Abstract
Background
Rotavirus (RV) is a major agent of gastroenteritis and an important cause of child death worldwide. Immunization (RVI) has been available since 2006, and the Federation of International Societies of Gastroenterology Hepatology and Nutrition (FISPGHAN) identified RVI as a top priority for the control of diarrheal illness. A FISPGHAN working group on acute diarrhea aimed at estimating the current RVI coverage worldwide and identifying barriers to implementation at local level.
Methods
A survey was distributed to national experts in infectious diseases and health-care authorities (March 2015–April 2016), collecting information on local recommendations, costs and perception of barriers for implementation.
Results
Forty-nine of the 79 contacted countries (62% response rate) provided a complete analyzable data. RVI was recommended in 27/49 countries (55%). Although five countries have recommended RVI since 2006, a large number (16, 33%) included RVI in a National Immunization Schedule between 2012 and 2014. The costs of vaccination are covered by the government (39%), by the GAVI Alliance (10%) or public and private insurance (8%) in some countries. However, in most cases, immunization is paid by families (43%).
Elevated cost of vaccine (49%) is the main barrier for implementation of RVI. High costs of vaccination (rs = −0.39, p = 0.02) and coverage of expenses by families (rs = 0.5, p = 0.002) significantly correlate with a lower immunization rate. Limited perception of RV illness severity by the families (47%), public-health authorities (37%) or physicians (24%) and the timing of administration (16%) are further major barriers to large-scale RVI programs.
Conclusions
After 10 years since its introduction, the implementation of RVI is still unacceptably low and should remain a major target for global public health. Barriers to implementation vary according to setting. Nevertheless, public health authorities should promote education for caregivers and health-care providers and interact with local health authorities in order to implement RVI.
Keywords: Rotavirus, Diarrhea, Immunization, Vaccine, Implementation
1. Introduction
Rotavirus (RV) is the most common agent of acute gastroenteritis (AGE) in children under five years of age, and the most severe independent of age [1,2]. Despite a progressive decrease in diarrhea-related deaths, RV is still a major cause of mortality mainly in developing countries [3]. RV disease can be prevented by vaccination, and 95% of RV-induced deaths occurred in 72 countries, which were all eligible to receive GAVI Alliance support. As of January 1, 2016, 80 of the 193 countries worldwide have introduced RV vaccines in their National Immunization Programs (NIP) [4]. Although there are public health barriers to the implementation of RV immunization (RVI), WHO and other authorities recommend universal immunization and considered it a priority in countries with high rotavirus gastroenteritis-associated fatality rates, such as in south and south-eastern Asia and sub-Saharan [5]. Two oral vaccines with high efficacy and good safety profiles are currently available: Rotarix™ administered in a 2-dose schedule, and RotaTeq® administered in a 3-dose schedule. Both vaccines aim to prime broad immune responses followed by progressively broader protection developing through successive natural rotavirus infections [6–9].
Thus far, RV vaccines have been introduced in United States, some European countries, and Australia and are being implemented in selected countries in Asia.
Limited time frame (six to eight weeks following birth) for administration was considered a potential barrier to large-scale immunization. Therefore, even if early immunization is still favored, WHO loosened its recommendation and allowed infants to receive RV vaccine (either RotaTeq® or Rotarix™) together with DTP [5].
The likelihood of intussusception following RVI is low based on the results of both large clinical trials and post-marketing surveillance data. Furthermore, the benefit in lives saved by broadening age restrictions for immunization may well exceed the risk of potential deaths related to intussusception [10].
In 2012, the Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition (FISPGHAN) identified the spread of RV vaccination as a top priority for the control of diarrheal illness in childhood [11].
In order to estimate current RVI coverage and identify the major barriers to local implementation, the FISPGHAN Working Group (WG) on AGE conducted a global survey aimed at collecting information on RVI worldwide.
2. Methods
2.1. Working group and survey
The WG on AGE was created during the FISPGHAN World Congress held in Taiwan in 2012 and encompasses two experts of each continental Society of Pediatric Gastroenterology, Hepatology and Nutrition: European (ESPGHAN), Asian Pan Pacific (APPSGHAN), Commonwealth Association (CAPGHAN), Latin American (LASPGHAN) and North American (NASPGHAN) Societies.
In order to identify and promote practical interventions that will help to reduce the burden of AGE in children worldwide, the WG on AGE collaborated with experts in the field of RVI actively involved in the dissemination of RVI around the world.
The WG coordinators developed a survey including information on the availability of RV vaccines, inclusion in the NIP, immunization coverage according to local available data, costs and financial support, main perceived barriers to implementation, possible interventions to achieve >90% global coverage (see Supplemental material).
2.2. Study design
National experts in infectious diseases and vaccination from several countries in the world were contacted between October 2015 and May 2016. Experts were identified among the members of national institutes for health, panels for local immunization programs, scientific societies working and/or reporting data on RVI (see Supplemental material).
All of them were asked to fill-in a survey to give information on inclusion of RV vaccination in their country’s NIP, implementation programs, costs and their perception of local barriers to implementation. All participants were encouraged to provide original local evidence supporting their data and to report the source of information (see Supplemental material).
2.3. Data analysis
The WG planned to reach at least one referral expert for each world country. When more than one expert from the same country participated to the survey, the data were discussed and combined and analyzed as a single source.
Since data and opinions about local barriers may vary slightly according to the setting, rough data were analyzed and reported according to the Human Development Index (HDI) list of countries with advanced economy (http://hdr.undp.org/en/content/human-development-index-hdi) and countries were differentiated into high HDI countries, medium HDI countries and low HDI countries.
Data were summarized as means ± SD for continuous variables and as percentage and frequencies for categorical variables. Comparison of groups was performed using one-way analysis of variance (ANOVA) for multiple group comparisons. Chi-square test with Fisher’s correction was used to address any differences for categorical variables, as needed. A p value of 0.05 or less was considered as significant. Results were updated in December 2015.
3. Results
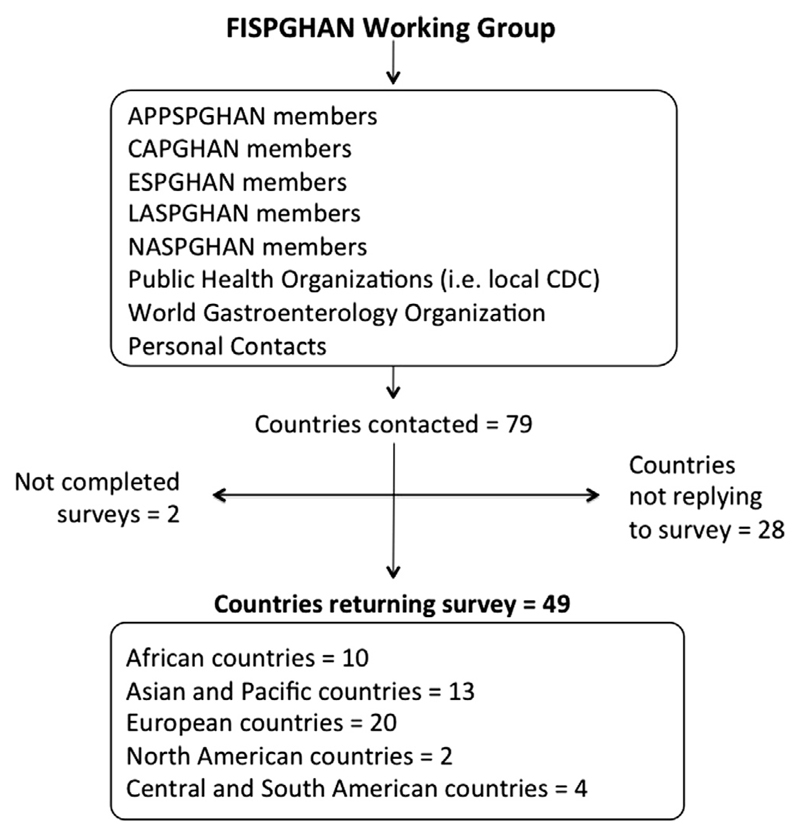
Ninety-one experts in the field were contacted by e-mail or met personally at medical meetings, symposia and workshops worldwide. Among the 79 countries contacted, 49 provided a survey eligible for analysis (response rate 62%) (Fig. 1). Forty-two of the 49 responders provided data for all required fields, but for other seven countries the data on RVI coverage were not available (Ireland, Lithuania, Portugal, Romania, Singapore, Slovenia, Switzerland) although the survey was completed in all other fields.
Fig. 1.
Flowchart of study methodology. FISPGHAN: Federation of International Societies of Pediatric Gastroenterology Hepatology and Nutrition, APPSGHAN: Asian Pan Pacific Society of Pediatric Gastroenterology Hepatology and Nutrition, CAPGHAN: Commonwealth Association of Paediatric Gastroenterology & Nutrition, ESPGHAN: European Society of Pediatric Gastroenterology Hepatology and Nutrition, LASPGHAN: Latin American Society of Pediatric Gastroenterology Hepatology and Nutrition, NASPGHAN: North American Society of Pediatric Gastroenterology Hepatology and Nutrition. CDC: Center for Disease Control and Prevention – Atlanta United States.
Responders were equally distributed between low- (23, 47%) and medium/high-income countries (26, 53%).
3.1. Rotavirus immunization coverage and costs
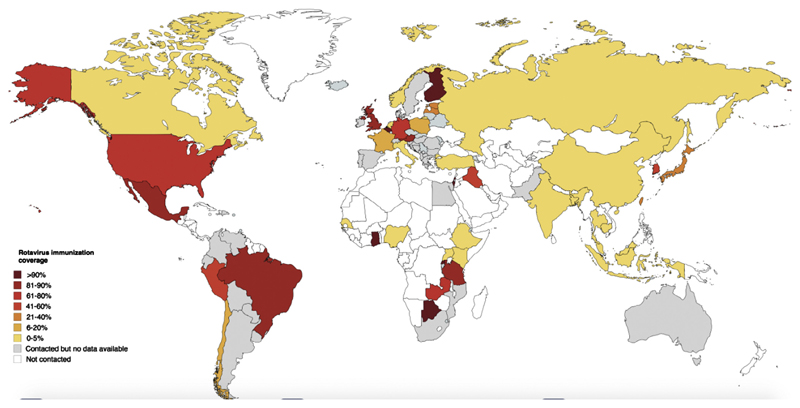
RVI was recommended in 27 out of the 49 countries (55%) participating in the global survey (eTable 3). Although some countries have recommended RVI since 2006, most countries (16/49, 33.3%) first included RVI in the NIP between 2012 and 2014 (eTable 3). RVI rates showed a scattered pattern from 0 to over 90% according to different countries. Overall RVI coverage is reported in Fig. 2.
Fig. 2.
Global coverage for Rotavirus Immunization: estimate 2015** (data collected up to May 2016).
Rotarix™ and Rotateq® are both distributed worldwide, with 40 (81.6%) and 38 (77.5%) countries respectively, but Rotarix® resulted to be prevalent in African countries (Table 1).
Table 1. Inclusion of Rotavirus Immunization in national immunization plans according to different countries.
| Continent | Country | Recommendation by national immunization plan | Year of start |
|---|---|---|---|
| Africa | Botswana | Recommended | 2012 |
| Ethiopia | Recommended | 2013 | |
| Ghana | Recommended | 2012 | |
| Kenya | Recommended | 2014 | |
| Nigeria | Not recommended | ||
| Rwanda | Recommended | 2012 | |
| Senegal | Recommended | 2014 | |
| Tanzania | Recommended | 2012 | |
| Uganda | Not recommended | ||
| Zambia | Recommended | 2013 | |
| Asia and Oceania | Bangladesh | Not recommended | |
| Cambodia | Not recommended | ||
| China | Not recommended | ||
| Japan | Recommendeda | 2011 | |
| India | Recommendeda | 2016 | |
| Indonesia | Not recommended | ||
| Iraq | Recommended | 2012 | |
| Israel | Recommended | 2011 | |
| Malaysia | Not recommended | ||
| Singapore | Not recommended | ||
| South Korea | Not recommended | ||
| Taiwan | Recommended | 2006 | |
| Thailand | Not recommended | ||
| Europe | Austria | Recommended | 2007 |
| Belgium | Recommended | 2006 | |
| Estonia | Recommended | 2014 | |
| Finland | Recommended | 2009 | |
| France | Recommended | 2013 | |
| Germany | Recommended | 2013 | |
| Ireland | Not recommended | ||
| Italy | Not recommended | ||
| Latvia | Recommended | 2010 | |
| Lithuania | Not recommended | ||
| Netherlands | Not recommended | ||
| Norway | Recommended | 2014 | |
| Poland | Not recommended | ||
| Portugal | Not recommended | ||
| Romania | Not recommended | ||
| Russia | Not recommended | ||
| Slovenia | Not recommended | ||
| Switzerland | Not recommended | ||
| Turkey | Not recommended | ||
| United Kingdom | Recommended | 2013 | |
| North America | Canada | Recommendeda | 2006 |
| Mexico | Recommended | 2013 | |
| USA | Recommended | 2006 | |
| South America | Brazil | Recommended | 2006 |
| Chile | Not recommended | ||
| Peru | Recommended | 2009 |
RV vaccination is recommended only in some regions of the country.
The costs of vaccines are substantially different, being higher in European and American and lower in Asian and African countries (Table 2). Costs reported for a complete vaccination cycle with Rotateq® were slightly higher than those of RotarixTM in average (176.8 vs 103.8USD, p = 0.14). In most countries these costs were charged directly to families (42.8%) (Table 2). In other nations the costs are covered by the government (38.7%), by international organization such as the GAVI Alliance (10.2%) or public and private insurance (8.1%). However, in the countries in which financial support by public authorities is limited or related to by family income, brand distribution, age of administration or geographic location, it’s possible for families to obtain one or both vaccines in the private sector. Anyway, uptake is usually substantially less in the private than in the public sector.
Table 2. Rotavirus vaccines and relative costs according to continents.
| Questions | Total (n = 49) | Africa (n = 10) | Asia (n = 13) | Europe (n = 20) | North America (n = 3) | South America (n = 3) | p |
|---|---|---|---|---|---|---|---|
| Inclusion in NIS (n, %) | 27 (55.1) | 8 (80) | 5 (38.4)b | 9 (45)b | 3 (100) | 2 (66.6) | 0.116 |
| Available vaccine | |||||||
| Rotarix (n, %) | 40 (81.6) | 7 (70) | 10 (76.9) | 17 (85) | 3 (100) | 3 (100) | 0.627 |
| RotaTeq (n, %) | 38 (77.5) | 3 (30) | 11 (84.6) | 18 (90) | 3 (100) | 3 (100) | 0.002 |
| Other (n, %) | 2 (4) | 0 (0) | 2 (15.3) | 0 (0) | 0 (0) | 0 (0) | 0.217 |
| None (n, %) | 3 (6.1) | 1 (10) | 2 (18.2) | 0 (0) | 0 (0) | 0 (0) | 0.422 |
| Costs in USD | |||||||
| Rotarix (mean + SD) | 103.8 (70⋅1) | 12.6 (18⋅5) | 80 (56.1) | 140.6 (56.4) | 182 (45.3) | 57⋅5 (61.5) | <0.001 |
| RotaTeq (mean + SD) | 176.8 (267⋅4) | 15 (NA) | 84.9 (47⋅8) | 253.2 (363⋅3) | 187.8 (53⋅4) | 125.5 (44.5) | 0.64 |
| Other (mean + SD) | 37.5 (48⋅8) | – | 37.5 (48.8) | – | – | – | NA |
| Payment charged to: | |||||||
| Family (n, %) | 21 (42.8) | 2 (20) | 8 (61.5) | 10 (50) | 0 (0)a | 1 (33.3) | 0.149 |
| Government (n, %) | 19 (38.7) | 4 (40) | 3 (23) | 7 (35) | 3 (100) | 2 (66.6) | 0.054 |
| GAVI Alliance (n, %) | 5 (10.2) | 4 (40) | 1 (7.6) | 0 (0) | 0 (0)a | 0 (0) | 0.014 |
| Insurance (n, %) | 4 (8.1) | 0 (0) | 1 (9) | 3 (15) | 0 (0)a | 0 (0) | 0.638 |
| No answer | 1 (2.0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | NA |
NIS = National Immunization Schedule, NA = Not assessable.
Private Insurance may cover the cost of vaccination.
One country changed recommendation in 2015–2016 (see text).
It should be noted that national policies for reimbursement are often reassessed. For example, in Latvia, Europe, the government has partially reimbursed RV vaccination since September 2012 and full reimbursement was introduced in January 2015. In France the vaccination was available since 2006 and has been successively included in the NIP in 2013 at expenses of families. However, due to a safety warning released by the National Agency of Drug Safety, at the moment the survey is collected, French government was reviewing RVI recommendations and reimbursement. In the United States of America, beginning September 2010, children 0 through 18 years that are enrolled in new private health plans are eligible to receive vaccines (including RV). Children covered by government insurance were already fully eligible.
These major changes in local policy for RVI may well significantly impact future immunization coverage.
3.2. Barriers to local implementation
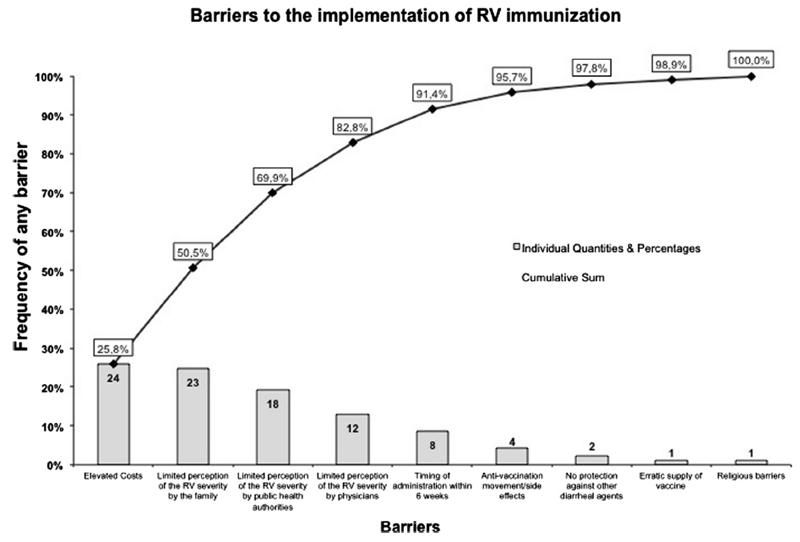
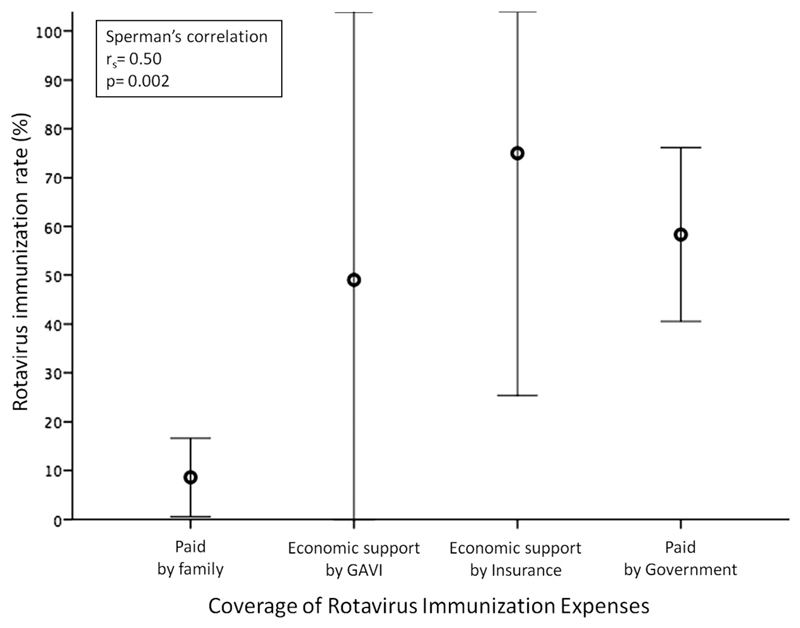
The direct and indirect costs for immunization and the limited perception of RV severity by the families are two major barriers for large-scale implementation of RVI programs (Fig. 3, eTable 4). High costs of vaccination (rs = -0.39, p = 0.02) and its charge to families (rs = 0.5, p = 0.002) significantly correlate with a lower immunization rate (Fig. 4). Only eight countries reported the timing of first administration as a potential barrier (eTable 4).
Fig. 3.
Barriers to local implementation of Rotavirus immunization (Pareto Chart).
Fig. 4.
Correlation between Rotavirus immunization rate and its coverage expenses.
The underestimation of RV severity by public health authorities is a common barrier worldwide, however, it is particularly relevant in developing countries with low and medium HDI (eTable 4).
An impact of anti-vaccination movements and a general fear about vaccines’ side effects was reported in the United States, Latvia, and Iraq. In France, the notification of serious side effects after RVI had a relevant impact on national agency recommendations and on local immunization coverage.
In Malaysia, the government did not consider RVI as a health priority and restricted funds for RVI implementation and reimbursement. In addition, two experts reported that the finding of porcine circovirus DNA fragments in vaccines available on the market [12,13] had a relevant negative impact on local implementation of RV vaccines for religious reasons.
In Zambia, the overall erratic distribution of vaccines has been reported as a major barrier to large-scale implementation of RVI.
3.3. Role of Scientific Societies and Health-care authorities
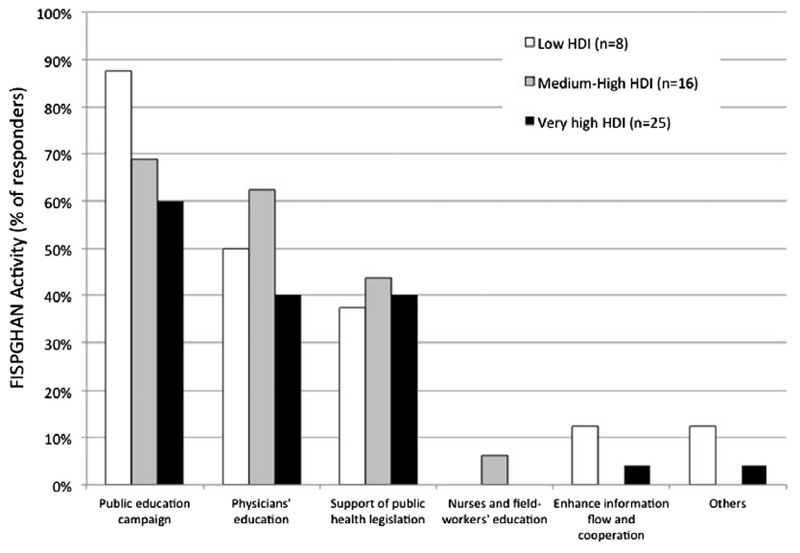
To the question “How could Scientific Societies and Health-care authorities help to achieve the goal?” most experts suggested educational initiatives to be adopted as primary intervention (Fig. 5). The majority of responders identified the need of public educational campaigns (33/49, 67.3%) or educational programs addressed to local health-care providers (24/49, 48.9%).
Fig. 5.
Activities to improve rates of Rotavirus immunization coverage.
This is particularly true in low-income countries where the majority of responders indicated the education directed to care-givers (88%) or physicians (50%) as the major interventions to be promoted (Fig. 5). According to the result of our survey, the education programs for caregivers could be started in the third trimester of gestation or alternatively during first well-baby visits. The latter may also provide an optimal opportunity to administer the vaccine.
Countries with medium-high or very high income suggested to provide support for public health legislation aiming to reduce vaccination costs and simplify access to vaccination (Fig. 5). In addition, scientific societies should interact with national health authorities to promote the inclusion of RVI in the NIP and to enhance the cooperation amongst the active stakeholders to improve the spread of information.
4. Discussion
This study provides a worldwide overview of the status of RVI and outlines the main barriers to local implementation of RV vaccination with the final aim of identifying possible interventions that may help to reach a goal of global coverage. Our results showed that common barriers to the implementation of RV vaccine included its costs and the perception of a low disease burden as observed in a previous publication in European countries where potential safety concerns represented a third relevant barrier[14].
4.1. RVI coverage and costs
Oral RV vaccines can prevent severe cases of infection. Even if RV vaccination is currently recommended by WHO and all authoritative guidelines [5,15–19], RVI coverage significantly varies in the world, and even within the same geographical area (from 0 to over 90%). In Europe only few countries such as Austria, Belgium and Finland have reached adequate vaccination coverage as high as 90%, despite the specific recommendations of European guidelines since 2008 [18]. The inclusion in those countries of the RV vaccine in the NIP since 2006–2007 and the implementation of clinical studies conducted by local researchers [20–22] may well have positively impacted the current rate of immunization coverage.
The scenario in North and South America seems to be different: in almost all countries included in the survey the vaccine is recommended and the coverage rates range from 50% (Peru) to over 90% (United States). The only exception is represented by Canada, where recommendations vary according to regional provinces and coverage has been estimated <10%. Coverage in Asian countries is even lower and only Japan reported values above 10%, probably because in most Asian countries the RV vaccine is not nationally recommended by health authorities, with exception of Taiwan and Iraq, and more recently India.
High coverage rates have been reached in African countries where GAVI alliance supported RV vaccination campaign (Rwanda, Ghana, Tanzania and Botswana).
In addition, we observed that, after approval of the two RV vaccines and early endorsement by some countries in 2006, most countries recommended RVI very recently between 2012 and 2014. This two-peak distribution might be affected by the emergence of data on potential side effects, including the risk of intestinal intussusception (2008–2012) [23] and the presence of porcine circovirus in Rotarix™ (2009–2010) [24].
According to our results there was no significant difference in the distribution of the two main types of vaccines among the participating countries. However RotaTeq® tended to be less employed in African countries confirming previous reports that identified Rotarix™ as a preferred choice due to a better cost-effectiveness, the requirement of fewer doses, less storage space, and proven thermo-stability [25]. For similar reasons, the GAVI Alliance subsidizes much more 2-dose rather than 3-dose RV vaccine. Significant differences emerged in relation to costs, which are higher in European and American countries and lower in Asia and Africa. This represents a commitment that developed countries could support RVI in developing areas especially due to GAVI international support [26]. As of mid-2016, GAVI supported the introduction of RVI in the NIP of 43 African and South American countries [26].
4.2. Barriers to RVI implementation
Elevated costs of immunization and a misperception regarding the potential severity of RV infection and its consequences have been identified as the major barriers to universal dissemination of RVI. However, the factors limiting local implementation vary greatly between countries and even within the same geographic area. In Europe, the opinion of experts varied country by country, from Finland where no barriers to implementation were reported to Slovenia where RVI “is not recognized by far as a priority among vaccine-preventable diseases”. Awareness of disease burden can drive vaccination uptake, as suggested in several studies [14,27,28]. According to our results it was felt that a large percentage of caregivers are simply not aware of risks of RV infection probably because they received inadequate information. Many parents are aware of the risk of hospitalization or death, but most do not know about the advantages and availability of RV vaccines in their own country. Counseling can be an integral part of health education to the public and can provide useful information against vaccine-preventable diseases to families who accept to receive information by health-care personnel [27].
Unfavorable cost-effectiveness has been put forward as a reason not to implement universal RV vaccination in several HDI-countries [28]. Since in HDI countries the RV infection is managed with high efficacy, the ratio between admission and sick children is low. In addition, either health-related costs and social costs (working day loss) are underscored and, as a consequence, health authorities consider RVI expensive [29].
Universal vaccination has been estimated to be cost-effective from a wider societal perspective, in particular in relation to the beneficial effects coming from herd immunity [30]. In keeping with our results, previous studies also identified the concerns about reimbursement issues and parental acceptance of the vaccine as major barriers to optimal implementation of RV vaccination. Copayment systems or funding by sickness funds have been implemented as an alternative to national funding in several high-income and medium-income countries [14]. However, guidelines were against the hypothesis of immunizing at risk populations only [18,19].
A further barrier is represented by the overall concern of vaccine-related side effects that, together with anti-vaccination movements, impact on immunization campaign worldwide. In France, the notification in December 2014 of three deaths and about 50 intussusceptions after RV vaccine administration significantly changed government’s attitude towards routine RVI and national agency recommendations (letter reported at http://www.bmj.com/content/350/bmj.h2867/rr-1).
Historically the time of administration of RV vaccine was seen as a barrier. According to our results this seems to be a minor issue, also because WHO changed its recommendation in 2013, introducing the first vaccination dose together with DTP vaccination starting from the second month of life. This intervention could enhance RVI and reach children who were previously excluded from the benefits of RVI.
Other barriers have been reported by experts as a result of the direct interaction that FISPGHAN had with local experts: one of these is related to a religious matter. In Malaysia, a country with a predominance of people practicing Islam (over 60%), the finding of DNA fragments of porcine circoviruses type-1 in RV vaccines Rotarix™ has been reported as a relevant barrier for local implementation of large-scale immunization programs.
The Strategic Advisory Group of Experts on immunization in 2010 reported to the World Health Organization that porcine circovirus type-1 is not known to cause disease in humans and is often found in food products, confirming the safety of Rotarix™ [5,24]. However, according to our data, in an Islamic-prevalent country the potential assumption of pig derivate seems to overweigh safety reasons and this significantly impacts on regional health authorities, religious leaders and families’ beliefs.
4.3. Future prospective for RVI implementation
Educational initiatives directed at health-care providers and caregivers have been identified as primary interventions that should be adopted and promoted. However the role of FISPGHAN is likely to vary according to setting and countries needs. Countries with medium-high or very-high income asked scientific societies to support public health legislation aimed at a reduction in vaccination costs and simplified access to vaccination. Systems involving different health-care workers were effective. For example, in Norway the nurses, who are responsible for the child immunization program, provide information to caregivers using printed information material and web-pages. In developing areas, some experts suggested a role by scientific societies (including FIPS-GHAN) in supporting public health legislation and the introduction of RVI in NIP as well as in promoting the enhancement of cooperation and information flow between local health-care practitioners, regulatory authorities and field workers. More recently National Immunization Technical Advisory Groups (NITAGs) have been established in the majority of countries to advise Ministries of Health on the value of new vaccines and to develop strategies through the analysis and discussion of scientific evidence [30]. By the end of 2012, half of the countries in the world reported the existence of a NITAG with a formal legislative or administrative basis (with a high of 86% in the Eastern Mediterranean Region) [31].
This survey was supposed to cover as many countries as possible to ensure a better view of RVI scenario in the world. Unfortunately, despite our commitment and efforts, we have been able to obtain reliable information from only a quarter of world countries, and this represents a limitation of our study. However, we observed a balanced distribution between high- and low-income countries and provided useful new information about RVI coverage and barriers to implementation of RVI. A further limitation of our survey is that data about RVI coverage are based on single person reports. However it should be considered that all enrolled healthcare workers are experts in the field and provided supporting data including material from local literature, websites or published material (see Online-only material).
In conclusion, immunization is the best approach for preventing RV infection [3,5]
After approximately 10 years since the introduction of RVI, the implementation of this major life saving intervention is still unacceptably low and remains a major target for reaching the Millennium Developmental Goal.
Barriers to implementation vary according to setting and local conditions, but the costs of RVI programs and perceptions about disease burden are major barriers for global dissemination of RVI. In order to sustain and implement RVI, medical professional societies and public health authorities should promote education for caregivers and physicians and interact with local health organizations to enhance networking among stakeholders and develop strategies to reduce RVI-related costs.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.01.082.
Acknowledgments
The FIPSGHAN Working Group is grateful to all experts who collaborate by completing this survey and providing local data on immunization coverage and personal perspectives on the barriers that affect local programs of implementation. Their effort and competence has been the core of our work.
Footnotes
Declaration of interests
The authors have no conflicts of interest relevant to this article to disclose.
None of the authors received any honorarium, grant or other form of payment to produce the manuscript. In case of acceptance, the costs for open-access publication would be covered by the Bill & Melinda Gates Foundation.
All the authors have substantially contributed to the conception and design of the study, to the acquisition, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and approved its final version.
References
- [1].Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- [2].Albano F, Bruzzese E, Bella A, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166(3):241–7. doi: 10.1007/s00431-006-0237-6. [DOI] [PubMed] [Google Scholar]
- [3].Tate JE, Burton AH, Boschi-Pinto C, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programs: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- [4].http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet [accessed on Aug 04 2016].
- [5].WHO. Weekly Epidemiological Record. 2013;88(5):49–64. [Google Scholar]
- [6].Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- [7].Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- [8].Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX 4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–43. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- [9].Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- [10].Patel MM, Clark AD, Glass RI, et al. Broadening the age restriction for initiating rotavirus vaccination in regions with high rotavirus mortality: benefits of mortality reduction versus risk of fatal intussusception. Vaccine. 2009;27:2916–22. doi: 10.1016/j.vaccine.2009.03.016. [DOI] [PubMed] [Google Scholar]
- [11].Guarino A, Winter H, Sandhu B, et al. Acute gastroenteritis disease: report of the FISPGHAN Working Group. J Pediatr Gastroenterol Nutr. 2012;55:621–6. doi: 10.1097/MPG.0b013e318272b5e2. [DOI] [PubMed] [Google Scholar]
- [12].Dubin G, Toussaint JF, Cassart JP, et al. Investigation of a regulatory agency enquiry into potential porcine circovirus type 1 contamination of the human rotavirus vaccine, Rotarix: approach and outcome. Hum Vaccin Immunother. 2013;9(11):2398–408. doi: 10.4161/hv.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baylis SA, Finsterbusch T, Bannert N, Blümel J, Mankertz A. Analysis of porcine circovirus type 1 detected in Rotarix vaccine. Vaccine. 2011;29(4):690–7. doi: 10.1016/j.vaccine.2010.11.028. [DOI] [PubMed] [Google Scholar]
- [14].Parez N, Giaquinto C, Du Roure C, et al. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis. 2014;14:416–25. doi: 10.1016/S1473-3099(14)70035-0. [DOI] [PubMed] [Google Scholar]
- [15].Prevention ACIP. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58:1–25. [PubMed] [Google Scholar]
- [16].Buttery JP, Danchin MH, Lee KJ, et al. for the PAEDS/APSU Study Group Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29:3061–6. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- [17].de Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis J. 2011;30(suppl 1):61–6. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- [18].Vesikari T, Van Damme P, Giaquinto C, et al. European society for paediatric infectious diseases/European society for paediatric gastroenterology, hepatology, and nutrition evidence-based recommendations for rotavirus vaccination in Europe. J Pediatr Gastroenterol Nutr. 2008;46(suppl 2):38–48. doi: 10.1097/MPG.0b013e31816f7a10. [DOI] [PubMed] [Google Scholar]
- [19].Vesikari T, Van Damme P, Giaquinto C, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015 Jun;34(6):635–43. doi: 10.1097/INF.0000000000000683. [DOI] [PubMed] [Google Scholar]
- [20].Sabbe M, Berger N, Blommaert A, et al. Sustained low rotavirus activity and hospitalisation rates in the post-vaccination era in Belgium, 2007 to 2014. Euro Surveill. 2016;7(27):21. doi: 10.2807/1560-7917.ES.2016.21.27.30273. [DOI] [PubMed] [Google Scholar]
- [21].Braeckman T, Van Herck K, Meyer N, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ. 2012 Aug 8;345:e4752. doi: 10.1136/bmj.e4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hemming M, Räsänen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172(6):739–46. doi: 10.1007/s00431-013-1945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haber P, Patel M, Izurieta HS, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008;121(6):1206–12. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- [24].Payne DC, Humiston S, Opel D, et al. A multi-center, qualitative assessment of pediatrician and maternal perspectives on rotavirus vaccines and the detection of Porcine circovirus. BMC Pediatr. 2011 Sep;26(11):83. doi: 10.1186/1471-2431-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Hoek AJ, Ngama M, Ismail A, et al. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines. PLoS One. 2012;7(10):e47511. doi: 10.1371/journal.pone.0047511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].<http://www.gavi.org/support/nvs/rotavirus/> [accessed Aug 03 2016].
- [27].Kannan Kutty P, Pathmanathan G, Salleh NM. Analysis of factors in response to rotavirus vaccination counselling in a private paediatric clinic. Med J Malaysia. 2010 Jun 65;2:127–32. [PubMed] [Google Scholar]
- [28].VENICE2. Finalised report on the decision making process, modalities of implementation and current country status for the introduction of human papilloma virus and rotavirus vaccination into national immunisation programmes in Europe. 2010. [accessed Feb 18, 2014]. < http://venice.cineca.org/Venice2_WP3_Report_December2010.pdf>. [Google Scholar]
- [29].Plosker GL. Rotavirus vaccine RIX4414 (Rotarix™): a pharmacoeconomic review of its use in the prevention of rotavirus gastroenteritis in developing countries. Pharmacoeconomics. 2011;29:989–1009. doi: 10.2165/11207210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [30].Blau J, Sadr-Azodi N, Clementz M, et al. Indicators to assess national immunization technical advisory groups (NITAGs) Vaccine. 2013;31:2653–7. doi: 10.1016/j.vaccine.2013.01.047. [DOI] [PubMed] [Google Scholar]
- [31].Takla A, Wichmann O, Carrillo-Santisteve P, Cotter S, Lévy-Bruhl D, Paradowska-Stankiewicz I, Valentiner-Branth P, D’Ancona F, the VENICE III NITAG Survey Group Characteristics and practices of National Immunisation Technical Advisory Groups in Europe and potential for collaboration, April 2014. Euro Surveill. 2015;20(9) doi: 10.2807/1560-7917.ES2015.20.9.21049. [pii=21049] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.