Summary
Background
Safe and effective vaccines against Ebola could prevent or control outbreaks. The safe use of replication-competent vaccines requires a careful dose-selection process. We report the first safety and immunogenicity results in volunteers receiving 3 × 105 plaque-forming units (pfu) of the recombinant vesicular stomatitis virus-based candidate vaccine expressing the Zaire Ebola virus glycoprotein (rVSV-ZEBOV; low-dose vaccinees) compared with 59 volunteers who had received 1 × 107 pfu (n=35) or 5 × 107 pfu (n=16) of rVSV-ZEBOV (high-dose vaccinees) or placebo (n=8) before a safety-driven study hold.
Methods
The Geneva rVSV-ZEBOV study, an investigator-initiated phase 1/2, dose-finding, placebo-controlled, double-blind trial conducted at the University Hospitals of Geneva, Switzerland, enrolled non-pregnant, immunocompetent, and otherwise healthy adults aged 18–65 years. Participants from the low-dose group with no plans to deploy to Ebola-affected regions (non-deployable) were randomised 9:1 in a double-blind fashion using randomly permuted blocks of varying sizes to a single injection of 3 × 105 pfu or placebo, whereas deployable participants received single-injection 3 × 105 pfu open-label. Primary safety and immunogenicity outcomes were the incidence of adverse events within 14 days of vaccination and day-28 antibody titres, respectively, analysed by intention to treat. After viral oligoarthritis was observed in 11 of the first 51 vaccinees (22%) receiving 107 or 5 × 107 pfu, 56 participants were given a lower dose (3 × 105 pfu, n=51) or placebo (n=5) to assess the effect of dose reduction on safety and immunogenicity. This trial is ongoing with a follow-up period of 12 months; all reported results are from interim databases. This study is registered with ClinicalTrials.gov, number NCT02287480.
Findings
Between Jan 5 and Jan 26, 2015, 43 non-deployable participants received low-dose rVSV-ZEBOV (3 × 105 pfu) or placebo in a double-blind fashion, whereas 13 deployable participants received 3 × 105 pfu open-label. Altogether, in the low-dose group, 51 participants received rVSV-ZEBOV and five received placebo. No serious adverse events occurred. At 3 × 105 pfu, early-onset reactogenicity remained frequent (45 [88%] of 51 compared with 50 [98%] of 51 high dose and two [15%] of 13 placebo recipients), but mild. Objective fever was present in one (2%) of 51 low-dose versus 13 (25%) of 51 high-dose vaccinees receiving at least 1 ×107 pfu (p<0·0001). Subjective fever (p<0·0001), myalgia (p=0·036), and chills (p=0·026) were significantly reduced and their time of onset delayed, reflecting significantly lower viraemia (p<0·0001) and blood monocyte-activation patterns (p=0·0233). Although seropositivity rates remained similarly high (48 [94%] of 51), day-28 EBOV-glycoprotein-binding and neutralising antibody titres were lower in low-dose versus high-dose vaccinees (geometric mean titres 344·5 [95% CI 229·7–516·4] vs 1064·2 [757·6–1495·1]; p<0·0001; and 35·1 [24·7–50·7] vs 127·0 [86·0–187·6]; p<0·0001, respectively). Furthermore, oligoarthritis again occurred on day 10 (median; IQR 9–14) in 13 (25%) of 51 low-dose vaccinees, with maculopapular, vesicular dermatitis, or both in seven (54%) of 13; arthritis was associated with increasing age in low-dose but not high-dose vaccinees. Two vaccinees presented with purpura of the lower legs; histological findings indicated cutaneous vasculitis. The presence of rVSV in synovial fluid and skin lesions confirmed causality.
Interpretation
Reducing the dose of rVSV-ZEBOV improved its early tolerability but lowered antibody responses and did not prevent vaccine-induced arthritis, dermatitis, or vasculitis. Like its efficacy, the safety of rVSV-ZEBOV requires further definition in the target populations of Africa.
Funding
Wellcome Trust through WHO.
Introduction
Despite unprecedented public health interventions, the Ebola virus disease (EVD) epidemic has infected more than 27 000 people, more than 11 000 of whom did not survive.1 Safe and effective vaccines could prevent future outbreaks.
The live-attenuated recombinant vesicular stomatitis virus (rVSV) vaccine expressing the glycoprotein of Zaire Ebola virus (ZEBOV) was identified as a promising candidate:2 one injection of 1 × 107 plaque-forming units (pfu) of rVSV-ZEBOV protected all (≥17) challenged non-human primates3–6 with no apparent safety concerns.2,5,7,8 After the Public Health Agency of Canada’s donation of 800 vials of rVSV-ZEBOV to WHO, the latter created an African and European consortium (VEBCON [VSV-EBola CONsortium]) to initiate dose-escalation phase 1 trials of rVSV-ZEBOV in Germany (NCT02283099), Kenya (NCT02296983), and Gabon (PACTR2014000089322), as well as a double-blind phase 1/2 randomised, controlled trial in Geneva, Switzerland (NCT02287480). Preliminary results from these9 and parallel US trials10 indicate that rVSV-ZEBOV is immunogenic but reactogenic. In Geneva, 13 (25%) of 51 volunteers vaccinated with at least 1×107 pfu had fever and 11 (22%) had oligoarthritis, the latter leading to a study hold. Meanwhile, preliminary data from Gabon suggested that lower vaccine doses might be better tolerated and remain immunogenic.9 The Geneva trial thus resumed at the significantly lower dose of 3 × 105 pfu. Here, we report the first safety and immunogenicity results in volunteers receiving 3 × 105 pfu of rVSV-ZEBOV (low-dose vaccinees) compared with those receiving 1 × 107 or 5 × 107 pfu (high-dose vaccinees) before the study hold.
Methods
Study design and participants
In the Geneva rVSV-ZEBOV study, an investigator-initiated phase 1/2, dose-finding, placebo-controlled, double-blind trial conducted at the University Hospitals of Geneva, Switzerland, we enrolled non-pregnant, immunocompetent and otherwise healthy adults aged 18–65 years. Additional inclusion and exclusion criteria are detailed at ClinicalTrials.gov (NCT02287480). All participants provided written informed consent. The study and all protocol amendments were reviewed and approved by the ethics committees of the Canton of Geneva and WHO as well as by the Swiss Agency for Therapeutic Products (Swissmedic).
Randomisation and masking
As previously described,9 the first 19 study participants formed a run-in group, receiving a single injection of 1×107 pfu of rVSV-ZEBOV without blinding (open-label) under close clinical observation. Thereafter, participants planning to deploy to Ebola-affected regions (deployables) were randomly assigned (1:1) in a double-blind fashion to receive either single-injection 1 × 107 pfu or 5 × 107 pfu of vaccine, whereas non-deployables were randomly assigned (1:1:1) to receive either vaccine dose or placebo as a single injection. The emergence of arthritides in vaccinees prompted a study hold on Dec 9, 2014.9 The trial resumed on Jan 5, 2015, to test the safety and immunogenicity of a sharply reduced dose; non-deployable participants were randomised (9:1) in double-blind fashion to a single injection of 3 × 105 pfu or placebo, whereas deployable participants received single-injection 3 × 105 pfu open-label (figure 1). The blind was intentionally lifted on April 7, 2015, for all high-dose volunteers (study day 84 [D84] visits completed) and for the 15 low-dose volunteers with vaccine-related arthritis, skin lesions, or both (D28 visits completed), and on May 18 for the remaining low-dose volunteers (D84 visits completed).
Figure 1. Trial profile.
14 (10%) of 142 participants were excluded or withdrawn before study intervention due to ineligibility and 13 (9%) of 142 withdrew during the study hold. *The deployable participant and one non-deployable participant were withdrawn due to concerns regarding ability to follow the study protocol and the other non-deployable participant for uncontrolled hypertension just before the planned randomisation. rVSV-ZEBOV=recombinant vesicular stomatitis virus-based Zaire Ebola virus vaccine. pfu=plaque-forming units.
Procedures
The rVSV-ZEBOV candidate vaccine is replication-competent; the VSV vector’s G gene was replaced by the glycoprotein gene of Zaire Ebola virus.3 The vaccine (BPSC1001, lot number 003 0513) was manufactured at IDT Biologika (Dessau-Rosslau, Germany) and dispensed in single-dose vials as 1×108 pfu per mL. The 3 × 105 pfu in 0·5 mL dose was achieved by dilution with normal saline (appendix p 8). Placebo syringes containing 0·5 mL normal saline were packaged identically. Injections were given intramuscularly over 30 s into the deltoid.
Clinical and laboratory assessments were done at baseline and on days 1, 3, 7, 14, and 28 after injection. Participants were given a diary for the first 2 weeks and asked to record all adverse events, both solicited (prompted in the diary and by investigators at study visits) and unsolicited. Solicited adverse events were local pain, swelling and erythema, chills, myalgia, subjective and objective fever, loss of appetite, headache, and fatigue; after the study hold, arthralgia and skin lesions were added as solicited events for low-dose vaccinees. Adverse events occurring early (≤7 days after injection) are referred to as reactogenicity and do not include arthritis, dermatitis, and vasculitis. Severity was assessed according to US Food and Drug Administration toxicity scales:11 mild symptoms not interfering with usual activity are grade 1, moderate symptoms leading to some interference with activity are grade 2, and major symptoms preventing usual activity are grade 3.
Laboratory analyses included full blood count, creatinine, and liver function tests. Adverse events were listed per participant and are reported individually and in aggregate. All participants with swollen joint involvement were referred to a rheumatologist (AF) and all but two underwent ultrasound imaging. Arthritis was confirmed if the study team noted swelling or if imaging showed joint effusion(s), or both. Participants with skin lesions underwent biopsy sampling, swabbing, or puncture of lesions, as appropriate.
Detection of rVSV RNA was done by a quantitative RT-PCR assay targeting the nucleoprotein gene of VSV-Indiana, as previously described.9 RT-PCR was done on days 1, 3, and 7 post injection on all plasma specimens (limit of detection: 30 copies per mL), and occasionally on swabs of oral or skin lesions or on synovial fluid, or both.
Fresh whole blood was treated with lysis buffer to remove erythrocytes and 1–2 × 106 cells were stained with a panel of monoclonal antibodies including against the CD169 monocyte activation marker12,13 before data acquisition by flow cytometry (appendix p 8). Monocyte activation was characterised by the geometric mean fluorescence intensity ratio compared with isotype control.
Sera were assessed on days 0 and 28 after injection. ELISA for EBOV-glycoprotein-specific antibodies used the homologous Zaire-Kikwit strain glycoprotein following the US Army Medical Research Institute for Infectious Diseases’ (USAMRIID) standard operating procedure (SOP AP-03-35-00; USAMRIID ELISA) or inactivated whole virions of the Zaire-Makona strain, as described.9 EBOV glyocprotein-specific antibodies were also assessed by endpoint dilutions with a commercial ELISA assay (Alpha Diagnostic, TX, USA; ADI ELISA), according to the manufacturer’s instructions (quantification method C, appendix p 9). The relative amounts of EBOV-glycoprotein-specific antibodies were reported as endpoint titres or the geometric mean (logarithm base 10) titres (GMT) or concentrations (GMC) of arbitrary ELISA units per mL with 95% CIs, as indicated. Neutralising antibodies (NTAb) were detected with rVSV pseudovirions expressing the luciferase reporter gene complemented by glycoprotein from the ZEBOV 95 Kikwit strain, as described.9
Outcomes
Primary safety and immunogenicity outcome measures are the incidence of adverse events within 14 days and quantitative EBOV-specific IgG antibody responses measured by ELISA across dose groups on day 28, respectively. The incidence of adverse events occurring more than 14 days after intervention and cellular and humoral immunogenicity of rVSV-ZEBOV across dose groups are assessed secondarily.
Statistical analysis
The combined target sample size for all VEBCON studies was roughly 250 participants, because WHO estimates had concluded that roughly 74–124 participants would be needed in each dose arm to show a greater than twofold difference in EBOV glycoprotein-specific antibody titres. The Geneva proportion of roughly 115 volunteers was based primarily on recruitment capacity. At the time of the study hold, 59 of the 115 participants had been included, thus 56 inclusions were scheduled upon study resumption.
Comparisons between groups were done with χ2 or Fisher’s exact tests or by Mann-Whitney or Kruskal-Wallis tests for continuous factors for independent groups; McNemar and Wilcoxon tests were used for measures repeated over time. Unadjusted associations between predefined factors and the most common adverse events were tested. Associations between age and dose and arthritis risk were assessed with a logistic regression model with an interaction term between age and dose. Antibody responses are reported as GMT or GMC with 95% CIs and are represented by reverse cumulative distributions (RCD). Antibody response patterns across doses were assessed with Cuzick’s test for trend.14 Associations with antibody titres were assessed by means of linear regression models in the low-dose group (unadjusted associations) and in all vaccinees (associations adjusted for vaccine dose). Because the distributions of antibody titres were skewed, a logarithm transformation (base 10) was applied and associations were expressed as ratios of geometric means. Correlations between assays were assessed (Spearman’s coefficient). All statistical tests were two-sided with an α risk of 0·05.
In the absence of relevant differences in baseline characteristics and safety outcomes between recipients of 1 × 107 and 5 × 107 pfu (high-dose participants),9 between placebo recipients from both groups, and between randomised and open-label recipients of a given vaccine dose (table 1; appendix pp 12–13), their respective results are presented together.
Table 1. Baseline demographic and clinical characteristics by intervention groups.
| All | Intervention groups |
||||
|---|---|---|---|---|---|
| 3 × 105 pfu | 1 × 107 pfu* | 5 × 107 pfu* | Placebo† | ||
| Group size, n | 115 | 51 | 35 | 16 | 13 |
| Baseline demographics | |||||
| Age, years | 41 (12) | 40 (12) | 42 (11) | 43 (14) | 41 (12) |
| Sex | |||||
| Female | 53 (46%) | 27 (53%) | 14 (40%) | 5 (31%) | 7 (54%) |
| Male | 62 (54%) | 24 (47%) | 21 (60%) | 11 (69%) | 6 (46%) |
| Ethnic origin | |||||
| White | 108 (94%) | 51 (100%) | 29 (83%) | 15 (94%) | 13 (100%) |
| Other | 7 (6%) | 0 | 6 (17%) | 1 (6%) | 0 |
| Deployability | |||||
| Deployable | 38 (33%) | 13 (26%) | 18 (51%) | 7 (44%) | 0 |
| Non-deployable | 77 (67%) | 38 (75%) | 17 (49%) | 9 (56%) | 13 (100%) |
| Clinical characteristics | |||||
| Haemoglobin, g/L | 143·1 (13·1) | 143·0 (13·7) | 143·1 (14·1) | 144·3 (11·2) | 142·1 (10·7) |
| Platelets, g/L | 244·7 (54·6) | 249·7 (60·7) | 246 (47·6) | 234·6 (45·3) | 234·4 (60·5) |
| Leucocytes, g/L | 6·21 (1·8) | 6·44 (2·1) | 5·9 (1·2) | 6·6 (2·1) | 5·7 (1·4) |
| Lymphocytes, g/L | 2·0 (0·5) | 2·0 (0·6) | 2·0 (0·5) | 2·0 (0·5) | 2·0 (0·7) |
| Neutrophils, g/L | 3·5 (1·5) | 3·7 (1·7) | 3·3 (1·0) | 3·8 (1·8) | 3·0 (1·0) |
| Monocytes, g/L | 0·5 (0·2) | 0·5 (0·2) | 0·5 (0·2) | 0·6 (0·2) | 0·5 (0·1) |
| Creatinine, mg/dL | 76·2 (12·3) | 73·7 (11·9) | 78·2 (11·2) | 81·7 (13·8) | 73·6 (12·9) |
| AST, U/L | 15·3 (5·5)‡ | 15·0 (5·5)‡ | 15·3 (5·3) | 15·4 (6·5) | 16·1 (5·6) |
| ALT, U/L | 18·9 (11·1) | 18·3 (8·3) | 21·1 (14·8) | 15·5 (6·3) | 19·8 (13·0) |
Data are n (%) or mean (SD). pfu=plaque-forming units. AST=aspartate aminotransferase. ALT=alanine aminotransferase.
As previously described.9
Placebo recipients from both dose groups did not differ significantly (appendix p 13) and were pooled.
Data missing for one participant.
Role of the funding source
The funder (the Wellcome Trust Foundation) had no role in the study design, data collection, data analysis or data interpretation, or writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
The trial is ongoing with a follow-up period of 12 months; all reported results are from interim databases.
At the time of the study hold between Dec 9, 2014, and Jan 5, 2015, 59 high-dose participants had received rVSV-ZEBOV at 1 × 107 pfu (n=35) or 5 × 107 pfu (n=16) or placebo (n=8).9 After trial resumption, 43 non-deployable participants were randomly assigned (9:1) to receive low-dose rVSV-ZEBOV (3 × 105 pfu) or placebo in double-blind fashion, while 13 deployable participants received 3 × 105 pfu open-label (figure 1). Altogether, in the low-dose group 51 participants received rVSV-ZEBOV and five received placebo. Median age was 40 years (IQR 30–50, range 20–63). Like high-dose participants, all low-dose participants have been followed up for at least 12 weeks (median 96 days, range 83–104). Baseline characteristics of low-dose vaccinees were similar to those of placebo recipients and high-dose vaccinees9 (table 1; appendix pp 12–13).
There were no serious adverse events. In the first 14 days post injection, solicited reactogenicity, unsolicited events (median three events [IQR 2–6; range 0–13] per participant), or both were noted in 45 (88%) of low-dose vaccinees (figure 2; appendix pp 14–15) compared with 50 (98%) of 51 high-dose vaccinees and two (15%) of 13 placebo recipients (p=0·141).9 Most events were mild or moderate: their incidence and intensity did not differ significantly between low-dose vaccinees and placebo recipients. Local pain (11 [22%] of 51 in the low-dose group vs 39 [76%] of 51 in the high-dose group), subjective fever (14 [27%] vs 33 [65%]), objective fever (≥38°C axillary temperature, one [2%] vs 13 [25%]), and myalgia (20 [39%] vs 34 [67%]) were significantly less common (p<0·0001 for pain, p=0·001 for subjective and objective fever, and p=0·004 for myalgia) and less intense (figure 2; appendix p 14) in low-dose vaccinees than in high-dose vaccinees. Grade 3 symptoms were reported by slightly fewer low-dose vaccinees than high-dose vaccinees (seven [14%] of 51 vs 11 [22%] of 51, not significant; p=0·28]. The onset of reactogenicity was significantly later in low-dose vaccinees than in high-dose vaccinees: 25 (19%) of 133 vs 12 (5%) of 248 solicited adverse events with onset ≤7 days after injection began after day 2 (p<0·0001; appendix p 16). Duration of reactogenicity was similarly short (median 1 day, IQR <24 h–3 days). Reactogenicity was not affected by age or sex (data not shown).
Figure 2. Comparison of local and systemic adverse events after receipt of rVSV-ZEBOV or placebo.
Solicited adverse events reported in the first 14 days after injection and their severity are reported following injection of 3 × 105 plaque-forming units (pfu; low dose, n=51), 1×107 pfu or 5 × 107 pfu (high dose, n=51), or placebo (n=13). p values for comparisons between low-dose and high-dose recipients (shown on the right) show a significant dose effect on pain, subjective and objective fever, and myalgia, with similar trends for chills, fatigue, and headaches. rVSV-ZEBOV=recombinant vesicular stomatitis virus-based Zaire Ebola virus vaccine.
Between days 1 and 3 after injection, lymphocyte, neutrophil, and platelet counts decreased in low-dose vaccinees compared with high-dose vaccinees, whereas monocytes increased (appendix pp 17–18). The decline in circulating lymphocytes, neutrophils, and platelets occurred on the same days, but was significantly milder in low-dose versus high-dose vaccinees (appendix p 19). Monocytosis was associated with increased expression of the CD169 monocyte-activation marker12 on classical, intermediate, and non-classical blood monocytes15 from a random subset of 46 participants (appendix p 20). The intensity of CD169 expression was significantly weaker in low-dose versus high-dose vaccinees, suggesting a dose effect on monocyte activation. Liver profiles and creatinine levels remained unchanged (data not shown).
Following rVSV-ZEBOV RNA quantification by RT-PCR, only ten (20%) of 51 low-dose vaccinees had detectable RNA levels at any time compared with 46 (90%) of 51 high-dose vaccinees (p<0·0001; appendix pp 21–22). When detected, viraemia was minimal and significantly reduced (p<0·0001) among low-dose vaccinees. No correlation was noted between viraemia occurrence or peak value and age, sex, lymphopaenia, and adverse event frequency or intensity.
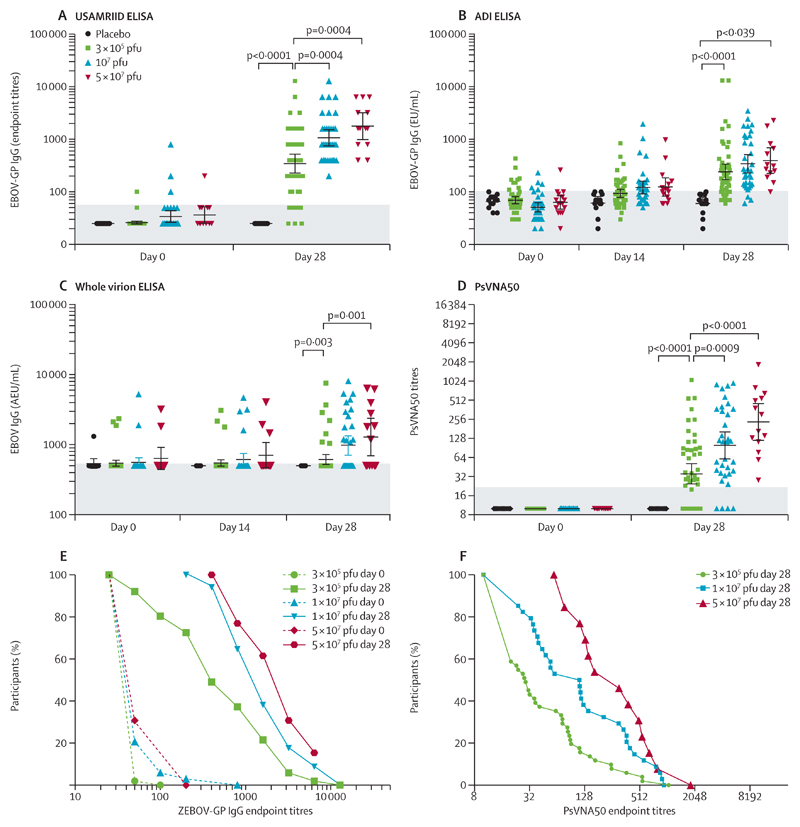
Serum antibodies induced by rVSV-ZEBOV were assessed using four distinct assays. Baseline antibodies, occasionally detected above quantification thresholds, increased to significantly higher titres after immunisation (figure 3; appendix pp 23–27). In low-dose vaccinees, EBOV-glycoprotein-binding antibody responses were already observed on day 14, and on day 28 seropositivity rates reached 48 (94%) of 51 and 37 (73%) of 51 with anoptimised in-house (USAMRIID) or commercial (ADI) EBOV-glycoprotein ELISA, respectively, and seven (14%) of 51 with whole virions (figure 3A–C). GMCs were also highest with the USAMRIID, intermediate with the ADI, and lowest with whole-virion ELISA, presumably reflecting various glycoprotein contents (figure 3; table 2; appendix pp 23–27). With the USAMRIID glycoprotein-ELISA assay, similar seropositivity rates but significantly lower GMTs were noted in low-dose versus high-dose vaccinees (344·5 [95% CI 229·7–516·4] vs 1064·2 [758·6–1495·1], p=0·002; table 2; appendix p 28). The pseudovirion neutralisation assay (PsVNA50) identified NTAb in 30 (59%) of 51 low-dose versus 44 (92%) of 48 high-dose vaccinees (p=0·0004; figure 3D; table 2; appendix pp 26–27). Their GMT reflected significantly lower titres in low-dose vs high-dose responders (p<0·0001, figure 3D–F; table 2; appendix pp 26–27). Significant correlations were noted between results obtained with the three assays (appendix p 28). Univariate (appendix p 29) and multivariate (appendix p 30) analyses identified no significant correlation with age, sex, reactogenicity, haematological changes, or peak viraemia.
Figure 3. Glycoprotein antibody titres by vaccine dose and assay.
Individual antibody titres assessed at baseline and 28 days after injection by dose group. Results from high-dose vaccinees, previously reported,9 are provided for comparison. Antibodies were measured by ELISA against the homologous glycoprotein of Zaire-Kikwit strain (USAMRIID [A], ADI [B]) or inactivated whole virions of the Zaire-Makona strain (C). Data are geometric mean concentration of endpoint titres (A) or of AEUs per mL (B, C) with 95% CIs. The shaded zones indicate the quantification thresholds. Neutralising antibodies were detected with rVSV pseudovirions complemented by homologous glycoprotein (D). Geometric mean titres and 95% CIs are shown for each dose group and timepoint assessed. The shaded zone indicates the quantification threshold. The results of USAMRIID glycoprotein-ELISA (E) and pseudovirion neutralisation assay (F) were expressed as the reciprocal of the highest dilution showing a positive result. The curves represent the distribution of individual antibody titres in each dose group. Dotted lines indicated baseline titres (E). USAMRIID=US Army Medical Research Institute of Infectious Diseases. ADI=Alpha Diagnostic International. EU=ELISA units. AEU=arbitrary ELISA units. PsVNA50=pseudovirion neutralisation assay. pfu=plaque-forming units. ZEBOV-GP=Zaire Ebola virus glycoprotein.
Table 2. Geometric mean titres and concentrations by treatment groups.
| Placebo | 3 × 105 pfu | 1 × 107 pfu‡ | 5 × 107 pfu‡ | ≥1 × 107 pfu | p value* | p value† | |
|---|---|---|---|---|---|---|---|
| EBOV-GP ELISA USAMRIID (GMT) | |||||||
| Day 0 | 25 | 26·0 (24·5–27·6) | 33·9 (26·6–43·4) | 36·3 (26·1–50·5) | 34·6 (28·4–42·1) | .. | .. |
| Day 28 | 25 | 344·5 (229·7–516·4) | 1064·2 (757·6–1495·1) | 1780·1 (1048·3–3022·5) | 1227·0 (917·3–1641·2) | <0·0001 | <0·0001 |
| EBOV-GP ELISA ADI (EU per mL) | |||||||
| Day 0 | 66·0 (56·5–77·2) | 69·6 (59·7–81·0) | 51·2 (42·2–62·0) | 63·1 (48·0–83·0) | 54·6 (46·6–64·0) | .. | .. |
| Day 28 | 60·0 (46·4–77·5) | 241·4 (173·8–335·4) | 342·3 (232·9–503·0) | 392·8 (237·2–650·5) | 355·6 (261·0–484·4) | 0·022 | 0·032 |
| Whole-virion ELISA (EU per mL) | |||||||
| Day 28 | 500 | 614·7 (524·3–720·5) | 982·9 (721·1–1339·6) | 1285·1 (738·9–2235·3) | 1058·6 (807·9–1387·1) | 0·0005 | 0·001 |
| PsVNA50 (GMT) | |||||||
| Day 0 | 10 | 10 | 10 | 10 | 10 | .. | .. |
| Day 28 | 10 | 35·4 (24·7–50·7) | 99·1 (61·9–158·9) | 231·8 (126·7–424·3) | 127·0 (86·0–187·6) | <0·0001 | <0·0001 |
Data are geometric endpoint titres (GMT) or mean concentrations (GMC) of ELISA units per mL with 95% CIs. pfu=plaque-forming units. EBOV-GP=Zaire Ebolavirus glycoprotein. USAMRIID=US Army Medical Research Institute of Infectious Diseases. ADI=Alpha Diagnostic International. EU=ELISA units. PsVNA50=pseudovirion neutralisation assay.
Cuzick’s test for three group comparisons of recipients of 3 × 105 pfu, 1 × 107 pfu, and 5 × 107 pfu.
Mann-Whitney test for two group comparisons of low-dose (3 × 105) and high-dose (≥ 1 × 107) vaccinees.
Results from participants injected with 1 × 107 pfu and 5 × 107 pfu, previously reported,9 are provided for comparison.
At a median of 10 days after immunisation (IQR 9–14), 13 (25%) of 51 low-dose vaccinees had arthralgia, as observed in 11 (22%) of 51 high-dose vaccinees.9 Arthritis was confirmed by clinical examination (12 [92%] of 13) or by ultrasound showing (teno)synovitis or bursitis (11 [85%] of 13), of at least one joint (table 3). A median of four joints per participant were affected (IQR 2–6; range 1–10). Pain initially reached grade 3 in six (46%) of 13 participants, although only briefly (median <24 h [IQR <24 h–3 days]). Nine participants (81%) had at least grade 2 pain (median duration 2 days [IQR 1–4]). Overall, pain lasted a median of 18 days (IQR 8–30), with one participant reporting ongoing mild pain upon movement (>80 days; table 3). Therapeutic arthrocentesis with lidocaine and betamethasone or triamcinolone infiltration was required in three of 13 participants. Functional impact remained moderate, with a median score of 2·7 (IQR 1·0–3·7) on the Routine Assessment of Patient Index Data (RAPID3).16 PCR detected rVSV in the synovial fluid of two of three participants assessed, at 1337 and 5000 copies per mL, respectively. rVSV viraemia remained negative in all participants at diagnosis and at all times in ten (76%) of 13 participants. No association of arthritis with sex or reactogenicity was noted. The median age of participants with arthritis (51·8 years [IQR 47·3–54·0]) was significantly higher than that of vaccinees without arthritis (36·7 [29·7–42·7; p=0·006]). In a logistic regression model including high-dose and low-dose vaccinees, age was significantly associated with the risk of arthritis in low-dose vaccinees (odds ratio [OR] 2·43 [95% CI 1·24–4·78; p=0·010] per 10 additional years), but not in high-dose vaccinees (OR 0·76 per 10 additional years [95% CI 0·43–1·35], p=0·356; appendix pp 31–32). The two ORs were significantly different (p=0·011), identifying an association between age and arthritis only in low-dose vaccinees.
Table 3. Description of arthritis cases among recipients of 3 × 105 plaque-forming units (low-dose) rVSV-ZEBOV by case number.
| Age, years | Sex | Day of onset post injection | Joints affected (total number) | Imaging: type, findings (study day done) | Pain |
Morning stiffness, >30 min | Skin lesion(s) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total duration, days | Grade* (days) | ||||||||
| 1 | 62 | Female | 9 | Right wrist, right MCP4, left DIP2 (finger), left knee (4) | US: right hand flexor tenosynovitis (right carpal tunnel syndrome), right MCP4 tenosynovitis (14) | 24 | 3 (6); 2 (8); 1 (10) |
Present | Generalised maculopapular rash, one vesicle |
| 2 | 54 | Male | 10 | Right PIP3, right PIP4 (fingers), right MTP1, right elbow, right knee, right ankle (6) | US: right MTP1 arthritis, right PIP3 synovitis, right knee arthritis | 19† | 2 (4); 1 (15) |
Present | Vesicles on first right toe |
| 3 | 53 | Male | 6 | Bilateral wrists, TMJ, ankles, knees, hips (10) | US: left radiocarpal synovitis | 25 | 3 (3); 2 (5); 1 (17) |
Present | None |
| 4 | 55 | Male | 14 | Right DIP2, right DIP5 (fingers), right wrist, right knee (4) | US D17: right carpal synovitis, right extensor tenosynovitis, right long flexor tenosynovitis, left discrete carpal synovitis | 37 | 3 (5); 2 (1); 1 (31) |
Present | Plantar vesicles on toes; left tibial petechial rash (cutaneous vasculitis) with dermal swab positive for rVSV RNA |
| 5 | 47 | Male | 15 | Left wrist (1) | US D17: left para-articular ulnar cyst, carpal arthritis | 2 | 1 (2) | Present | None |
| 6 | 52 | Male | 8 | Right elbow, bilateral shoulders, left knee, left ankle (5) | US D9: right elbow bursitis | >80 (pain ongoing in left ankle only) | 3 (2); 2 (2); 1 (>76) |
0 | Diffuse maculopapular rash with vesicles |
| 7 | 31 | Female | 10 | Right elbow (1) | US D13: NSF | 9 | 1 (9) | 0 | None |
| 8 | 25 | Female | 10 | Right wrist, right knee (2) | US D16: right knee effusion; tapped therapeutically | 8‡ | 2 (1); 1 (7) |
Present | 2 macular erythematous lesions on fingers |
| 9 | 54 | Female | 18 | Right tarsometatarsal, left distal metatarsal 2-3-4 (6) | US D30: NSF | 82 | 2 (2); 1 (80) |
Present | Localised macular rash on dorsum of left foot |
| 10 | 49 | Female | 16 | Right wrist, right elbow, right knee (3) | US D18: significant right knee effusion (participant refused tap), radial flexor tenosynovitis | 6 | 3 (2); 2 (2); 1 (2) |
Present | None |
| 11 | 50 | Female | 12 | Right MCP4 (1) | Not done | 4 | 2 (3); 1(1) |
Present | None |
| 12 | 51 | Male | 7 | Left ankle, left wrist, left cervical, right MCP3, right PIP3, right DIP3 (fingers; 6) | US D15: left ankle arthritis, left wrist arthritis, right extensor tenosynovitis MCP3 and PIP3 | 30 | 3 (4); 2 (1); 1 (25) |
Present | Hyperkeratosis and erythematous patches on both wrists |
| 13 | 29 | Female | 9 | Left PIP2 (finger), right wrist (2) | Not done | 13 | 2 (5); 1 (8) |
Present | None |
rVSV-ZEBOV=recombinant vesicular stomatitis virus-based candidate expressing the Zaire Ebola virus. MCP=metacarpophalangeal joint. DIP=distal interphalangeal joint. PIP=proximal interphalangeal joint. MTP=metatarsophalangeal joint. US=ultrasound. NSF=no significant findings. TMJ=temporomandibular joint.
Pain intensity: grade 1=no interference with activity; 2=some interference with activity; 3=significant, prevents daily activity; 4=medical consultation, admission to hospital, or both required.
This participant had a suspected relapse on day 59 with arthralgia and discrete swelling in his third right finger PIP; symptoms were mild and self-limited, with full resolution after 4 weeks.
This participant had a suspected relapse on day 52 with arthralgia and mild swelling of the first right MTP; symptoms were mild and self-limited, with full resolution after 46 days. Data reported are as of April 19, 2015.
In the second and third weeks after immunisation, seven (54%) of 13 low-dose vaccinees with arthritis developed the maculopapular (n=5) and/or vesicular (n=4) dermatitis previously described in three (27%) of 11 high-dose vaccinees with arthritis.9
In addition, two vaccinees presented with purpura on the lower legs. The first is a 55-year-old man with onset of arthralgia on day 14 (table 3; case number 4). On day 17, a painless, non-palpable petechial and purpuric rash (10 cm × 4 cm) was noted on his left leg (figure 4A). Histological examination showed a dense CD4+ T-cell lymphocytic infiltrate surrounding dermal blood vessels with swollen endothelial cells and extravasated erythrocytes, consistent with lymphocytic vasculitis (figure 4B, C).17 A deep dermal swab taken during skin biopsy confirmed the presence of rVSV RNA. The second participant is a 48-year-old woman who developed a painless, pruritic, non-palpable petechial and purpuric rash of both lower legs (figure 4D) on day 12, with a macular rash on both arms and a papule on one finger, but no arthritis. A skin biopsy of a purpuric lesion showed similar alterations (figure 4E, F), leading to the same diagnosis of cutaneous lymphocytic vasculitis. A swab at the biopsy site remained negative for rVSV-ZEBOV. Platelet counts, urinalysis, and creatinine levels were within normal limits in both participants. The clinical course was favourable for both, with spontaneous resolution of the vasculitis after 14 days and 10 days, respectively.
Figure 4. Cutaneous vasculitis after rVSV-ZEBOV immunisation.
Purpuric lesions on the pretibial area (A) and lower legs (D) of two vaccinees. Haematoxylin and eosin stain (original magnification × 2) shows swollen endothelial cells and a dense perivascular lymphocytic infiltrate with numerous extravasated erythrocytes, but no fibrinoid necrosis or thrombi (B, E). Examination of skin biopsy samples were done on days 2 and 5 after the onset of purpura, respectively. Immunostaining identifies the lymphocytic infiltrate as composed mainly of CD4+ T cells (C, F). Complement C3 was detected in the vessel walls and at the dermal epidermal junction (not shown), without IgG, IgM, or IgA deposits. rVSV-ZEBOV=recombinant vesicular stomatitis virus-based Zaire Ebola virus vaccine.
Discussion
High-dose rVSV-ZEBOV vaccination led to detectable viraemia in almost all vaccinees and viral dissemination with secondary arthritis in up to 22% of Geneva vaccinees.9 Here, we show that a major dose reduction substantially decreased viraemia and reactogenicity, but did not preclude the viral dissemination to joints and skin leading to arthritis, dermatitis, and vasculitis. Neither of these observations was anticipated.
Replicating vaccines classically remain infectious and thus trigger similar vaccine-specific reactogenicity across a large dose range. Reduction of the 17D yellow-fever vaccine dose from 2000 pfu to 20 pfu did not change its reactogenicity,18 and adverse reactions to a live-attenuated dengue virus type 4 candidate remained unchanged between 1 × 105 pfu and 1 pfu.19 The same was expected for rVSV-ZEBOV: reduction of its dose from 2000 pfu to 2 pfu did not reduce its efficacy in mice,8 and reactogenicity and viraemia have been similar in human beings receiving doses between 3 × 106 pfu and 5 × 107 pfu.9,10 Weaker reactogenicity was noted in 20 Gabonese participants immunised with 3 × 105 pfu;9 this could have reflected differences in baseline immunity,20 study populations, or both. Our findings now show a strong dose effect of rVSV-ZEBOV on acute reactogenicity: at 3 × 105 pfu, early reactogenicity was similarly low in vaccinees and placebo recipients and significantly stronger in high-dose vaccinees (figure 2).
A rVSV-ZEBOV dose reduction to 3 × 105 pfu also reduced the occurrence and magnitude of viraemia, which remained transient (appendix p 22). This finding again contrasts with the 17D yellow-fever vaccine: reduction of the dose delayed the onset of viraemia, which lasted longer and reached higher levels—an effect presumed to reflect the induction of weaker innate responses and thus protracted viral replication.18 Whether resulting from direct infection or indirect activation, both of which are currently indistinguishable, monocyte activation (defined by CD169 expression) was indeed reduced in low-dose vaccinees, and the onset of acute reactogenicity was significantly delayed. Thus, the replication pattern of the rVSV-ZEBOV-vector vaccine differs from that of live-attenuated vaccines. In-depth analyses of innate responses will be needed to unravel the correlations between rVSV-ZEBOV dose, viraemia, and viral replication in the periphery, immune activation, and the onset of inflammatory symptoms.
The data also show a dose effect on the immunogenicity of rVSV-ZEBOV. Titres of EBOV-glycoprotein-binding and neutralising antibodies were significantly weaker in low-dose recipients, affecting the seropositivity rates of the less sensitive assays. Their distribution (figure 3E, F) was remarkably similar to that noted in the 20 Gabonese recipients of 3 × 105 pfu,9 suggesting a dose rather than a population effect on immunogenicity. Until correlates of protection against EBOV are established, any prediction as to whether this reduction in antibody titres would have a detrimental effect on protection remains speculative. Assay sensitivities vary substantially, and correlations between antibody titres elicited in rVSV-ZEBOV-immunised non-human primates and human beings have not yet been established. Similarly, direct correlations between immune responses elicited in humans by rVSV-ZEBOV and the chimpanzee adenovirus 3 (ChAd3)-vectored vaccine21 are yet lacking. At high doses (≥1 × 107 pfu and 1 × 1011 pfu, respectively), both vaccines seem to elicit initial EBOV-glycoprotein-specific responses of a similar magnitude. An advantage of rVSV-ZEBOV, which contributed to its selection for further trials in west Africa, is its anticipated potential as a single-dose vaccine.
The decision to resume the trial at 3 × 105 pfu was motivated by the hope that a substantial dose reduction would obviate the viral dissemination leading to arthritis and dermatitis, neither of which had been reported at 3 × 105 pfu.9 Yet rVSV-ZEBOV remained equally arthritogenic, with the recovery of vaccine RNA in synovial fluid (in this paper and a previous report9) confirming viral arthritis.
This study adds important information. First, arthritis is not dose dependent, but intrinsic to rVSV-ZEBOV. Arthritis has not been reported after wild-type VSV infection22–26 or vaccination with rVSV with non-EBOV-glycoprotein inserts,27 whereas polyarthralgia is frequent after Ebola virus disease,28 suggesting immune reactions to EBOV antigens in rVSV-ZEBOV infected joints. Arthritis was not reported after immunisation with the replication-deficient ChAd3-ZEBOV vaccine expressing the same glycoprotein,21 suggesting the pathophysiological role of viral replication. Second, viraemia was never detected in ten of 13 low-dose participants with arthritis, reflecting the capacity of rVSV-ZEBOV to target the joints even when replication in the blood is apparently limited. Our current hypothesis is that the highly expressed29 EBOV-glycoprotein mediates the entry of rVSV-ZEBOV into blood monocytes,30 which transport rVSV-ZEBOV to the joints, where replication occurs and local inflammation is triggered, persisting until viral antigens are eventually cleared by the immune system. Whether vaccine-induced arthritis might be obviated by further attenuation of the rVSV backbone or modification of the EBOV-glycoprotein, or both, is unknown. Third, arthritis was associated with dermatitis in more than 50% of low-dose vaccinees with arthritis. Further analyses will determine whether this results from the weaker innate responses facilitating viral dissemination to peripheral tissues, delayed viral clearance, or both. Finally, a significant age effect was observed: low-dose vaccinees with arthritis were significantly older, as previously reported for rubella vaccine-induced arthritis:31,32 the relative risk of arthritis increased from 5% at 20 years to 60% at 60 years (appendix p 32). This association was not noted in high-dose vaccinees. Further investigation is required to determine whether young age exerts a protective effect through faster viral clearance in peripheral tissues or attenuated inflammatory joint responses, or both, at low—but not high—doses, and whether joints become more vulnerable to viral seeding by infected inflammatory cells with age, increasing viral load, or both. Regardless of the mechanism, the determinants of rVSV-ZEBOV-induced oligoarthritis thus include host factors. One case of arthritis has been reported from Africa9 and cases of transient low-grade oligoarthritis and skin lesions have now been identified (but at lower frequency) in a US multicentre trial (Merck/NewLink, personal communication). Thus, the frequency of vaccine-related arthritis, dermatitis, and vasculitis remains higher in the Geneva population than in other sites. Potential contributing factors include differences in reporting or clinical investigative approaches; because these events are generally mild or moderate, and not readily attributed to vaccination, they could escape notification. Host factors regulating inflammatory or immune responses probably differ between study populations and include age, sex, fitness and physical activity, body-mass index, baseline immunity, and human leucocyte antigen types. Their relative contributions could affect the frequency and severity of arthritis, requiring safety evaluation in the target populations of Africa.
The occurrence of rVSV-ZEBOV-associated lymphocytic vasculitis was unexpected, as it also does not occur in wild-type VSV infection. Purpuric areas were free of dermatitis and lymphocytic infiltration was perivascular, absent from the dermoepidermal junction (as observed in rVSV-ZEBOV-induced dermatitis9), but concentrated around swollen endothelial cells.17 These clinical and histological patterns are reminiscent of the so-called gloves-and-socks syndrome caused by parvovirus B19,33 which results from the infection of endothelial cells in dermal capillaries.34 This suggests that rVSV-ZEBOV might target and infect dermal endothelial cells, possibly mediated by EBOV-glycoprotein in view of its noted effects on endothelial cells.35 The delayed onset of purpura, not associated with early or delayed viraemia, and the perivascular CD4+ T-cell infiltrate suggest a pathophysiological role for vaccine-induced T cells.
This study has limitations. It had to include open-label immunisations to meet the request of international agencies not to impose placebo on deployable individuals, and was interrupted by a safety-driven study hold. Thus, participants were not simultaneously randomised to low-dose or high-dose vaccine, but injected before or after a 3-week study hold; nonetheless, we identified no relevant disparities in baseline characteristics that could explain the different outcomes observed. Sample size was largely based on logistic capacities, such that the study was not powered to examine modest specific correlations. Thus, the absence of association does not exclude its existence. Information about the first arthritis cases was disseminated to all volunteers, thus an awareness bias cannot be excluded. However, arthritis, dermatitis, and vasculitis were confirmed by objective findings in all participants.
The rVSV-ZEBOV vaccine was selected for further phase 3 testing in Guinea, Sierra Leone, and Liberia. Its safety and efficacy require further definition in these target populations. As low-grade, transient symptoms would not affect the use of an effective Ebola vaccine, the further characterisation of frequency and severity of arthritis-related, dermatitis-related, or vasculitis-related symptoms (or sequelae, if any) in African populations will critically inform the development of rVSV-ZEBOV.
The study provides a first opportunity to directly compare the effects of various doses of rVSV-ZEBOV. This dose effect is strong: reduction of the dose of rVSV-ZEBOV from 107 pfu or greater to 3 × 105 pfu substantially reduced viraemia and acute reactogenicity, but it negatively impacted antibody responses and did not prevent the homing of rVSV-ZEBOV towards the joints, skin, and endothelium. These findings do not support a strategy of rVSV-ZEBOV dose reduction to prevent vaccine-induced arthritis, dermatitis, or vasculitis.
Supplementary Material
Research in context.
Evidence before this study
The ClinicalTrials.gov database was searched on July 24, 2015, for studies including “Ebola” and “vaccine” as keywords. This search yielded 34 clinical trials using vaccines expressing the Ebola virus (EBOV) glycoprotein by recombinant vesicular stomatitis virus (rVSV-EBOV), chimpanzee adenovirus (ChAd3-EBO-Z), human adenovirus (Ad5-EBOV, Ad26-ZEBOV), or modified vaccinia virus Ankara (MVA-BN Filo) vectored vaccines, DNA vaccines, or nanoparticles. The rVSV-ZEBOV vaccine is considered a promising candidate against Ebola virus disease; it has been selected for further phase 3 testing by WHO, the US Centers for Disease Control, the US National Institutes of Health, and the national health authorities of Guinea, Sierra Leone, and Liberia. Its safety and immunogenicity, however, require further definition. Two preliminary reports from a total of six phase 1 studies were recently published; these indicate that rVSV-ZEBOV is immunogenic, but reactogenic: vaccine viraemia was common and associated with frequent mild-to-moderate acute inflammatory reactions and, in some vaccinees, with viral dissemination leading to arthritis and occasional dermatitis.
Added value of this study
This study shows that a significant rVSV-ZEBOV dose reduction from 1–5 × 107 to 3 × 105 pfu successfully decreases the occurrence and magnitude of viraemia, monocyte activation, and early reactogenicity. This dose reduction, however, negatively affects antibody responses, fails to prevent viral seeding of peripheral tissues, and thus does not decrease the risk of vaccine-induced arthritis, dermatitis, and cutaneous vasculitis.
Implications of all the available evidence
These results suggest that reducing the dose of rVSV-ZEBOV is not a useful strategy to prevent vaccine-induced arthritis, dermatitis, or vasculitis. Like its efficacy, the safety of rVSV-ZEBOV needs further definition in the target populations of Africa.
Acknowledgments
We thank all volunteers for their generous participation in this trial, and in particular those who consented to undergo skin biopsies or arthrocentesis to help us understand rVSV-ZEBOV pathogenesis; the Wellcome Trust for funding; Public Health Agency of Canada for donating the vaccine vials to WHO; the entire team of the Clinical Trials Unit of the HUG–Hôpitaux Universitaires de Genève for exceptional assistance, dedication, and performance; Khaled Mostaguir and Serenella Ferro Rojas for enabling electronic data management in record-breaking time; Jocelyne Chabert and Tanya Scharton-Kersten for regulatory guidance and assistance; Jean-Pierre Petchot-Bacqué, Pauline Vetter and Basile Darbellay for clinical assistance; Angèle Gayet-Ageron for randomisation procedures; Lucie Bouchoud and the HUG pharmacy for the daily preparation of syringes; Bruno Di Lenardo, Laurence Morandi, Stéphane Grillet, and all laboratory members who contributed to the proper handling of a large number of clinical samples; Marie-Estelle Gaignard, Lena Groenendijk, Hadrien Komaromi, Ani Nigolian, and Amit Ramsahye for daily data collection; Jason Regules (WRAIR), Tom Monath and Gray Heppner (NewLink Genetics), and Mark Feinberg (Merck) for sharing precious information; Karin Marescotti (HUG) and Guido Torelli (WHO) for arranging contracts in an exceptionally rapid manner; the CCER (chair: Bernard Hirschel), the WHO research ethics committee (chair: Melba Gomes), and the DSMB (chair: Markus Müller) for exceptionally rapid reviews. USAMRIID’s research was funded by MCS-JVAP workplan B.30 and B.31 under USAMRIID project numbers 18836631, 17336629, and 17336630. Opinions, interpretations, and conclusions expressed in this manuscript are those of the authors and do not necessarily represent the position or policies of the World Health Organization and are not necessarily endorsed by the US Army.
Footnotes
Contributors
AH, J-AD, SY, AF, AM, and C-AS collected the data. AH, SY, CC, FA, ME, ARG, JWH, GK, VK, SK, BL, PS, SB, LK, and C-AS analysed and interpreted the data. AH, SY, JD, PEF, VM, MPK, LK, and C-AS made substantial contributions to the conception and design of the study. AH, CC, SY, and C-AS wrote the report. All authors contributed to the revision of the report.
Declaration of interests
We declare no competing interests.
Contributor Information
Angela Huttner, Infection Control Programme, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; Division of Infectious Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Julie-Anne Dayer, Division of Infectious Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Sabine Yerly, Virology Laboratory, Geneva University Hospitals, Geneva, Switzerland.
Christophe Combescure, Division of Clinical Epidemiology, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Floriane Auderset, WHO Collaborating Centre for Vaccinology, Faculty of Medicine, Geneva, Switzerland.
Prof Jules Desmeules, Centre for Clinical Research, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Markus Eickmann, Philipps University Marburg, Institute for Virology, Marburg, Germany.
Prof Axel Finckh, Division of Rheumatology, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Ana Rita Goncalves, Virology Laboratory, Geneva University Hospitals, Geneva, Switzerland.
Jay W Hooper, Department of Molecular Virology and Department of Translational Sciences, US Army Medical Research Institute for Infectious Diseases (USAMRIID), Frederick, MD, USA.
Gürkan Kaya, Division of Dermatology, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Verena Krähling, Philipps University Marburg, Institute for Virology, Marburg, Germany.
Steve Kwilas, Department of Molecular Virology and Department of Translational Sciences, US Army Medical Research Institute for Infectious Diseases (USAMRIID), Frederick, MD, USA.
Barbara Lemaître, WHO Collaborating Centre for Vaccinology, Faculty of Medicine, Geneva, Switzerland.
Alain Matthey, Centre for Clinical Research, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Peter Silvera, Department of Molecular Virology and Department of Translational Sciences, US Army Medical Research Institute for Infectious Diseases (USAMRIID), Frederick, MD, USA.
Prof Stephan Becker, Philipps University Marburg, Institute for Virology, Marburg, Germany.
Marie Paule Kieny, World Health Organization, Geneva, Switzerland.
Prof Laurent Kaiser, Division of Infectious Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; Virology Laboratory, Geneva University Hospitals, Geneva, Switzerland.
Prof Claire-Anne Siegrist, WHO Collaborating Centre for Vaccinology, Faculty of Medicine, Geneva, Switzerland; Center for Vaccinology, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
References
- 1.WHO. WHO Situation Report. [accessed June 22, 2015]; http://apps.who.int/ebola/currentsituation/ebola-situation-report-17-june-2015.
- 2.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204:S1075–81. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X, Fernando L, Alimonti JB, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong Ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4:e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of Ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mire CE, Miller AD, Carville A, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012;6:e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SM, Stroher U, Fernando L, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196:S404–12. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 9.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe—preliminary report. N Engl J Med. 2015 doi: 10.1056/NEJMoa1502924. published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine—preliminary report. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414216. published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. [accessed April 5, 2015]; http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf.
- 12.York MR, Nagai T, Mangini AJ, et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–20. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 13.Kwissa M, Nakaya HI, Oluoch H, et al. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012;119:2044–55. doi: 10.1182/blood-2011-10-388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pincus T. RAPID3, an index of only 3 patient self-report core data set measures, but not ESR, recognizes incomplete responses to methotrexate in usual care of patients with rheumatoid arthritis. Bull Hosp Joint Dis. 2013;71:117–20. [PubMed] [Google Scholar]
- 17.Kossard S. Defining lymphocytic vasculitis. Australas J Dermatol. 2000;41:149–55. doi: 10.1046/j.1440-0960.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 18.Monath TP, Gershman J, Staples E, et al. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: Elsevier; 2013. pp. 870–968. [Google Scholar]
- 19.Durbin AP, Whitehead SS, McArthur J, et al. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191:710–18. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 20.Muyanja E, Ssemaganda A, Ngauv P, et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest. 2014;124:3147–58. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine—preliminary report. [published online Nov 26];N Engl J Med. 2014 doi: 10.1056/NEJMoa1410863. [DOI] [Google Scholar]
- 22.Hanson RP, Rasmussen AF, Jr, Brandly CA, et al. Human infection with the virus of vesicular stomatitis. J Lab Clin Med. 1950;36:754–58. [PubMed] [Google Scholar]
- 23.Johnson KM, Vogel JE, Peralta PH. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am J Trop Med Hyg. 1966;15:244–46. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- 24.Fields BN, Hawkins K. Human infection with the virus of vesicular stomatitis during an epizootic. N Engl J Med. 1967;277:989–94. doi: 10.1056/NEJM196711092771901. [DOI] [PubMed] [Google Scholar]
- 25.Reif JS, Webb PA, Monath TP, et al. Epizootic vesicular stomatitis in Colorado, 1982: infection in occupational risk groups. Am J Trop Med Hyg. 1987;36:177–82. doi: 10.4269/ajtmh.1987.36.177. [DOI] [PubMed] [Google Scholar]
- 26.Quiroz E, Moreno N, Peralta PH, et al. A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am J Trop Med Hyg. 1988;39:312–14. doi: 10.4269/ajtmh.1988.39.312. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs JD, Frank I, Kochar N, et al. First-in-human phase I clinical trial of a recombinant vesicular stomatitis virus (rVSV)-based preventive HIV-1 vaccine. Retrovirology. 2012;9(suppl 2):P134. [Google Scholar]
- 28.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)70152-0. published online April 21. [DOI] [PubMed] [Google Scholar]
- 29.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez O, Johnson JC, Honko A, et al. Ebola virus exploits a monocyte differentiation program to promote its entry. J Virol. 2013;87:3801–14. doi: 10.1128/JVI.02695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weibel RE, Stokes J, Jr, Buynak EB, et al. Influence of age on clinical response to HPV-77 duck rubella vaccine. JAMA. 1972;222:805–07. [PubMed] [Google Scholar]
- 32.Swartz TA, Klingberg W, Goldwasser RA, et al. Clinical manifestations, according to age among females given HPV-77 duck rubella vaccine. Am J Epidemiol. 1971;94:246–51. doi: 10.1093/oxfordjournals.aje.a121318. [DOI] [PubMed] [Google Scholar]
- 33.Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet. 2011;378:198. doi: 10.1016/S0140-6736(11)60554-0. [DOI] [PubMed] [Google Scholar]
- 34.Santonja C, Nieto-Gonzalez G, Santos-Briz A, et al. Immunohistochemical detection of parvovirus B19 in “gloves and socks” papular purpuric syndrome: direct evidence for viral endothelial involvement. Report of three cases and review of the literature. Am J Dermatopathol. 2011;33:790–95. doi: 10.1097/DAD.0b013e318221bc41. [DOI] [PubMed] [Google Scholar]
- 35.Wahl-Jensen VM, Afanasieva TA, Seebach J, et al. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J Virol. 2005;79:10442–50. doi: 10.1128/JVI.79.16.10442-10450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.