Abstract
Background and study aims There are numerous studies published on the diagnostic yield of the new fine-needle biopsy (FNB) needles in pancreas masses. However, there are limited studies in suspected gastrointestinal stromal tumors (GIST lesions). The aim of this study was to evaluate the diagnostic yield of a new fork-tip FNB needle.
Patients and methods This was a multicenter retrospective study of consecutive patients from prospectively maintained databases comparing endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) versus endoscopic ultrasound-guided FNB (EUS-FNB) using the fork-tip needle. Outcomes measured were cytopathology yield (ability to obtain tissue for analysis of cytology), ability to analyze the tissue for immunohistochemistry (IHC yield), and diagnostic yield (ability to provide a definitive diagnosis).
Results A total of 147 patients were included in the study of which 101 underwent EUS-FNB and 46 patients underwent EUS-FNA. Median lesion size in each group was similar (21 mm vs 25 mm, P = 0.25). Cytopathology yield, IHC yield, and diagnostic yield were 92 % vs 46 % ( P = 0.001), 89 % vs 41 % ( P = 0.001), and 89 % vs 37 % ( P = 0.001) between the FNB and FNA groups, respectively. Median number of passes was the same between the two groups at 3.5.
Conclusion EUS-FNB is superior to EUS-FNA for diagnostic yield of suspected GIST lesions. This should be confirmed with a prospective study.
Introduction
Incidental detection of subepithelial lesions of the gastrointestinal tract is common owing to widespread use of endoscopy 1 2 3 . These lesions are found in approximately 1 in 300 of all upper endoscopy procedures, with the majority of them being gastrointestinal stromal tumors (GIST) 2 3 . Traditionally, endoscopic ultrasound-guided tissue acquisition (EUS-TA) has been the gold standard for identifying the layer in which the subepithelial lesion resides, obtaining accurate measurements, but also obtaining tissue. The goal of tissue acquisition is to obtain not only a sample that can yield spindle cells, but also a sample in which immunohistochemistry (IHC) can be performed. IHC staining for CD117, DOG1, S100, CD34, and PDGFRA can differentiate GISTs from other subepithelial lesions 4 .
Traditionally EUS-guided fine-needle aspiration (EUS-FNA) was the mainstay of obtaining tissue. Diagnostic yield is variable and has been reported to be anywhere from 46 % to 93 % 3 5 6 7 8 . The yield of EUS-FNA has been suboptimal and overall, it has been limited in its ability to provide a quantity of tissue sufficient for IHC 3 9 . To help overcome this issue for sampling of GIST and other lesions, core needles were developed. However, trials showed mixed results and the upwards diagnostic yield of 75 % was still not optimal 10 11 . Recently newer core needles have been developed for EUS-guided fine-needle biopsy (EUS-FNB) that can help optimize diagnostic yield for stromal tumors. Most of these data have been evaluated for diagnostic yield of fork-tip FNB needle in pancreatic masses 12 13 14 15 16 17 . The fork-tip needle has six cutting surfaces that help obtain a core tissue. Studies comparing the fork-tip needle to FNA have shown an increased diagnostic yield, sufficient aspirated material for histology compared to cytology for FNA needles, and need for fewer passes.
Although an abundant body of literature now exists for use of the newer-generation core needles for pancreatic masses, data are lacking on their use for suspected GIST. There is one retrospective study comparing EUS-FNA to EUS-FNB with the fork-tip needle 9 . That study compared 91 patients with EUS-FNA using a 22-gauge needle to only 15 patients for EUS-FNB using the 22-gauge fork-tip needle. The study found a significant difference in diagnostic yield (53 % vs 87 %, P = 0.01). Although these data are encouraging, only 15 patients were included in the study who underwent FNB with a fork-tip needle. Thus more data are needed on the diagnostic yield of the fork-tip core needle before it can be considered as the primary needle for use in suspected GIST. The aim of this study was to report the diagnostic yield of this needle in a large multicenter study for consecutive patients undergoing EUS-FNB sampling versus EUS-FNA sampling.
Patients and methods
This was a retrospective multicenter study of five endosonographers from five academic tertiary care hospitals in the United States. Each endosonographer primarily used the fork-tip FNB needle since its inception as their primary needle for suspected GIST. Prior to this, EUS-FNA was performed with standard FNA needles. Institutional Review Board approval was obtained from each participating center. Procedures were in accordance with ethical standards as they are considered the standard of care and with the Helsinki Declaration of 1975. Informed consent was obtained for every procedure.
Data were abstracted from prospectively maintained endoscopic databases. Patients were included if they: 1) had a suspected GIST based on EUS characteristics (i. e., lesions that were fourth-layer lesions, hypoechoic, and heterogeneous) 18 ; 2) underwent EUS-guided sampling using a fork-tip FNB needle (Sharkcore, Medtronic, United States) or with standard FNA needles; and 3) were age18 or older. Indications for EUS-FNB were to obtain a diagnosis to justify surgery or differentiate from a benign lesion such as a leiomyoma that would not require further endoscopic follow-up. This was especially the case for lesions smaller than 2 cm. Patients were excluded if they: 1) underwent sampling with both a FNA and FNB needle, as it would be unclear which needle contributed to a positive yield; 2) were younger than age 18; or 3) had repeat procedures that were also negative on the first session, so as to not artificially decrease the yield of the FNA group (most of repeat procedures occurred in FNA group). Procedure characteristics that were abstracted included lesion size and location, echogenicity, homogeneity, size of needle used, number of passes, and presence of rapid onsite cytology. Cytopathology characteristics abstracted were ability to detect spindle cells or diagnostic cells and ability to perform IHC. All reports were de-identified and made available to all investigators among all five sites to ensure data accuracy. De-identified reports were uploaded to an institutional encrypted server to which all investigators had access. None of the cases in this study have been published elsewhere in full manuscript form.
The technique used for FNB was the slow-pull stylet technique or suction technique. For the slow-pull technique, once the needle was advanced into the lesion under endosonographic guidance, the stylet was slowly removed by an assistant, while at least five throws of the needle were made into the mass. The goal was to obtain a visible core of tissue ( Fig. 1 ). EUS-FNA technique was at the discretion of the endoscopist but included standard suction, wet suction, or no suction technique. Number of passes taken in each group was at the discretion of the endoscopist. This was mainly decided based on the yield of material that was being obtained.
Fig. 1.
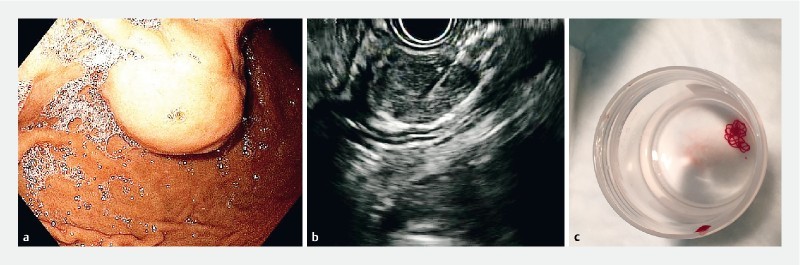
Example of a lesion from this study. a Endoscopic view of a subepithelial lesion in the stomach suspected of being a gastrointestinal stromal tumor (GIST). b Fine-needle biopsy under endoscopic ultrasound guidance. c Visible core of tissue obtained after one pass placed in formalin. Histology and immunohistochemistry were consistent with a GIST.
For cases in which a cytotechnologist was present, a portion of the FNA or FNB specimen was placed on a slide. The slide was air dried and then stained with Diff-Quik. This was used to determine adequacy of the sample. A second slide was fixed with 95 % ethyl alcohol and polyethylene glycol and dipped into Papanicolaou stain for more detailed cytologic examination. For cases in which a cytotechnologist was not present, the sample was placed in formalin and processed as a typical cell block. If a sample had spindle cells on cytopathology, it was then stained for IHC (CD117 and DOG-3).
The study had three primary outcomes. Ability to yield material for evaluation (e. g.: obtain spindle cells) was defined as the cytopathology yield. Technically in FNB, a core sample is yielded that can be analyzed by pathology for histology rather than cytology. However. for purposes of the study, to be consistent between both groups, we termed this cytopathologic yield. Ability to perform IHC in order to obtain a diagnosis of a GIST was defined as the IHC yield. Ability to yield a distinct diagnosis (when combining cytology and IHC) was defined as the diagnostic yield. If cytology showed spindle cells but there was not enough material to perform IHC, then that patient was defined as someone for whom a cytologic yield was possible but not a diagnostic yield. However, when a sample had spindle cells on cytopathology and stained positive for IHC markers of a GIST (CD117, DOG-3), then it had both a cytologic and diagnostic yield.
Chi square testing was performed for categorical variables. A student t test was performed for continuous variables. Statistical significance was defined as P < 0.05. All statistics were performed using SAS version 9.4 (Cary, North Carolina, United States).
Results
A total of 147 patients were included in the study, of whom 101 underwent EUS-FNB using a Fork-tip needle and 46 underwent EUS-FNA. Thirty-two patients were excluded as multiple needles were used in the procedure. Patient and lesion characteristics are shown in Table 1 . Median age of patients was 66 in each group. Both groups had a high percentage of males (57 % in FNA vs 52 % in FNB, P = 0.57). There were no statistically significant differences between the two groups in regards to lesion size or median number of passes taken. The majority of suspected lesions were in the stomach in both groups. In both groups, the 22-gauge needle was the most common needle used (85 % in FNB group vs 61 % in the FNA group, P = 0.001) although the FNA group also had higher usage of the 25-gauge and 19-gauge needle versus the FNB group (20 % vs 9 %, P = 0.07 and 20 % vs 6 %, P = 0.01). There were no reported immediate AEs recorded in the procedure reports for either group. No reported delayed AEs were abstracted from the charts in either group.
Table 1. Patient and lesion characteristics.
| Characteristics | All patients N = 147 | FNA N = 46 | FNB N = 101 | P value |
| Age, median | 66 | 66 | 66 | 0.31 |
| Gender, male: female | 78:69 | 26:20 | 52:49 | |
|
69 (47 %) | 20 (43.5 %) | 49 (48.5 %) | 0.57 |
|
78 (53 %) | 26 (56.5 %) | 52 (51.5 %) | 0.57 |
| Size of mass, median mm | 23 | 20.5 | 25 | 0.25 |
| Lesion location, no. (%) | ||||
|
18 (12.3 %) | 7 (15.2 %) | 11 (10.9 %) | 0.46 |
|
115 (78.2 %) | 31 (67.4 %) | 84 (83.2 %) | 0.03 |
|
5 (3.4 %) | 2 (4.4 %) | 3 (3.0 %) | 0.70 |
|
6 (4.1 %) | 4 (8.7 %) | 2 (2.0 %) | 0.06 |
|
3 (2.0 %) | 2 (4.4 %) | 1 (1 %) | 0.18 |
| Distribution of lesion type (%) | ||||
|
40 (27.2 %) | 29 (63 %) | 11 (10.9 %) | 0.0001 |
|
87 (59.2 %) | 12 (26.1 %) | 75 (74.3 %) | 0.0001 |
|
14 (9.52 %) | 3 (6.5 %) | 11 (10.9 %) | 0.32 |
|
6 (4.1 %) | 2 (4.4 %) | 4 (4 %) | 0.91 |
| Lesion EUS features, no. (%) | ||||
|
106 (72.11 %) | 36 (78.3 %) | 70 (69.3 %) | 0.2635 |
|
41 (27.9 %) | 10 (21.7 %) | 31 (30.7 %) | 0.2635 |
|
133 (88.7 %) | 38 (82.6 %) | 90 (89.11 %) | 0.2777 |
|
17 (11.3 %) | 8 (17.4 %) | 11 (10.9 %) | 0.2777 |
Table 2 contains information on procedure characteristics and outcomes. Ninety-three patients in the FNB group had material aspirated with a cytopathology yield while 21 patients had a cytopathology yield in the FNA group (92 % vs 46 %, P = 0.001). Subgroup analysis was performed by lesion size ( Table 3 ). The groups analyzed were < 10 mm, 11 to 15 mm, 16 to 20 mm, 21 to 30 mm, and > 30 mm. FNB had a statistically significant higher cytopathology yield in every size group except for lesions < 10 mm. No difference was observed in diagnostic yield based on location in the stomach. Lesions that underwent FNB in the fundus, body, and cardia had a diagnostic yield of 87.5 %, 88 %, and 89 %, respectively ( P values not significant).
Table 2. Procedure characteristics and outcomes.
| Characteristics | All patients N = 147 | FNA N = 46 | FNB N = 101 | P value |
| Needle size, gauge, and no. (%) | ||||
|
15 (10.2 %) | 9 (19.6 %) | 6 (5.9 %) | 0.01 |
|
114 (77.6 %) | 28 (60.9 %) | 86 (85.2 %) | 0.001 |
|
18 (12.2 %) | 9 (19.6 %) | 9 (8.9 %) | 0.07 |
| No. of passes, median | 3.5 | 3.5 | 3.5 | NS |
| Diagnostic yield (%) | 107 (72.8 %) | 17 (37 %) | 90 (89.1 %) | 0.0001 |
| Adequate tissue obtained to perform IHC (IHC yield) | 109 (74.2 %) | 19 (41.3 %) | 90 (89.1 %) | 0.0001 |
| Use of rapid onsite cytology | 34 (22.7 %) | 15 (32.6 %) | 19 (18.3 %) | 0.06 |
| Cytopathology yield | 112 (74.7 %) | 21 (45.7 %) | 93 (92.1 %) | 0.0001 |
Table 3. Subgroup analysis based on lesion size.
| < 10 mm | P value | 11 – 15 mm | P value | 16 – 20 mm | P value | 21 – 30 mm | P value | > 30 mm | P value | |
| Diagnostic yield for FNB N = 90 | 4/7 (57.1 %) | NS | 11/13 (84.6 %) | NS | 17/19 (89.5 %) | 0.0005 | 29/30 (96.7 %) | 0.0001 | 29/32 (90.6 %) | 0.002 |
| Diagnostic yield for FNA N = 17 | 3/6 (50 %) | 4/8 (50 %) | 2/9 (22.2 %) | 4/14 (28.6 %) | 4/9 (44.4 %) | |||||
| GISTs in FNB group N = 75 | 3/7 (42.9 %) | NS | 6/13 (46.2 %) | NS | 15/19 (79 %) | 0.0009 | 24/30 (80 %) | 0.0002 | 27/32 (84.4) | 0.02 |
| GIST in FNA group N = 12 | 0 | 4/8 (50 %) | 1/9 (11.1 %) | 3/14 (21.4 %) | 4/9 (44.4 %) | |||||
| Leiomyoma in FNB group N = 11 | 1/7 (14.3 %) | NS | 4/13 (30.8 %) | NS | 2/19 (10.5 %) | NS | 2/30 (6.7 %) | NS | 2/32 (6.3 %) | NS |
| Leiomyoma in FNA group N = 3 | 2/6 (33.3 %) | 0 | 1/9 (11.1 %) | 0 | 0 | |||||
| Other in FNB group N = 4 | 0/7 | NS | 1/13 (7.7 %) | NS | 0 | NS | 3/30 (10 %) | NS | 0 (0 %) | NS |
| Other in FNA group N = 2 | 1/6 (16.7 %) | 0 | 0 | 1/14 (7.1 %) | 0 (%) | |||||
| IHC in FNB group N = 90 | 4/7 (42.9 %) | NS | 11/13 (84.6 %) | NS | 17/19 (89.5 %) | 0.0005 | 29/30 (96.7 %) | 0.0001 | 29/32 (90.6 %) | 0.01 |
| IHC in FNA group N = 19 | 3/6 (50 %) | 4/8 (50 %) | 2/9 (22.2 %) | 5/14 (35.7 %) | 5/9 (55.6 %) | |||||
| Cytopathology yield for FNB N = 93 | 4/7 (57.1 %) | NS | 12/13 (92.3) | 0.03 | 17/19 (89.5 %) | 0.0005 | 29/30 (96.7 %) | 0.0001 | 31/32 (96.9 %) | 0.008 |
| Cytopathology yield for FNA N = 21 | 4/6 (66.7 %) | 4/8 (50 % ) | 2/9 (22.2 %) | 5/14 (35.7 %) | 6/9 (66.7 %) |
FNB, fine-needle biopsy; GIST, gastrointestinal stromal tumor; FNA, fine-needle aspiration. NS, not significant or cannot calculate due to null values in one group.
An IHC yield was obtained in 90 patients in the FNB group and 19 patients in the FNA group (89 % vs 41 %, P < 0.001). Subgroup analysis was also performed by size for IHC yield ( Table3 ). FNB had a statistically higher IHC yield for lesions > 16 mm. Lesions ≤ 15 mm had no statistical difference. Overall when incorporating the cytology and IHC, the EUS-FNB group had an overall diagnostic yield of 89 % vs 37 % for the EUS-FNA group ( P < 0.0001). Subgroup analysis was also performed by size for diagnostic yield ( Table 3 ). FNB had a statistically higher diagnostic yield for lesions > 16 mm. Lesions ≤ 15 mm had no statistical difference.
Rapid On-Site Evaluation (ROSE) was utilized in 18 % of patients who underwent FNB and 32 % of patients with FNA ( P = 0.06). There were no specific features of a lesion (eg lesion size, location, etc.) that predicted when ROSE was used.
Discussion
In this multicenter retrospective study, we showed that EUS-FNB has a statistically higher diagnostic yield for suspected GIST lesions than EUS- FNA. Diagnostic yield for FNB was more than double that of FNA. These data provide compelling evidence to support new core needles, specifically the fork-tip needle used in this study, as the standard of care needle for suspected GIST lesions.
Most of the data for the new fine needle biopsy needles have been from studies looking at diagnostic yield in pancreas masses. Both a recent randomized controlled trial 19 and an updated meta-analysis 20 of EUS-FNA vs EUS-FNB for pancreatic masses show similar diagnostic yields between the two needles. Thus the exact role of FNB needles in regards to diagnostic yield of pancreatic masses is unclear 21 . Per expert opinion, EUS-FNA would suffice for most tissue acquisition for most pancreatic masses 21 . The additional benefit of FNB may be for personalized medicine to obtain histologic grade tissue for genotyping.
The role of FNB for suspected GIST lesions, unlike with pancreatic masses, seems more straightforward. From the available literature on FNB for suspected GIST lesions (the aforementioned smaller study 9 and this current study) the diagnostic yield is significantly higher for FNB. This correlates with our personal experience where it was common to bring a patient back for additional tissue for a non-diagnostic result from EUS-FNA. However, because of use of the fork-tip FNB needles, it is now uncommon to bring patients back for repeat sampling. It should be noted that a previous prospective study was performed with a reverse bevel core needle on all suspected subepithelial lesions on 70 patients from 2012 to 2015 22 . Patients also underwent FNA at the same session. This study showed a higher diagnostic yield for the FNB needle. Although this study did not evaluate one of the newer FNB needles, it did support use of FNB over FNA.
In the current study, 63 % of patients in the FNA group had a non-diagnostic result. These patients either underwent another repeat procedure or if clinical suspicion was high, went to surgery for lesion removal. Although this was not a cost-effective analysis study, use of a FNB needle prevented repeat EUS procedures or unnecessary surgery on lesions found to be benign at surgery. Thus, increased cost of the FNB needle may be justified in EUS sampling cases of suspected GIST lesions.
This study had limitations. First, it was retrospective. However, the cases were abstracted from prospective endoscopic databases of consecutive patients. Thus the study design was adequate to show the difference between FNA and FNB. It should also be noted that the cases were performed in academic tertiary care centers, thus the study results are applicable only to tertiary care centers. Another limitation is that the FNA and FNB groups were heterogeneous in regards to sampling techniques (for both FNA and FNB groups) and needle gauge (FNA group). The needles used in the FNA group included needles from three different manufacturers (Medtronic Beacon needle, Boston Scientific Expect needle, and Cook Medical EchoTip needle). However, all sampling techniques were standard best practices so as to obtain a high diagnostic result, and thus reflect a real-world situation. A prospective study would likely use the same sampling techniques and gauge needles, as these are standard-of-care practices. Finally, because the procedures were performed at tertiary care centers, the patients were referred back to their local gastroenterologists and surgeons, thus surgical follow-up is lacking.
Conclusion
In conclusion, in this large multicenter study, we showed that FNB needles for suspected GIST lesions are superior to FNA needles for diagnostic yield. Although the evidence in this study strongly supports use of FNB needles for suspected GIST lesion, they ideally require confirmation with a prospective study.
Footnotes
Competing interests Dr. Trindade is a consultant for Pentax Medical. Dr. Benias is a consultant for Medtronic and Fuji Film. Dr. Ryou is a consultant for Medtronic, Pentax Medical, Fuji Film, and GI Windows.
References
- 1.Hedenbro J L, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 2.Polkowski M, Gerke W, Jarosz D et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329–334. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 3.Obuch J, Wani S. EUS-guided tissue acquisition in GI stromal tumors. Gastrointest Endosc. 2017;86:516–518. doi: 10.1016/j.gie.2017.03.1515. [DOI] [PubMed] [Google Scholar]
- 4.Nishida T, Blay J-Y, Hirota S et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akahoshi K. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoda K M, Rodriguez S A, Faigel D O. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–1223. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Philipper M, Hollerbach S, Gabbert H et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–305. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 8.Watson R R, Binmoeller K F, Hamerski C M et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757–1762. doi: 10.1007/s10620-011-1646-6. [DOI] [PubMed] [Google Scholar]
- 9.El Chafic A H, Loren D, Siddiqui A et al. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2017;86:510–515. doi: 10.1016/j.gie.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Esparrach G, Sendino O, Solé M et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292–299. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 11.Korean EUS Study Group . Kim G H, Cho Y K, Kim E Y et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347–354. doi: 10.3109/00365521.2013.867361. [DOI] [PubMed] [Google Scholar]
- 12.Jovani M, Abidi W M, Lee L S. Novel fork-tip needles versus standard needles for EUS-guided tissue acquisition from solid masses of the upper GI tract: a matched cohort study. Scand J Gastroenterol. 2017;52:784–787. doi: 10.1080/00365521.2017.1306879. [DOI] [PubMed] [Google Scholar]
- 13.Bang J Y, Hebert-Magee S, Navaneethan U et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–1438. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Kandel P, Tranesh G, Nassar A et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016;84:1034–1039. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 15.Abdelfatah M M, Grimm I S, Gangarosa L M et al. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018;87:495–500. doi: 10.1016/j.gie.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Naveed M, Siddiqui A A, Kowalski T E et al. A multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCoreTM) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018;7:34–40. doi: 10.4103/eus.eus_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa T, Mohamed R, Heitman S J et al. Diagnostic yield of small histological cores obtained with a new EUS-guided fine needle biopsy system. Surg Endosc. 2017;31:5143–5149. doi: 10.1007/s00464-017-5580-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim G H, Park D Y, Kim S et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? 2009;15:3376–3381. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagula S, Pourmand K, Aslanian H et al. New York Endoscopic Research Outcomes Group (NYERO). Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018;16:1307–1313. doi: 10.1016/j.cgh.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Machicado J D, Wani S, Thosani N. Will Abandoning fine-needle aspiration increase diagnostic yield from tissues collected during endoscopic ultrasound? Clin Gastroenterol Hepatol. 2018;16:1203–1206. doi: 10.1016/j.cgh.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Wani S, Shah R J. EUS-guided tissue acquisition: Do we need to shoot for a “core” to score? Gastrointest Endosc. 2016;84:1047–1049. doi: 10.1016/j.gie.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Bengt H M, Akif N. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions : a prospective, comparative study. 2018;21:1304–1313. doi: 10.1007/s00464-017-5808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


