Abstract
Focal cartilage defects lead to significant pain and disability, prompting the development of various options for biologic restoration of the articular surface. Although each technique and biologic implant provides various advantages and associated limitations, matrix-induced autologous chondrocyte implantation (MACI) has gained popularity given promising long-term results. We present a technique for the facile implantation of MACI membranes using cookie cutter instrumentation to aid in defect debridement and graft preparation. The technique described allows for efficient operative workflow while ensuring the creation of vertical, stable defect edges and a form-fitting MACI membrane in a readily implemented fashion.
Articular cartilage defects can impair quality of life in a fashion similar to severe osteoarthritis and cause long-term dysfunction and joint degeneration.1 These defects have limited healing potential because of the poor regenerative capacity and avascular nature of cartilage. As a result, chondral lesions can be a source of pain and mechanical symptoms, as well as a risk factor for posttraumatic osteoarthritis.
Given that palliative treatment options offer limited and short-term symptom relief and arthroplasty is reserved for end-stage arthritic disease states, articular cartilage restoration has demonstrated effectiveness in reducing pain and functional disability.2 A variety of surgical options are available to treat cartilage lesions, including microfracture, osteochondral autograft transfer, osteochondral allograft transplantation, and matrix-induced autologous chondrocyte implantation (MACI).3, 4 In several large studies and randomized controlled trials, cell-based strategies such as MACI have demonstrated better durability than microfracture, owing to formation of hyaline-like cartilage over fibrocartilage.4, 5, 6
In placing the MACI membrane, defect edges are debrided freehand to stable and vertical walls. Surgeons subsequently cut the membrane to approximate the size and shape of the defect. In this Technical Note, we present a technique for the facile implantation of MACI membranes (Vericel) using cookie cutter instrumentation to aid in efficient defect debridement and graft preparation, along with a video demonstrating the technique described (Video) and a list of pearls and pitfalls the authors have found helpful (Table 1). The technique described allows for the creation of true, vertical defect edges and a form-fitting membrane cutout for exact and complete defect fill at the time of implantation.
Table 1.
Pearls and Pitfalls of the Cookie Cutter Technique
| Pearls | Pitfalls |
|---|---|
| Match cookie cutter tool to defect size and shape | Choosing a template that is a poor match to the defect and leads to inadequate or overzealous cartilage debridement |
| Debride cartilage to stable vertical borders and remove diseased surrounding cartilage | Failure to debride cartilage to calcified layer for subsequent MACI membrane application |
| Take care to gently mallet cookie cutter to the calcified cartilage layer | Excessive malleting, which can create subchondral violation using the cookie cutter |
| Allow the fibrin glue sufficient time to cure before testing implant stability and proceeding with arthrotomy closure | Early implant stability testing or arthrotomy closure, which may lead to implant dislodgement and failure |
Surgical Technique
Patient Positioning and Preparation
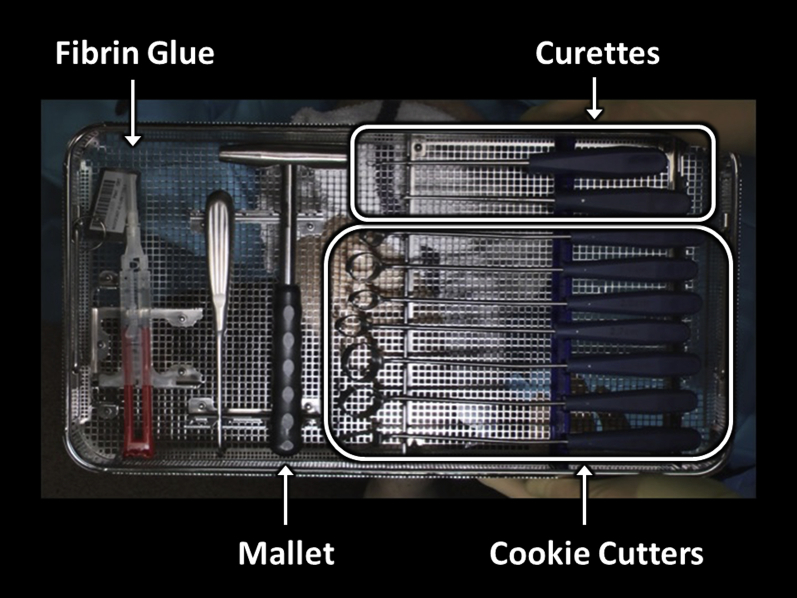
The patient is positioned supine with the knee over the edge of the operating table. The lower extremity is prepared and draped in the standard fashion for mini–open knee arthrotomy. The extremity is exsanguinated, and the tourniquet is inflated to 250 mm Hg. Appropriate supplies for MACI implantation are confirmed in the operating room, including sharp curettes, a complete set of cookie cutter instrumentation for various defect shapes and sizes, the manufactured MACI membrane itself, and fibrin glue (4 mL; Tisseel; Baxter) for membrane fixation (Figure 1).
Fig 1.
Surgical supplies and instrumentation necessary for matrix-induced autologous chondrocyte implantation with the described technique, namely curettes, cookie cutters of various sizes, and fibrin glue.
Arthrotomy and Graft Site Preparation
A 6- to 8-cm medial- or lateral-based longitudinal incision is used to gain access to the knee, depending on defect location. A parapatellar arthrotomy is performed with preservation of the meniscus and care taken to avoid damage to the underlying articular cartilage (Figure 2). The patella is subluxed medially or laterally to allow surgical visualization of the defect, and retractors are placed (Figure 3).
Fig 2.
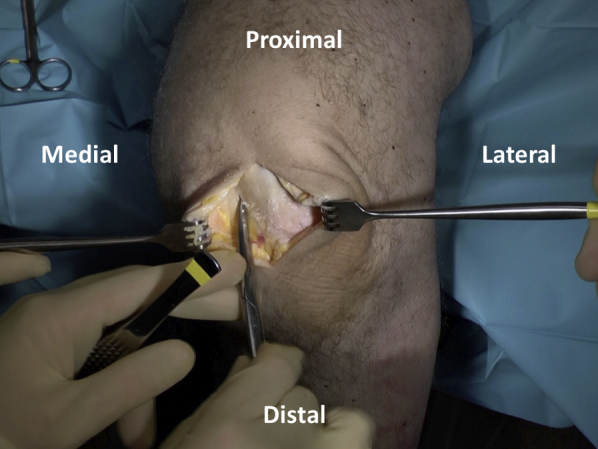
A longitudinal incision is used to gain access to the knee and focal cartilage defect location. Scissors are used to perform a parapatellar arthrotomy while preserving the meniscus and avoiding damage to the underlying cartilage.
Fig 3.
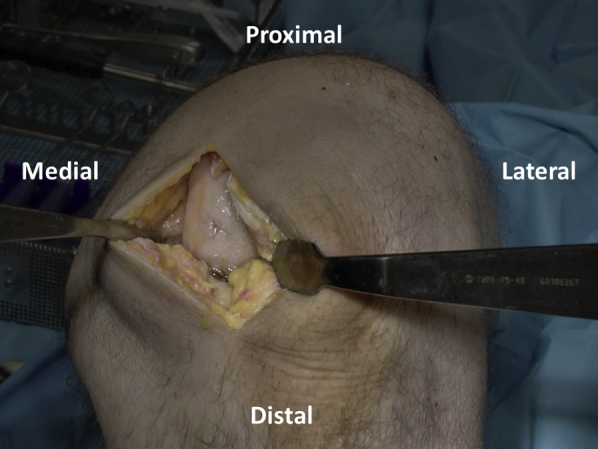
The patella is subluxed medially to allow visualization of the defect. Retractors are placed to provide a clear view and surgical access to the defect.
Cookie cutters of various sizes and shapes are tried to determine the ideal selection for defect matching (Figure 4). Once chosen, the appropriate size cutter (with defect size marked on the handles) is gently malleted down to the level of the calcified cartilage layer to create a contained defect with stable and vertical edges. A ringed curette is then carefully used to remove the pathologic cartilage from within the defect (Figure 5). Final preparation is made using fine strokes to ensure preparation up to but not beyond the calcified tide mark layer of cartilage. Rolled gauze on curved surgical hemostats may then be used to atraumatically clear debrided cartilage from the base of the prepared area (Figure 6).
Fig 4.
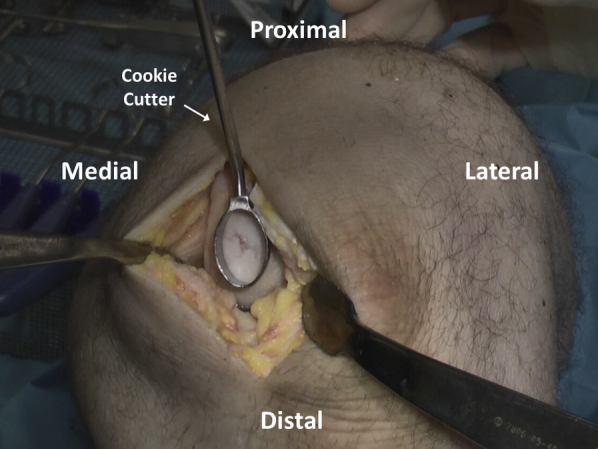
Cookie cutters are tried by placing them over the defect to determine the ideal selection for defect matching.
Fig 5.
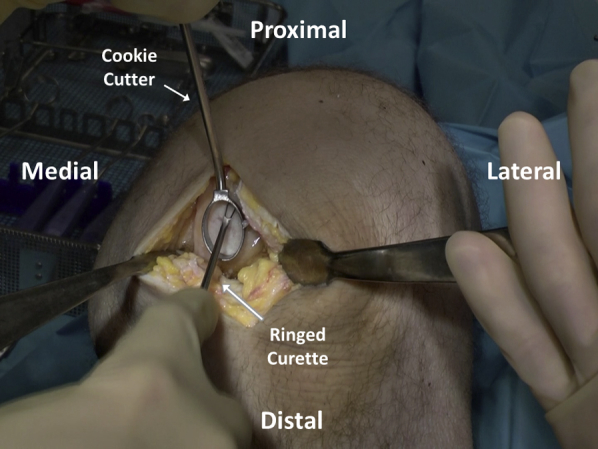
The focal cartilage defect is debrided using a ringed curette, which is contained using a cookie cutter that has been malleted into place to provide stable, vertical walls.
Fig 6.
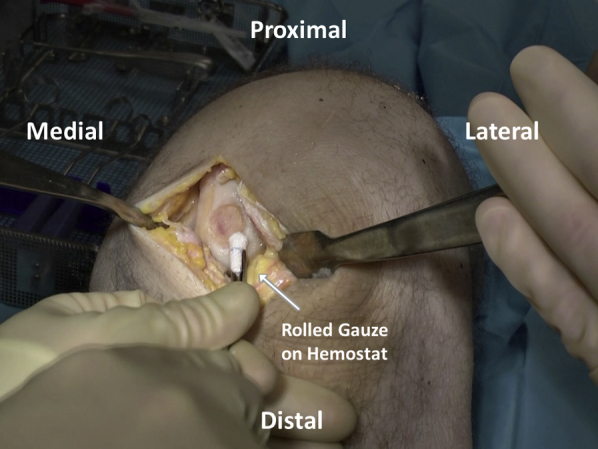
After cartilage defect debridement, final preparation is carried out using rolled gauze on curved surgical hemostats to atraumatically clear the base of the prepared area.
Graft Preparation
After satisfactory defect debridement to stable edges, attention is turned to graft preparation. The authors' chosen technique is to place the membrane onto a piece of sterile foil, such as that from suture packaging, to facilitate preparation (Figure 7). It is key to note the orientation of the membrane to ensure that the matted cellular layer will come to rest on the debrided defect base. With this technique, the membrane is placed smooth, shiny side down and matted side up onto the sterile foil. Subsequently, the cookie cutter that had been previously used to debride the articular defect is placed over the membrane and gently malleted down (Figure 8). The resulting construct provides the membrane on a piece of foil, ready for transfer, while protecting the membrane from forceps-induced damage as it is moved to the articular surface (Figure 9).
Fig 7.
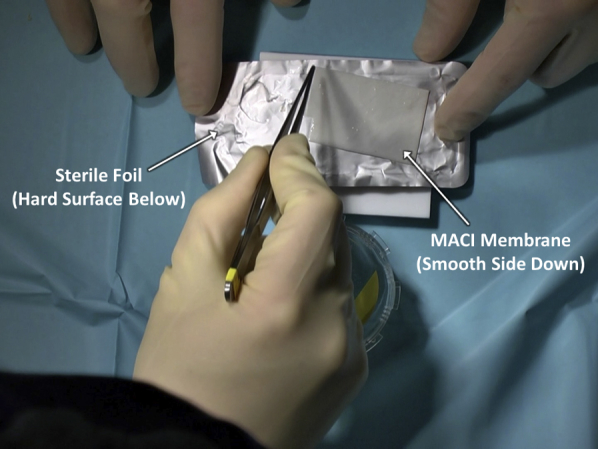
The matrix-induced autologous chondrocyte implantation (MACI) membrane is placed, smooth and shiny side down and matted side up, onto a sterile piece of foil, such as that found in suture packaging. A hard surface is present under the foil in preparation for membrane sizing with the cookie cutter and a mallet.
Fig 8.
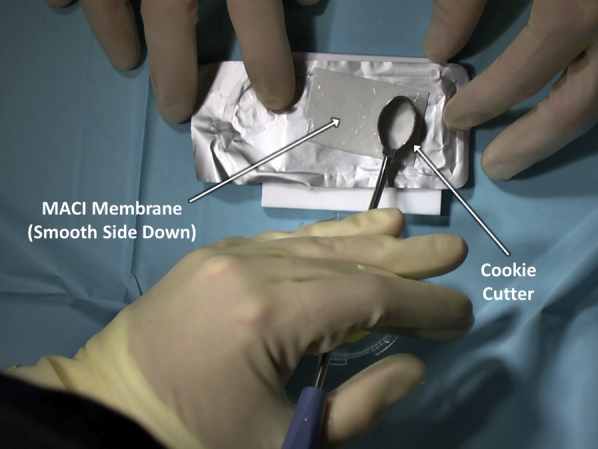
The cookie cutter that had been previously used to debride the articular defect is placed over the matrix-induced autologous chondrocyte implantation (MACI) membrane and gently malleted down.
Fig 9.
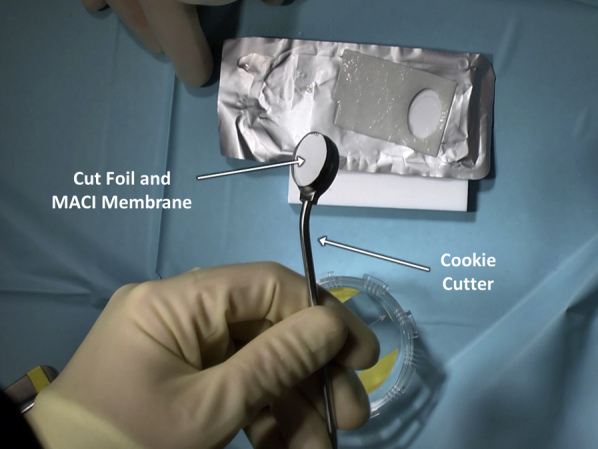
By malleting down the cookie cutter that had been previously used to debride the articular defect, a piece of matrix-induced autologous chondrocyte implantation (MACI) membrane and underlying foil for handling is generated, ready for subsequent implantation.
Graft Implantation
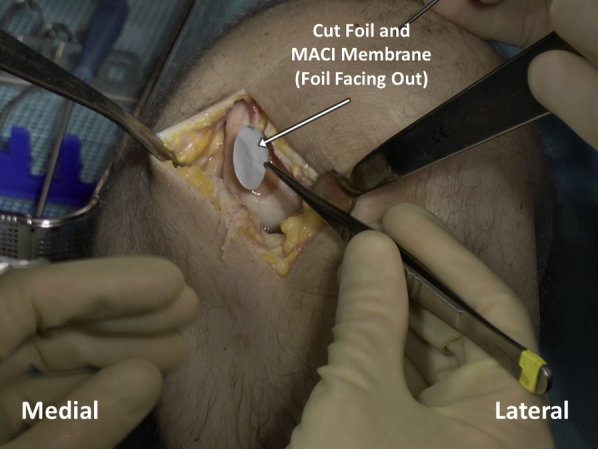
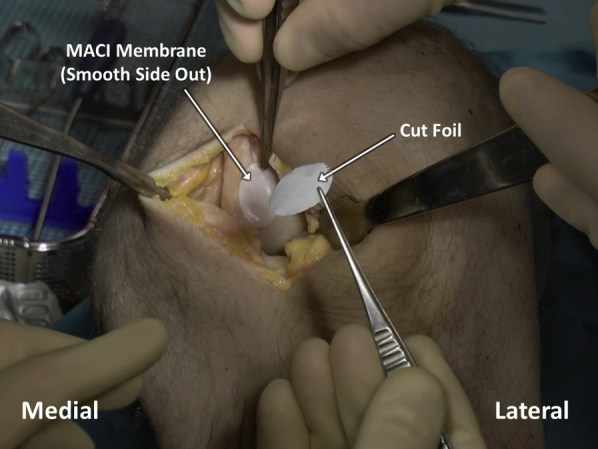
Fibrin glue is applied within the defect bed in an even, thin layer (Figure 10) and allowed to cure for 3 minutes. The membrane is placed in the defect, taking care to handle the membrane gently and to grasp it by the supporting foil as opposed to by the membrane itself (Figure 11). The final membrane should come to rest with the matted side on the defect base and the foil facing outwards. The foil carrier is then removed, exposing the smooth surface of the MACI implant (Figure 12). The membrane is gently compressed down against the defect for 3 minutes, and an additional, even layer of fibrin glue is applied over the membrane and allowed to cure for another 3 minutes (Figure 13).
Fig 10.
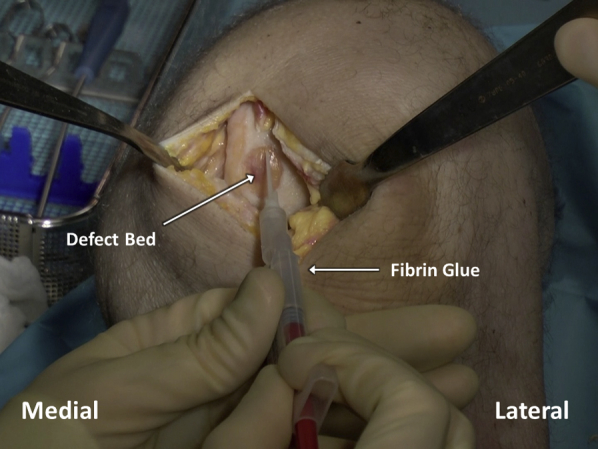
After appropriate sizing and cutting of the matrix-induced autologous chondrocyte implantation membrane with the cookie cutter, fibrin glue is applied in a thin and even layer in the debrided articular cartilage defect bed.
Fig 11.
The foil and the matrix-induced autologous chondrocyte implantation (MACI) membrane, which have been cut with the cookie cutter to match the debrided defect, are placed within the defect bed, which has been prepared with fibrin glue. Care is taken to handle the membrane gently and to grasp it by the supporting foil as opposed to by the membrane itself.
Fig 12.
After the cut matrix-induced autologous chondrocyte implantation (MACI) membrane and overlying foil are placed atop the debrided defect bed, which has been prepared with fibrin glue, the foil carrier is then removed, exposing the smooth surface of the MACI implant.
Fig 13.
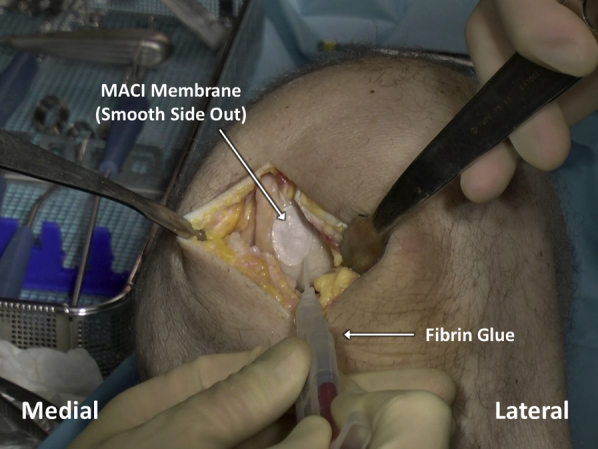
Once the matrix-induced autologous chondrocyte implantation (MACI) membrane has been placed over the articular cartilage defect bed and left for 3 minutes to cure to the underlying fibrin glue, an additional, even layer of fibrin glue is applied over the membrane and allowed to cure for another 3 minutes.
After the fibrin glue has cured, a final graft check is performed. Knee retractors are gently removed, and the patella is allowed to relocate. The knee is subsequently cycled 5-10 times, and the graft is once again visualized to ensure stability (Figure 14).
Fig 14.
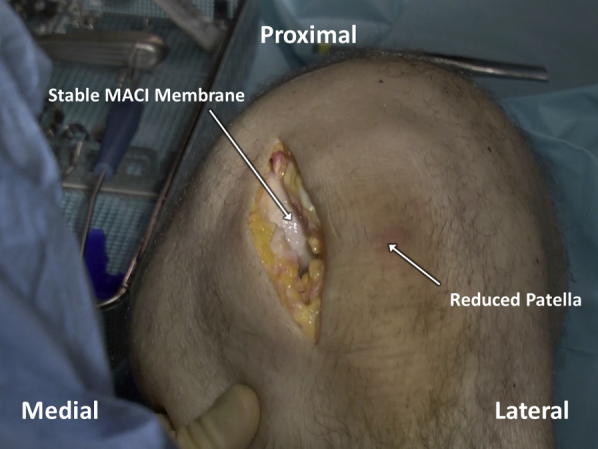
After the fibrin glue overlying the matrix-induced autologous chondrocyte implantation (MACI) membrane has cured, a final graft check is performed by gently removing any retractors and allowing the patella to relocate. The knee is subsequently cycled 5-10 times, and the graft is visualized to ensure stability.
Closure
The wound is closed in layers using 0 Vicryl for the deep fascia, 2-0 Monocryl for the subcutaneous tissue, and running 3-0 Monocryl for the skin. Steri-Strips are applied to the incision, followed by sterile dressing. A hinged knee brace is fitted and locked in full extension.
Rehabilitation
For the first 6 weeks, the patient is allowed toe-touch weightbearing with the knee brace locked in full extension. Active and passive range of motion without resistance is initiated and gently progressed as tolerated. From 6 to 12 weeks, weightbearing status is gradually increased, and the brace is discontinued. No knee loading is allowed beyond 90° of flexion. Closed chain exercises are initiated at 10 weeks postoperatively at 0°-40° of flexion. After 12 weeks, activity is advanced as tolerated. Return to sport is allowed at 4-6 months, pending clinical assessment and restoration of gait mechanics and strength symmetry.
Discussion
Articular cartilage defects cause significant pain, impairments to quality of life, and risk of posttraumatic arthritis.1 Modern biologic treatment using MACI has demonstrated positive short- and long-term outcomes in prospective randomized controlled trials, with better durability and outcomes compared with microfracture.4, 6 In a series of 144 patients by Saris et al.,4 patients undergoing MACI demonstrated significantly better Knee Injury and Osteoarthritis Outcome Score values in the pain, function, activities of daily living, and knee-related quality of life subscales. At 5 years of follow up, of the remaining 128 patients who continued participating in the trial, those treated with MACI demonstrated continued superior subjective outcomes in terms of pain, function, and activities of daily living.6 No unexpected safety events were reported over the midterm observation period, and only 1 additional MACI patient was found to have a treatment failure by the adjudication committee, compared with 3 microfracture patients. These outcomes support the safety and efficacy of MACI in the treatment of focal cartilage defects.
Although MACI has demonstrated good clinical outcomes, it remains highly dependent on surgeon skill at the time of implantation, requiring defect edges to be debrided freehand with the membrane subsequently cut using surgical scissors in an effort to match defect contour and size. The cookie cutter technique offers various advantages throughout MACI implantation (Table 2). Namely, it allows for the creation of true, vertical defect walls and containment of curetting to protect the abutting cartilage. This has positive effects on workflow, given that vertical walls are no longer made by hand using a scalpel, and the surgeon is free to curette effectively within the cookie cutter. After defect debridement, the cookie cutters can be used to efficiently and accurately create a punched-out portion of membrane that exactly matches the size and shape of the defect. Conversely, special care must be taken to ensure that cookie cutters are selected to satisfactorily match the defect shape as well as the size of available MACI membrane. Additionally, the sharp nature of the cookie cutters bears attention during malleting to ensure that the subchondral plate is not perforated.
Table 2.
Advantages and Disadvantages, Including Risks and Limitations, of the Cookie Cutter Technique
| Advantages | Disadvantages, Risks, and Limitations |
|---|---|
| Cookie cutter enables creation of circumferential stable vertical borders | The cutting tools available may not perfectly match defect borders |
| Cookie cutting template provides containment of curettage, enabling smooth, focal workflow | The flat profile of the cookie cutting blade may not conform well to highly convex areas of the articular surface (i.e., near the intracondylar notch) |
| Various cutting tools can be chosen and matched to the contour of the defect | The cookie cutter represents a sharp tool that must be used with caution to avoid risk of subchondral plate violation and collapse |
| The cookie cutter tool allows for the generation of a perfectly matching MACI membrane to fill the debrided defect | The MACI membrane must be adequately large to enable templating with the cookie cutter, especially in the case of multiple defects and defect sizes |
MACI, matrix-induced autologous chondrocyte implantation.
Conclusions
By providing a facile approach for reproducible, efficient, and accurate MACI implantation using cookie cutter instrumentation, we believe the technique described provides a valuable addition to the surgeon's toolbox in focal cartilage defect restoration.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: D.B.F.S. is a board member of Cartilage; is a consultant for Cartiheal, Smith & Nephew, Vericel; and has received research support from Arthrex, Ivy Sports, Smith and Nephew. A.J.K. is a board member of American Journal of Sports Medicine, International Cartilage Research Society, International Society of Arthroscopy, Knee Surgery and Orthopedic Sports Medicine, Minnesota Orthopedic Society, Musculoskeletal Transplantation Foundation; is a consultant for Arthrex, Vericel; has received research support from Aesculap/B.Braun, Arthritis Foundation, Ceterix, Histogenics; and has received royalties from Arthrex. M.H. reports other from Depuy Synthes Sales. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Open treatment of cartilage defects with the matrix-induced autologous chondrocyte implantation (MACI) cookie cutter technique. Demonstration is provided for the necessary instrumentation including curettes and cookie cutter templates (step 1); defect exposure with the patient positioned supine (step 2); defect site preparation through cookie cutter selection, placement, and subsequent defect curettage (step 3); MACI graft preparation using a cookie cutter (step 4); graft implantation using fibrin glue and the cut MACI membrane (step 5); and a final graft check in anticipation of arthrotomy closure (step 6).
References
- 1.Heir S., Nerhus T.K., Rotterud J.H. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: A comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2.Minas T. Chondrocyte implantation in the repair of chondral lesions of the knee: Economics and quality of life. Am J Orthop (Belle Mead NJ) 1998;27:739–744. [PubMed] [Google Scholar]
- 3.Krych A.J., Hevesi M., Desai V.S., Camp C.L., Stuart M.J., Saris D.B.F. Learning from failure in cartilage repair surgery: An analysis of the mode of failure of primary procedures in consecutive cases at a tertiary referral center. Orthop J Sports Med. 2018;6 doi: 10.1177/2325967118773041. 2325967118773041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saris D., Price A., Widuchowski W. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384–1394. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 5.Pareek A., Carey J.L., Reardon P.J., Peterson L., Stuart M.J., Krych A.J. Long-term outcomes after autologous chondrocyte implantation: A systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7:298–308. doi: 10.1177/1947603516630786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittberg M., Recker D., Ilgenfritz J., Saris D.B.F. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018 doi: 10.1177/0363546518756976. 363546518756976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Open treatment of cartilage defects with the matrix-induced autologous chondrocyte implantation (MACI) cookie cutter technique. Demonstration is provided for the necessary instrumentation including curettes and cookie cutter templates (step 1); defect exposure with the patient positioned supine (step 2); defect site preparation through cookie cutter selection, placement, and subsequent defect curettage (step 3); MACI graft preparation using a cookie cutter (step 4); graft implantation using fibrin glue and the cut MACI membrane (step 5); and a final graft check in anticipation of arthrotomy closure (step 6).