), IM | Excision of the tumor, tapering of the dilated duodenal segment after explorative duodenotomy |
Death after cardiac surgery |
| 3 | F | 1997 | Term, VD | 2,660 | Lower sigma | Trisomy 21 syndrome, VSD | (1) Resection of the dilated segment, blind rectum closure, and terminal sigmoidostomy (2) End-to-end anastomosis between descending colon and rectum |
Alive, frequent mechanical dilatation of the anastomosis during the first 2 y of life, growth delay |
| 4 | F | 2008 | 39th, CS* | 3,700 | Ileum | AVC, unroofed CS, AAD, AL, LCVP, left liver, IM | Resection of dilated ileum segment, end-to-end ileoileostomy | Alive, uneventful, apart from multiple (7) CV operations |
Abbreviations: AAD, arcus aortae dexter (right aortic arch); AL, arteria lusoria; ASD, atrial septal defect; AVC, atrioventricular channel; BW, birth weight (g); CS*, cesarean section; CS, coronary sinus; CSID, congenital segmental intestinal dilatation; CV, cardiovascular; IM, intestinal malrotation; LCVP, persistence of the left caval vein; VD, vaginal delivery; VSD, ventricular septal defect; VUR III, third degree of vesical-urethral reflux.
Notes : Ileoileostomy is a surgical anastomosis between two segments of the ileum. The pyloric “tumor” was a gastric adenomyoma of the pylorus. Pathological examination revealed glandular structures lined by cuboidal to columnar epithelium surrounded by hypertrophic smooth muscle bundles. Gastric adenomyoma should be considered a differential diagnosis of hypertrophic pyloric stenosis and gastric duplication in newborns and children.
Fig. 1.

(a) Patient 1: Scheme of the operation site with the malformations of the lower gastrointestinal tract (dilatation of the ascending colon, atresia of the descending colon, blindly closed rest of the sigmoid colon and the rectum, as well as anal atresia) after a double-barreled colostomy at the transverse colon. (b) Patient 1: Operative site of the CSID of the ascending colon. (c) Patient 2: Plain abdominal X-ray showing a sizeable gas-filled bowel loop in right upper abdominal quadrant. (d) Patient 2: Operative site of the mobile dilated duodenum. (e) Patient 3: Scheme of the circumscribed dilatation of the lowest part of the sigmoid colon. (f) Patient 3: Operative site of the dilated segment of the sigmoid colon (diameter of 10 cm), the oral colon with standard configuration. (g) Patient 4: Upper gastrointestinal X-ray series showing pooling of the contrast media in a dilated loop of the ileum in the right hemiabdomen. (h) Patient 4: Intraoperative photograph of an 18-cm-long segmental dilatation of the middle ileum. The arrow in (h) points to the segmental dilatation of the portion of the intestine. The transition of normal bowel on both ends did not show any sign of mechanical obstruction. CSID, congenital segmental intestinal dilatation.
Fig. 2.
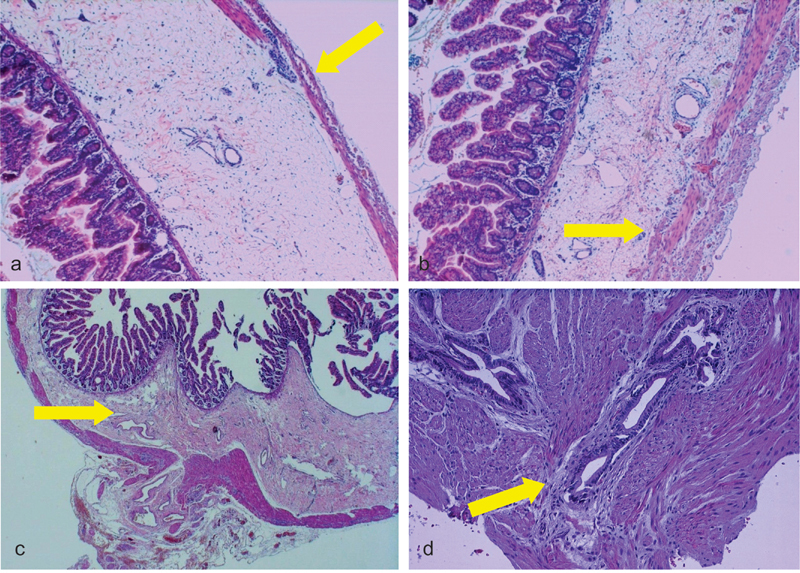
(a, b) Microphotograph of CSID (Patient 4) with thin external muscle layer (hematoxylin and eosin ×40) and dilated lymphatic vessels of the submucosa (hematoxylin and eosin staining, ×40 original magnification); (c) microphotograph of CSID (Patient 3) showing sclerosis of the submucosa (hematoxylin and eosin staining, ×20 original magnification); and (d) microphotograph of the “pyloric tumor” showing hypertrophic muscle layers with some scattered glandular proliferations corresponding to a gastric adenomyoma of the pyloric region (hematoxylin and eosin staining, ×100 original magnification). The yellow arrows in (a) and (b) point to the very thin muscularis propria, while the yellow arrows in (c) and (d) point to the sclerosis of the submucosa and to the gastric adenomyoma of the pylorus, respectively. The gastric adenomyoma of the pylorus is characterized by glandular structures lined by cuboidal to columnar epithelium surrounded by hypertrophic smooth muscle bundles by histological examination. CSID, congenital segmental intestinal dilatation.
Noteworthy, all patients presented with a cardiac/cardiovascular defect. Two of the four defects required multiple cardiac surgical interventions. Two patients had each an atrial septum defect and a ventricular septal defect (VSD), while the other two patients had complex congenital heart disease. Three patients recovered completely. To date, these three infants are alive. One patient died of cardiac surgery complications.
Discussion
CSID has been defined as a circumscribed dilatation of the lumen with an abrupt transition between normal and dilated bowel and neither intrinsic nor extrinsic barrier distal of the dilatation. There is a sharply defined and markedly enlarged segment of the intestine flanked by normal caliber afferent and efferent bowel segments. 7 CSID can involve the gastrointestinal tract anywhere from duodenum to the distal colon. The ileum and colon are the most commonly affected sites, while duodenum and jejunum are less frequently involved (Swenson and Rathauser [1959] [colon], Rossi and Giacomoni [1973] [jejunum], Sjölin and Thoren [1962] [ileum], Ueda and Okamoto [1972] [ileum], Irving and Lister [1977] [ileum], and Rovira et al [1989] [duodenum]). 7 9 10 11 12 13 Since the entity may be described differently, the precise number of all published cases of CSID may be challenging to identify. In consideration of it, Ben Brahim et al reported 125 literature cases of CSID published up to 2006 1 and added eight of their own, of which seven were newborn. However, Elemen et al 14 indicated 2 years later that “slightly more than 100 cases” have been reported up to 2008. It seems that there are ∼110 to 120 cases adequately reported in around 50 years of reviewed literature. In consideration of the number of cases reported in the literature, two to three cases may present to the clinical attention yearly. In the last couple of decades, the criteria became stricter and, since 2008, to the best of our knowledge, 20 adequately described cases have been reported in the English literature. 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Here, we report on four infants with CSID with segmental dilatation located in the duodenum, ileum, ascending colon, and sigmoid colon. Two of our infants showed additional intestinal anomalies, but remarkably, all four patients had cardiovascular defects, and this is the first report demonstrating such a consistent association in four consecutive cases. In the review of the CSID cases, it is manifest that the associated malformations, which are found in more than 50% of the cases, are quite diverse, although the gastrointestinal tract and the omphalomesenteric duct are mostly involved. 12 13 33 34 35 36 37 38 39
Histopathological findings of cases described in the literature range from partial or complete absence of lamina propria in the affected segment to partial hypertrophy of muscle layers with or without an irregular muscular network, minimal thinning of the external muscle layer, degenerated myocytes filled with vacuoles, and distended submucosal vessels up to angiodysplasia. 1 12 13 28 40 41 42 43 44 45 In most cases, the histology of the resected segment is normal. Occasionally, a supernumerary intestinal muscle coat in the dilated segment, without any evidence of neurological abnormality, was observed. Histopathological findings of the resected ileal portion are reported to discuss the role of developmental malformation of muscularis propria as a cause of CSID. 46 The presence of ganglion cells was reported in all cases of CSID as part of the criteria. Cheng et al performed an extended histochemical and immunohistochemical analyses of one instance of a jejunal CSID and postulated that segmental dilatations of the small bowel might not be in origin of the enteric nervous system but accuse a localized myopathy of the smooth muscle of being at least part of the sources of this entity. 40 A differential diagnosis may reside in a segmental aganglionosis, an entity which is controversially discussed in the literature. 47 48 49 In the case reported by Soyer et al, 46 a 3-day-old male neonate presenting with clinical findings of intestinal obstruction was diagnosed to have CSID and had supernumerary intestinal muscle coat in the dilated segment, without any evidence of neurological abnormality. Histopathological findings identify a striking similarity to our cases with duodenum dilatation or colon dilatation with anal atresia.
Other than hypertrophic or very thin muscle layers in the involved segment, ectopic tissue, such as the gastric or pancreatic tissue, was demonstrated. 8 27 50 Gastric adenomyoma of the pyloric region should be considered a differential diagnosis of hypertrophic pyloric stenosis and gastric duplication in newborns and children. 51 52
Nonspecific inflammatory or erosive changes can also occur. In the case reported by Elemen et al, erosive changes and nonspecific inflammation of the lamina propria were also found. 14
In consideration of the clinical and histological presentations, it is unlikely to have only one pathogenesis for this condition. Three theories are frequently postulated, including the theory of Irving and Lister (1977), 12 the theory of Mathé et al (1982), 53 and the theory of Heller and Waag (1987). 54 Irving and Lister suggested embryological damages implicate an extrinsic intrauterine intestinal compression with the intestine being strangulated by an umbilical ring, vitelline blood vessels, or omphalomesenteric bands. 12 Mathe et al suggested a primitive (embryonic) neuromuscular dysfunction of the intestine, 53 while Heller and Waag focused on an early disturbance during splitting of the notochord from the entoderm. 54 These last authors pointed out that parts of the entoderm remain adherent to the ectoderm with the organization of cysts and/or tubes impairing the healthy development of the spine and eliciting other associated defects. 54
In consideration of the cardiovascular anomalies encountered in our patients, we may consider our CSID infants to be a part of combined developmental disorders rather than a separate entity. In consideration of the development of the gut muscle layers and identification of pancreatic islets at 12 weeks of embryonal age (crown-rump length of 8.8 cm), we may expect that a developmental disorder limited to the gut may involve these infants at this age. In case of an associated cardiovascular defect, the time of embryonic damage should be located earlier. The time of susceptibility for the development of atrial septal defect, VSD, aortic arch hypoplasia/coarctation of the aorta, and atrioventricular septal defects occurs between 6- and 8-week postfertilization. Thus, the insult should be prolonged enough to reach the 12th week of postfertilization age. 55 Congenital defects of the cardiovascular system are among the most common congenital birth defects estimated to occur in ∼1 in 100 living births. 56 Congenital malformations of the gut, urinary, and musculoskeletal systems are the most frequently seen extracardiac defects in patients with congenital defects of the cardiovascular system. 57 Intestinal malrotation has been observed in 2.8 to 4.1% of all the patients with congenital defects of the cardiovascular system. 58 59 In embryogenesis, the gut is developed at a later stage than the cardiovascular system does. Changes in intra-abdominal space caused by congenital heart defects might also increase the risk of abnormal gut development during embryogenesis. 60 61 A common genetic cause might be another explanation in our patients. The left–right asymmetry is present in both the gut and the heart, and a specific biochemical cascade during embryogenesis has been demonstrated. 62 Nodal growth differentiation factor ( NODAL ) and paired-like homeodomain 2 transcription factor 2 isoform c ( PITX2c ) are two signaling molecules derived from NODAL and PITX2c genes. Both NODAL and PITX2c are inevitable for this cardiac and gastrointestinal asymmetry and hypertrophy of the cardiomyocytes. 63 In animal studies, an abnormal (reduced, diffuse, or absent) expression of PITX2c in the embryo causes abnormal looping from both heart and gut, 64 65 66 67 resulting mainly in abnormal outflow tract orientation (e.g., unseptated and misaligned great artery trunks, double outlet right ventricle, symmetrical outflow tract cushions, and common atrioventricular trunk). 65 66 The disturbance of the expression of PITX2c could cause a spectrum of isomerism. 68
Concerning the diagnosis, there are challenges both during pregnancy and in the postpartum. CSID can either become symptomatic during the intrauterine life or after birth. However, the opinion that a CSID in the newborn is associated with subileus or ileus is not always correct. Depending on the extent of the enlargement, the intestinal transit is more or less affected, which means that a CSID can be entirely asymptomatic explaining the report of cases in older children. CSID is a malformation that becomes symptomatic at any age or even is an incidental finding at laparotomy. 10 14 69 As a CSID can already become symptomatic in a fetus and/or in a newborn, a wide range of possibilities arises regarding diagnostics. To date, prenatal diagnosis has also increasingly diagnosed minor cystic changes in the abdomen of fetuses, for example, duodenal atresia, meconium ileus, intestinal duplication, and mesenteric cyst. The problem that arises is that while these structures are usually easy to recognize, they often cause diagnostic challenges when associated with CSID. Apart from a single case diagnosed prenatally, CSID is most commonly identified after birth because of the inaccuracy of the fetal intestinal echography. Paradiso et al 19 found that two of their patients had a prenatal ultrasonographic suspicion of intestinal abnormality, which was confirmed in postpartum surgery.
However, the diagnosis of a CSID is not easy even postpartum, mainly if other intestinal malformations are associated. The clinical polymorphism and the glazing nonspecificity of radiological investigations complicate the diagnostic procedure. This entity remains enigmatic and challenging because of the ambiguous clinical findings, nonspecific radiological examinations, and mimickers of the urinary tract. 50 70
In our four patients, two patients had ileal symptoms, and an exploratory laparotomy was scheduled on the second postpartum day (after deriving the gastric contents via a nasogastric tube). In the third patient, a cystic structure was detected in the fetus in the pelvis as part of sonographic pregnancy monitoring, but an assignment was not made. In the postnatal period, Hirschsprung's disease was suspected, although the megacolon appeared “spherical.” Only in the fourth newborn could the prenatal diagnosis of a presumed cystic duplication be preoperatively revised in the direction of a CSID. Noteworthy, all four patients had congenital heart disease.
The relationship between CSID and heart failure remains to be questioned, although the occurrence of a heart defect in a child with trisomy 21 or with a high level of anal atresia is well known in the literature. Probably, the use of trisomy 16 mice, the animal model for human trisomy 21, may be useful in the future.
The surgical procedure for correcting a CSID depends on its position. Most often, a segment resection in the sense of a one-step procedure with end-to-end anastomosis is possible. 28 36 44 50 71 72 73 74 In our four patients, however, this was only possible in the third and fourth patients. In the third patient, because of the macroscopically unclear findings with suspicion of aganglionosis or neuronal intestinal dysplasia, such an approach was only feasible in two phases. Postoperative courses and outcomes, although dependent on the associated malformations, are mostly reported to be without complaints. 9 28 71 Regarding therapy, low-dose vasopressin may improve cardiac function as identified in previous experiments in newborn piglets with acute hypoxia–reoxygenation. 75
In conclusion, CSID is a rare intestinal malformation, which can be associated with other anomalies, mostly of intestinal type. An association with cardiovascular defects should be considered an extreme rarity and may suggest that genetic counseling may be appropriate. The final diagnosis relies on the intraoperative picture and the exclusion of a segmental aganglionosis. Standard therapy is the total resection of the affected segment and end-to-end anastomosis or, depending on the position of the intestinal malformation, using specific surgical procedures. Postoperative course and outcome depend on the associated defects, but CSID itself is a condition which is mostly free of complications.
Footnotes
Conflict of Interest None.
References
- 1.Ben Brahim M, Belghith M, Mekki M et al. Segmental dilatation of the intestine. J Pediatr Surg. 2006;41(06):1130–1133. doi: 10.1016/j.jpedsurg.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Takawira C, D'Agostini S, Shenouda S, Persad R, Sergi C. Laboratory procedures update on Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2015;60(05):598–605. doi: 10.1097/MPG.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 3.Burtelow M A, Longacre T A. Utility of microtubule associated protein-2 (MAP-2) immunohistochemistry for identification of ganglion cells in paraffin-embedded rectal suction biopsies. Am J Surg Pathol. 2009;33(07):1025–1030. doi: 10.1097/PAS.0b013e31819b23f2. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm K M, Longacre T A. Utility of peripherin versus MAP-2 and calretinin in the evaluation of Hirschsprung disease. Appl Immunohistochem Mol Morphol. 2016;24(09):627–632. doi: 10.1097/PAI.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 5.Yang W I, Oh J T. Calretinin and microtubule-associated protein-2 (MAP-2) immunohistochemistry in the diagnosis of Hirschsprung's disease. J Pediatr Surg. 2013;48(10):2112–2117. doi: 10.1016/j.jpedsurg.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 6.Oyachi N, Suzuki T, Emura T et al. Segmental absence of intestinal musculature with metachronous bowel perforations in an infant. J Pediatr Surg Case Rep. 2018;30:1–3. [Google Scholar]
- 7.Swenson O, Rathauser F. Segmental dilatation of the colon; a new entity. Am J Surg. 1959;97(06):734–738. doi: 10.1016/0002-9610(59)90338-1. [DOI] [PubMed] [Google Scholar]
- 8.Porreca A, Capobianco A, Terracciano C, D'Onofrio V. Segmental dilatation of the ileum presenting with acute intestinal bleeding. J Pediatr Surg. 2002;37(10):1506–1508. doi: 10.1053/jpsu.2002.35434. [DOI] [PubMed] [Google Scholar]
- 9.Rossi R, Giacomoni M A. Segmental dilation of the jejunum. J Pediatr Surg. 1973;8(02):335–336. doi: 10.1016/s0022-3468(73)80107-1. [DOI] [PubMed] [Google Scholar]
- 10.Sjolin S, Thoren L. Segmental dilatation of the small intestine. Arch Dis Child. 1962;37:422–424. doi: 10.1136/adc.37.194.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda T, Okamoto E. Segmental dilatation of the ileum. J Pediatr Surg. 1972;7(03):292–293. doi: 10.1016/0022-3468(72)90127-3. [DOI] [PubMed] [Google Scholar]
- 12.Irving I M, Lister J. Segmental dilatation of the ileum. J Pediatr Surg. 1977;12(01):103–112. doi: 10.1016/0022-3468(77)90303-7. [DOI] [PubMed] [Google Scholar]
- 13.Rovira J, Morales L, Parri F J, Juliá V, Claret I. Segmental dilatation of the duodenum. J Pediatr Surg. 1989;24(11):1155–1157. doi: 10.1016/s0022-3468(89)80103-4. [DOI] [PubMed] [Google Scholar]
- 14.Elemen L, Inanc D, Oz F, Erdogan E. Segmental dilatation of the ileum accompanying hypoproteinemia. J Pediatr Surg. 2008;43(07):e15–e18. doi: 10.1016/j.jpedsurg.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 15.Johnson L, Simone K, Cullen J, Talley A, Cohen E B.Radiographic features of congenital segmental dilation of the intestine in a German shepherd dogVet Radiol Ultrasound2017. DOI: https://doi.org/10.1111/vru.12581 [DOI] [PubMed]
- 16.Taguchi T, Ieiri S, Miyoshi K et al. The incidence and outcome of allied disorders of Hirschsprung's disease in Japan: results from a nationwide survey. Asian J Surg. 2017;40(01):29–34. doi: 10.1016/j.asjsur.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser M, Castellani C, Singer G, Marterer R, Ratschek M, Till H. Huge congenital segmental dilatation of the sigmoid colon in a neonate: a “rarity to meet” and a “challenge to treat”. Case Rep Pediatr. 2016;2016:9.685307E6. doi: 10.1155/2016/9685307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai B K, Mirza B, Hashim I, Saleem M. Varied presentation of congenital segmental dilatation of the intestine in neonates: report of three cases. J Neonatal Surg. 2016;5(04):55. doi: 10.21699/jns.v5i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paradiso F V, Coletta R, Olivieri C et al. Antenatal ultrasonographic features associated with segmental small bowel dilatation: an unusual neonatal condition mimicking congenital small bowel obstruction. Pediatr Neonatol. 2013;54(05):339–343. doi: 10.1016/j.pedneo.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Sam C J. Segmental ileal dilatation in a child. Trop Gastroenterol. 2011;32(03):221–223. [PubMed] [Google Scholar]
- 21.DeBenedet A T, Saini S D, Takami M, Fisher L R. Do clinical characteristics predict the presence of small bowel angioectasias on capsule endoscopy? Dig Dis Sci. 2011;56(06):1776–1781. doi: 10.1007/s10620-010-1506-9. [DOI] [PubMed] [Google Scholar]
- 22.Harjai M M, Katiyar A, Negi V, Yadav D, Sharma M. Congenital segmental dilatation of jejunoileal region in a newborn: unusual clinical and radiologic presentation. J Indian Assoc Pediatr Surg. 2010;15(03):96–97. doi: 10.4103/0971-9261.71752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue M, Uchida K, Otake K, Koike Y, Miki C, Kusunoki M. Congenital segmental dilatation of the duodenum: report of a case. Pediatr Int. 2010;52(04):e184–e186. doi: 10.1111/j.1442-200X.2010.03117.x. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Sasaki F, Honda S et al. Disorders of interstitial cells of Cajal in a neonate with segmental dilatation of the intestine. J Pediatr Surg. 2010;45(06):e11–e14. doi: 10.1016/j.jpedsurg.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Shah A D, Kovanlikaya A, Beneck D, Spigland N, Brill P W. Segmental dilatation of the ileum in a healthy adolescent. Pediatr Radiol. 2009;39(12):1350–1353. doi: 10.1007/s00247-009-1383-6. [DOI] [PubMed] [Google Scholar]
- 26.Saha S, Konar H, Chatterjee P et al. Segmental ileal obstruction in neonates--a rare entity. J Pediatr Surg. 2009;44(09):1827–1830. doi: 10.1016/j.jpedsurg.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Brown B R, Hennessey I, Lansdale N, Humphrey G. Pancreatic tissue in congenital segmental dilatation of intestine: case presentation and recommendation for treatment. J Pediatr Surg. 2008;43(11):e9–e11. doi: 10.1016/j.jpedsurg.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Daher P, Ghanimeh J, Riachy E, Zeidan S, Eid B. Congenital segmental dilatation of the small bowel (CSD) Eur J Pediatr Surg. 2007;17(04):289–291. doi: 10.1055/s-2007-965535. [DOI] [PubMed] [Google Scholar]
- 29.Kella N, Rathi P K. Segmental defect of intestinal musculature: a rare cause of intestinal obstruction in children. J Coll Physicians Surg Pak. 2006;16(08):551–552. [PubMed] [Google Scholar]
- 30.Kothari P, Rastogi A, Dipali R, Kulkarni B; Gowrishankar.Congenital segmental dilatation of colon with colonic atresia Indian J Gastroenterol 20052403123–124. [PubMed] [Google Scholar]
- 31.Katsura S, Kudo T, Enoki T, Taguchi T, Hamano K. Congenital segmental dilatation of the duodenum: report of a case. Surg Today. 2011;41(03):406–408. doi: 10.1007/s00595-010-4257-5. [DOI] [PubMed] [Google Scholar]
- 32.Mathur P, Mogra N, Surana S S, Bordia S. Congenital segmental dilatation of the colon with anorectal malformation. J Pediatr Surg. 2004;39(08):e18–e20. doi: 10.1016/j.jpedsurg.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 33.Doody D, Nguyen L T. Congenital atresia of the colon combined with segmental dilatation of the ileum: a case report. J Pediatr Surg. 1987;22(09):804–805. doi: 10.1016/s0022-3468(87)80639-5. [DOI] [PubMed] [Google Scholar]
- 34.Ratcliffe J, Tait J, Lisle D, Leditschke J F, Bell J. Segmental dilatation of the small bowel: report of three cases and literature review. Radiology. 1989;171(03):827–830. doi: 10.1148/radiology.171.3.2655007. [DOI] [PubMed] [Google Scholar]
- 35.Ratan S K, Kulsreshtha R, Ratan J. Cystic duplication of the cecum with segmental dilatation of the ileum: report of a case. Surg Today. 2001;31(01):72–75. doi: 10.1007/s005950170225. [DOI] [PubMed] [Google Scholar]
- 36.Bell M J, Ternberg J L, Bower R J. Ileal dysgenesis in infants and children. J Pediatr Surg. 1982;17(04):395–399. doi: 10.1016/s0022-3468(82)80497-1. [DOI] [PubMed] [Google Scholar]
- 37.Brown R L, Azizkhan R G. Gastrointestinal bleeding in infants and children: Meckel's diverticulum and intestinal duplication. Semin Pediatr Surg. 1999;8(04):202–209. doi: 10.1016/s1055-8586(99)70027-2. [DOI] [PubMed] [Google Scholar]
- 38.Leinster S J, Hughes L E. Segmental mega-ileum presenting as anaemia. Br J Surg. 1981;68(06):417–419. doi: 10.1002/bjs.1800680618. [DOI] [PubMed] [Google Scholar]
- 39.Morewood D J, Cunningham M E. Case report: segmental dilatation of the ileum presenting with anaemia. Clin Radiol. 1985;36(03):267–268. doi: 10.1016/s0009-9260(85)80057-x. [DOI] [PubMed] [Google Scholar]
- 40.Cheng W, Lui V C, Chen Q M, Tam P K. Enteric nervous system, interstitial cells of cajal, and smooth muscle vacuolization in segmental dilatation of jejunum. J Pediatr Surg. 2001;36(06):930–935. doi: 10.1053/jpsu.2001.23979. [DOI] [PubMed] [Google Scholar]
- 41.Huang S F, Vacanti J, Kozakewich H. Segmental defect of the intestinal musculature of a newborn: evidence of acquired pathogenesis. J Pediatr Surg. 1996;31(05):721–725. doi: 10.1016/s0022-3468(96)90687-9. [DOI] [PubMed] [Google Scholar]
- 42.Kuint J, Avigad I, Husar M, Linder N, Reichman B. Segmental dilatation of the ileum: an uncommon cause of neonatal intestinal obstruction. J Pediatr Surg. 1993;28(12):1637–1639. doi: 10.1016/0022-3468(93)90127-7. [DOI] [PubMed] [Google Scholar]
- 43.Machens A, Thonke F, Kluth D, Lambrecht W. An unusual case of segmental dilatation of the sigmoid colon. Eur J Pediatr Surg. 1997;7(06):369–370. doi: 10.1055/s-2008-1071195. [DOI] [PubMed] [Google Scholar]
- 44.Mboyo A, Aubert D, Massicot R, Destuynder O, Lassauge F, Lorin A. Antenatal finding of intestinal obstruction caused by isolated segmental jejunal dilatation: a case report. J Pediatr Surg. 1996;31(10):1454–1456. doi: 10.1016/s0022-3468(96)90856-8. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y, Hamada Y, Taguchi T. Berlin, Germany: Springer; 2017. Congenital segmental dilatation of the intestine; pp. 1–7. [Google Scholar]
- 46.Soyer T, Talim B, Tanyel F C. Segmental ileal dilatation with supernumerary intestinal muscle coat in a neonate. Surg Case Rep. 2015;1(01):16. doi: 10.1186/s40792-015-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bălănescu R N, Bălănescu L, Moga A A, Drăgan G C, Djendov F B. Segmental aganglionosis in Hirschsprung's disease in newborns - a case report. Rom J Morphol Embryol. 2015;56(02):533–536. [PubMed] [Google Scholar]
- 48.Moore S W, Sidler D, Schubert P A. Segmental aganglionosis (zonal aganglionosis or “skip” lesions) in Hirschsprung's disease: a report of 2 unusual cases. Pediatr Surg Int. 2013;29(05):495–500. doi: 10.1007/s00383-013-3286-8. [DOI] [PubMed] [Google Scholar]
- 49.Martin L W, Buchino J J, LeCoultre C, Ballard E T, Neblett W W. Hirschsprung's disease with skip area (segmental aganglionosis) J Pediatr Surg. 1979;14(06):686–687. doi: 10.1016/s0022-3468(79)80245-6. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Uchida N, Shiojima M et al. Segmental dilatation of the ileum covered almost entirely by gastric mucosa: report of a case. Surg Today. 2007;37(12):1102–1104. doi: 10.1007/s00595-007-3526-4. [DOI] [PubMed] [Google Scholar]
- 51.Arslan E E, Demir T A, Güney L H, Tepeoğlu M, Akıllı M S, Hiçsönmez A. A rare case of a gastric adenomyoma mimicking a gastric duplication cyst. Turk J Gastroenterol. 2018;29(05):613–615. doi: 10.5152/tjg.2018.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeyama J, Sato T, Tanaka H, Nio M. Adenomyoma of the stomach mimicking infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2007;42(11):E11–E12. doi: 10.1016/j.jpedsurg.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 53.Mathé J C, Khairallah S, Phat Vuoung N P, Boccon-Gibod L, Rey A, Costil J. [Segmental dilatation of the ileum in a neonate. Study of the myenteric plexus with a silver staining preparation (author's transl)] Nouv Presse Med. 1982;11(04):265–266. [PubMed] [Google Scholar]
- 54.Heller K, Waag K L. [Formal genesis of segmental intestinal dilatation] Langenbecks Arch Chir. 1987;372:735–738. doi: 10.1007/BF01297921. [DOI] [PubMed] [Google Scholar]
- 55.Hill M A.Embryology Paper - Teratogenicity in the setting of cardiac development and maldevelopmentAvailable at:https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Teratogenecity_in_the_setting_of_cardiac_development_and_maldevelopment. Accessed March 1, 2019
- 56.Hoffman J I, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 57.Calzolari E, Garani G, Cocchi G et al. Congenital heart defects: 15 years of experience of the Emilia-Romagna Registry (Italy) Eur J Epidemiol. 2003;18(08):773–780. doi: 10.1023/a:1025312603880. [DOI] [PubMed] [Google Scholar]
- 58.Pradat P, Francannet C, Harris J A, Robert E. The epidemiology of cardiovascular defects, part I: a study based on data from three large registries of congenital malformations. Pediatr Cardiol. 2003;24(03):195–221. doi: 10.1007/s00246-002-9401-6. [DOI] [PubMed] [Google Scholar]
- 59.Harris J A, Francannet C, Pradat P, Robert E. The epidemiology of cardiovascular defects, part 2: a study based on data from three large registries of congenital malformations. Pediatr Cardiol. 2003;24(03):222–235. doi: 10.1007/s00246-002-9402-5. [DOI] [PubMed] [Google Scholar]
- 60.Torres A M, Ziegler M M. Malrotation of the intestine. World J Surg. 1993;17(03):326–331. doi: 10.1007/BF01658699. [DOI] [PubMed] [Google Scholar]
- 61.Kouwenberg M, Severijnen R S, Kapusta L. Congenital cardiovascular defects in children with intestinal malrotation. Pediatr Surg Int. 2008;24(03):257–263. doi: 10.1007/s00383-007-2086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beddington R S, Robertson E J. Axis development and early asymmetry in mammals. Cell. 1999;96(02):195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 63.Essner J J, Branford W W, Zhang J, Yost H J. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127(05):1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- 64.Campione M, Steinbeisser H, Schweickert A et al. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126(06):1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- 65.Dagle J M, Sabel J L, Littig J L, Sutherland L B, Kolker S J, Weeks D L. Pitx2c attenuation results in cardiac defects and abnormalities of intestinal orientation in developing Xenopus laevis. Dev Biol. 2003;262(02):268–281. doi: 10.1016/s0012-1606(03)00389-0. [DOI] [PubMed] [Google Scholar]
- 66.Liu C, Liu W, Lu M F, Brown N A, Martin J F. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128(11):2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- 67.Ryan A K, Blumberg B, Rodriguez-Esteban Cet al. Pitx2 determines left-right asymmetry of internal organs in vertebrates Nature 1998394(6693):545–551. [DOI] [PubMed] [Google Scholar]
- 68.Logan M, Pagán-Westphal S M, Smith D M, Paganessi L, Tabin C J. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94(03):307–317. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- 69.Javors B R, Gold R P, Ghahremani G G et al. Idiopathic localized dilatation of the ileum in adults: findings on barium studies. AJR Am J Roentgenol. 1995;164(01):87–90. doi: 10.2214/ajr.164.1.7998575. [DOI] [PubMed] [Google Scholar]
- 70.Metcalfe P D, Bascom A, Sergi C.Mimickers and tumours in the lower urinary tract: do we need more efficient vigilance? Can Urol Assoc J 20137(5-6):E421–E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.al-Salem A H, Grant C. Segmental dilatation of the colon. Report of a case and review of the literature. Dis Colon Rectum. 1990;33(06):515–518. doi: 10.1007/BF02052150. [DOI] [PubMed] [Google Scholar]
- 72.Brawner J, Shafer A D. Segmental dilatation of the colon. J Pediatr Surg. 1973;8(06):957–958. doi: 10.1016/0022-3468(73)90023-7. [DOI] [PubMed] [Google Scholar]
- 73.Peña A, el Behery M. Megasigmoid: a source of pseudoincontinence in children with repaired anorectal malformations. J Pediatr Surg. 1993;28(02):199–203. doi: 10.1016/s0022-3468(05)80275-1. [DOI] [PubMed] [Google Scholar]
- 74.Ravasse P, Petit T, Cau D, Delmas P. Volvulus of the sigmoid colon as a complication of segmental dilatation of the colon. Report of 2 cases. Eur J Pediatr Surg. 1996;6(06):375–377. doi: 10.1055/s-2008-1071021. [DOI] [PubMed] [Google Scholar]
- 75.Pelletier J S, LaBossiere J, Dicken B et al. Low-dose vasopressin improves cardiac function in newborn piglets with acute hypoxia-reoxygenation. Shock. 2013;40(04):320–326. doi: 10.1097/SHK.0b013e3182a4284e. [DOI] [PubMed] [Google Scholar]


