Summary
Grass pollen allergy affects approximately 40% of allergic patients. Subcutaneous allergen immunotherapy (SCIT) is the only allergen-specific and disease-modifying treatment available. Currently available therapeutic vaccines for the treatment of grass pollen allergy are based on natural grass pollen extracts which are either made from pollen of one cross-reactive grass species or from several related grass species. Clinical studies have shown that SCIT performed with timothy grass pollen extract is effective for the treatment of grass pollen allergy. Moreover, it has been demonstrated that recombinant timothy grass pollen allergens contain the majority of relevant epitopes and can be used for SCIT in clinical trials. However, recent in vitro studies have suggested that mixes consisting of allergen extracts from several related grass species may have advantages for SCIT over single allergen extracts. Here, we review current knowledge regarding the disease-relevant allergens in grass pollen allergy, available clinical studies comparing SCIT with allergen extracts from timothy grass or from mixes of several related grass species of the Pooideae subfamily, in vitro cross-reactivity studies performed with natural allergen extracts and recombinant allergens and SCIT studies performed with recombinant timothy grass pollen allergens. In vitro and clinical studies performed with natural allergen extracts reveal no relevant advantages of using multiple grass mixes as opposed to single grass pollen extracts. Several studies analysing the molecular composition of natural allergen extracts and the molecular profile of patients’ immune responses after SCIT with allergen extracts indicate that the major limitation for the production of a high quality grass pollen vaccine resides in intrinsic features of natural allergen extracts which can only be overcome with recombinant allergen-based technologies.
Introduction
Grass pollen is a major cause for IgE-mediated allergy throughout the world. A survey carried out in Switzerland has shown that more than 12% of the population is sensitized to grass pollen allergens [1]. A recent European study confirmed the high prevalence of grass pollen allergy demonstrating that more than 30% of allergic patients showed positive skin prick test reactions to grass pollen [2]. In sensitized patients, grass pollen contact triggers IgE-mediated symptoms of acute inflammation, such as allergic rhinitis, conjunctivitis and asthma [3]. Due to a relatively long flowering period and the release of heavy loads of pollen, grasses are also among the clinically most relevant allergen sources and can induce severe symptoms of allergy such as asthma [4].
Although many patients achieve satisfactory disease control with symptomatic medication, subcutaneous allergen-specific immunotherapy (SCIT) is the only allergen-specific and disease-modifying treatment available [5, 6]. In fact, the first reported SCIT trial was performed with grass pollen extract in 1911 [7]. Since then numerous clinical studies have documented the efficacy of grass pollen SCIT [8]. Furthermore, it has been demonstrated that SCIT has long-lasting effects even after discontinuation of treatment and prevents the progression from allergic rhinitis to allergic asthma [9, 10]. Current forms of grass pollen immunotherapy are still based on crude allergen extracts, which unfortunately are often of poor quality [11]. However, new allergy vaccines based on purified recombinant allergens have been used successfully in SCIT trials and recombinant allergen-based tests have become available as routine diagnostic tests for improved patients selection and monitoring of SCIT [12, 13]. Still the selection of therapeutic extracts is often based on patients’ clinical history, IgE testing to crude allergen extracts from different sources and patients’ responses to skin prick tests with allergen extracts [14, 15].
Due to high cross-reactivity among related grass species diagnostic tests and SCIT are frequently conducted with single extracts derived from one grass species [16].
Two more recent studies provided additional evidence for extensive IgE cross-reactivity towards Pooideae grasses and it has been suggested that timothy grass pollen allergens are sufficient for SCIT against allergy to Pooideae [17, 18]. However, another study suggested that there may be a considerable variability in cross-reactivity even among the major group 1 and 5 allergens of the Pooideae [19]. This study suggested that a mix of grass pollen extracts from five grasses of the Pooideae would be better suited for grass pollen SCIT. The question whether one should use one or a mix of several grass pollen extracts for immunotherapy is not a new one but has been investigated since the concept of immunotherapy has been described [7, 20]. Two more recent studies, one in favour of single and another one in favour of mixed allergen extracts, rekindled the interest in this question [21, 22]. Here, we review scientific evidence from in vitro and in vivo data regarding advantages and disadvantages of using multiple grass mixes as opposed to single grasses for SCIT of grass pollen allergy. However, after more than one hundred years of clinical practice, the drawbacks of allergen extract-based immunotherapy come increasingly into view. In many points, these drawbacks are identical regardless whether single or mixed natural extracts are used because they are due to intrinsic features of natural allergen extracts. The authors of this article are convinced that major improvement for SCIT can only be achieved with synthetic or recombinant allergen-based vaccine technologies.
Grass pollen allergens
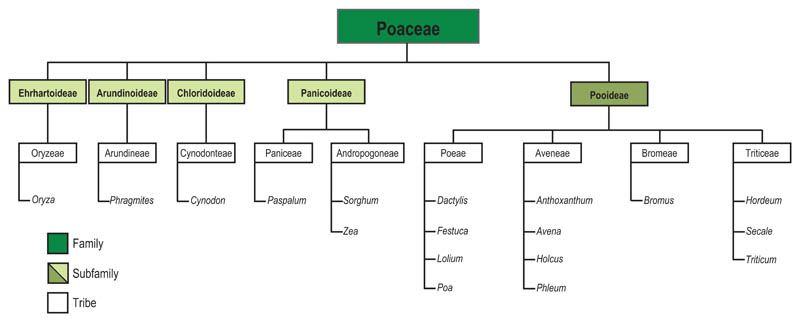
Allergenic grasses are unequally distributed in different geographical regions. Most allergenic grasses belong to the Poaceae family, which comprises about 9000 species; three of its subfamilies [Chloridoideae e.g. Bermuda grass (Cynodon dactylon); Arundinoideae e.g. Common Reed (Phragmites communis), Panicoideae e.g. Corn (Zea mays)] have their centres of distribution in the Southern hemisphere, whereas in the Northern hemisphere the sub-family of Pooideae grasses is widely distributed in the temperate regions [17, 23, 24]. Well-known allergenic members of this latter subfamily include Timothy grass (Phleum pratense), orchard grass (Dactylis glomerata), Fescue grass (Festuca pratensis), Ryegrass (Lolium perenne), Kentucky blue grass (Poa pratensis), sweet vernal grass (Anthoxatum odoratum), wheat (Triticum aestivum) and rye (Secale cereale) [17, 23, 24]. (Fig. 1). The geographical distribution of selected representative grass species is shown in Table 1.
Fig 1.
Phylogenetic relationship between important allergenic grasses [adapted from Andersson and Lidholm 2003 (23) and modified according Grass World 2011 (24)]
Table 1. Geographical distribution of selected Poaceae grasses [Regions according to http://grassworld.myspecies.info/content/maps-tdwg at the Database Grass World 2011 (24)].
| Subfamily | Grass species | Geographical distribution |
|---|---|---|
| Ehrhartoideae | Oryza sativa | Africa, Temperate Asia, Tropical Asia, Australasia, North America, Pacific, South America |
| Arunidinoideae | Phragmites ssp | Europe, Africa, Temperate Asia, Tropical Asia, Australasia, North America, Pacific, South America |
| Chloridoideae | Cynodon dactylon | Europe, Africa, Temperate Asia, Tropical Asia, Australasia, North America, Pacific, South America, Antarctica |
| Panicoideae | Paspalum notatum | Europe, Africa, Temperate Asia, Tropical Asia, Australasia, North America, Pacific, South America, Antarctica |
| Pooideae | Phleum pratense | Europe, Temperate Asia, Tropical Asia, North America, South America, Antarctica. |
| Lolium perenne | Europe, Africa, Temperate Asia, Tropical Asia, Australasia, North America, Pacific, South America, Antarctica | |
| Triticum aestivum | Europe, Africa, Temperate Asia, Tropical Asia, Australasia, North America, South America |
All grass species are wind pollinated and release allergenic pollen grains in great abundance in their peak flowering season [23–25].
The allergenic activity of grass pollen can be attributed to a limited number of proteins and glycoproteins, which are rapidly released from the pollen grain upon hydration [4, 26, 27]. Individual allergenic pollen proteins have been identified, described and classified (see websites of the WHO/IUIS Allergen Nomenclature Sub-Committee at http://www.allergen.org/; Allergome at http://www.allergome.org/). Allergen molecules from different grass species have been grouped according to IgE-binding frequencies and physicochemical properties in extracts of related grasses [17, 23, 28, 29]. To date, 10 different groups of grass pollen allergens are officially recognized. Some of these allergens are exclusively present in the pollen of grasses, whereas other allergens also are expressed in other botanically unrelated plants (e.g. trees, weeds) and thus represent a source of inter-species cross-reactivity [30, 31]. Table 2 shows that grass pollen allergens can be grouped according to their distribution across the grasses as allergens which are present in all grasses, allergens which are present only in some grasses, and allergens, which are expressed in grasses and other botanically unrelated plants. Highly cross-reactive allergens occurring in many species have been designated as pan-allergens [32].
Table 2. Presence of groups 1–13 grass pollen allergens in pollen of different grass species as determined by cloning, specific antisera/antibodies and IgE-binding/inhibition experiments.
| Allergens | MW | Phl p | Poa p | Lol p | Dac g | Fes p | Ant o | Ave s | Hol l | Sec c | Pas n | Zea m | Cyn d | Phr a | Ory s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergens found in all grasses | |||||||||||||||
| Group 1 | 31–35 kDa | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Group 13 | ~55 kDa | + | + | + | + | + | + | + | + | + | + | + | + | + | ± |
| Allergens found in some grasses | |||||||||||||||
| Group 2/3 | 10–12 kDa | + | + | + | + | − | + | + | + | + | n.d. | − | − | − | ± |
| Group 5 | 27–33 kDa | + | + | + | + | + | + | + | + | + | n.d. | − | − | + | − |
| Group 6 | ~13 kDa | + | + | − | − | − | + | − | − | − | n.d. | − | − | − | − |
| Group 11 | ~20 kDa | + | n.d. | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | + | n.d. | n.d. | + |
| Allergens, not grass-specific | |||||||||||||||
| Group 4 | 50–67 kDa | + | + | + | + | + | + | + | + | + | n.d. | + | + | + | n.d. |
| Group 7 | ~9 kDa | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | + | n.d. | + |
| Group 12 | ~14 kDa | + | + | + | n.d. | n.d. | + | + | n.d. | + | n.d. | + | + | + | + |
MW, molecular weight; +, allergen cloned or found by specific antiserum/antibody probe or IgE-binding/inhibition experiment; −, allergen not cloned or found by specific antiserum/antibody probe or IgE-binding/inhibition experiment; ±, presence of allergen predicted by in silico sequence analysis; n.d., no data. Phl p, Phleum pratense; Poa p, Poa pratensis; Lol p, Lolium perenne; Dac g, Dactylis glomerata; Fes p, Festuca pratensis; Ant o, Anthoxantum odoratum; Ave s, Avena sativa; Hol l, Holcus lanatus; Sec c, Secale cereale; Pas n, Paspalum notatum; Zea m, Zea mays; Cyn d, Cynodon dactylon; Phr a, Phragmites australis; Ory s, Oryza sativa.
Allergens found in most grasses
Group 1 and group 13 allergens seem to be present in most allergenic grasses. Group 1 allergens have been isolated or cloned from more than 19 species [23, 33– 35]. About 90% of grass pollen allergic patients show IgE reactivity to group 1 allergens [17, 23, 34, 36–39]. In several species, IgE reactivity to Group 1 allergens accounts for a considerable amount of grass pollen specific IgE and in vivo allergenic activity of group 1 allergens is well proven [23, 35]. The Phl p 1 allergen shows a sequence identity of ca 90% to allergens from other members of the Pooideae subfamily and extensive IgE cross-reactivity [23, 36, 38].
Group 13 allergen was described in P. pratense as a by-product of fractionating P. pratense extract using chromatography and found to be a 55 kDa protein distinct from Phl p 4 but with a similar molecular weight [40, 41]. The frequencies of group 13-specific IgE responses in patients were found to be at 50% of the sera that were examined; however, the purified protein is not stable to protease digestion and degrades rapidly [41, 42]. Group 13 allergens show almost no cross-reactivity with allergens from other plants and are hence specific for grasses [42, 43]. They display relatively low clinical allergenic reactivity as demonstrated in basophil activation experiments and in a skin test study in allergic patients [44, 45]. In summary, group 1 and 13 allergens can be considered markers of genuine grass pollen sensitization [43].
Allergens found in some grasses
Group 5 allergens are the most important allergens in this group, as 65% to 85% of grass pollen allergic patients from temperate regions display IgE reactivity to group 5 allergens and their allergenic activity is extremely high [23, 46–48]. Homologous allergens were found in all grasses from the Pooideae subfamily, such as timothy grass (P. pratense), rye (S. cereale), Kentucky bluegrass (Poa pratense) and rye grass (L. perenne); however, group 5 allergens are not present in all grasses. No group 5 allergens were found in Z. mays and C. dactylon [49] and not in Oryza sativa, which are members of the Panicoideae, Chloridoideae and Bambusoideae subfamily respectively. The latter subfamilies are highly prevalent in the Southern hemisphere. In fact, it has been found that patients in such areas are preferentially sensitized to group 1, 4 and 13 allergens but show less frequently reactivities to group 2, 5 and 6 allergens [50]. Phl p 5, one of the best characterized group 5 allergen molecule, is one of several allergens within a species, of which exist different isoallergens, in this case Phl p 5a (i.e. Phl p 5.01) and Phl p 5b (i.e. Phl p 5.02); isoallergens differ in sequence but most patients show extensive IgE cross-reactivity to Phl p 5 isoallergens [23].
Group 2 allergens have been found to react with IgE from 40% to 60% of grass pollen allergic patients with varying prevalence in different reports and display high allergenic activity [23, 51]. IgE-reactive group 2 allergens are not expressed in pollens of all subfamilies of grasses; for instance, they were not detected in C. dactylon, Z. mays and Phragmites australis [49]. Group 3 allergens have a sequence identity of 58% with group 2 allergens and were traditionally assigned a separate group number; however, they show similar 3-D structures and conformational epitopes to group 2 grass pollen allergen as well as cross-reactivity to group 2 grass pollen allergens in grass pollen allergic patients [23, 52]. Although group 2 allergens exhibit significant sequence identities with the C-terminal portions of group 1 grass pollen allergens, there seems to be no relevant IgE cross-reactivity in patients [51].
Group 6 allergens have been shown to react with 60% to 70% of sera of grass pollen allergic patients [23, 53]. They have been identified in P. pratense and Poa pratensis and may occur also in A. odoratum [49, 53].
Group 10 grass pollen allergens have been purified from P. pratense, L. perenne, P. pratensis and C. dactylon; however, the results could not be reproduced. Although this allergen group is mentioned in several data bases, it is not recognized by the WHO/IUIS Allergen Nomenclature Sub-Committee as an official allergen group [23] (http://www.allergen.org/).
Group 11 allergens have been found in other plants than grasses but IgE cross-reactivity is very limited [54]. Group 11 grass pollen allergens have been purified as 18 kDa glycoproteins from L. perenne [55] and P. pratense [54]. IgE responses to recombinant non-glycosylated Phl p 11 were found in approximately 32% of examined grass pollen allergic patients. Phl p 11 is therefore not regarded as a major allergen [23]. Reactivity to group 11 allergens is not confined to grasses, as homologues include also Ole e 1, maize (Zea m 13) and tomatoes but cross-reactivity is very limited.
Allergens from groups 1, 2, 5 and 6 have been found exclusively in grass pollen [3, 23, 30]. IgE antibodies to these allergens are therefore considered diagnostic markers for true grass pollen sensitization [30].
Pan-Allergens, not grass-specific
The third group of allergens, designated ‘pan-allergens’, occurs not only in grass pollen but also in other botanically unrelated plants. (Table 2).
Group 7 grass pollen allergens are proteins containing two binding sites for calcium, termed EF-hands. Not only have they been identified in pollens of several grasses (C. dactylon, P. pratense) but also in numerous pollens from other plant species (trees, weeds). Because of cross-reactive IgE epitopes in these allergens patients with sera containing antibodies, which react with group 7 allergens, are at risk of experiencing symptoms when confronted with pollen from a large number of plants [30]. Only approximately 10% of grass pollen-sensitized patients show IgE responses to Phl p 7. However, Phl p 7 possesses high allergenic activity [23, 56]. It is a marker for broad sensitisation against pollen from various sources.
Group 12 grass pollen allergens are members of the profilin protein family. Profilin was first identified in birch pollen [57] and since then has been found in many pollens of grasses, trees and weeds and in plant-derived foods [23, 30]. As a consequence extensive cross-reactivity of allergic patients’ IgE antibodies with profilins from different sources has been described [30]. Specific IgE binding occurs in 15–30% of pollen allergic individuals. Profilin is considered a marker allergen for broad allergic sensitization.
Up to 85% of grass pollen sensitized individuals show IgE reactivity to group 4 allergens, which represent oxidoreductases [58, 59]. However, average levels of IgE to natural group 4 allergens (e.g. Phl p 4) are relatively low [23]. Moreover, allergenic reactivity of Phl p 4 was found to be low by skin prick testing and patients who react in vitro exclusively to group 4 allergens, often do not display clinical symptoms of grass pollen allergy [48]. Group 4 homologues have been found in timothy grass, mugwort and birch pollen, but also in peanut, apple, celery root and carrot root, and thus occurring in pollen of unrelated plants and in plant foods [58, 59]. IgE cross-reactivity seems to be primarily due to the presence of cross-reactive carbohydrates and the term ‘pan-allergen’ may therefore not be fully applicable for group 4 allergens.
If one reviews the data regarding contents of the so far described allergens in various grass species, it is evident that only timothy grass pollen has been shown to contain each of the so far described grass pollen allergens. Based on the analysis of the allergen-distribution in the various grass species, timothy grass pollen seems to be sufficient for grass pollen SCIT but it is also important to consider the degree of cross-reactivity among the grass species, results of clinical data and the question what allergens need to be present in a grass pollen vaccine.
Cross-reactivity among grasses
Clinical and in vitro studies regarding cross-reactivity obtained with allergen extracts and natural allergens
Almost thirty years ago, clinical studies directly compared subcutaneous injection immunotherapy with single pollen extracts and mixtures reaching the conclusion that timothy grass pollen extract and birch pollen extract are equally effective or even better than SCIT performed with mixes of extracts from various grasses and trees respectively.
These studies and their findings are summarized in Table 3. As for tree pollen one study found no difference in the effect of subcutaneous immunotherapy with birch pollen extract compared to a mixture from four different tree pollen [60]. Another study compared 3 years of subcutaneous immunotherapy with birch pollen only to treatment with a combination of alder, birch and hazel and found no significant difference between the two groups concerning decrease in reactivity in skin prick tests and specific sensitivity of the nasal mucosa [61–63]. Concerning grass pollen, one study compared refined Timothy grass pollen extract, in which high molecular weight non-allergenic components and all low molecular weight components had been removed by gel filtration and diafiltration, with crude aqueous Timothy grass pollen extract and with a four grass pollen mix and found that patients treated with the refined extract needed significantly less antihistaminic medication than patients having received the crude extract or the four grass mix [64]. Another study comparing a tyrosine-bound grass pollen extract mixture with mixed grass pollen extract and refined timothy pollen extract found the tyrosine-bound mixture to be less active in reducing symptom and medication scores, whereas results obtained with mixed grass and with refined timothy pollen were comparable, and even in favour of the refined timothy pollen extract [65].
Table 3. Immunotherapy trials comparing single extract to multiple extract immunotherapy.
| References | Study objective | Design/participants | Intervention and outcome measures | Results | Possible study limitations |
|---|---|---|---|---|---|
| Henzgen et al. [60] | Comparison of SCIT with single tree pollen extract and a mixture of tree pollen extracts in tree pollen allergic patients | Open, randomized, Single centre N = 40 Intervention group 1: N = 18/20 completed Intervention group 2: N = 18/20 completed |
Intervention: Intervention group 1: SCIT with birch pollen extract for 3 years Intervention group 2: SCIT with mixture of tree pollen extracts (hornbeam/oak/alder/hazel) Outcome measure: Symptom score Number of symptomatic days |
Significant improvement in both groups No significant difference in outcome measures between both groups |
No placebo No objective outcome measures |
| Petersen et al. [61] Wihl et al. [62] Ipsen et al. [63] |
Comparison of SCIT with single tree pollen extracts and a mixture of tree pollen extracts in tree pollen allergic patients | Double-blind, matched pair, single centre N = 54 Intervention group 1: N = 23/27 completed Intervention group 2: N = 22/27 completed |
Intervention: Intervention group 1: SCIT with standardized birch pollen extract for 3 years Intervention group 2: SCIT with a mixture of birch, alder and hazel extract for 3 years Outcome measure: Symptom score Medicine consumption score SPT Nasal Provocation Test Patients’ specific IgE |
Significant improvement in both groups No significant difference in outcome measures between both groups |
No placebo |
| Frostad et al. [64] | Comparison of SCIT with crude and refined timothy grass pollen extract and with a mixture of four grass pollen extracts in grass pollen allergic patients | Single-blind for treated groups, randomized, single centre N = 90 Intervention group 1: N = 14/17 completed Intervention group 2: N = 20/24 completed Intervention group 3: N = 16/19 completed Control Group: N = 30 |
Intervention: Intervention group 1: SCIT with crude aqueous timothy grass pollen extract for 3 years Intervention group 2: SCIT with refined aqueous timothy grass pollen extract for 3 years Intervention group 3: SCIT with crude aqueous extract from equal amounts of four grass pollens (Dactylis glomerata, Festuca elatior, Phleum pratense, Lolium perenne) Control group: No SCIT, but symptomatic medication Outcome measure: Symptom score and symptomatic medication score Patient self-evaluation Nasal Provocation Patients’ specific IgE |
Significant improvement in all intervention groups compared to the control group Refined timothy grass pollen extract at least equally effective as crude pollen extract and grass pollen mixture |
Crude extract possibly not stable in storage, No real placebo |
| Verstraeten and Verstraeten [65] | Comparison of SCIT with tyrosine-bound grass pollen mixture, aluminium hydroxide-adsorbed grass pollen mixture, purified timothy grass pollen extract for 4 years | Open, Randomized, single centre N = 60 Intervention group 1: N = 20 Intervention group 2: N = 17 Intervention group 3: N = 23 |
Intervention: Intervention group 1: SCIT with crude extract of 12 grass pollens adsorbed with aluminium hydroxide (bent, brome, cocksfoot, dogstail, fescue, foxtail, meadow, rye, vernal, timothy, false oat, Yorkshire fog) for 4 years Intervention group 2: SCIT with glutaraldehyde-pollen-tyrosine adsorbate of 12 grass pollens (bent, brome, cocksfoot, dogstail, fescue, foxtail, meadow, rye, vernal, timothy, false oat, Yorkshire fog) for 4 years Intervention group 3: SCIT with purified standardized timothy pollen extract for 4 years Outcome measure: Symptom score as correlated with pollen count Supplementary medication score Patient self-evaluation |
Significant improvement in all intervention groups Timothy grass pollen extract at least equally effective as grass pollen mixture |
No placebo |
Quite recently several studies again brought up the question whether one extract or a mix of extracts should be used for SCIT. These studies were not based on clinical data but were performed only by serological IgE-cross-reactivity testing with allergen extracts, natural allergens and by comparing sequences of major allergens regarding similarities. Yet, these studies have generated interest because different companies offer either immunotherapy based on one grass pollen extract or based on vaccines consisting of mixtures of several allergen extracts.
One large scale study reports that specific IgE response in sera from grass pollen allergic subjects mainly from Northern Europe to four different combined extracts (mixes) was correlated with the response to timothy grass pollen extract and was found to be of the same magnitude for pollen mixes and timothy pollen alone. In IgE inhibition studies, timothy grass pollen extract completely inhibited the binding of specific IgE to mixed allergen extracts from different related grass species. Also, cross-reactivity of SCIT-induced IgG4 was found to be complete [18]. In yet another study, specific IgE binding to extracts of various grass pollen was found to be of the same magnitude when correlated with specific IgE binding to extract of timothy grass pollen in population samples ranging from 1075 to 3293 subjects. Complete cross-inhibition of binding to extracts of nine different grass pollen species in 49 sera from grass pollen allergic patients with extract of timothy grass pollen was found [17].
On the other hand, it was shown in ELISA competition experiments with sera from 27 patients that there are two groups of patients, one group where complete cross-inhibition can be achieved using only P. pratense extract to inhibit binding to natural group 1 or group 5 species-specific allergens, whereas in another group of patients this complete inhibition can only be achieved with a mix of extracts from five related grasses. This result was considered as evidence that patients have both cross-reactive and species-restricted epitopes [19].
Another recent study sought to investigate differences in RAST-inhibition patterns in sera from SCIT-naïve patients from central Italy comparing binding inhibitions to single grass pollen extracts using timothy grass pollen extract and a 5 grass pollen mix as inhibitors. The study found a significantly higher inhibition of binding of patients’ sera to A. odoratum and P. pratensis using the 5 grass mix than using timothy grass pollen extract and concluded that this was evidence favouring clinical immunotherapy with a mix of several extracts [21]. In yet another recent study, grass pollen allergic patients received a short course of injection immunotherapy with timothy grass pollen extract and were tested for IgE and IgG4 levels and cutaneous sensitivity to a five grass-mix extract and to timothy grass pollen extract before and after the intervention. Cutaneous sensitivity to both extracts was equally reduced, and IgG4 levels to both extracts increased significantly in the intervention group. A positive and high correlation between IgG4 levels to the major group 5 allergen of timothy and rye grass was found. The results indicate that the response induced by SCIT with one grass induces cross-protective IgG responses [22].
Problems occurring when cross-reactivity is determined with allergen extracts and natural allergens
The above-mentioned serological cross-reactivity studies have been performed mainly with crude allergen extracts, which represent relatively ill-defined mixtures of allergens and non-allergenic components [11]. It is therefore not possible to determine the specificity of IgE and IgG antibodies for the clinically relevant allergen molecules.
In fact, several studies demonstrate that grass pollen contains on the one hand allergens, which elicit strong allergic reactions in patients, but also components, which show only in vitro IgE reactivity but elicit poor or no clinical symptoms [45, 48]. In this context, it should be mentioned that carbohydrate epitopes with poor or without clinical relevance represent major IgE-reactive structures in grass pollens [66, 67].
A recent study demonstrated that a considerable percentage of grass pollen allergic patients show specific IgE to cross-reactive carbohydrate-bearing epitopes [68]. Several naturally occurring grass pollen allergens (group 1, 4, 11, 13) contain one or several carbohydrate residues, and the most heavily glycosylated grass pollen group 4 allergens are thought to carry 10–15% carbohydrate moieties [23]. About 20–40% of individuals sensitized to group 4 grass pollen allergen exhibit reactivity to carbohydrate epitopes [58, 59]. However, earlier studies showed that in vitro serum reactivity with carbohydrate epitopes, while widespread in grass pollen allergic patients, is not an indicator of clinical symptoms in sensitized patients [45, 69, 70]. Glycoproteins seem to elicit only a monovalent IgE-binding, which yields positive results in serum-based assays without having clinical significance [69, 70]. Therefore, carbohydrate cross-reactive determinants are generally considered of little clinical importance in grass pollen allergy [31, 71] and it has been suggested to designate carbohydrate allergens as ‘IgE reactive antigens’ to prevent confusion with biologically active allergens [71].
Another grass pollen allergen family with low clinical relevance are group 13 allergens showing high frequency of IgE recognition (56%) but poor clinical reactivity by SPT. Sensitization to group 13 allergen is practically never found without patient reactivity to other major grass pollen allergens [45].
The clinical relevance of group 11 allergens has not been investigated in detail, but they are recognized only by approximately 30–40% of grass pollen allergic patients [54, 55].
For group 1, 2 and 5 allergens, it has been demonstrated that they induce strong allergic reactions in a high percentage of grass pollen allergic patients [23, 48]. Group 6 allergens are major grass pollen allergens, which induce basophil activation in grass pollen allergic patients and thus seem to be clinically relevant [53]. Therefore, reactivity to these allergens is a marker of true grass pollen sensitization [23, 30, 72].
Cross-reactivity studies should therefore take group 1, 2, 5 and 6 allergens in consideration. Another cause for confusion is the presence of highly cross-reactive allergens such as the pan-allergens, group 7 and 12 in grass pollen, which are responsible for extensive cross-reactivity with other non-related plants and may yield a false positive result for grass pollen sensitization in extract-based diagnostic tests.
In our opinion, recombinant allergen-based inhibition data or data with purified allergen molecules with defined protein and carbohydrate epitopes are needed to identify clinically relevant major allergens and settle the question, which allergens need to be used in grass pollen immunotherapy [49, 50].
Cross-reactivity as measured with recombinant allergens
For grass pollen allergy, several IgE cross-reactivity studies were performed with recombinant allergens and the allergenic activity of several allergens was assessed. In experiments with purified recombinant grass pollen allergens, a mixture of a few major allergens of one grass species (Phl p 1, Phl p 2, Phl p 5, Profilin) was sufficient to diagnose most grass pollen allergic patients in different populations, thus confirming earlier results that were achieved using purified natural and recombinant allergens [37, 73–75]. Indeed, group 1 and group 5 allergens accounted for 60–80% of grass pollen allergic patients’ IgE even in different populations from different geographical areas [75].
Recombinant allergen-based inhibition experiments with purified allergens have demonstrated extensive IgE cross-reactivity for major allergens. Recombinant Phl p 1 inhibited binding of patients’ sera to extracts of eight different grasses (P. pratense, A. odoratum, A. sativa, C. dactylon, L. perenne, P. communis, P. pratensis, S. cereale) yielding an average inhibition of 76% [36]. Extensive cross-inhibition of patients’ IgE binding to nine different grass pollen extracts (A. odoratum, A. sativa, C. dactylon, L. perenne, P. australis, P. pratensis, S. cereale, Triticum sativum, Z. mays) was achieved with a panel of several purified recombinant grass pollen allergens (Phl p 1, Phl p 2, Phl p 5) and purified recombinant cross-reactive profilin, Bet v 2 [49]. Group 6 allergen, although it has been described only in P. pratense and Poa pratensis, is a major allergen with modest cross-reactivity to group 5 allergen despite extensive sequence homology, and thus seems to be a necessary candidate for inclusion in a grass pollen vaccine formulation, as it elicits basophil activation in sensitized patients [53].
A recent study examined sequence differences and post-translational modifications in group 1 and 5 isoforms of L. perenne, P. pratense, D. glomerata, A. odoratum and P. pratensis by mass spectrometry analysis of purified natural allergens, sequence alignment and computer modelling [19]. Although this study contained only very limited IgE-binding data from 27 patients, the authors argued that better allergen coverage is achieved by mixing allergen extracts from different grasses. In fact, earlier, many attempts were made to quantify the percentage of sequence identity predictive of cross-reactivity [76]. However, sometimes the substitution of just a few amino acids results in non-allergenic isoforms of allergen molecules, whereas in other cases relatively low sequence homology may be sufficient for structural similarity leading to conserved conformational epitopes [77–79]. As a consequence, sequence analysis or pure structural data are not sufficient to predict or exclude immunological cross-reactivity, but IgE cross-reactivity needs to be established by testing large groups of patients [71].
One such study was performed with a large number of patients (i.e. more than 1000 patients) and reconfirmed extensive IgE cross-reactivity [17]. Furthermore, the authors highlighted extensive sequence homology of a large portion of the Phl p 1 surface (aa 1–240) and found that the surface is relatively unaffected by the amino acid substitutions identified in both Phl p 1 isoforms and group 1 allergens from other species within the Pooideae subfamily (Hol l 1, Poa p 1 and Lol p 1) [17]. Two other studies using Phl p 1-specific antibodies found extensive cross-reactivity towards natural group 1 allergens from different Pooideae species: A recent study described a strong reaction of recombinant human Phl p 1–specific IgE Fabs with natural group 1 allergens from sweet vernal grass, rye grass, common reed, Kentucky blue grass and rye and pointed out that IgE epitopes on Phl p 1 cluster in one region of the molecule [80]. In another study, monoclonal antibodies were raised against Phl p 1 and defined at least four distinct epitopes on Phl p 1. These antibodies recognized most wild grasses from the temperate regions of the Poeae tribe family, in particular L. perenne, P. pratensis, F. pratensis and D. glomerata, with similar immuno-logical strength [81].
Limited cross-reactivity thus does not seem to be a major bottleneck when it comes to the choice of allergens for treating allergies to Pooideae (i.e. temperate grasses). However, there seems to be a considerable difference regarding the composition of allergens between Pooideae and Panicoideae/Chloridoideae (i.e. subtropical grasses).
Different allergen repertoires in subtropical grasses and temperate grasses
As for the Southern Hemisphere, subtropical grasses may deserve special attention. Subtropical grasses from the Chloridoideae, for example, Bermuda grass (C. dactylon); Arundinoideae, for example, Common Reed (P. communis), Panicoideae such as Bahia grass (Paspalum notatum) reportedly lack allergenic group 2/3, group 5 and group 6 allergens (Table 2). In those tropical grasses, only groups 1, 4 and 13 seem to occur. Cross-reactivity towards group 1 allergen is lower between P. pratense and C. dactylon or Paspalum notatum [18, 23]. Also, a lower degree of sequence homology between group 1 allergens from Pooideae grasses and the other three subfamilies has been described [17, 82]. In patients from temperate climate zones, P. notatum extract could not inhibit binding to P. pratense extract, but in patients from a subtropical region complete cross-inhibition between P. pratense and P. notatum extract was found, indicating different sensitization patterns in patients from different regions [82]. Furthermore, in patients from a subtropical region in Australia, complete cross-inhibition of patients sera binding to L. perenne extract could be achieved with C. dactylon or P. notatum extract, but not the other way round indicating species-specific IgE epitopes [83]. Whether species-specific IgE epitopes in subtropical grasses represent carbohydrates or protein epitopes has not been investigated in detail but there is preliminary evidence that carbohydrates are frequently recognized but clinically irrelevant in subtropical areas (Cabauatan & Valenta, unpublished observations). Therefore, further studies are warranted to define the allergens, which are required for treating patients, who are sensitized to subtropical grasses.
Features of natural extracts for immunotherapy
To date, grass pollen immunotherapy is carried out with natural extracts, as recombinant allergen-based therapeutic vaccines for respiratory and food allergy are still in clinical trials [reviewed in 84–92]. There are several common features of natural allergen extracts, which are responsible for the problems when natural allergens are used for the production of vaccines (Table 4).
Table 4. Features of natural extracts for grass pollen immunotherapy.
| Traditionally used for SIT |
| Clinical efficacy documented by several studies |
| But, |
| Contain in addition to allergens undefined allergen source-derived materials |
| May lack important allergens |
| Amounts of different allergens may vary |
| Precise contents of many allergens unknown |
| Variations regarding allergen composition depending on extraction and production protocols and different raw materials |
| Contaminating allergens from other allergen sources |
| Degradation of allergens due to presence of proteases |
| High allergenic activity requires tedious up-dosing protocols |
| Unsuitable for vaccine production according to current quality guide lines |
Natural allergen extracts are by definition mixtures of allergenic and undefined non-allergenic components, which are obtained from natural sources and therefore subject to variation [6, 11]. Interestingly, some of the non-allergenic components in pollen extracts are not just innocuous bystanders but have been shown to prime Th2 responses [93]. Moreover, it is by no means guaranteed that natural grass pollen extracts contain even sufficient quantities of the clinically important allergens such as group 1, group 2, group 5 and group 6 grass pollen allergens. Most manufacturers measure only group 1 and/or group 5 allergens in their extracts using house-made assays which in most cases have not been compared between different manufacturers. Efforts have been made to establish reliable assays, which can be used by all manufacturers, but these efforts include mainly group 5 allergens and are not yet in routine use [94]. In fact, it has been demonstrated that timothy grass pollen extracts from different manufacturers differ greatly regarding allergen content and often lack important allergens [11]. Similar results were obtained for other Pooideae grass pollen extracts which were found to contain varying amounts of group 1 and group 5 allergens [18, 95].
The precise content of most allergens in natural extracts is therefore not known and will vary from batch to batch depending on the used source. Nowadays, measurement of allergen content in commercial pollen extracts frequently is also done by biochemical analysis of allergen content [11, 96]. Traditionally, it was done by determination of IgE reactivity of extracts with pooled sera of allergic subjects or determination of certain allergens in extracts by monoclonal antibodies [11, 14, 81, 94, 97–99]. None of the methods currently employed on a routine basis is very precise (i.e. tests often cannot discriminate between intact allergens, less IgE-reactive isoforms and allergen fragments), and usually economical and practical considerations lead to the measurement of one or few allergen only for which assays are available, leaving doubt about the concentration of most other important allergens in the mixture.
Pollen is a product from a natural source. Therefore, it is evident that pollen quality and allergen content varies over the different pollen seasons and depending on environmental factors [100]. As a consequence variation in allergen content may be substantial between batches of natural extracts from the same company and even more so between natural extracts from different companies, which are produced according to different production protocols [11, 99]. Most importantly, if allergens are lacking or present in too low quantities, there are no possibilities for adding these components once an extract has been prepared. In this sense, standardization means at best that the concentrations of certain allergens have been measured. Furthermore, companies use different forms of units for the measured allergens which therefore cannot be compared between different manufacturers [101].
Contaminations of natural pollen extracts from unrelated allergen sources or with bacterial toxins have been described [102, 103]. Storage of pollen extracts over time also leads to degradation depending on the presence of proteases [11, 104].
Also, production according to current quality guidelines is not possible as many natural pollen extracts were introduced to the market at a time when quality guidelines were not as stringent as today.
Finally, natural grass pollen extracts are highly allergenic and may induce anaphylactic side-effects if the concentration of certain components is too high. As a consequence, grass pollen allergy vaccination with natural extracts requires tedious up-dosing protocols and many administrations, which is a costly and time-consuming procedure.
What has been said for extracts in general makes it already difficult to produce vaccines based on single allergen sources. Mixing different grass pollen extracts from related Pooideae grasses will certainly exacerbate these problems while diluting the overall dose of individual important allergens from the individual sources in the mixture.
In summary, a number of problematic features are related to the intrinsic nature of natural extracts in immunotherapy, which will certainly exacerbate if different extracts are mixed.
Recombinant allergens for diagnosis and grass pollen immunotherapy
Recombinant allergen-based vaccines can address and solve many, if not all of the problems which occur with natural allergen extracts [84, 101, 105, 106]. Some of the advantages of recombinant allergen-based vaccines are listed in Table 5. Recombinant allergen-based vaccines contain defined amounts of well-characterized molecules without contaminations with unwanted non-allergenic material or unwanted allergenic substances. For each of the components, detailed production protocols are established and the physicochemical, immunological and allergenic properties of the active ingredients are known. Thus, the components can be produced at any time and in any place in the same quality. Allergenic and immunogenic activity of recombinant allergen-based vaccines is not limited to characteristics of wild-type pure molecules, but can be modified by various molecular biology methods with the aim of minimizing unwanted side-effects of immunotherapy and enhancing properties which are of advantage for SCIT (e.g. Reduction in allergenic activity, increase in immunogenicity). Thus, so-called hypoallergenic derivatives have been produced as fragments of recombinant allergens, reassembly of fragments in mosaics and mutants [107–115] and hybrid molecules containing most of the epitopes of major grass pollen allergens [116–118]. One of the most recent techniques is the strategy of coupling allergenic fragments of grass pollen allergens to unrelated carrier proteins (e.g. from virus proteins), so that T cell help from T cell epitopes of carrier proteins induces a strong IgG antibody response against the IgE epitopes used in the molecule without unwanted IgE- or T cell induced side-effects [119, 120]. Recombinant allergen molecules and hypoallergenic derivatives can be routinely produced in bacteria or insect cells in huge amounts at relatively low costs according to high quality standards. Therefore, they meet current quality guidelines required for vaccines in the European Union [121]. Moreover, these techniques allow the production of compound vaccines for large populations as well as the production of patient-tailored vaccines.
Table 5. Features of recombinant allergen-based vaccines.
| Contain defined amounts of well-characterized molecules |
| Allergenic and immunogenic activity of components is known |
| Formulation of composite vaccines for large populations but also of vaccines for patient-tailored therapy possible |
| Can be produced in large amounts, at low costs, according to high quality standards |
| Meet current quality guidelines required for vaccines |
| But, |
| So far only few clinical studies performed |
| No registered product available |
While several clinical studies with recombinant allergens from different sources have proven the general efficacy of recombinant allergen-based vaccines [84–87, 122, 123], in the context of grass pollen allergy studies need to be highlighted, which provided a proof of principle for the effectiveness of immunotherapy with defined recombinant allergens from one grass pollen species. In a double-blind, placebo-controlled Phase II clinical vaccination study, grass pollen allergic patients received subcutaneous immunotherapy with a mixture of 5 recombinant grass pollen allergens from P. pratense (Phl p 1, Phl p 2, Phl p 5a, Phl p 5b, Phl p 6) covering group 1, 2, 5 and 6 allergens and experienced a substantial reduction in clinical symptoms and the need for symptomatic medication in comparison to the placebo group [124]. Results from a dose-finding and safety study have just become available and document that patients tolerated also high doses very well and mounted immune responses associated with success of SCIT towards the natural allergens [125]. A therapeutic hypoallergenic vaccine for grass pollen allergy containing epitopes of the most important grass pollen allergens (Phl p 1, Phl p 2, Phl p 5 and Phl p 6) bound to a virus carrier protein was tested for safety by skin testing and in a phase II study and currently is being evaluated for efficacy in a large multi-centre vaccination study with promising results [92].
Today, the prescription and monitoring of SCIT can be effectively achieved with recombinant allergen-based tests which in the format of micro-arrayed allergens allow establishing the sensitization profile against the clinically relevant grass pollen allergens and the monitoring of the vaccine-induced immune response [13, 30, 126]. The recombinant allergen-based diagnostic tests allow identifying different sensitization profiles which are indicative for sensitization to temperate and/or subtropical grasses, and/or to clinically less relevant carbohydrate structures. Population-wide testing with recombinant allergen-based diagnostic test will therefore be a basis for the development of new generations of allergy vaccines, which meet the needs of patients best [105].
In summary, we have given an overview of the known grass pollen allergens and have reviewed clinical and in vitro studies showing that immunotherapy with one grass species containing all important allergen groups, that is, P. pratense is as effective as immunotherapy with several related grass pollen species in temperate climate zones. Moreover, we have enumerated a number of problems, which are associated with the use of natural grass pollen extracts in immunotherapy and have given an overview of the advantages of recombinant allergens. Instead of further fruitless discussion of the advantages and disadvantages of using one or more than one natural grass pollen extract for immunotherapy, we recommend to focus on the development of recombinant allergen-based vaccines because the sophisticated diagnostic and therapeutic possibilities of recombinant allergen technology provide better options for diagnosis and therapy of grass pollen allergy than allergen extracts.
Acknowledgements
We acknowledge Julia Eckl-Dorna, MD, PhD, Medical University of Vienna for assistance with the layout of Fig. 1. This study was supported by research grants from the Christian Doppler Research Association, BIO-MAY AG, Vienna, Austria and by grants F4605, 4613 of the Austrian Science Fund (FWF).
Footnotes
Conflicts of interest
Rudolf Valenta has received funds for research from Biomay AG, Vienna, Austria and from Thermofisher, Uppsala, Sweden and serves as a consultant for both companies. All other authors have no conflict of interest to declare.
References
- 1.Wüthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss study on air pollution and lung diseases in adults. Int Arch Allergy Immunol. 1995;106:149–56. doi: 10.1159/000236836. [DOI] [PubMed] [Google Scholar]
- 2.Burbach GJ, Heinzerling LM, Edenharter G, et al. GA(2)LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–15. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 3.Suphioglu C. What are the important allergens in grass pollen that are linked to human allergic disease? Clin Exp Allergy. 2000;30:1335–41. doi: 10.1046/j.1365-2222.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- 4.Suphioglu C, Singh MB, Taylor P, et al. Mechanism of grass-pollen-induced asthma. Lancet. 1992;339:569–72. doi: 10.1016/0140-6736(92)90864-y. [DOI] [PubMed] [Google Scholar]
- 5.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 7.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;I:1572–3. [Google Scholar]
- 8.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheik A, Druham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001936.pub2. CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 10.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 11.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 12.Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 13.Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy therapy. FASEB. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 14.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125:S306–13. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 15.Nelson HS. Allergen immunotherapy: where is it now? J Allergy Clin Immunol. 2007;119:767–77. doi: 10.1016/j.jaci.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Weber R. Patterns of pollen cross-allergenicity. J Allergy Clin Immunol. 2003;112:229–39. doi: 10.1067/mai.2003.1683. [DOI] [PubMed] [Google Scholar]
- 17.Johansen N, Weber RW, Ipsen H, Barber D, Broge L, Hejl C. Extensive IgE cross-reactivity towards the Pooideae grasses substantiated for a large number of grass-pollen-sensitized subjects. Int Arch Allergy Immunol. 2009;150:325–34. doi: 10.1159/000226233. [DOI] [PubMed] [Google Scholar]
- 18.Hejl C, Wurtzen PA, Kleine-Tebbe J, Johansen N, Broge L, Ipsen H. Phleum pratense alone is sufficient for allergen specific immunotherapy against allergy to Pooideae grass pollens. Clin Exp Allergy. 2009;39:752–9. doi: 10.1111/j.1365-2222.2008.03195.x. [DOI] [PubMed] [Google Scholar]
- 19.Chabre H, Gouyon B, Huet A, et al. Molecular variability of group 1 and 5 grass pollen allergens between Pooideae species: implications for immunotherapy. Clin Exp Allergy. 2010;40:505–19. doi: 10.1111/j.1365-2222.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- 20.Grubb GD, Vaughan WT. Evidence of group specific and species specific sensitization to pollen. J Allergy. 1938;9:211–26. [Google Scholar]
- 21.Marcucci F, Sensi L, Di Cara G, et al. Which allergen extract for grass pollen immunotherapy? An in vitro study. Immunol Invest. 2010;39:635–44. doi: 10.3109/08820131003796876. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Cócera C, Sastre J, Cimarra M, et al. Immunotherapy with a Phleum pratense allergen extract induces an immune response to a grass-mix allergen extract. J Investig Allergol Clin Immunol. 2010;20:13–9. [PubMed] [Google Scholar]
- 23.Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003;130:87–107. doi: 10.1159/000069013. [DOI] [PubMed] [Google Scholar]
- 24.Simon BK, Clayton WD, Harman KT, et al. Grass World. [Accessed on 12 November 2012];2011 http://grassworld.myspecies.info/
- 25.Knox RB. Grass pollen, thunderstorms and asthma. Clin Exp Allergy. 1993;23:354–9. doi: 10.1111/j.1365-2222.1993.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 26.Vrtala S, Grote M, Duchene M, et al. Properties of tree and grass pollen allergens: reinvestigation of the link-age between solubility and allergenicity. Int Arch Allergy Immunol. 1993;102:160–9. doi: 10.1159/000236567. [DOI] [PubMed] [Google Scholar]
- 27.Grote M, Vrtala S, Niederberger V, Wiermann R, Valenta R, Reichelt R. Release of allergen-bearing cytoplasm from hydrated pollen: a mechanism common to a variety of grass (Poaceae) species revealed by electron microscopy. J Allergy Clin Immunol. 2001;108:109–15. doi: 10.1067/mai.2001.116431. [DOI] [PubMed] [Google Scholar]
- 28.Løwenstein H. Immunological partial identity and in vitro inhibitory effect of two major timothy pollen allergens to whole pollen extract of four grasses. Int Arch Allergy Appl Immunol. 1978;57:379–83. doi: 10.1159/000232128. [DOI] [PubMed] [Google Scholar]
- 29.Chakrabarty S, Løwenstein H, Ekramoddoullah AK, Kisil FT, Sehon AH. Detection of cross-reactive allergens in Kentucky bluegrass pollen and six other grasses by crossed radioimmunoelectrophoresis. Int Arch Allergy Appl Immunol. 1981;66:142–57. doi: 10.1159/000232813. [DOI] [PubMed] [Google Scholar]
- 30.Kazemi-Shirazi L, Niederberger V, Linhart B, Lidholm J, Kraft D, Valenta R. Recombinant marker allergens: diagnostic gatekeepers for the treatment of allergy. Int Arch Allergy Immunol. 2002;127:259–68. doi: 10.1159/000057742. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira F, Hawranek T, Gruber P, Wopfner N, Mari A. Allergic cross-reactivity: from gene to clinic. Allergy. 2004;59:243–67. doi: 10.1046/j.1398-9995.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- 32.Valenta R, Duchene M, Ebner C, et al. profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175:377–85. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson P, Marsh DG. ‘Isoallergens’ from rye grass pollen. Nature. 1965;206:935–7. doi: 10.1038/206935b0. [DOI] [PubMed] [Google Scholar]
- 34.Perez M, Ishioka GY, Walker LE, Chesnut RW. cDNA cloning immunological characterization of the rye grass allergen Lol p I. J Biol Chem. 1990;265:16210–5. [PubMed] [Google Scholar]
- 35.Griffith IJ, Smith PM, Pollock J, et al. Cloning and sequencing of Lol pI, the major allergenic protein of rye-grass pollen. FEBS Lett. 1991;279:210–5. doi: 10.1016/0014-5793(91)80151-r. [DOI] [PubMed] [Google Scholar]
- 36.Laffer S, Valenta R, Vrtala S, et al. Complementary DNA cloning of the major allergen Phl p I from timothy grass (Phleum pratense); recombinant Phl p I inhibits IgE binding to group I allergens from eight different grass species. J Allergy Clin Immunol. 1994;94:689–98. doi: 10.1016/0091-6749(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 37.Van Ree R, van Leeuwen WA, Aalberse RC. How far can we simplify in vitro diagnostics for grass pollen allergy? A study with 17 whole pollen extracts and purified natural and recombinant allergens. J Allergy Clin Immunol. 1998;102:184–90. doi: 10.1016/s0091-6749(98)70084-3. [DOI] [PubMed] [Google Scholar]
- 38.Laffer S, Duchene M, Reimitzer I, et al. Common IgE-epitopes of recombinant Phl p I, the major timothy grass pollen allergen and natural group I grass pollen isoallergens. Mol Immunol. 1996;33:417–26. doi: 10.1016/0161-5890(95)00152-2. [DOI] [PubMed] [Google Scholar]
- 39.Laffer S, Vrtala S, Duchêne M, et al. IgE-binding capacity of recombinant timothy grass (Phleum pratense) pollen allergens. J Allergy Clin Immunol. 1994;94:88–94. doi: 10.1016/0091-6749(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 40.Suck R, Hagen S, Cromwell O, Fiebig H. Rapid and efficient purification of Phleum pratense major allergens Phl p 1 and group Phl p 2/3 using a two-step procedure. J Immunol Methods. 1999;229:73–80. doi: 10.1016/s0022-1759(99)00101-5. [DOI] [PubMed] [Google Scholar]
- 41.Suck R, Hagen S, Cromwell O, Fiebig H. The high molecular mass allergen fraction of timothy grass pollen (Phleum pratense) between 50–60 kDa is comprised of two major allergens: Phl p 4 and Phl p 13. Clin Exp Allergy. 2000;30:1395–1402. doi: 10.1046/j.1365-2222.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- 42.Petersen A, Suck R, Hagen S, Cromwell O, Fiebig H, Becker W-M. Group 13 grass allergens: structural variability between different grass species and analysis of proteolytic stability. J Allergy Clin Immunol. 2001;107:856–62. doi: 10.1067/mai.2001.114114. [DOI] [PubMed] [Google Scholar]
- 43.Grote M, Swoboda I, Valenta R, Reichelt R. Group 13 allergens as environmental and immunological markers for grass pollen allergy: studies by immunogold field emission scanning and transmission electron microscopy. Int Arch Allergy Immunol. 2005;136:303–10. doi: 10.1159/000083975. [DOI] [PubMed] [Google Scholar]
- 44.Swoboda I, Grote M, Verdino P, et al. Molecular characterization of polygalacturonases as grass pollen-specific marker allergens: expulsion from pollen via submicronic respirable particles. J Immunol. 2004;15:172. doi: 10.4049/jimmunol.172.10.6490. [DOI] [PubMed] [Google Scholar]
- 45.Westritschnig K, Horak F, Swoboda I, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–7. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 46.Vrtala S, Sperr WR, Reimitzer I, et al. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl pV allergen. J Immunol. 1993;151:4773–81. [PubMed] [Google Scholar]
- 47.Flicker S, Vrtala S, Steinberger P, et al. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: molecular, immunological, and structural characterization of the epitope-containing domain. J Immunol. 2000;165:3849–59. doi: 10.4049/jimmunol.165.7.3849. [DOI] [PubMed] [Google Scholar]
- 48.Niederberger V, Stubner P, Spitzauer S, et al. Skin test results but not serology reflect immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001;117:848–51. doi: 10.1046/j.0022-202x.2001.01470.x. [DOI] [PubMed] [Google Scholar]
- 49.Niederberger V, Laffer S, Fröschl R, et al. IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5 andBet v 2) account for a high percentage of grass pollen-specific IgE. J Allergy Clin Immunol. 1998;101:258–64. doi: 10.1016/s0091-6749(98)70391-4. [DOI] [PubMed] [Google Scholar]
- 50.Westritschnig K, Sibanda E, Thomas W, et al. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. 2003;33:22–7. doi: 10.1046/j.1365-2222.2003.01540.x. [DOI] [PubMed] [Google Scholar]
- 51.Dolecek C, Vrtala S, Laffer S, et al. Molecular characterization of Phl p II, a major timothy grass (Phleum pratense) pollen allergen. FEBS Lett. 1993;335:299–304. doi: 10.1016/0014-5793(93)80406-k. [DOI] [PubMed] [Google Scholar]
- 52.Petersen A, Suck R, Lindner B, et al. Phl p 3: structural and immunological characterization of a major allergen of timothy grass pollen. Clin Exp Allergy. 2006;36:840–9. doi: 10.1111/j.1365-2222.2006.02505.x. [DOI] [PubMed] [Google Scholar]
- 53.Vrtala S, Fischer S, Grote M, et al. Molecular, immunological, and structural characterization of Phl p 6, a major allergen and P-particle-associated protein from Timothy grass (Phleum pratense) pollen. J Immunol. 1999;163:5489–96. [PubMed] [Google Scholar]
- 54.Marknell DeWitt A, Niederberger V, Lehtonen P, et al. Molecular and immunological characterization of a novel timothy grass (Phleum pratense) pollen allergen, Phl p 11. Clin Exp Allergy. 2002;32:1329–40. doi: 10.1046/j.1365-2222.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 55.van Ree R, Hoffman DR, van Dijk W, et al. Lol p XI, a new major grass pollen allergen, is a member of a family of soybean trypsin inhibitor-related proteins. J Allergy Clin Immunol. 1995;95:970–8. doi: 10.1016/s0091-6749(95)70097-8. [DOI] [PubMed] [Google Scholar]
- 56.Niederberger V, Hayek B, Vrtala S, et al. Calcium-dependent immunoglobulin E recognition of the apo- and calcium-bound form of a cross-reactive two EF-hand timothy grass pollen allergen, Phl p 7. FASEB J. 1999;13:843–56. doi: 10.1096/fasebj.13.8.843. [DOI] [PubMed] [Google Scholar]
- 57.Valenta R, Duchêne M, Pettenburger K, et al. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991;253:557–60. doi: 10.1126/science.1857985. [DOI] [PubMed] [Google Scholar]
- 58.Stumvoll S, Lidholm J, Thunberg R, et al. Purification, structural and immunological characterization of a timothy grass (Phleum pratense) pollen allergen, Phl p 4, with cross-reactive potential. Biol Chem. 2002;383:1383–96. doi: 10.1515/BC.2002.157. [DOI] [PubMed] [Google Scholar]
- 59.DeWitt AM, Andersson K, Peltre G, Lidholm J. Cloning, expression and immunological characterization of full-length timothy grass pollen allergen Phl p 4, a berberine bridge enzyme-like protein with homology to celery allergen Api g 5. Clin Exp Allergy. 2006;36:77–86. doi: 10.1111/j.1365-2222.2006.02399.x. [DOI] [PubMed] [Google Scholar]
- 60.Henzgen M, Wenz W, Strümpfel R. Experiences with desensitization of early spring pollen allergy using 2 tree pollen extracts. Z Gesamte Inn Med. 1989;44:691–3. [PubMed] [Google Scholar]
- 61.Petersen BN, Janniche H, Munch EP, et al. Immunotherapy with partially purified and standardized tree pollen extracts. I. Clinical results from a three-year double-blind study of patients treated with pollen extracts either of birch or combinations of alder, birch and hazel. Allergy. 1988;43:353–62. doi: 10.1111/j.1398-9995.1988.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 62.Wihl JA, Ipsen H, Petersen BN, Munch EP, Janniche H, Løwenstein H. Immunotherapy with partially purified and standardized tree pollen extracts. II. Results of skin prick tests and nasal provocation tests from a three-year double-blind study of patients treated with pollen extracts either of birch or combinations of alder, birch and hazel. Allergy. 1988;44(Suppl):1–42. doi: 10.1111/j.1398-9995.1988.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 63.Ipsen H, Schwartz B, Wihl JA, et al. Immunotherapy with partially purified and standardized tree pollen extracts. III. Specific IgE response to the major allergens of alder, birch and hazel pollen during immunotherapy. Allergy. 1988;43:370–7. doi: 10.1111/j.1398-9995.1988.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 64.Frostad AB, Grimmer O, Sandvik L, Moxnes A, Aas K. Clinical effects of hyposensitization using a purified allergen preparation from Timothy pollen as compared to crude aqueous extracts from Timothy pollen and a four-grass pollen mixture respectively. Clin Allergy. 1983;13:337–57. doi: 10.1111/j.1365-2222.1983.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 65.Verstraeten JM, Verstraeten AF. Clinical evaluation of hyposensitization in allergic rhinitis: comparison of the results obtained with three different extracts. Material from a 3-year study. Ann Allergy. 1987;58:416–20. [PubMed] [Google Scholar]
- 66.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356–64. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- 67.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–95. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 68.Manduzio H, Fitchette AC, Hrabina M, et al. Glycoproteins are species-specific markers and major IgE reactants in grass pollens. Plant Biotechnol J. 2012;10:184–94. doi: 10.1111/j.1467-7652.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 69.van der Veen MJ, van Ree R, Aalberse RC, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–34. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 70.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 71.Valenta R. Biochemistry of allergens and recombinant allergens. In: Kay AB, Kaplan AP, Bousquet J, Holt PG, editors. Allergy and allergic diseases. 2nd edition. London: Wiley-Blackwell; 2008. pp. 895–911. [Google Scholar]
- 72.Mari A, Scala E. Allergenic extracts for specific immunotherapy: to mix or not to mix? Int Arch Allergy Immunol. 2006;141:57–60. doi: 10.1159/000094254. [DOI] [PubMed] [Google Scholar]
- 73.Løwenstein H, Wihl JA, Bache Billesbølle K, Bøwadt H. Rationale for specific immunotherapy of grass pollen allergy with extracts of rye pollen. Skin test reactivity and immunochemical relationship between pollen allergens from rye and other common grasses. Allergy. 1984;39:421–32. doi: 10.1111/j.1398-9995.1984.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 74.Valenta R, Vrtala S, Ebner C, Kraft D, Scheiner O. Diagnosis of grass pollen allergy with recombinant timothy grass (Phleum pratense) pollen allergens. Int Arch Allergy Immunol. 1992;97:287–94. doi: 10.1159/000236135. [DOI] [PubMed] [Google Scholar]
- 75.Laffer S, Spitzauer S, Susani M, et al. Comparison of recombinant timothy grass pollen allergens with natural extract for diagnosis of grass pollen allergy in different populations. J Allergy Clin Immunol. 1996;98:652–8. doi: 10.1016/s0091-6749(96)70099-4. [DOI] [PubMed] [Google Scholar]
- 76.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–8. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 77.Ferreira F, Hirtenlehner K, Jilek A, et al. Dissection of Immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J Exp Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gehlhar K, Petersen A, Schramm G, Becker WM, Schlaak M, Bufe A. Investigation of different recombinant isoforms of grass group-V allergens (timothy grass pollen) isolated by low-stringency cDNA hybridization - antibody binding capacity and allergenic activity. Eur J Biochem. 1997;247:217–23. doi: 10.1111/j.1432-1033.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 79.Fedorov AA, Ball T, Mahoney NM, Valenta R, Almo SC. The molecular basis for allergen cross-reactivity: crystal structure and IgE-epitope mapping of birch pollen profilin. Structure. 1997;5:33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 80.Flicker S, Steinberger P, Ball T, et al. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J Allergy Clin Immunol. 2006;117:1336–43. doi: 10.1016/j.jaci.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Duffort O, Quintana J, Ipsen H, Barber D, Polo F. Antigenic similarity among group 1 allergens from grasses and quantitation ELISA using monoclonal antibodies to Phl p 1. Int Arch Allergy Immunol. 2008;145:283–90. doi: 10.1159/000110887. [DOI] [PubMed] [Google Scholar]
- 82.Davies JM, Dang TD, Voskamp A, et al. Functional immunoglobulin E cross-reactivity between Pas n 1 of Bahia grass pollen and other group 1 grass pollen allergens. Clin Exp Allergy. 2011;41:281–91. doi: 10.1111/j.1365-2222.2010.03670.x. [DOI] [PubMed] [Google Scholar]
- 83.Davies JM, Li H, Green M, Towers M, Upham JW. Subtropical grass pollen allergens are important for allergic respiratory diseases in subtropical regions. Clin Transl Allergy. 2012;2:4. doi: 10.1186/2045-7022-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Focke-Tejkl M, Valenta R. Safety of engineered allergen-specific immunotherapy vaccines. Curr Opin Allergy Clin Immunol. 2012;12:555–63. doi: 10.1097/ACI.0b013e328357ca53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linhart B, Valenta R. Vaccines for allergy. Curr Opin Immunol. 2012;24:354–60. doi: 10.1016/j.coi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cromwell O, Niederberger V, Horak F, Fiebig H. Clinical experience with recombinant molecules for allergy vaccination. Curr Top Microbiol Immunol. 2011;352:27–42. doi: 10.1007/82_2011_129. [DOI] [PubMed] [Google Scholar]
- 88.Rancitelli P, Hofmann A, Burks AW. Vaccine approaches for food allergy. Curr Top Microbiol Immunol. 2011;352:55–69. doi: 10.1007/82_2011_126. [DOI] [PubMed] [Google Scholar]
- 89.Valenta R, Niespodziana K, Focke-Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–4. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Pauli G, Malling HJ. Allergen-specific immunotherapy with recombinant allergens. Curr Top Microbiol Immunol. 2011;352:43–54. doi: 10.1007/82_2011_125. [DOI] [PubMed] [Google Scholar]
- 91.Cromwell O, Häfner D, Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011;127:865–72. doi: 10.1016/j.jaci.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 92.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–83. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 93.Gilles S, Mariani V, Bryce M, et al. Pollen allergens do not come alone: pollen associated lipid mediators (PALMS) shift the human immune systems towards a T(H)2-dominated response. Allergy Asthma Clin Immunol. 2009;5:3. doi: 10.1186/1710-1492-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapman MD, Ferreira F, Villalba M, et al. CREATE consortium, The European Union CREATE project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008;122:882–9. doi: 10.1016/j.jaci.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 95.Moingeon P, Hrabina M, Bergmann KC, et al. Specific immunotherapy for common grass pollen allergies: pertinence of a five grass pollen vaccine. Int Arch Allergy Immunol. 2008;146:338–342. doi: 10.1159/000121468. [DOI] [PubMed] [Google Scholar]
- 96.Seppälä U, Dauly C, Robinson S, Hornshaw M, Larsen JN, Ipsen H. Absolute quantification of allergens from complex mixtures: a new sensitive tool for standardization of allergen extracts for specific immunotherapy. J Proteome Res. 2011;10:2113–22. doi: 10.1021/pr101150z. [DOI] [PubMed] [Google Scholar]
- 97.Arilla MC, Ibarrola I, Eraso E, Aguirre M, Martínez A, Asturias JA. Quantification in mass units of group 1 grass allergens by a monoclonal antibody-based sandwich ELISA. Clin Exp Allergy. 2001;31:1271–8. doi: 10.1046/j.1365-2222.2001.01166.x. [DOI] [PubMed] [Google Scholar]
- 98.Gavrović MD, Trtić T, Vujcić Z, Petrović S, Jankov RM. Comparison of allergenic potentials of timothy (Phleum pratense) pollens from different pollen seasons collected in the Belgrade area. Allergy. 1997;52:210–4. doi: 10.1111/j.1398-9995.1997.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 99.Esch R. Evaluation of allergen vaccine potency. Curr Allergy Asthma Rep. 2006;6:402–6. doi: 10.1007/s11882-996-0013-8. [DOI] [PubMed] [Google Scholar]
- 100.Eckl-Dorna J, Klein B, Reichenauer TG, Niederberger V, Valenta R. Exposure of rye (Secale cereale) cultivars to elevated ozone levels increases the allergen content in pollen. J Allergy Clin Immunol. 2010;126:1315–7. doi: 10.1016/j.jaci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 101.Calderón M, Cardona V, Demoly P. EAACI 100 Years of Immunotherapy Experts Panel, One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012;67:462–76. doi: 10.1111/j.1398-9995.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 102.Van der Veen MJ, Mulder M, Witteman AM, et al. False-positive skin prick test responses to commercially available dog dander extract caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98:1028–34. doi: 10.1016/s0091-6749(96)80187-4. [DOI] [PubMed] [Google Scholar]
- 103.Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–83. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- 104.Baeyens-Volant D, M’Rabet N, El Mahyaoui R, Wattiez R, Azarkan M. Contaminant trypsin-like activity from the timothy grass pollen is responsible for the conflicting enzymatic behavior of the major allergen Phl p1, Biochim. Biophys Acta. 2013;1834:272–83. doi: 10.1016/j.bbapap.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 105.Valenta R, Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007;119:826–30. doi: 10.1016/j.jaci.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 107.Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;30:4328–35. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swoboda I, De Weerd N, Bhalla PL, et al. Mutants of the major ryegrass pollen allergen, Lol p5, with reduced IgE-binding capacity: candidates for grass pollen-specific immunotherapy. Eur J Immunol. 2002;32:270–80. doi: 10.1002/1521-4141(200201)32:1<270::AID-IMMU270>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 109.Westritschnig K, Focke M, Verdino P, et al. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, Phl p 7. J Immunol. 2004;172:5684–92. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- 110.Vrtala S, Focke M, Kopec J, et al. Genetic engineering of the major timothy grass pollen allergen, Phl p 6, to reduce allergenic activity and preserve immunogenicity. J Immunol. 2007;179:1730–9. doi: 10.4049/jimmunol.179.3.1730. [DOI] [PubMed] [Google Scholar]
- 111.Westritschnig K, Linhart B, Focke-Tejkl M, et al. A hypoallergenic vaccine obtained by tail-to-head restructuring of timothy grass pollen profilin, Phl p 12, for the treatment of cross-sensitization to profilin. J Immunol. 2007;179:7624–34. doi: 10.4049/jimmunol.179.11.7624. [DOI] [PubMed] [Google Scholar]
- 112.Ball T, Linhart B, Sonneck K, et al. Reducing allergenicity by altering allergen fold: a mosaic protein of Phl p 1 for allergy vaccination. Allergy. 2009;64:569–80. doi: 10.1111/j.1398-9995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 113.Mothes-Luksch N, Stumvoll S, Linhart B, et al. Disruption of allergenic activity of the major grass pollen allergen Phl p 2 by reassembly as a mosaic protein. J Immunol. 2008;181:4864–73. doi: 10.4049/jimmunol.181.7.4864. [DOI] [PubMed] [Google Scholar]
- 114.Linhart B, Mothes-Luksch N, Vrtala S, Kneidinger M, Valent P, Valenta R. A hypoallergenic hybrid molecule with increased immunogenicity consisting of derivatives of the major grass pollen allergens, Phl p 2 and Phl p 6. Biol Chem. 2008;389:925–33. doi: 10.1515/BC.2008.105. [DOI] [PubMed] [Google Scholar]
- 115.Wald M, Kahlert H, Reese G, et al. Hypoallergenic mutants of the Timothy grass pollen allergen Phl p 5 generated by proline mutations. Int Arch Allergy Immunol. 2012;159:130–42. doi: 10.1159/000336651. [DOI] [PubMed] [Google Scholar]
- 116.Linhart B, Jahn-Schmid B, Verdino P, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. FASEB J. 2002;16:1301–3. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 117.Linhart B, Hartl A, Jahn-Schmid B, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 118.Metz-Favre C, Linhart B, Focke-Tejkl M, et al. Skin test diagnosis of grass pollen allergy with a recombinant hybrid molecule. J Allergy Clin Immunol. 2007;120:315–21. doi: 10.1016/j.jaci.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 119.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 120.Edelmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. In: Valenta R, Coffman RL, editors. Vaccines against allergies, current topics in microbiology and immunology. Vol. 352. Berlin-Heidelberg: Springer Verlag; 2011. pp. 121–40. [DOI] [PubMed] [Google Scholar]
- 121.Lorenz AR, Luttkopf D, Seitz R, Vieths S. The regulatory system in europe with special emphasis on allergen products. Int Arch Allergy Immunol. 2008;147:263–75. doi: 10.1159/000146074. [DOI] [PubMed] [Google Scholar]
- 122.Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci. 2004;101(Suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 124.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 125.Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immunotherapy with recombinant grass pollen allergens: first randomized dose-ranging safety study. Clin Exp Allergy. 2012;42:936–45. doi: 10.1111/j.1365-2222.2012.03971.x. [DOI] [PubMed] [Google Scholar]
- 126.Sastre J, Landivar ME, Ruiz-García M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–11. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]